Abstract
The growth plate is an essential component of endochondral bone development. Not surprisingly, the growth plate and its surrounding structure, the perichondrium, contain a wealth of skeletal stem cells (SSCs) and progenitor cells that robustly contribute to bone development. Recent in vivo lineage-tracing studies using mouse genetic models provide substantial insight into the diversity and versatility of these skeletal stem and progenitor cell populations, particularly shedding light on the importance of the transition from cartilage to bone. Chondrocytes and perichondrial cells are inseparable twins that develop from condensing undifferentiated mesenchymal cells during the fetal stage; although morphologically and functionally distinct, these cells ultimately serve for the same goal, that is, to make bone bigger and stronger. Even in the postnatal stage, a small subset of growth plate chondrocytes can transform into osteoblasts and marrow stromal cells; this is in part fueled by a unique type of SSCs maintained in the resting zone of the growth plate, which continue to self-renew for the long term. Here, we discuss diverse skeletal stem and progenitor cell populations in the growth plate and the perichondrium and their transition from cartilage to bone.
Keywords: Skeletal stem cells (SSCs), growth plate, perichondrium, bone development, in vivo cell-lineage analysis, bone marrow stromal cells (BMSCs), parathyroid hormone-related protein (PTHrP), transdifferentiation
1. Introduction
The growth plate, a specialized cartilaginous structure located at the ends of growing endochondral bones, is a crucial component of endochondral bone development [1, 2]. Continuous activities of the growth plate into early adulthood are essential for establishing fundamental functions of bones as a pivotal organ, which include protection of vital organs, locomotion, support of hematopoiesis and regulation of mineral metabolism. Particularly, the growth plate contributes substantially to elongation of long bones [1]. As a result, injuries or disorders of the growth plate cause shortening of long bones [3, 4]. For example, epiphyseal bone fractures involving the growth plate in children often cause inhibition on long bone growth [3, 4]. Achondroplasia, one of the most common genetic skeletal dysplasia associated with dwarfism, is caused by activating mutations in fibroblast growth factor receptor 3 (FGFR3) [5, 6]. These mutations suppress proliferation and maturation of growth plate chondrocytes, and cause shortening of the limb [7]. In addition, the growth plate can provide a source of cartilage tumors; in Ollier diseases, asymmetrically distributed multiple enchondromas develop in close proximity to the growth plate and cause a limb length discrepancy [8].
It has been long speculated that the growth plate contains “stem cells” that support bone elongation. The pioneering study in early 2000s demonstrated using a surgical transplantation model that the resting zone of the rabbit growth plate contains stem-like cells that give rise to clones of proliferating chondrocytes [9]. Skeletal stem cells (SSCs) are a type of somatic stem cells (also known as tissue-specific stem cells) dedicated to bones, which generate a variety of cells of the skeletal lineage including, but not limited to, osteoblasts, chondrocytes, adipocytes and marrow stromal cells [10]. SSCs play important roles in skeletal growth and regeneration, based on their two hallmark capabilities of (1) self-renewal, which is the ability to reproduce stem cells, and (2) multipotency, which is the ability to generate multiple types of differentiated cells. SSCs were originally described in adult bone marrow. The classical gold standard to define SSCs is a combination of in vitro colony-forming unit-fibroblast (CFU-F) assays and cell transplantation experiments; in this paradigm, SSCs are defined as self-renewing cells with a “trilineage” differentiation potential into chondrocytes, osteoblasts and adipocytes [11–13]. A variety of SSCs have been identified in bone marrow based on combinations of cell surface markers for fluorescence-activated cell sorting (FACS); these include PDGFRα+Sca1+CD45−TER119− cells [14], CD73+CD31− cells [15, 16], CD271+CD45− cells [17–20] and CD106+ cells [21].
