Abstract
Background
Glucocorticoids used to treat childhood leukemia and lymphoma can result in osteonecrosis, leading to physical dysfunction and pain. Improving survival rates warrants research into long-term outcomes among this population.
Objective
The objective of this study was to compare the physical function and quality of life (QOL) of survivors of childhood cancer who had an osteonecrosis history with that of survivors who had no osteonecrosis history and with that of people who were healthy (controls).
Design
This was a cross-sectional study.
Methods
This study included St Jude Lifetime Cohort Study participants who were ≥ 10 years from the diagnosis of childhood leukemia or lymphoma and ≥ 18 years old; 135 had osteonecrosis (52.5% men; mean age = 27.7 [SD = 6.08] years) and 1560 had no osteonecrosis history (52.4% men; mean age = 33.3 [SD = 8.54] years). This study also included 272 people who were from the community and who were healthy (community controls) (47.7% men; mean age = 35.1 [SD = 10.46] years). The participants completed functional assessments and questionnaires about QOL.
Results
Survivors with osteonecrosis scored lower than other survivors and controls for dorsiflexion strength (mean score = 16.50 [SD = 7.91] vs 24.17 [SD = 8.61] N·m/kg) and scored lower than controls for flexibility with the sit-and-reach test (20.61 [SD = 9.70] vs 23.96 [SD = 10.73] cm), function on the Physical Performance Test (mean score = 22.73 [SD = 2.05] vs 23.58 [SD = 0.88]), and mobility on the Timed “Up & Go” Test (5.66 [SD = 2.25] vs 5.12 [SD = 1.28] seconds). Survivors with hip osteonecrosis requiring surgery scored lower than survivors without osteonecrosis for dorsiflexion strength (13.75 [SD = 8.82] vs 18.48 [SD = 9.04] N·m/kg), flexibility (15.79 [SD = 8.93] vs 20.37 [SD = 10.14] cm), and endurance on the 6-minute walk test (523.50 [SD = 103.00] vs 572.10 [SD = 102.40] m).
Limitations
Because some eligible survivors declined to participate, possible selection bias was a limitation of this study.
Conclusions
Survivors of childhood leukemia and lymphoma with and without osteonecrosis demonstrated impaired physical performance and reported reduced QOL compared with controls, with those requiring surgery for osteonecrosis most at risk for impairments. It may be beneficial to provide strengthening, flexibility, and endurance interventions for patients who have pediatric cancer and osteonecrosis for long-term function.
Survival rates continue to improve among children treated for leukemia and lymphoma such that the vast majority of these children will survive their disease.1 Five-year relative survival rates for childhood-onset acute lymphoid leukemia, Hodgkin lymphoma, and non-Hodgkin lymphoma are 91%, 98%, and 93%, respectively.2 Unfortunately, survivors are at risk for acute and long-term treatment-related toxicities,3,4 which may result in persistent impairment of body structure and function. Osteonecrosis is one of these toxicities,5–8 affecting up to one-third of children with acute lymphoid leukemia9 and up to 44% of children treated with hematopoietic stem cell transplantation.10
Glucocorticoids are an essential part of treatment for leukemia and lymphoma and are used to manage graft-vs-host disease in children treated with hematopoietic stem cell transplantation.11,12 It is well documented that glucocorticoid treatment places cancer patients at an increased risk for developing osteonecrosis, with numerous studies describing the effect of steroid dosage and type, among other risk factors.7,11–19 Glucocorticoids are toxic to osteocytes and promote lipid infiltration into bone marrow, which results in increased intramedullary pressure and decreased blood flow to bone.13 Concomitant damage to vascular epithelial and smooth muscle cells furthers stasis and ischemia.14,15 One study describing steroid-induced osteonecrosis in mice indicated that arteriopathy is likely the initial event.16 Damage may be transient; many children with radiographic evidence of early osteonecrosis are asymptomatic. However, continued use of glucocorticoids, particularly when combined with asparaginase,16–18 perpetuates damage, eventually resulting in decreased range of motion, increased pain with weight bearing, and gait abnormalities.9,13,14,20
During cancer therapy, in efforts to improve function and decrease pain, conservative treatment options, including discontinuation of glucocorticoid therapy, the use of analgesics, limitation of weight bearing, physical therapy, and rarely the use of hyperbaric oxygen therapy, prostacyclin, and bisphosphonates, are implemented on a case by case basis.13,21 If conservative treatment fails, surgical intervention may be necessary. Core decompression is used in an attempt to preserve the joint for patients at risk for collapse,22–24 whereas bone grafting, resurfacing, or total joint arthroplasty are required for those with joint collapse.8,21 Although interventions may offer acute relief, persistent osteonecrosis-associated pain or the effects of needed surgeries may interfere with participation in life roles and perceived quality of life (QOL).5,6
Unfortunately, the available literature describing long-term functional outcomes, risk factors for long-term functional loss, and the impact of functional loss on participation and QOL among survivors of childhood leukemia and lymphoma with a history of osteonecrosis is very limited.23,25–29 Because these data are necessary to document the scope of the problem, understand the potential rehabilitation needs of this vulnerable population, and identify those at greatest risk for suboptimal function, we aimed to describe long-term functional outcomes among childhood leukemia and lymphoma survivors with a history of symptomatic osteonecrosis compared with survivors without a history of osteonecrosis and to people who were from the community and who were healthy (community controls). Symptomatic osteonecrosis was defined as a patient requiring a pain-related orthopedic clinical consult. In addition to our primary aim of describing long-term functional outcomes among survivors with osteonecrosis, we also characterize survivors with osteonecrosis at greatest risk for functional loss and report associations between functional loss, participation in social roles, and QOL.
Methods
Study Population
Participants were survivors of childhood leukemia and lymphoma (with or without osteonecrosis) and community controls with no cancer or osteonecrosis history. The survivors were members of the St Jude Lifetime Cohort Study (SJLIFE) and completed an on-campus functional assessment as a component of their SJLIFE visits. The details (eligibility, recruitment, and evaluation) of SJLIFE, a study designed to assess long-term late effects of childhood cancer and its treatment, were previously reported.30,31 In brief, SJLIFE participants in these analyses were survivors of leukemia or lymphoma who were previously treated at St Jude Children’s Research Hospital, at least 18 years of age, and at least 10 years after diagnosis. The comparison group (community controls) consisted of adult friends of SJLIFE members as well as parents and relatives of current pediatric patients frequency matched by age, sex, and race.
