Abstract
Purpose
The COVID-19 pandemic has been responsible for thousands of deaths worldwide. Testing remains at a premium, and criteria for testing remains reserved for those with lower respiratory infection symptoms and/or a known high-risk exposure. The role of imaging in COVID-19 is rapidly evolving; however, few algorithms include imaging criteria, and it is unclear what should be done in low-suspicion patients with positive imaging findings.
Methods
From 03/01/2020–03/20/2020, a retrospective review of all patients with suspected COVID-19 on imaging was performed. Imaging was interpreted by a board-certified, fellowship-trained radiologist. Patients were excluded if COVID-19 infection was suspected at the time of presentation, was the reason for imaging, or if any lower respiratory symptoms were present.
Results
Eight patients with suspected COVID-19 infection on imaging were encountered. Seven patients received testing due to suspicious imaging findings with subsequent lab-confirmed COVID-19. No patients endorsed prior exposure to COVID-19 or recent international travel. COVID-19 was suggested in six patients incidentally on abdominal CT and two on chest radiography. At the time of presentation, no patients were febrile, and seven endorsed gastrointestinal symptoms. Five COVID-19 patients eventually developed respiratory symptoms and required intubation. Two patients expired during the admission.
Conclusions
Patients with imaging findings suspicious for COVID-19 warrant prompt reverse transcription polymerase chain reaction (RT-PCR) testing even in low clinical suspicion cases. The prevalence of disease in the population may be underestimated by the current paradigm of RT-PCR testing with the current clinical criteria of lower respiratory symptoms and exposure risk.
Keywords: COVID-19, Incidental findings
Introduction
The COVID-19 pandemic has been responsible for more than one million infections and thousands of deaths worldwide [1, 2]. In the USA, testing remains limited and at a premium. Criteria for testing even at many tertiary academic centers remain reserved for those with lower respiratory infection symptoms and a known primary exposure to a reverse transcription polymerase chain reaction (RT-PCR)-confirmed COVID-19 patient or travel to a high-risk area [3]. Despite the Centers for Disease Control and Prevention (CDC) releasing an immediate statement that community spread (i.e., infection without relevant travel history or exposure to another known patient) has been present since February, 2020; testing has not expanded significantly to include communally affected patients [4]. Furthermore, many patients who are RT-PCR-positive for COVID-19 remain minimally symptomatic and can transmit the virus without prolonged close contact with other individuals [5]. Together, data suggest that by limiting lab testing to only those with lower respiratory symptoms and a known exposure increases risk to unsuspecting health care workers without personal protective equipment (PPE), results in nosocomial transmission, continued propagation in the community, and potentially delay in care. Indeed, reports now indicate high rates of hospital spread, both to healthcare workers and hospitalized patients, many of which are in the group at highest risk of mortality [6].
The role of imaging has rapidly evolved with the progression of the pandemic, along with the availability and improvements in RT-PCR. Early in the COVID-19 outbreak in Wuhan, China, insufficient test kits and poor PCR sensitivity resulted in a role of computer tomography (CT) imaging for disease detection [7, 8]. Many studies from this cohort have established a high sensitivity (up to 97%) in the diagnosis of COVID-19. While major concerns arose from the low reported specificity of CT (25%), recent studies have suggested that radiologists with experience or artificial intelligence may be able to distinguish COVID-19 from other pneumonias with up to 80–90% accuracy [9, 10].
Despite many studies illustrating the benefits of CT, many centers do not include imaging on their algorithm for PCR testing. In this cohort study, we present a number of patients with incidentally discovered COVID-19 on imaging with subsequent positive RT-PCR at a major tertiary center at a time point where only 30 total infections were confirmed. This highlights the potential of undetected nosocomial and community spread even with low confirmed population prevalence, as well as calls for inclusion of patients with positive imaging for subsequent RT-PCR testing even in the absence of symptoms or exposure.
Methods
From 03/01/2020 to 03/20/2020, a retrospective review at a single center of all patients with suspected COVID-19 on imaging was performed. This study was reviewed and approved by the institutional review board. Image interpretation was performed by a board-certified, fellowship-trained radiologist. Each patient’s chart was reviewed to identify the chief complaint at presentation. Patients were included in the study if the primary presenting symptom was not lower respiratory in nature or if the imaging was performed for a non-respiratory reason. Patients were further excluded if COVID-19 infection was suspected at the time of presentation or if the patient was included in the active “rule-out” census. At the time of data abstraction, a total of 137 RT-PCR were performed at the institution, with a total of 30 lab-confirmed infections, nine of which remained inpatient.