It was not until recently that the idea of SSCs was extrapolated from bone marrow to other bone tissues. The breakthrough came when SSCs were isolated from the developing growth plate using a defined set of cell surface markers, initially in perinatal mice as CD51+CD90−CD105−CD200+ non-hematopoietic mesenchymal cells [22], and later in human fetus as PDPN+CD146−CD73+CD164+ cells [23]. These studies provided the premise that the growth plate contains its own population of SSCs; this was somewhat at odds with the generally accepted concept at the time that growth plate chondrocytes mostly die after “terminal” differentiation into hypertrophic chondrocytes. Until recently, hypertrophic chondrocytes were considered to be unanimously replaced by new bone cells that originate from unknown sources during bone growth [24, 25]. However, a series of in vivo lineage-tracing studies clearly demonstrate that at least some of growth plate chondrocytes do not die by apoptosis, but instead differentiate into osteoblasts, bone marrow stromal cells (BMSCs) and marrow adipocytes [26–29]. These hypertrophic chondrocytes are descendants of chondrocytes in the resting zone, which play important roles in maintaining the growth plate [10, 27, 30]. A special subset of resting zone chondrocytes are now identified to function as “growth plate SSCs”, which continue to self-renew within the resting zone through asymmetric divisions; their differentiating progeny first become proliferating and hypertrophic chondrocytes, and subsequently “transform” into osteoblasts and BMSCs at the post-mitotic stage, including those termed as C-X-C motif chemokine 12 (CXCL12, also known as stromal cell-derived factor 1, SDF1)-abundant reticular (CAR) cells [27]. These studies highlight the new role of the growth plate as a reservoir of SSCs that orchestrate endochondral bone development.
In this review, we discuss diverse groups of SSCs associated with the growth plate and the perichondrium, and how they potentially regulate endochondral bone development.
2. The formation of the growth plate
Endochondral bone development is initiated by condensation of undifferentiated mesenchymal cells in defined locations during the embryonic stage [31, 32]. These condensing mesenchymal cells differentiate into two distinct types of cells in the subsequent stage: chondrocytes that form the cartilage template, and their surrounding perichondrial cells that stay outside the cartilage template. Chondrocytes at the center of the cartilage template differentiate into hypertrophic chondrocytes as the cartilage template enlarges [1]. Subsequently, perichondrial cells located immediately outside the hypertrophic zone of the cartilage template invade into the template with blood vessels and differentiate into osteoblasts [33–35]. The resultant primary ossification center is composed of a nascent bone marrow stromal compartment accompanied by trabecular bones, which provides a new niche for hematopoietic cells. The origin of the perichondrium remains largely undefined, although it has been long proposed that cells at the outer edges of mesenchymal condensations become perichondrial cells with distinct properties and functions [36]. After formation of the primary ossification center, chondrocytes persist at the both ends of the template; these cells are aligned in a highly organized manner and form three distinct layers of resting, proliferating and hypertrophic zones; as a result, the growth plate is formed [1].
Indian hedgehog (Ihh) and parathyroid hormone-related protein (PTHrP) signaling pathways collaboratively regulate growth plate activities and endochondral bone growth by forming a negative feedback loop [37, 38]. PTHrP is secreted from resting chondrocytes, binds to the receptors expressed by proliferating chondrocytes and suppresses their hypertrophic “terminal” differentiation; as a result, PTHrP delays the production of Ihh from pre-hypertrophic chondrocytes. After the distance between the resting and the hypertrophic zone become increased, Ihh is produced and increases the rate of chondrocyte proliferation, differentiation and columnar chondrocyte formation [1, 38, 39]. Thus, the PTHrP–Ihh negative feedback loop serves as the primary mechanism maintaining the growth plate.
3. Fates of chondrocytes and perichondrial cells in endochondral bone development
Current knowledge on cell fates pertaining to bone development is primarily defined by in vivo fate-mapping studies using constitutive active cre and, more importantly, lineage-tracing studies using tamoxifen-inducible creER, which are combined with reporter strains. Expression of cre or creER is driven by a promoter/enhancer of a particular gene that is typically active in a specific group of cells. The creER-based reporter system is a powerful tool for analyzing cell fates of a particular group of cells at a given time under unperturbed conditions; although the potential weakness of this system is that creER protein might be carried over to other cells by inter-cell transfer via matrix vesicles, which may result in “leakiness” of creER activities. It is therefore imperative to define the specificity of a given creER line using a rigorous short-chase analysis, before making any assumption on the cell lineage.