To study the relationship between the survivor groups, we additionally analyzed the osteonecrosis group by joint affected, intervention type, and involvement percentage. Among the group of 135 survivors with osteonecrosis, 110 (81%) had osteonecrosis confirmed by diagnostic imaging; the majority (n = 105; 78%) had osteonecrosis involving the hips, knees, or both, consistent with previous reports.5,32 Those without imaging were clinically diagnosed at time of active treatment by an experienced orthopedic oncology surgeon whose examination yielded positive findings of osteonecrosis symptoms and glucocorticoid history. Positive findings include hip pain with weight bearing and/or physical activity; pain at end ranges of passive hip motion, particularly internal rotation; and recent exposure to corticosteroids as part of a cancer treatment protocol. The group of 1560 survivors without osteonecrosis had no positive diagnostic imaging for osteonecrosis and no history of osteonecrosis symptoms requiring orthopedic consultation.
Outcomes
The primary outcomes for these analyses were physical function, social attainment, and QOL. Physical function measures included range of motion, flexibility, muscular strength, physical performance, mobility, and endurance.
Range of Motion and Flexibility
Active and passive ankle plantar flexion and dorsiflexion range of motion were measured using a goniometer while the participant was sitting with the knee flexed to 90 degrees and the ankle unsupported. Range-of-motion data were collected from 2 trials, unless there was a score differential of > 5 degrees; in the latter case, a third trial was performed, and the 2 closest scores were recorded. The fulcrum of the goniometer was placed just distal to the lateral malleolus, the stationary arm parallel to the fibula, and moving arm parallel to fifth metatarsal.33,34 Low back and hamstring flexibility was measured using a Flex-Tester sit and reach box (Novel Products Inc, Rockton, IL, USA). With patient sitting upright with lower extremities extended (long-sitting position) with the plantar surfaces of the feet flat on the box and the knees in full extension, participants reached forward with both hands as far as possible. The better of 2 trials (centimeters) was used for analysis.35
Muscular Strength
Lower extremity strength was measured using an isokinetic dynamometer (Biodex System IV; Biodex, Shirley, NY, USA). While seated with the spine and thigh completely supported, participants were instructed to provide maximum effort against the dynamometer. Knee extension strength was measured bilaterally as peak torque (N·m/kg) during 5 repetitions at 60 degrees, 10 repetitions at 180 degrees, and 15 repetitions at 300 degrees per second. Ankle dorsiflexion and plantar flexion strength were measured as peak torque during 5 repetitions at 60 and 90 degrees per second.36 Isometric handgrip strength was measured using a Baseline handheld dynamometer (Fabrication Enterprises Inc, White Plains, NY, USA) in the sitting position while maintaining 0 to 10 degrees of shoulder flexion, 90 degrees of elbow flexion, and neutral supination. Of the data recorded from 2 trials for each hand, the maximum value (kilograms) was analyzed.37
Physical Performance
The 7-item version of the Physical Performance Test (PPT) was used to evaluate abilities to perform daily tasks. Items included writing a sentence, simulated eating, lifting a book and placing it on shelf, donning and doffing a jacket, picking up a coin from the floor, turning 360 degrees, and walking approximately 15 m (50 ft). Items were observed and/or timed and scored on a scale from 0 to 4; 28 points were possible, with higher scores indicating better performance.38,39
Mobility
General mobility was measured using the Timed “Up & Go” Test.40,41 Participants were instructed to rise from a standard chair, walk 3 m, turn, walk back to chair, and resume sitting as quickly as possible. The time (seconds) taken to complete the second trial was used for analysis.
Endurance
The 6-minute walk test (6MWT) was used to characterize endurance. Participants were instructed to walk as quickly as possible along a corridor for 6 minutes. Stopping and starting were allowed and use of an assistive device permitted if necessary. Encouragement was given according to the American Thoracic Society Guidelines; heart rate and rate of perceived exertion using the Borg Scale of Perceived Exertion were recorded at baseline, every 2 minutes, and after 2 minutes of recovery. Distance covered in meters over the 6 minutes was used for analysis.42
Social Attainment
Participation in social roles was captured via questionnaire and characterized for educational attainment, employment, marital status, ability to live independently, and annual household income. Variables were dichotomized for analysis and compared as follows: high school graduate or less versus some college or higher; employed, caring for home/family, or student versus unemployed, retired, unable to work, or looking for work; married or living as married versus unmarried; ability to live independently (living with spouse, roommate, or living alone) versus inability to live independently (living with parent, sibling, or other relative); and an annual household income of < $40,000 (just below the lower limit of middle income, which is defined by the Pew Research Center as 67–200% of the median income in the United States)43 versus $40,000 or higher.
Quality of Life
Self-reported QOL was measured by having participants complete the Medical Outcomes Study 36-Item Health Survey Questionnaire (SF-36).44 This instrument includes physical and mental component summary scales and 8 subscales: physical function, role physical, role emotional, vitality, mental health, social function, bodily pain, and general health. For these analyses, a cutoff score of ≤ 40 was used to identify impaired QOL.
Data Analysis
Descriptive statistics, including means and SDs, medians and ranges, and frequencies and percentages, were used to characterize the study population and controls. Paired t tests or Wilcoxon rank sum tests were used to compare function between survivors and community controls, social outcomes, and QOL outcomes. Among the survivors, the association between SF-36 items and functional outcomes was analyzed by a paired t test or Wilcoxon rank sum test. Generalized linear models were used to analyze the associations between the SF-36 physical component summary scale scores with the PPT score adjusted for survivor status, age, and sex. All analyses were performed in SAS version 9.3 (SAS Institute Inc, Cary, NC, USA).
Role of the Funding Source
This study received funding support from the National Cancer Institute (U01 CA195547) (to M.M. Hudson and L.L. Robison), a Cancer Center Support (CORE) grant (P30 CA21765), and support from the American Lebanese-Syrian Associated Charities (ALSAC). This support was provided for the SJLIFE Cohort Study, from which data were collected to perform this study. Funding sources helped provide support for data collection and multidisciplinary collaboration for this study’s design.
Results
Characteristics of Study Participants
Among the 2726 survivors of leukemia and lymphoma who were potentially eligible, 107 were not yet recruited, 227 were interested but had not made a campus visit by the time of analysis, 240 declined participation, 88 were lost to follow-up, 193 were passive nonparticipants, and 137 elected to complete a survey (Fig. 1). Of the 1734 eligible consenting survivors who completed a campus visit, 39 were excluded because they did not complete a functional assessment within 60 days of the visit. The resulting 1695 eligible participants completed a functional assessment between 2007 and 2015, and the results were divided between 2 groups: 135 survivors with a history of osteonecrosis (110 with osteonecrosis confirmed by diagnostic imaging and 25 with symptomatic osteonecrosis diagnosed by clinical examination) and 1560 with no history of osteonecrosis. Additionally, 272 participants with no history of cancer, frequency matched by age, sex, and race, were included as a noncancer comparison group (controls). The characteristics of the study population are shown in Table 1.
Figure 1.