The COVID-2019 assay utilized primer, probes and reagents and the procedure designated by the CDC and the Institutional Diagnostic Molecular Biology Laboratory using Real Time PCR method. FDA approval for these tests is currently pending.
Results
A total of eight (five females, three males) lab-confirmed COVID-19 patients met the inclusion criteria for this study (Table 1). All of these patients presented with no or very low suspicion for COVID-19 infection, with subsequent laboratory RT-PCR testing initiated after the time of imaging suspicion. No patients presented with known contact to an RT-PCR-confirmed COVID-19 patient, and no patients endorsed recent international travel to a high-level region. Seven out of eight patients were found to be COVID-19 RT-PCR positive, with one patient diagnosed with influenza A. The mean age among PCR-positive patients was 65 years (SD 8.9, range 54–80). At the time of presentation, no patients presented with fever, and the mean maximum recorded temperature was 36.7 °C (range 36.2–37.6 °C). One patient presented with hypertension; otherwise, no other vital sign derangements including abnormal respiratory rate or oxygen saturation were present at the time of initial triage. Six patients received CT imaging of the abdomen and pelvis, with findings suspicious for COVID-19 identified at the lung bases (Fig. 1). Of these six patients, three patients presented with subjective fevers, low energy, abdominal pain, and diarrhea, and the CT was obtained to evaluate for intra-abdominal pathology. A fourth patient presented with abdominal distention and acute kidney injury in the setting of chemotherapy, and a stone-protocol CT scan was ordered to evaluate for obstruction and hydronephrosis. A sixth patient was recently post-operative from an iliac bypass surgery and presented with anemia. The seventh patient presented with colostomy stoma prolapse and leg swelling and was known to have recent influenza infection; this was the only patient found to be COVID-19 RT-PCR negative. The possibility of COVID-19 infection was raised on chest radiograph in two patients (Fig. 2). Both of these patients presented with subjective fevers, low energy, and diarrhea.
Table 1.
Patient demographics, presentation, and clinical course
| Age | Sex | Presenting symptoms | Incidentally detected imaging modality | COVID-19 PCR | Clinical course | Documented recent (<1 week) visit to healthcare center | |
|---|---|---|---|---|---|---|---|
| Patient 1 | 65 | F | Abdominal pain | CT | Positive | Escalation to critical care, intubation | No |
| Patient 2 | 80 | F | Anemia, post-operative | CT | Positive | Expired during admission | Yes |
| Patient 3 | 56 | F | Abdominal pain, nausea, diarrhea | CT | Positive | Never developed COVID-19 symptoms | No |
| Patient 4 | 69 | M | Diarrhea, subjective fevers, fatigue | Radiograph | Positive | Escalation to critical care, intubation | Yes |
| Patient 5 | 62 | F | Diarrhea, subjective fevers, vomiting | Radiograph | Positive | Escalation to critical care, intubation | Yes |
| Patient 6 | 68 | M | AKI, abdominal distention | CT | Positive | Expired during admission | Yes |
| Patient 7 | 54 | M | Abdominal pain, subjective fevers | CT | Positive | Never developed COVID-19 symptoms | Yes |
| Patient 8 | 59 | F | Stoma prolapse, lower extremity swelling | CT | Negative | Discharged without follow-up | Yes |
Fig. 1.