A master transcription factor for chondrocyte differentiation, SRY-box gene 9 (Sox9), becomes expressed in mesenchymal condensations at the initial stage of endochondral bone development [40–42]. These Sox9-cre+ cells contribute to all skeletal cells in the subsequent stages, therefore behave as osteo-chondro-progenitor cells [43]. The identity of precursor cells for these Sox9-cre+ cells has not been clearly defined. Prrx1 is initially expressed in the lateral plate mesoderm during the embryonic stage [44]; as a result, Prrx1-cre marks all skeletal cells in the appendicular skeleton [45]. However, because Prrx1 is expressed by both chondrocytes and perichondrial cells in the subsequent stages of development, this fate-mapping study cannot unambiguously define the lineage relationship between Prrx1+ and Sox9+ cells. Our recent findings demonstrate that a Notch effector gene Hes1 is abundantly expressed in a manner surrounding the condensation; as a result, Hes1-creER marks cells in mesenchymal condensations that do not overlap with Sox9+ cells. These peri-condensation Hes1-creER + cells at this early stage robustly contribute to chondrocytes within the cartilage template, osteoblasts of the primary ossification center and the bone collar, as well as marrow stromal cells in the subsequent stages [46]. These findings may pose a challenge to the established concept that all osteo-chondroprogenitors are derived from Sox9+ cells. There are two possible explanations. The first possibility is that Hes1-creER marks the precursors for Sox9+ cells, supporting the notion that Hes1+ cells function as earlier perichondrial skeletal progenitor cells. The second possibility is that Hes1-creER marks perichondrial cells after these cells have turned off Sox9 expression. Identifying the precise role of Hes1+ cells and their relationship with Sox9+ cells will be important to identify the origin and the function of early perichondrial cells that appear at the onset of endochondral bone development.
As the cartilage template is established, other representative chondrocyte markers such as type II collagen alpha 1 chain (Col2a1), aggrecan (Acan) and fibroblast growth factor receptor 3 (FGFR3), become abundantly expressed by cells therein. These intra-template cells expressing these markers (Sox9-creER, Col2a1-creER, Acan-creER or Fgfr3-creER) contribute not only to a majority of growth plate chondrocyte, but also to a large number of bone cells in the postnatal stage, including osteoblasts and osteocytes both in cortical and trabecular bones, BMSCs and marrow adipocytes [26]. This concept is reconfirmed by our recent in vivo lineage-tracing studies using Fgfr3-creER, which has a significant advantage over Acan-creER and Col2a1-creER in terms of specificity to chondrocytes. Fgfr3-creER almost exclusively marks Sox9+ cells within the cartilage template, marking little in the perichondrium [46]. Furthermore, Col10a1-creER+ hypertrophic chondrocytes, which lie at the bottom of the growth plate with abundant type X collagen alpha 1 (Col10a1) expression, contribute to cells of the osteoblast lineage [28, 29]. Therefore, chondrocytes and their precursors within the cartilage template, as well as those in the more-developed growth plate, can provide an important source of bone cells. Moreover, Pthrp-creER+ chondrocytes in the resting zone of the postnatal growth plate eventually differentiate into the osteoblasts and BMSCs in the metaphysis [27]. These studies collectively establish the concept that at least some of growth plate chondrocytes escape apoptosis and transform into cells in bone and bone marrow.