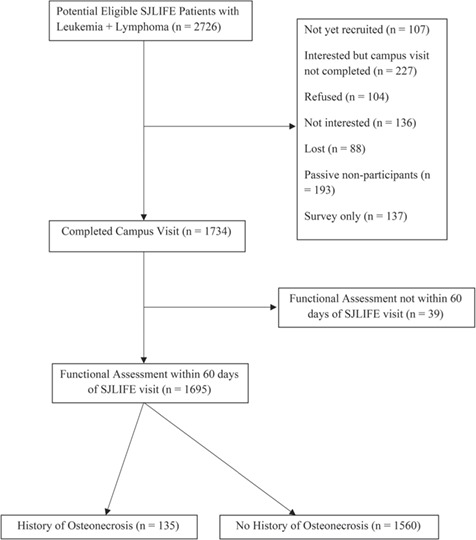
CONSORT diagram.
Table 1.
Characteristics of Adult Survivors of Childhood Cancer With or Without Symptomatic Osteonecrosis and People Who Were Healthy (Controls)a
| Characteristic |
Survivors With
Osteonecrosis (n = 135) |
Survivors Without
Osteonecrosis (n = 1560) |
Controls (n = 272) |
|---|---|---|---|
| Demographic | |||
| Age group, y | |||
| <35 | 120 (88.8) | 931 (59.6) | 141 (51.8) |
| 35–54 | 15 (11.1) | 610 (39.1) | 117 (43.0) |
| ≥55 | 19 (1.2) | 14 (5.1) | |
| Sex | |||
| Women | 64 (47.4) | 742 (47.5) | 142 (52.2) |
| Men | 71 (52.5) | 818 (52.4) | 130 (47.7) |
| Race/ethnicity | |||
| Non-Hispanic white | 107 (79.2) | 1342 (86.0) | 230 (84.5) |
| Non-Hispanic black | 19 (14.0) | 158 (10.1) | 21 (7.7) |
| Hispanic | 4 (2.9) | 42 (2.6) | 12 (4.4) |
| Other | 5 (3.7) | 18 (1.1) | 9 (3.3) |
| Clinical | |||
| Cancer diagnosis | |||
| Leukemia | 110 (81.4) | 1008 (64.6) | |
| Lymphoma | 25 (18.5) | 552 (35.3) | |
| Age at cancer diagnosis, y | |||
| 0–4 | 13 (9.6) | 545 (34.9) | |
| 5–9 | 36 (26.6) | 397 (25.4) | |
| 10–14 | 52 (38.5) | 347 (22.2) | |
| 15+ | 34 (25.1) | 271 (17.3) | |
| Steroid treatment | |||
| Dexamethasone | |||
| No | 40 (29.6) | 1385 (88.7) | |
| Yes | 95 (70.3) | 175 (11.2) | |
| Cumulative dose, mg/m2, mean (range) | 551.76 (177.78–1085.71) | 556.63 (1.69–3400.0) | |
| Prednisone | |||
| No | 9 (6.6) | 308 (19.7) | |
| Yes | 126 (93.3) | 1252 (80.2) | |
| Cumulative dose, mg/m2, mean (range) | 5163.03 (82.19–10,243.86) | 4072.49 (148.15–25,414.40) | |
| Bone marrow transplant | |||
| No | 123 (91.1) | 1477 (94.6) | |
| Yes | 12 (8.8) | 83 (5.3) | |
| Autologous | 2 (16.6) | 33 (39.7) | |
| Allogeneic | 10 (83.3) | 49 (59.0) | |
| Unknown | 1 (1.2) | ||
| Age at osteonecrosis diagnosis, y | |||
| 0–6 | 2 (1.4) | ||
| 7–16 | 83 (61.4) | ||
| ≥17 | 50 (37.0) | ||
| Osteonecrosis location | |||
| Unifocal | 27 (20.0) | ||
| Bilateral involvement | 57 (42.2) | ||
| Multifocal | 51 (37.7) | ||
| Joint affected | |||
| Hip only | 19 (14.0) | ||
| Knee only | 28 (20.7) | ||
| Ankle only | 3 (2.2) | ||
| Hip and knee | 28 (20.7) | ||
| Hip, knee, or both and ankle | 23 (17.0) | ||
| Hip, knee, or both and shoulder | 7 (5.1) | ||
| Shoulder only | 2 (1.4) | ||
| No imaging | 25 (18.5) | ||
| Severity of hip involvement | |||
| <30% of femoral head | 16 (38.0) | ||
| ≥30% of femoral head | 26 (61.9) | ||
| Severity of knee involvement | |||
| <30% of articular surface | 35 (77.7) | ||
| ≥30% of articular surface | 10 (22.2) | ||
| Osteonecrosis treatment (nonsurgical) | |||
| Nonsteroidal antiinflammatory drug | |||
| No | 94 (69.6) | ||
| Yes | 41 (30.3) | ||
| Opioid | |||
| No | 88 (65.1) | ||
| Yes | 47 (34.8) | ||
| Steroid injection | |||
| No | 128 (94.8) | ||
| Yes | 7 (5.1) | ||
| Physical therapy | |||
| No | 101 (74.8) | ||
| Yes | 34 (25.1) | ||
| Surgical intervention | |||
| Core decompression/scope/graft | |||
| Hip | |||
| No | 122 (90.3) | ||
| Yes | 13 (9.6) | ||
| Knee | |||
| No | 131 (97.0) | ||
| Yes | 4 (2.9) | ||
| Shoulder | |||
| No | 133 (98.5) | ||
| Yes | 2 (1.4) | ||
| Ankle | |||
| No | 133 (98.5) | ||
| Yes | 2 (1.4) | ||
| Resurfacing | |||
| Hip | |||
| No | 127 (94.0) | ||
| Yes | 8 (5.9) | ||
| Shoulder | |||
| No | 131 (97.0) | ||
| Yes | 4 (2.9) | ||
| Total joint arthroplasty | |||
| Hip | |||
| No | 124 (91.8) | ||
| Yes | 11 (8.1) | ||
| Knee | |||
| No | 134 (99.2) | ||
| Yes | 1 (0.7) | ||
| Total joint arthroplasty revision | |||
| Hip | |||
| No | 132 (97.7) | ||
| Yes | 3 (2.2) | ||
| Hip fusion | |||
| No | 134 (99.2) | ||
| Yes | 1 (0.7) | ||
| Behavioral | |||
| Smoking status | |||
| Current | 27 (20.0) | 362 (23.2) | 54 (19.8) |
| Prior | 12 (8.8) | 192 (12.3) | 44 (16.1) |
| Never | 95 (70.3) | 980 (62.8) | 167 (61.3) |
| Unknown | 1 (0.7) | 26 (1.6) | 7 (2.5) |
| Body mass index | |||
| Underweight (<18.5) | 3 (2.2) | 43 (2.7) | 8 (2.9) |
| Normal weight (18.5–24.9) | 48 (35.5) | 468 (30.0) | 89 (32.7) |
| Overweight (25–29.9) | 44 (32.5) | 445 (28.5) | 72 (26.4) |
| Obese | |||
| I (30.0–34.9) | 20 (14.8) | 325 (20.8) | 46 (16.9) |
| II (35–39.9) | 13 (9.6) | 139 (8.9) | 31 (11.3) |
| III (40+) | 7 (5.1) | 139 (8.9) | 26 (9.5) |
| Unknown | 1 (0.1) | ||
| Occupation activity level | |||
| Active | 108 (80.0) | 1171 (75.0) | 212 (77.9) |
| Sedentary | 22 (16.2) | 340 (21.7) | 50 (18.3) |
| Missing | 5 (3.7) | 49 (3.1) | 10 (3.6) |
| Low physical activity | |||
| No | 81 (60.0) | 909 (58.2) | 176 (64.7) |
| Yes | 52 (38.5) | 637 (40.8) | 81 (29.7) |
| Unknown | 2 (1.4) | 14 (0.8) | 15 (5.5) |
| Current use of assistive device | |||
| None | 115 (85.1) | 1397 (89.5) | 259 (95.2) |
| Cane or single crutch | 7 (5.1) | 60 (3.8) | 4 (1.4) |
| Walker | 7 (5.1) | 58 (3.7) | 2 (0.7) |
| Crutches | 1 (0.7) | 12 (0.7) | |
| Wheelchair | 3 (0.1) | ||
| Missing | 5 (3.7) | 30 (1.9) | 7 (2.5) |
aData are reported as numbers (percentages) of participants unless otherwise indicated.