Imaging findings of COVID-19 incidentally detected on CT. a A 54-year-old presented to clinic with right lower quadrant pain with concern for appendicitis. A CT of the abdomen and pelvis was obtained revealing rounded, peripheral ground glass opacities (GGO; arrows) with associated areas of vascular engorgement (arrow heads) at the lung bases. b A chest radiograph was obtained for the same patient. Subtle, peripheral ground glass opacities are identified at the lung bases (arrows). c A 56-year-old lady presented with abdominal pain, nausea, and diarrhea. An initial CT of the abdomen revealed findings consistent with COVID-19. A CT chest was obtained, revealing bilateral, diffuse, rounded GGOs in the lungs (arrows)
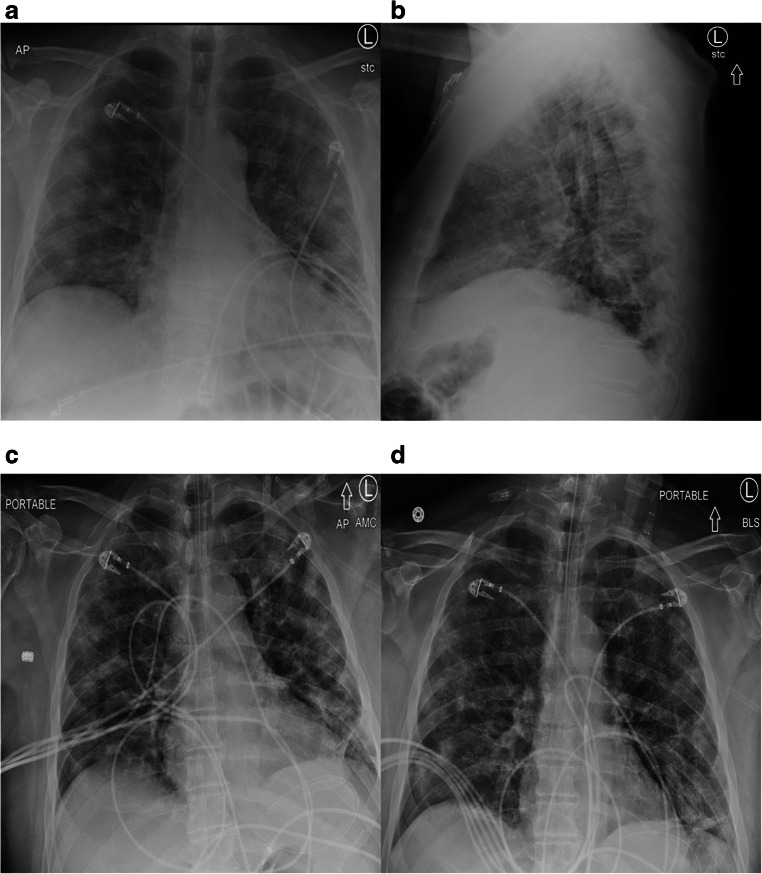
Fig. 2.
a A 62-year-old lady presented with subjective fevers, vomiting, and diarrhea. A chest radiograph was obtained to evaluate for etiology of fever, revealing bilateral, hazy, peripheral patchy grand glass opacities. b Lateral view chest radiograph of the same patient. c Chest radiograph two days after admission demonstrates increasing bilateral airspace opacities, with signs of developing acute respiratory distress syndrome. d Chest radiograph four days after admission demonstrate an intubated patient with progressive lung involvement, bilateral consolidations with septal thickening, and florid ARDS
Five COVID-19-infected patients in this cohort had a documented visit to a health care facility (i.e., hospital, urgent care); however, none of these patients endorsed a respiratory or gastrointestinal related issue at that time.
While no patients endorsed lower respiratory symptoms at presentation, five of the seven COVID-19 patients eventually developed respiratory symptoms, all of which eventually required escalation to critical care and intubation due to respiratory failure. Initially, two patients were triaged to home quarantine with a pending RT-PCR, though both returned for admission and rapidly required escalation to critical care and intubation. Two out of seven patients in this cohort subsequently expired after admission to the intensive care unit.
Laboratory values at presentation are included in Table 2. At the time of presentation, one patient presented with leukopenia (0.3 K/UL) and thrombocytopenia and one patient with leukocytosis (17.4 K/UL). The remaining patients presented with normal white blood cell and platelet counts. D-Dimer data was available for four patients, all of which eventually required intubation and one of which did not survive the admission. D-Dimer rates were markedly elevated (median 2580 DDU ng/mL, range 1396–3140, reference range 0–230) among these admitted COVID-19 patients. C-reactive protein was elevated in all patients (median 20.6 mg/dL, range 0.8–159, reference range 0–0.5). Lactate dehydrogenase (LDH) and pro-calcitonin levels were elevated in all but one patient (median LDH 300 U/L, range 218–341, reference range 0–271, median pro-calcitonin 0.31 ng/mL, range 0–7.8, reference range 0–0.05).
Table 2.