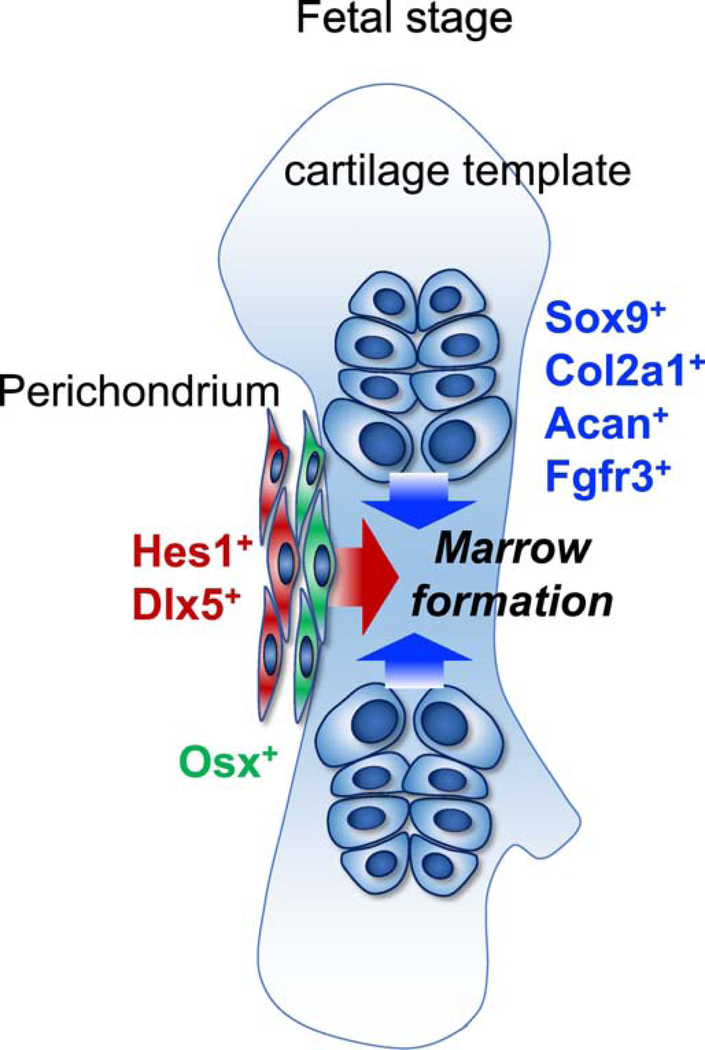
The perichondrium, composed of multiple layers of mesenchymal cells surrounding the cartilage template, is equally important as a source of bone cells. For example, perichondrial cells expressing osterix (Osx)-creER, located in the innermost layer of the perichondrium adjacent to pre-hypertrophic chondrocytes, invade into the cartilage template and function as transient osteoblast precursor cells [33, 35]. Our recent lineage-tracing studies demonstrate that Hes1-creER+ and Dlx5-creER+ cells, located in the outer layers of the perichondrium of the cartilage template, contribute to a substantial fraction of osteoblasts and BMSCs within the marrow space, particularly in the diaphysis of postnatal bones. Hes1-creER and Dlx5-creER are highly specific to the perichondrium and mark precursors for Osx-creER+ cells in the perichondrium. Interestingly, Hes1-creER+ and Dlx5-creER+ fetal perichondrial cells and Fgfr3-creER+ fetal chondrocytes appear to contribute to functionally distinct stromal compartments of postnatal bone marrow [46]. Taken together, highly coordinated actions of the cartilage template and the perichondrium are essential to endochondral bone development (Fig. 1).
Figure 1. Coordinated actions of the cartilage template and the perichondrium in fetal endochondral bone development.
Endochondral bone development is executed by various types of cells in the fetal cartilage template and the perichondrium. The intra-template cells (Sox9+, Col2a1+, Acan+ or Fgfr3+ cells) contribute to a majority of growth plate chondrocyte, and then robustly contribute to a large number of bone cells. The outer layers of the perichondrial cells (Hes1+ or Dlx5+) contribute specifically to a substantial fraction of bone cells, while the innermost layer cells marked by Osx are transient osteoblast precursors.
4. Growth plate skeletal stem cells (SSCs) in the postnatal stage
An important question is whether the growth plate of the postnatal stage can also provide a major source of bone cells during active bone elongation. Lines of evidence support that “stem cell” populations exist in the postnatal growth plate. Early evidence was provided by a surgical transplantation study that indicated that the resting zone of growing rabbits contains stem-like cells [9]. Importantly, more recent studies demonstrate that the skeletal stem cell niche is established postnatally within the epiphysis in conjunction with formation of the highly-vascularized secondary ossification center (SOC), which supports a specific subset of chondrocytes expressing PTHrP in the resting zone of the growth plate [27, 30]. PTHrP+ chondrocytes, marked by Pthrp-mCherry knock-in allele or Pthrp-creER transgenic line upon tamoxifen injection, express a set of markers for transplantable SSCs, and uniquely possess properties as SSCs in cultured conditions with a “trilineage” differentiation potential in vitro. Lineage-tracing studies using Pthrp-creER demonstrate that these PTHrP+ cells continue to stay within the resting zone through rounds of asymmetric divisions and form columnar chondrocytes for the long term. Moreover, some of the descendants of PTHrP+ cells contribute to a small number of osteoblasts and BMSCs beneath the growth plate [27]. Therefore, a special subset of PTHrP+ resting chondrocytes can behave as SSCs with self-renewability and multipotency, descendants of which transition into cells in bone marrow.