At assessment, the median age of survivors with an osteonecrosis history was 27 (range = 19–50) years, that of survivors with no osteonecrosis history was 32 (range = 18–64) years, and that of controls was 34 (range = 18–70) years. Among the 135 survivors with osteonecrosis, 28 required surgical intervention (49 separate surgeries); 22 (78.6%) of these required hip surgery (36 surgeries). Thirteen survivors had hip core decompressions (19 hips), with 8 survivors (61.5%) requiring a later, more invasive surgery (total hip arthroplasty [THA] or hip resurfacing). Two patients with bilateral hip resurfacing required conversion to bilateral THA (4 hips), and 2 patients with unilateral THA required a later THA revision (2 hips).
Functional Outcomes
As shown in Table 2, the survivor groups, with and without osteonecrosis, scored similarly in all functional outcomes except for dorsiflexion strength; however, survivors with osteonecrosis had significantly lower scores than controls for dorsiflexion strength, flexibility (sit-and-reach test), mobility (Timed “Up & Go” Test), and overall function (PPT). When comparing subgroups, we found that survivors who required surgery to treat hip osteonecrosis (median time since surgery: 10 years [range = 1–20 years]) were most vulnerable and demonstrated significantly lower scores for dorsiflexion strength, flexibility (sit-and-reach test), and endurance (6MWT) than adult survivors with no history of osteonecrosis (Tab. 3). The surgery group also scored lower than the noncancer comparison group for those same outcomes as well as for functional ability on the PPT and mobility on the Timed “Up & Go” Test. Additionally, we found that survivors requiring hip surgery for osteonecrosis had lower scores for flexibility (sit-and-reach test) and endurance (6MWT) than survivors who had osteonecrosis but no history of hip surgery (Tab. 4).
Table 2.
Comparison of Functional Outcomes Among Adult Survivors of Childhood Cancer With Osteonecrosis, Survivors Without Osteonecrosis, and People Who Were Healthy (Controls)a
| Outcome |
Survivors
With Osteonecrosis, Mean (SD) |
Survivors
Without Osteonecrosis, Mean (SD) |
Survivors With Osteonecrosis vs Survivors Without Osteonecrosis,
P |
Controls,
Mean (SD) |
Survivors With Osteonecrosis vs Controls,
P |
Survivors Without Osteonecrosis vs Controls,
P |
|---|---|---|---|---|---|---|
| Grip strength, kg | 39.04 (12.62) | 39.50 (13.63) | .71 | 39.75 (13.30) | .60 | .77 |
| Isokinetic strength | ||||||
| Knee extension | ||||||
| Peak torque at 60°/s | 143.52 (60.63) | 143.90 (56.33) | .94 | 151.08 (55.71) | .24 | .06 |
| Peak torque at 180°/s | 90.55 (39.69) | 90.49 (36.90) | .99 | 97.56 (38.05) | .10 | .01 |
| Peak torque at 300°/s | 65.44 (28.64) | 64.62 (26.82) | .75 | 67.54 (27.66) | .50 | .11 |
| Dorsiflexion | ||||||
| Peak torque at 60°/s | 16.50 (7.91) | 18.48 (9.04) | .02 | 24.17 (8.61) | <.0001 | <.0001 |
| Peak torque at 90°/s | 14.99 (6.33) | 16.19 (7.32) | .05 | 21.58 (7.50) | <.0001 | <.0001 |
| Plantar flexion | ||||||
| Peak torque at 60°/s | 53.82 (29.02) | 57.42 (29.49) | .20 | 52.39 (23.93) | .64 | .003 |
| Peak torque at 90°/s | 44.51 (24.82) | 46.37 (23.98) | .42 | 46.81 (22.39) | .37 | .78 |
| Functional ability: PPT score | 22.73 (2.05) | 22.77 (1.85) | .82 | 23.58 (0.88) | <.0001 | <.0001 |
| Flexibility | ||||||
| Sit-and-reach test score, cm | 20.61 (9.70) | 20.37 (10.14) | .79 | 23.96 (10.73) | .003 | <.0001 |
| Range of motion, degrees | ||||||
| Dorsiflexion | 7.94 (7.26) | 7.12 (8.08) | .26 | 8.60 (6.78) | .37 | .001 |
| Plantar flexion | 54.29 (7.88) | 54.21 (8.13) | .92 | 55.58 (6.58) | .10 | .003 |
| Mobility on Timed “Up & Go” Test, s | 5.66 (2.25) | 5.64 (1.67) | .95 | 5.12 (1.28) | .01 | <.0001 |
| Endurance on 6MWT, m | 577.78 (107.47) | 572.14 (102.37) | .55 | 589.15 (91.97) | .30 | .01 |
a6MWT = 6-minute walk test; PPT = Physical Performance Test.
Table 3.