Patient laboratory values at presentation
| D-Dimer (ng/mL; ref 0–230) | White blood cell count (K/UL; ref 3.5–10.5) | Platelet count (K/UL; ref 140–390) | C-reactive protein (mg/dL; ref 0–0.5) | Lactate dehydrogenase (U/L; ref 0–271) | Procalcitonin (ng/mL; 0–0.05) | Sedimentation rate (mm/h; ref 4–25) | |
|---|---|---|---|---|---|---|---|
| Patient 1 | 2560 | 7.6 | 253 | 159 | 451 | 0.14 | 63 |
| Patient 2 | N/A | 17.4 | 174 | N/A | N/A | 0.39 | N/A |
| Patient 3 | N/A | 4.9 | 284 | N/A | N/A | <0.05 | N/A |
| Patient 4 | 2600 | 7.2 | 299 | 16.6 | 278 | 0.23 | 99 |
| Patient 5 | 1396 | 7.2 | 311 | 24.6 | 322 | 0.82 | 117 |
| Patient 6 | 3140 | 0.3 | 27 | 0.8 | 218 | 7.8 | 15 |
| Patient 7 | N/A | 4.7 | 165 | N/A | N/A | N/A | N/A |
| Patient 8 | N/A | 7.8 | 378 | N/A | N/A | <0.05 | N/A |
Discussion
Accurate and prompt diagnosis of COVID-19 patients is of utmost importance in limiting spread of the disease as well as for identifying patients at risk for rapid deterioration. Epidemiological data from other countries, such as South Korea, China, and Italy have shown that increased testing, identification of infected individuals, and prompt medical attention can result in markedly lower rates of R0, disease propagation, and ultimately disease mortality [11]. With several treatments currently under development and investigation, diagnosis will be of increasing importance.
Testing in the USA remains limited, with most centers only testing patients with lower respiratory symptoms and positive exposure history. The cohort presented herein, along with many other published studies, show that this testing strategy may be overly restrictive as there is a significant rate of communal and sub-clinical spread [4, 5, 12–15]. It is well documented that many patients who are RT-PCR positive for COVID-19 may have an incubation period of 5 days and can transmit the virus without prolonged close contact with other individuals [5, 12]. Here, we show in seven patients without lower respiratory symptoms, documented fever, or known exposure that COVID-19 infection can not only be present, but subsequently result in high rates of intubation and mortality. Many centers do not incorporate imaging in their RT-PCR algorithms and there remains diagnostic uncertainty regarding patients with imaging consistent with COVID-19 but are otherwise of low clinical suspicion. We argue that patients with suspicious imaging findings, especially CT, even in the low-incidence and low clinical suspicion setting shown here, warrants prompt RT-PCR testing. Additionally, as detection of these patients is difficult, data support the institution of widespread quarantine protocols to contain disease propagation.
This patient cohort highlights an important potential blind spot in the use of PPE at the point-of-care and in the inpatient setting, potentially leading to increased nosocomial and healthcare worker transmission. The lab-confirmed disease incidence at the time of this report remained low, with only 30 lab-confirmed infections and nine in-hospital infections. Despite this, imaging was able to incidentally identify patients with suspected COVID-19 findings, ultimately resulting in seven additional diagnoses and prompt initiation of PPE and quarantine protocols. This suggests that even in the early stages of community spread, there may be a high rate of undetected disease and a much-higher-than-suspected population incidence than that established by RT-PCR, which has been suggested by other studies [13–15]. This is in part due to restrictive testing criteria, which would have excluded these patients without pulmonary symptoms. These patients present a unique risk to those at the point-of-care, as there is low suspicion for COVID-19 at presentation, and therefore, the appropriate PPE precautions are not taken. Had these incidental imaging findings not been identified and correctly reported, or if these patients were not subsequently tested despite the reporting of suspicious imaging findings, these individuals may have continued to spread the infection to healthcare workers and within the community. This is highlighted by the two patients in this cohort who never developed symptoms who would have been sent home with a presumed negative COVID-19 diagnosis.
The rate of escalation to critical care, intubation, and mortality in this cohort was alarmingly high, suggesting these patients may have rapidly decompensated if sent home without a known diagnosis and possible lost to follow-up. Two patients were discharged to home quarantine for “rule-out” with a pending RT-PCR after initial suspicious imaging, who subsequently required admission and intubation. More research regarding which patients are at risk for rapid deterioration will be important to identify these patients.