Interestingly, PTHrP+ chondrocytes stay dormant within the resting zone without forming columnar chondrocytes until SOC is formed, suggesting that SOC might provide an important cue to support self-renewal of SSCs. In fact, Newton et al. demonstrate that chondroprogenitors deplete themselves in the fatal and neonatal stage (consumption program), but when SOC is formed in the postnatal stage, these cells acquire self-renewability and establish large and stable monoclonal columns of chondrocytes [30]. Stem cells generally undergo either asymmetric division, which produces a new stem cell and a daughter cell, or symmetric division, which produces two new stem cells [47, 48]. The postnatal growth plate structure is maintained through the balance of these stem cell divisions, which are regulated by some of the critical signaling pathways. For example, activation of mammalian target of rapamycin complex 1 (mTORC1) signaling in chondrocytes disorganizes the resting zone, associated with an increase in the number and thickness of multi-columnar clones [30]. Hedgehog signaling also plays an essential role in regulating growth plate SSCs in a stage-specific manner. Administration of either Hedgehog antagonist LDE225 or agonist SAG before SOC formation results in significant reduction of PTHrP+ SSCs and their descendants [27]. In contrast, administration of Hedgehog antagonist vismodegib after SOC formation results in reduction of the clone size and premature fusion of the growth plate, whereas administration of SAG at the same stage increases proliferation of resting chondrocytes [30]. Together, growth plate SSCs are directly and indirectly regulated by a variety of well-described signaling pathways in a stage-specific manner.
5. Other cellular sources of the growth plate and their transition to bone
The interesting observation from Pthrp-creER-based lineage-tracing studies is that the descendants of PTHrP+ SSCs in the resting zone contribute only to a small fraction of osteoblasts and BMSCs. This finding supports the hypothesis that there are other skeletal stem and progenitor cell populations in the postnatal growth plate, which provide cells in bone and bone marrow through different routes (Fig. 2).
Figure 2. Growth plate skeletal stem/progenitor cells contribute to cells in bone and bone marrow through multiple routes.
During perinatal to early postnatal stage, skeletal stem/progenitor cells in the growth plate contribute to osteoblasts and BMSCs in bone and bone marrow through multiple routes. Growth plate skeletal stem/progenitor cells include, not not limited to, PTHrP+ skeletal stem cells in the resting zone and Axin2+ chondroprogenitor cells at the outermost layer. Col10a1+ hypertrophic chondrocytes and PTHrP+ borderline chondrocytes represent transient precursor cells that quickly move out of the growth plate.
The periphery of the growth plate may contain important skeletal progenitor and precursor cell populations. The Pthrp-creER line, which specifically marks chondrocytes in the resting zone at the early postnatal stage of postnatal day 6, marks a different population of “borderline chondrocytes” at the periphery of the growth plate immediately adjacent to the perichondrium, when pulsed in the neonatal stage shortly after birth. Cell-fate analyses reveal that these Pthrp-creER+ borderline chondrocytes contribute transiently to osteoblasts and BMSCs, indicating that chondrocyte-to-osteoblast transition also occurs in the peripheral route [49]. In addition, canonical Wnt-responsive gene Axin2 is expressed in the perichondrial groove of Ranvier adjacent to the resting zone, as well as in the outermost layer of the growth plate. In fact, Wnt/β-catenin signaling plays important roles in development of the growth plate [50–52], and lineage-tracing studies using Axin2-creER reveal that these cells contribute progressively to chondrocytes inside the growth plate [53]. Therefore, Wnt-responsive Axin2+ cells behave as chondroprogenitor cells that participate in circumferential growth of the growth plate.