Comparison of Functional Outcomes Among Survivors With a History of Hip Osteonecrosis Requiring Surgery, Survivors Without Osteonecrosis, and People Who Were Healthy (Controls)a
| Outcome |
Survivors
With Osteonecrosis and Hip Surgery, Mean (SD) |
Survivors
Without Osteonecrosis, Mean (SD) |
Survivors With Osteonecrosis and Hip Surgery vs Survivors Without Osteonecrosis,
P |
Controls,
Mean (SD) |
Survivors With Osteonecrosis and Hip Surgery vs Controls,
P |
|---|---|---|---|---|---|
| Grip strength, kg | 39.57 (12.87) | 39.50 (13.63) | .98 | 39.75 (13.30) | .95 |
| Isokinetic strength | |||||
| Knee extension | |||||
| Peak torque at 60°/s | 131.30 (54.62) | 143.90 (56.33) | .33 | 151.10 (55.71) | .14 |
| Peak torque at 180°/s | 79.98 (32.59) | 90.49 (36.91) | .22 | 97.56 (38.05) | .05 |
| Peak torque at 300°/s | 61.19 (23.57) | 64.62 (26.82) | .58 | 67.54 (27.66) | .33 |
| Dorsiflexion | |||||
| Peak torque at 60°/s | 13.75 (8.82) | 18.48 (9.04) | .02 | 24.17 (8.61) | <.0001 |
| Peak torque at 90°/s | 13.26 (8.32) | 16.19 (7.32) | .08 | 21.58 (7.50) | <.0001 |
| Plantar flexion | |||||
| Peak torque at 60°/s | 50.71 (27.01) | 57.42 (29.49) | .32 | 52.39 (23.93) | .77 |
| Peak torque at 90°/s | 39.45 (19.31) | 46.37 (23.98) | .21 | 46.81 (22.39) | .16 |
| Functional ability: PPT score | 22.38 (2.29) | 22.77 (1.85) | .34 | 23.58 (0.88) | .03 |
| Flexibility | |||||
| Sit-and-reach test score, cm | 15.79 (8.93) | 20.37 (10.14) | .04 | 23.96 (10.73) | .0008 |
| Range of motion, degrees | |||||
| Dorsiflexion | 6.57 (8.60) | 7.12 (8.08) | .76 | 8.60 (6.78) | .20 |
| Plantar flexion | 52.76 (9.28) | 54.21 (8.13) | .42 | 55.58 (6.58) | .19 |
| Mobility on the Timed “Up & Go” Test, s | 6.28 (1.82) | 5.64 (1.67) | .08 | 5.12 (1.28) | .01 |
| Endurance on the 6MWT, m | 523.50 (103.00) | 572.10 (102.40) | .03 | 589.20 (91.97) | .01 |
a6MWT = 6-minute walk test; PPT = Physical Performance Test.
Table 4.
Comparison of Functional Outcomes Among Survivors With a History of Osteonecrosis Requiring Hip Surgery and Survivors With a History of Osteonecrosis Not Requiring Hip Surgerya
| Outcome |
Survivors
With Osteonecrosis and Hip Surgery, Mean (SD) |
Survivors
With Osteonecrosis and No Hip Surgery, Mean (SD) |
P |
|---|---|---|---|
| Grip strength, kg | 39.57 (12.87) | 38.94 (12.62) | .83 |
| Isokinetic strength | |||
| Knee extension | |||
| Peak torque at 60°/s | 131.30 (54.62) | 145.80 (61.66) | .34 |
| Peak torque at 180°/s | 79.98 (32.59) | 92.52 (40.71) | .21 |
| Peak torque at 300°/s | 61.19 (23.57) | 66.23 (29.52) | .48 |
| Dorsiflexion | |||
| Peak torque at 60°/s | 13.75 (8.82) | 17.02 (7.66) | .10 |
| Peak torque at 90°/s | 13.26 (8.32) | 15.33 (5.87) | .31 |
| Plantar flexion | |||
| Peak torque at 60°/s | 50.71 (27.01) | 54.41 (29.48) | .61 |
| Peak torque at 90°/s | 39.45 (19.31) | 45.47 (25.71) | .33 |
| Functional ability: PPT score | 22.38 (2.29) | 22.79 (2.00) | .40 |
| Flexibility | |||
| Sit-and-reach test score, cm | 15.79 (8.93) | 21.53 (9.60) | .01 |
| Range of motion, ° | |||
| Dorsiflexion | 6.57 (8.60) | 8.19 (7.00) | .35 |
| Plantar flexion | 52.76 (9.28) | 54.58 (7.60) | .33 |
| Mobility on the Timed “Up & Go” Test, s | 6.28 (1.82) | 5.54 (2.31) | .17 |
| Endurance on the 6MWT, m | 523.50 (103.00) | 587.80 (105.70) | .01 |
a6MWT = 6-minute walk test; PPT = Physical Performance Test.
Social Outcomes and QOL
Both survivors with and without a history of osteonecrosis reported lower annual household incomes and were less likely to live independently compared with controls. Survivors without osteonecrosis and those with osteonecrosis who did not require surgical intervention had similar QOL outcomes on the SF-36 but scored lower than controls (Fig. 2) on the physical function, role physical, bodily pain, and physical component summary scales of the SF-36. Survivors who required surgery to treat hip osteonecrosis had the lowest QOL scores for those 4 scales of the SF-36.
Figure 2.
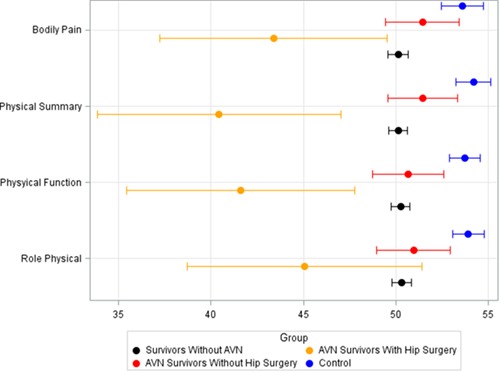
Comparison of QOL (Medical Outcomes Study 36-Item Health Survey Questionnaire) scores among adult survivors of childhood cancer, including subgroups with osteonecrosis, and people who were healthy (controls).
Association Between Function and Social Attainment
The results of multivariable models analyzing associations between function and social attainment among survivors with and without a history of symptomatic osteonecrosis are shown in Supplementary Table 1 (available at https://academic.oup.com/ptj). Among survivors with and without osteonecrosis, those with impaired physical performance and endurance (lower PPT score and shorter 6MWT distances) were less likely to have completed a level of education beyond high school and were more likely to report current unemployment than those who were not impaired. Additionally, those with shorter 6MWT distances were less likely to live independently.
Association Between QOL and Function
Supplementary Table 2 (available at https://academic.oup.com/ptj) shows the associations between function measures and QOL among survivors with symptomatic osteonecrosis and survivors without a history of osteonecrosis. For both survivor groups, impaired physical performance, mobility, and endurance were associated with scores of < 40 on the SF-36 physical function, role physical, bodily pain, and general health subscales.