This cohort identifies clinical findings that may be considered to be suspicious for COVID-19 infection in the absence of pulmonary or exposure history. Six out of seven (86%) of patients presented with abdominal complaints, consistent with an in-press publication suggesting nearly half of the patients in Hubei province of China endorsed gastrointestinal symptoms and that these patients had a more severe course of illness [16]. Classic pulmonary imaging findings in the absence of respiratory flu-like symptoms may increase the specificity for COVID-19 infection as the incidence of differential considerations (such as other respiratory viruses) is lower in this clinical context. COVID-19 in contrast is known to have an asymptomatic or sub-clinical period with positive chest CT findings [17]. Together, this may explain the high reported specificity of 88% in this small cohort of patients. Elevated inflammatory markers may be an important clinical correlate suggestive of a brewing COVID-19 infection, especially in the setting of leukopenia or a normal white blood cell count. Previous studies have shown that D-Dimer at the time of presentation may predict disease severity, which is concordant in this small cohort, with a markedly elevated D-Dimer range of 1396–3140 ng/mL among four patients, all of whom required eventual intubation and one of which expired during the admission [18]. These findings suggest that expansion of clinical suspicion beyond lower respiratory symptoms and exposure may be important to identify a larger proportion of infected patients and for appropriate triage and PPE at the point-of-care.
Of note, five of the seven patients were seen in a health care setting within seven days of infection for an unrelated complaint, suggesting a possibility of nosocomial spread. Studies from China have suggested rates of in-hospital transmission to be as high as 41% [6].
The classic CT findings of COVID-19 include bilateral, round, ground glass opacities in a peripheral distribution, “crazy paving”, and vascular engorgement [7, 19, 20]. Unlike other viral pneumonias which primarily affect the airway resulting in tree-in-bud nonduality in a bronchial distribution, COVID-19 affects the Type II pneumocyte and results in a unique CT appearance that lends increased specificity [21]. Emerging literature has shown that the initially reported rates of sensitivity may have been underestimated, with experienced radiologists and artificial intelligence successfully distinguishing COVID-19 from other pneumonias in upwards of 80% of the cases [9, 10]. In a recent study of over 1000 patients, CT resulted in higher sensitivity in detection of SARS-COV2 as compared with initial RT-PCR from pharyngeal swab samples [22]. Reported positive predictive values were upwards of 60%. Additional studies are emerging that further solidify this positive predictive value, with reports now uncovering a cohort of patients with high-suspicion CT and false-negative initial PCRs later discovered to have positive, concordant COVID-19-positive PCR [23, 24]. It remains to be seen how patients with positive cross-sectional imaging in the setting of high clinical suspicion, especially in the absence of other etiologies (for example, negative respiratory viral panel), should be managed, until higher rates of RT-PCR sensitivity and specificity are confirmed.
Chest radiography is not sensitive for the detection of ground-glass opacity and may demonstrate normal findings, especially in the early stages of infection [19]. Bilateral peripheral, multifocal consolidations may be seen. Those in the late stage may present with findings of “white lung” and acute respiratory distress syndrome. In this study, suggestion of COVID-19 on chest radiograph resulted in subsequent positive testing in two patients, which raises the question of whether there may be a role for radiography as a supplement to RT-PCR, or in areas where laboratory testing may be especially scarce.
This study has limitations. This is a retrospective study of a small cohort size at a single center. In order to fully understand the value of imaging in low clinical suspicion patients, an analysis of all patients with imaging consistent with COVID-19 in this cohort, including negative RT-PCRs should be analyzed to assess for specificity—data which is currently being collected and analysis ongoing.
Conclusion
As RT-PCR testing is limited and disease presentation varied, this study raises the possibility for an expanded role of imaging, such as chest radiography or CT as an adjunct to PCR in diagnosis.
Unique imaging findings in these patients present an important responsibility for radiologists and ordering providers in the COVID-19 pandemic. It is of utmost importance to be vigilant for findings which may suggest COVID-19 infection and to be diligent in follow-up to ensure the proper steps are taken in order to avoid any inadvertent delay in diagnosis, isolation, contact precautions, and treatment. Furthermore, these cases illustrate the importance of expanded testing criteria in the USA, especially in the setting of positive imaging findings.