It is important to note that the frequency of the transition from chondrocytes to osteoblasts or BMSCs in the postnatal stage appears to be modest. Historically, hypertrophic chondrocytes are considered to die massively by apoptosis or extended autophagy at the bottom of the growth plate [24, 25]. Cells marked by Col10a1-cre/creER alleles become osteoblasts in bone marrow within the primary spongiosa [28, 29]. However, descendants of these Col10a1-creER+ cells quickly disappear from the growth plate [28], indicating that Col10a1+ hypertrophic chondrocytes may behave as transient precursor cells with a limited lifespan. This is in sharp contrast with growth plate chondrocytes in the fetal stage, which exuberantly contribute to a large number of bone cells in the subsequent stage and persist widely in postnatal bones.
The identity of skeletal stem/progenitor cells that provide a robust source of osteoblasts and BMSCs in bone marrow during the postnatal stage remains largely undefined. BMSCs are undifferentiated mesenchymal cells of the bone marrow stromal compartment, which largely overlap with CAR cells and leptin receptor (LepR)+ cells (CXCL12+LepR+ cells) [54–56]. Of the two subsets of CAR cells (e.g. osteogenic and adipogenic), Adipo-CAR cells in the central marrow space, preferentially marked by Cxcl12-creER, are highly dormant and remain in the original domain of the marrow space during active bone growth, without producing new stromal cells in the newly formed domain of the marrow space in the metaphyseal region resulting from long-bone growth [57]. These findings indicate that as-yet unidentified skeletal stem/progenitor cells exist in the metaphyseal marrow space and provide a major source of dormant BMSCs in the growing portion of bone marrow. These bone marrow skeletal stem and progenitor cells might be at least partly derived from growth plate chondrocytes, and may overlap with Osx+ cells in the early postnatal stage, or Gli1+ cells in the juvenile stage that are predominantly located in the primary spongiosa [35, 58].
6. Conclusions and future directions
Taken together, current evidence from extensive in vivo lineage-tracing studies support the notion that diverse groups of chondrocytes and their precursors in the growth plate, including PTHrP+ chondrocytes in the resting zone, borderline chondrocytes, Col10a1+ hypertrophic chondrocytes (and their relatives) and Axin2+ cells in the outermost layer, collectively contribute to formation of bone cells, i.e. osteoblasts and BMSCs, particularly in the metaphyseal bone marrow. This establishes the concept that the transition from cartilage to bone is a biologically important process in the context of endochondral bone development. However, further research is needed to address remaining questions. Importantly, while fetal chondrocytes within the cartilage template can effectively transition to bone cells, PTHrP+ SSCs or other skeletal progenitor and precursor cells of the postnatal growth plate can contribute to bone cells much less effectively. It is possible that a majority of growth plate chondrocytes undergo apoptosis without arriving at the marrow space in the postnatal stage. In addition, borderline chondrocytes and hypertrophic chondrocytes are transient, as these cells quickly move away from the growth plate and translocate into the marrow space. Molecular mechanisms dictating their cell fate choice between survival and death during their transition to the marrow space remain largely undefined. In addition, cellular mechanisms underlying chondrocytes’ transition to osteoblasts or BMSCs are largely unclear. It is unknown whether chondrocytes revert into an intermediate skeletal progenitor cell-like state (dedifferentiation), or directly transform into osteoblasts or BMSCs (transdifferentiation) during their transition to the metaphyseal marrow space.
Thanks to the increasing availability of cell type-specific inducible creER mouse genetic tools, it is now possible to study closely the transition from growth plate chondrocytes to bone cells through more precise cell-fate analyses and functional studies. Future studies should elucidate cellular and molecular mechanisms underlying the transition of cartilage to bone and bone marrow.
Highlights.
PTHrP+ skeletal stem cells are maintained in the resting zone of the growth plate
Growth plate and perichondrium house distinct types of skeletal stem/progenitor cells
Growth plate stem/progenitor cells generate a variety of bone cells in bone marrow
Cartilage-to-bone transition is as an important mechanism to make new bone cells
Acknowledgement
This research was supported by grants from National Institute of Health (R01DE026666 to N.O., R03DE027421 to W.O., and the Japan Society for the Promotion of Science KAKENHI Grant JP19K19236 to Y.M.