Discussion
To the best of our knowledge, this is the first study to report the functional outcomes of pediatric cancer survivors with a history of osteonecrosis and to compare their outcomes with those of other survivors with similar diagnoses and treatment, and with a group of individuals without a history of cancer. Survivors overall had suboptimal function, social role attainment, and self-reported QOL compared with controls. However, survivors did not differ from each other on these outcomes based on their history of osteonecrosis but rather based on their need for surgical intervention to treat symptomatic osteonecrosis of the hip. Although previous studies have reported that osteonecrosis of the hip results in worse functional outcomes, higher rate of joint collapse, and a greater frequency of surgical intervention,5,9 this study clarifies that functional loss is greatest among those with hip osteonecrosis severe enough to require surgical intervention. Because survivors requiring surgery for hip osteonecrosis are at long-term risk, they should receive close monitoring for functional loss and be provided with appropriate rehabilitation to address impairments.
The loss of distal ankle strength, impaired low back and hamstring flexibility, and poor general physical performance that we identified in survivors of leukemia and lymphoma is likely multifactorial. Treatment-related risk factors include vincristine-related sensory and motor neuropathy36 as well as intrathecal methotrexate and cranial radiation exposures .3 In addition, loss of function, combined with sedentary days and altered nutrition during treatment, may initiate a cycle of inactivity among survivors, making it difficult for them to participate with their peers in activities at school or in sport.45 Inactivity over the life cycle is associated with reduced physical function46 and, importantly for these survivors whose lifetime risk for chronic disease is increased because of their previous treatment exposures,47,48 with health and longevity.49
Previous data indicate that chronic conditions in survivors of pediatric cancer are associated with suboptimal social role attainment and poor health-related QOL.50 Our data significantly expand this literature by reporting associations between specific performance limitation outcomes and both participation in social roles and health-related QOL among survivors, particularly those with a history of hip osteonecrosis who required surgical management of their lesion. Scores on the PPT and distance walked in 6 minutes were associated with educational attainment, employment, and independent living. This is consistent with a report that analyzed data from the National Health Interview Survey51 that reported that survivors who reported physical performance limitations, compared with those who did not, were less likely to have graduated high school or be employed. Lower scores on the PPT and shorter distances walked in 6 minutes were also associated with suboptimal scores on the physical function, role physical, bodily pain, and general health subscales of the SF-36. These results are similar to a study that reported a significant association between endurance, using the 6MWT, and health-related QOL in community-dwelling older adults where Wanderley et al52 reported a positive association between distance walked in 6 minutes and higher scores on the physical function, role physical, and vitality subscales of the SF-36. These results are interesting considering recent data that indicate childhood cancer survivors are at risk for accelerated aging and frailty.53,54
Perhaps the most novel finding in our study was that outcomes among survivors with osteonecrosis did not differ from those among survivors without osteonecrosis unless surgical intervention was required to treat hip osteonecrosis. This is important because it identifies a group of individuals at risk for adverse outcomes. Children and adolescents requiring surgical intervention for osteonecrosis may require special attention after surgery to identify functional limitations and provide rehabilitative treatment early in recovery. Physical therapy may play a special role in optimizing long-term function for these patients, through early postoperative evaluation, intervention, and periodic follow-up for monitoring.55 Included in a plan of care derived from evaluative findings, therapy programs including interventions targeting the long-term functional limitations identified here may be warranted. Programs should include components that address hamstring flexibility, ankle dorsiflexion strength, and endurance. These may include but are not limited to manual therapy techniques,56,57 strengthening programs,55 and instruction in home exercise programs58–60 specific to these impairments. In efforts to promote physical activity levels following discharge from physical therapy, it would be beneficial for therapists to educate patients on the long-term risks and to provide recommendations for home resources such as gym memberships, fitness programs, and athletic activities that are in line with postoperative precautions.57 Because these data indicate an association between impaired flexibility, strength, and endurance and overall physical performance and health-related QOL, periodic long-term follow-up evaluations may be beneficial to ensure that these patients are not experiencing these impairments.
The findings of these analyses should be considered in the context of some study limitations. Because not all eligible survivors agreed to participate, selection bias is a possibility. We do not know how outcomes of nonparticipants may have affected the results of this study. Other limitations include potential for error in interpretation of subjective data and the small sample of survivors with osteonecrosis relative to those without osteonecrosis. Social outcomes among survivors such as income at time of evaluation are difficult to interpret because it may have nothing to do with osteonecrosis and can change over time; thus, these outcomes should be considered within the limitations of cross-sectional analysis. Also, poor outcomes among the group that required hip surgery are likely not attributable to the surgery alone but also to disease severity that was extensive enough to require surgery. Additionally, our sample of 10-year survivors may not have had access to recent advancements in modern surgical techniques.61,62 Children treated more recently may have more favorable outcomes than the survivors we evaluated. This highlights a need for longitudinal follow-up of survivors treated on more recent protocols to determine if advances in surgical techniques have improved long-term functional outcomes.
Conclusion
This study found that adult survivors of childhood leukemia and lymphoma were at greater risk for long-term physical limitations than adults with no cancer history. Survivors with osteonecrosis did not differ greatly from survivors without a history of osteonecrosis except when surgery was required as treatment for hip osteonecrosis. This group was at greatest risk for deficits in strength, flexibility, and endurance compared with other survivors and adults who were otherwise healthy. These late effects were associated with poorer QOL outcomes, including the physical function, role physical, bodily pain, and general health subscales. Pediatric cancer patients currently being treated for osteonecrosis may require special attention to prevent long-term limitations into survivorship. It may be beneficial to implement physical therapy programs that include stretching, strengthening, and endurance training for all patients with symptomatic hip osteonecrosis. Based on our findings, future physical therapy programs for patients with osteonecrosis after hip surgery should include flexibility and endurance interventions in addition to traditional strength and gait protocols.
Supplementary Material
Author Contributions and Acknowledgments
Concept/idea/research design: B.M. DeFeo, S.C. Kaste, T.M. Brinkman, M.D. Neel, D.K. Srivastava, M.M. Hudson, L.L. Robison, K.K. Ness
Writing: B.M. DeFeo, S.C. Kaste, T.M. Brinkman, D.K. Srivastava,M.M. Hudson, S.E. Karol, K.K. Ness
Data collection: B.M. DeFeo, S.C. Kaste, M.M. Hudson, L.L. Robison, K.K. Ness
Data analysis: B.M. DeFeo, S.C. Kaste, Z. Li, D.K. Srivastava, M.M. Hudson, S.E. Karol, K.K. Ness
Project management: B.M. DeFeo, M.M. Hudson, K.K. Ness
Fund procurement: M.M. Hudson, L.L. Robison, K.K. Ness
Providing participants: M.D. Neel, M.M. Hudson, L.L. Robison, K.K. Ness
Providing facilities/equipment: L.L. Robison, K.K. Ness
Consultation (including review of manuscript before submitting): B.M. DeFeo, S.C. Kaste, M.D. Neel, S.E. Karol
Ethics Approval
This SJLIFE study was conducted with adherence to ethics guidelines established following full institutional review board evaluation and approval.