Ethics approval
This research study was conducted retrospectively from data obtained for clinical purposes. We consulted extensively with the IRB of Northwestern University who determined that our study did not need ethical approval. An IRB official waiver of ethical approval was granted from the IRB of Northwestern University.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Not Applicable
Availability of data and material
Not Applicable
Code availability
Not Applicable
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.CDC (2020) Coronavirus disease 2019 (COVID-19). Available from: https://www.cdc.gov/media/dpk/diseases-and-conditions/coronavirus/coronavirus-2020.html
- 2.WHO (2020) Coronavirus disease 2019 (COVID-19) Situation Report. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200317-sitrep-57-covid-19.pdf?sfvrsn=a26922f2_4
- 3.CDC (2020) Coronavirus disease 2019 (COVID-19) testing in U.S
- 4.CDC (2020) CDC confirms possible instance of community spread of COVID-19 in U.S. Available from: https://www.cdc.gov/media/releases/2020/s0226-Covid-19-spread.html
- 5.Cai J et al (2020) Indirect virus transmission in cluster of COVID-19 cases, Wenzhou, China, 2020. Emerg Infect Dis 26(6) [DOI] [PMC free article] [PubMed]
- 6.Wang D et al (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA [DOI] [PMC free article] [PubMed]
- 7.Li Y, Xia L, Disease C (2019) (COVID-19): role of chest CT in diagnosis and management. AJR Am J Roentgenol 1–7 [DOI] [PubMed]
- 8.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ (2020) Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology 200490 [DOI] [PMC free article] [PubMed]
- 9.Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, Pan I, Shi LB, Wang DC, Mei J, Jiang XL, Zeng QH, Egglin TK, Hu PF, Agarwal S, Xie F, Li S, Healey T, Atalay MK, Liao WH (2020) Performance of radiologists in differentiating COVID-19 from viral pneumonia on chest CT. Radiology 200823 [DOI] [PMC free article] [PubMed]
- 10.Lin Li LQ, Xu Z, Yin Y, Wang X, Kong B, Bai J, Lu Y, Fang Z, Song Q, Cao K, Lu D, Wang G, Xu Q, Fang X, Zhang S, Xia J, Xia J (2020) Artificial intelligence distinguishes COVID-19 from community acquired pneumonia on chest CT. Radiology [DOI] [PMC free article] [PubMed]
- 11.Cascella M et al (2020) Features, Evaluation and treatment coronavirus (COVID-19), in StatPearls. Treasure Island (FL) [PubMed]
- 12.Lauer SA, Grantz KH, Bi Q, Jones FK, Zheng Q, Meredith HR, Azman AS, Reich NG, Lessler J. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172:577–582. doi: 10.7326/M20-0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mizumoto K, Chowell G. Transmission potential of the novel coronavirus (COVID-19) onboard the diamond princess cruises ship, 2020. Infect Dis Model. 2020;5:264–270. doi: 10.1016/j.idm.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mizumoto K et al (2020) Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill 25(10) [DOI] [PMC free article] [PubMed]
- 15.Nishiura H (2020) Backcalculating the incidence of infection with COVID-19 on the Diamond Princess. J Clin Med 9(3) [DOI] [PMC free article] [PubMed]
- 16.Lei Pan MM, Ren HG, Yang P, Sun Y, Wang R, Yan J (2020) Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol [DOI] [PMC free article] [PubMed]
- 17.Shohei Inui AF, Jitsu M, Kunishima N, Watanabe S, Suzuki Y, Umeda S, Uwabe Y (2020) Chest CT findings in cases from the cruise ship “Diamond Princess” with coronavirus disease 2019 (COVID-19). Radiol: Cardiothorac Imaging [DOI] [PMC free article] [PubMed]
- 18.Zhou F, Yu T, du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salehi S et al (2020) Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 1–7 [DOI] [PubMed]
- 20.Kanne JP. Chest CT findings in 2019 novel coronavirus (2019-nCoV) infections from Wuhan, China: key points for the radiologist. Radiology. 2020;295(1):16–17. doi: 10.1148/radiol.2020200241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Zhang S, Cao T, Yang C, Li M, Guo G, Chen X, Chen Y, Lei M, Liu H, Zhao J, Peng P, Wang CY, du R. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172:629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L (2020) Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 200642 [DOI] [PMC free article] [PubMed]
- 23.Huang P, Liu T, Huang L, Liu H, Lei M, Xu W, Hu X, Chen J, Liu B. Use of chest CT in combination with negative RT-PCR assay for the 2019 novel coronavirus but high clinical suspicion. Radiology. 2020;295(1):22–23. doi: 10.1148/radiol.2020200330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie X, Zhong Z, Zhao W, Zheng C, Wang F, Liu J (2020) Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology 200343 [DOI] [PMC free article] [PubMed]