Footnotes
Declaration of interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Kronenberg HM, Developmental regulation of the growth plate, Nature 423(6937) (2003) 332–6. [DOI] [PubMed] [Google Scholar]
- [2].Long F, Ornitz DM, Development of the endochondral skeleton, Cold Spring Harb Perspect Biol 5(1) (2013) a008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cepela DJ, Tartaglione JP, Dooley TP, Patel PN, Classifications In Brief: Salter-Harris Classification of Pediatric Physeal Fractures, Clin Orthop Relat Res 474(11) (2016) 2531–2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Salter RB HW, Injuries involving the epiphyseal plate., The Journal of Bone and Joint Surgery, 1963, pp. 587–622. [Google Scholar]
- [5].Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer M, Munnich A, Mutations in the gene encoding fibroblast growth factor receptor-3 in achondroplasia, Nature 371(6494) (1994) 252–4. [DOI] [PubMed] [Google Scholar]
- [6].Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ, Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia, Cell 78(2) (1994) 335–42. [DOI] [PubMed] [Google Scholar]
- [7].Ornitz DM, Legeai-Mallet L, Achondroplasia: Development, pathogenesis, and therapy, Dev Dyn 246(4) (2017) 291–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Silve C, Jüppner H, Ollier disease, Orphanet J Rare Dis 1 (2006) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J, The role of the resting zone in growth plate chondrogenesis, Endocrinology 143(5) (2002) 1851–7. [DOI] [PubMed] [Google Scholar]
- [10].Matsushita Y, Ono W, Ono N, Skeletal Stem Cells for Bone Development and Repair: Diversity Matters, Curr Osteoporos Rep (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Friedenstein AJ, Piatetzky-Shapiro II, Petrakova KV, Osteogenesis in transplants of bone marrow cells, J Embryol Exp Morphol 16(3) (1966) 381–90. [PubMed] [Google Scholar]
- [12].Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY, The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine, Nat Med 19(1) (2013) 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bianco P, “Mesenchymal” stem cells, Annu Rev Cell Dev Biol 30 (2014) 677–704. [DOI] [PubMed] [Google Scholar]
- [14].Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, Shimmura S, Miyawaki A, Nakagawa T, Suda T, Okano H, Matsuzaki Y, Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow, J Exp Med 206(11) (2009) 2483–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Breitbach M, Kimura K, Luis TC, Fuegemann CJ, Woll PS, Hesse M, Facchini R, Rieck S, Jobin K, Reinhardt J, Ohneda O, Wenzel D, Geisen C, Kurts C, Kastenmüller W, Hölzel M, Jacobsen SEW, Fleischmann BK, In Vivo Labeling by CD73 Marks Multipotent Stromal Cells and Highlights Endothelial Heterogeneity in the Bone Marrow Niche, Cell Stem Cell 22(2) (2018) 262–276.e7. [DOI] [PubMed] [Google Scholar]
- [16].Cuthbert R, Boxall SA, Tan HB, Giannoudis PV, McGonagle D, Jones E, Single-platform quality control assay to quantify multipotential stromal cells in bone marrow aspirates prior to bulk manufacture or direct therapeutic use, Cytotherapy 14(4) (2012) 431–40. [DOI] [PubMed] [Google Scholar]
- [17].Álvarez-Viejo M, Menéndez-Menéndez Y, Otero-Hernández J, CD271 as a marker to identify mesenchymal stem cells from diverse sources before culture, World J Stem Cells 7(2) (2015) 470–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Das B, Kashino SS, Pulu I, Kalita D, Swami V, Yeger H, Felsher DW, Campos-Neto A, CD271(+) bone marrow mesenchymal stem cells may provide a niche for dormant Mycobacterium tuberculosis, Sci Transl Med 5(170) (2013) 170ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Boxall SA, Jones E, Markers for characterization of bone marrow multipotential stromal cells, Stem Cells Int 2012 (2012) 975871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Coutu DL, Kokkaliaris KD, Kunz L, Schroeder T, Three-dimensional map of nonhematopoietic bone and bone-marrow cells and molecules, Nat Biotechnol 35(12) (2017) 1202–1210. [DOI] [PubMed] [Google Scholar]