Funding
This study received funding support from the National Cancer Institute (U01 CA195547) (to M.M. Hudson and L.L. Robison), a Cancer Center Support (CORE) grant (P30 CA21765), and support from the American Lebanese-Syrian Associated Charities (ALSAC). This support was provided for the SJLIFE Cohort Study, from which data were collected to perform this study.
Disclosures
Dr Ness is a PTJ Editorial Board member. Dr Hudson is an associate editor of the Journal of Clinical Oncology, and member of the Patient Advisory Committee, Oncology Research Information Exchange Network (ORIEN). The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no other potential conflicts of interest.
References
- 1. Jemal A, Ward EM, Johnson CJ, et al. Annual report to the nation on the status of cancer, 1975-2014, featuring survival.J Natl Cancer Inst. 2017;109:djx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Cancer Society Cancer facts & figures 2018. American Cancer Society website. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2018.html. 2018. Accessed November 7, 2019.
- 3. Ness KK, Armenian SH, Kadan-Lottick N, Gurney JG. Adverse effects of treatment in childhood acute lymphoblastic leukemia: general overview and implications for long-term cardiac health. Expert Rev Hematol. 2011;4:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chavhan GB, Babyn PS, Nathan PC, Kaste SC. Imaging of acute and subacute toxicities of cancer therapy in children. Pediatr Radiol. 2016;46:9. [DOI] [PubMed] [Google Scholar]
- 5. Kaste SC, Karimova EJ, Neel MD. Osteonecrosis in children after therapy for malignancy. AJR. 2011;196:1011–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neel MD, Karimova EJ. Osteonecrosis of the femoral head in pediatric cancer patients. Semin Arthroplasty. 2007;18:203–210. [Google Scholar]
- 7. Inaba H, Pui C-H. Glucocorticoid use in acute lymphoblastic leukemia. Lancet Oncol. 2010;11:1096–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mont MA, Jones LC, Hungerford DS. Nontraumatic osteonecrosis of the femoral head: ten years later. J Bone Joint Surg. 2006;88:1117–1132. [DOI] [PubMed] [Google Scholar]
- 9. Karminova EJ, Rai SN, Howard SC, et al. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25:1525–1531. [DOI] [PubMed] [Google Scholar]
- 10. Sharma S, Leung WH, Deqing P, et al. Osteonecrosis in children after allogeneic hematopoietic cell transplantation: study of prevalence, risk factors and longitudinal changes using MR imaging. Bone Marrow Transplant. 2012;47:1067–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baker KS, Ness KK, Weisdorf D, et al. Late effects in survivors of acute leukemia treated with hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Leukemia. 2010;24:2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Majhail NS, Ness KK, Burns LF, et al. Late effects in survivors of Hodgkin and non-Hodgkin lymphoma treated with autologous hematopoietic cell transplantation: a report from the bone marrow transplant survivor study. Biol Blood Marrow Transplant. 2007;13:1153–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winkle ML, Pieters R, Wind E-J, Bessems J, Van den Heuvel-Eibrink MM. Management and treatment of osteonecrosis in children and adolescents with acute lymphoblastic leukemia. Haematologica. 2014;99:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barr RD, Sala A. Osteonecrosis in children and adolescents with cancer. Pediatr Blood Cancer. 2008;50:483–485. [DOI] [PubMed] [Google Scholar]
- 15. Saito S, Ohzono K, Ono K. Early arteriopathy and postulated pathogenesis of osteonecrosis of the femoral head: the intracapital arterioles. Clin Orthop Relat Res. 1992;277:98–110. [PubMed] [Google Scholar]
- 16. Janke LJ, Liu C, Vogel P, et al. Primary epiphyseal arteriopathy in a mouse model of steroid-induced osteonecrosis. Am J Pathol. 2013;183:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu C, Kawedia JD, Cheng C, et al. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26:2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu C, Janke LJ, Kawedia JD, et al. Asparaginase potentiates glucocorticoid-induced osteonecrosis in a mouse model. PLoS One. 2016;11:e0151433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mattano LAJ, Devidas M, Winick N, et al. Effects of dexamethasone (DEX) vs prednisone (PDN) and high-dose methotrexate (HD-MTX) vs Capizzi methotrexate/asparaginase (C-MTX/ASNase) on osteonecrosis (ON) incidence in children and young adults with high risk acute lymphoblastic leukemia (HR ALL): a report from the Children’s Oncology Group (COG) study AALL0232. Blood. 2012; ASH abstract 665. [Google Scholar]
- 20. Mayer SW, Mayer BK, Aldridge JM, Urbaniak JR, Fitch RD, Lark RK. Osteonecrosis of the femoral head in childhood malignancy. J ChildOrthop. 2013;7:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Banjeree S, Issa K, Pivec R, Kapadia BH, Khanuja HS, Mont MA. Osteonecrosis of the hip: treatment options and outcomes. Orthop Clin N Am. 2013;44:463–476. [DOI] [PubMed] [Google Scholar]
- 22. Steinberg ME, Larcom PG, Strafford B, et al. Core decompression with bone grafting for osteonecrosis of the femoral head. Clin Orthop Relat Res. 2001;386:71–78. [DOI] [PubMed] [Google Scholar]
- 23. Keizer SB, Kock NB, Dijkstra PDS, Taminiau AHM, Nelissen RGHH. Treatment of avascular necrosis of the hip by a non-vascularized cortical graft. J Bone Joint Surg. 2006;88B:460–466. [DOI] [PubMed] [Google Scholar]
- 24. Kaushik AP, Das A, Cui Q. Osteonecrosis of the femoral head: an update in year 2012. World J Orthop. 2012;3:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neumayr LD, Aguilar C, Earles AN, et al. Physical therapy alone compared with core decompression and physical therapy for femoral head osteonecrosis in sickle cell disease. J Bone Joint Surg. 2006;88:2573–2582. [DOI] [PubMed] [Google Scholar]
- 26. Mont MA, Marulanda GA, Seyler TM, Plate JF, Delanois RE. Core decompression and nonvascularized bone grafting for the treatment of early stage osteonecrosis of the femoral head. AAOS Instr Course Lect. 2007;56:213–220. [PubMed] [Google Scholar]
- 27. Steinberg ME, Steinberg DR. Classification systems for osteonecrosis: an overview. Orthop Clin N Am. 2004;35:273–283. [DOI] [PubMed] [Google Scholar]
- 28. Lee GC, Steinberg ME. Are we evaluating osteonecrosis adequately? Int Orthop. 2012;36:2433–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marchese VG, Connolly BH, Able C, et al. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescents, and young adults with acute lymphoblastic leukemia. Phys Ther. 2008;88:341–350. [DOI] [PubMed] [Google Scholar]
- 30. Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for classification and severity grading of long-term and late-onset health events among childhood cancer survivors in the St. Jude lifetime cohort. Cancer Epidemiol Biomarkers Prev. 2017;26:666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude lifetime cohort study. Pediatr Blood Cancer. 2011;56:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. te Winkel ML, Pieters R, Hop WC, et al. Prospective study on incidence, risk factors, and long-term outcome of osteonecrosis in pediatric acute lymphoblastic leukemia. J Clin Oncol. 2011;29:4143–4150. [DOI] [PubMed] [Google Scholar]
- 33. Boone DC, Azen SP, Lin CM, Spence C, Baron C, Lee L. Reliability of goniometric measurements. Phys Ther. 1978;58:1355–1390. [DOI] [PubMed] [Google Scholar]
- 34. Norkin CC, White DJ. Measurement of Joint Motion: A Guide to Goniometry. 5th ed. Philadelphia, PA, USA: FA Davis; 2016. [Google Scholar]
- 35. American College of Sports Medicine ACSM’s Guidelines for Exercise Testing and Prescription. Baltimore, MD: Lippincott Williams & Wilkins; 2013. [DOI] [PubMed] [Google Scholar]
- 36. Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226. [DOI] [PubMed] [Google Scholar]
- 38. Reuben DB, Siu AL. An objective measure of physical function of elderly outpatients: the physical performance test. J Am Geriatr Soc. 1990;38:1105–1112. [DOI] [PubMed] [Google Scholar]
- 39. Wilkins CH, Roe CM, Morris JC. A brief clinical tool to assess physical function: the mini-physical performance test. Arch Gerontol Geriatr. 2010;50:96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Isles RC, Choy NL, Steer M, Nitz JC. Normal values of balance tests in women aged 20-80. J Am Geriatr Soc. 2004;52:1367–1372. [DOI] [PubMed] [Google Scholar]
- 41. Davis JC, Bryan S, Best JR, et al. Mobility is a key predictor of changes in wellbeing among older fallers: evidence from the Vancouver falls prevention cohort. Arch Phys Med Rehabil. 2015;96:1634–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. [DOI] [PubMed] [Google Scholar]
- 43. Pew Research Center The lost decade of the middle class: fewer, poorer, gloomier Pew Research Center website. https://www.pewsocialtrends.org/2012/08/22/the-lost-decade-of-the-middle-class/. 2012. Accessed November 7, 2019.
- 44. Reulen RC, Zeegers MP, Jenkinson C, et al. The use of SF-36 questionnaire in adult survivors of childhood cancer: evaluation of data quality, score reliability, and scaling assumptions. Health Qual Life Outcomes. 2006;4:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wogksch MD, Howell CR, Wilson CL, et al. Physical fitness in survivors of childhood Hodgkin lymphoma: a report from the St. Jude lifetime cohort. Med Sci Sports Exerc. 2015;47:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Paterson DH, Warburton DE. Physical activity and functional limitations in older adults: a systematic review related to Canada's physical activity guidelines. Int J Behav Nutr Phys Act. 2010;7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mulrooney DA, Yeazel MW, Mertens AC, Stovall M, Sklar CA. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the childhood Cancer survivor study cohort. BMJ. 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the childhood cancer survivor study cohort: a review of published findings. J Clin Oncol. 2009;27:2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cupit-Link MC, Kirkland JL, Ness KK, et al. Biology of premature ageing in survivors of cancer. ESMO Open. 2017;2:e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yeh JM, Hanmer J, Ward ZJ, et al. Chronic conditions and utility-based health-related quality of life in adult childhood cancer survivors. J Natl Cancer Inst. 2016;108:djw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ness KK, Gurney JG, Zeltzer LK, et al. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the childhood Cancer survivor study. Arch Phys Med Rehabil. 2008;89:128–136. [DOI] [PubMed] [Google Scholar]
- 52. Wanderley FA, Silva G, Marques E, Oliveira J, Mota J, Carvalho J. Associations between objectively assessed physical activity levels and fitness and self-reported health-related quality of life in community-dwelling older adults. Qual Life Res. 2011;20:1371–1378. [DOI] [PubMed] [Google Scholar]
- 53. Schuitema I, Deprez S, Van Hecke W, et al. Accelerated aging, decreased white matter integrity, and associated neuropsychological dysfunction 25 years after pediatric lymphoid malignancies. J Clin Oncol. 2013;31:3378–3388. [DOI] [PubMed] [Google Scholar]
- 54. Ness KK, Armstrong GT, Kundu M, Wilson CL, Tchkonia T, Kirkland JL. Frailty in childhood cancer survivors. Cancer. 2015;121:1540–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sizer PS, McGalliard M, Azevedo E.. The hip: physical therapy patient management utilizing current evidence In: Current Concepts of Orthopaedic Physical Therapy. 3rd ed Hughes C. ed., Orthopedic Section APTA. Alexandria, VA: American Physical Therapy Association; 2012. [Google Scholar]
- 56. Marchese VG, Chiarello LA, Lange BJ. Effects of physical therapy intervention for children with acute lymphoblastic leukemia. Pediatr Blood Cancer. 2004;42:127–133. [DOI] [PubMed] [Google Scholar]
- 57. Wright MJ, Hanna SE, Halton JM, Barr RD. Maintenance of ankle range of motion in children treated for acute lymphoblastic leukemia. Pediatr Phys Ther. 2003;15:146–152. [DOI] [PubMed] [Google Scholar]
- 58. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301–1317. [PubMed] [Google Scholar]
- 59. Stefani L, Klika R, Mascherini G, et al. Effects of a home-based exercise rehabilitation program for cancer survivors. J Sports Med Phys Fitness. 2019;59:846–852. [DOI] [PubMed] [Google Scholar]
- 60. Okoro T, Whitaker R, Gardner A, Maddison P, Andrew JG, Lemmey A. Does an early home-based progressive resistance training program improve function following total hip replacement? Results of a randomized controlled study. BMC Musculoskelet Disord. 2016;17:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Moya-Angeler J, Gianakos AL, Villa JC, Ni A, Lane JM. Current concepts on osteonecrosis of the femoral head. World J Orthop. 2015;6:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Charissoux J-L, Asloum Y, Marcheix P-S. Surgical management of recurrent dislocation after total hip arthroplasty. Orthop Traumatol Surg Res. 2014;100:25–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