- [21].Yang ZX, Han ZB, Ji YR, Wang YW, Liang L, Chi Y, Yang SG, Li LN, Luo WF, Li JP, Chen DD, Du WJ, Cao XC, Zhuo GS, Wang T, Han ZC, CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties, PLoS One 8(3) (2013) e59354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu WJ, Senarath-Yapa K, Chung MT, Marecic O, Tran M, Yan KS, Upton R, Walmsley GG, Lee AS, Sahoo D, Kuo CJ, Weissman IL, Longaker MT, Identification and specification of the mouse skeletal stem cell, Cell 160(1–2) (2015) 285–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, Ransom RC, Reinisch A, Wearda T, Murphy M, Brewer RE, Koepke LS, Marecic O, Manjunath A, Seo EY, Leavitt T, Lu WJ, Nguyen A, Conley SD, Salhotra A, Ambrosi TH, Borrelli MR, Siebel T, Chan K, Schallmoser K, Seita J, Sahoo D, Goodnough H, Bishop J, Gardner M, Majeti R, Wan DC, Goodman S, Weissman IL, Chang HY, Longaker MT, Identification of the Human Skeletal Stem Cell, Cell 175(1) (2018) 43–56.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Gibson G, Active role of chondrocyte apoptosis in endochondral ossification, Microsc Res Tech 43(2) (1998) 191–204. [DOI] [PubMed] [Google Scholar]
- [25].Shapiro IM, Adams CS, Freeman T, Srinivas V, Fate of the hypertrophic chondrocyte: microenvironmental perspectives on apoptosis and survival in the epiphyseal growth plate, Birth Defects Res C Embryo Today 75(4) (2005) 330–9. [DOI] [PubMed] [Google Scholar]
- [26].Ono N, Ono W, Nagasawa T, Kronenberg HM, A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones, Nat Cell Biol 16(12) (2014) 1157–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mizuhashi K, Ono W, Matsushita Y, Sakagami N, Takahashi A, Saunders TL, Nagasawa T, Kronenberg HM, Ono N, Resting zone of the growth plate houses a unique class of skeletal stem cells, Nature 563(7730) (2018) 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang L, Tsang KY, Tang HC, Chan D, Cheah KS, Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation, Proc Natl Acad Sci U S A 111(33) (2014) 12097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B, Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice, PLoS Genet 10(12) (2014) e1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Newton PT, Li L, Zhou B, Schweingruber C, Hovorakova M, Xie M, Sun X, Sandhow L, Artemov AV, Ivashkin E, Suter S, Dyachuk V, El Shahawy M, Gritli-Linde A, Bouderlique T, Petersen J, Mollbrink A, Lundeberg J, Enikolopov G, Qian H, Fried K, Kasper M, Hedlund E, Adameyko I, Sävendahl L, Chagin AS, A radical switch in clonality reveals a stem cell niche in the epiphyseal growth plate, Nature 567(7747) (2019) 234–238. [DOI] [PubMed] [Google Scholar]
- [31].Eames BF, de la Fuente L, Helms JA, Molecular ontogeny of the skeleton, Birth Defects Res C Embryo Today 69(2) (2003) 93–101. [DOI] [PubMed] [Google Scholar]
- [32].Hall BK, Miyake T, All for one and one for all: condensations and the initiation of skeletal development, Bioessays 22(2) (2000) 138–47. [DOI] [PubMed] [Google Scholar]
- [33].Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM, Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels, Dev Cell 19(2) (2010) 329–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Colnot C, Lu C, Hu D, Helms JA, Distinguishing the contributions of the perichondrium, cartilage, and vascular endothelium to skeletal development, Dev Biol 269(1) (2004) 55–69. [DOI] [PubMed] [Google Scholar]
- [35].Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, Ono N, Kronenberg HM, Frenette PS, Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development, Dev Cell 29(3) (2014) 340–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kronenberg HM, The role of the perichondrium in fetal bone development, Ann N Y Acad Sci 1116 (2007) 59–64. [DOI] [PubMed] [Google Scholar]
- [37].Minina E, Wenzel HM, Kreschel C, Karp S, Gaffield W, McMahon AP, Vortkamp A, BMP and Ihh/PTHrP signaling interact to coordinate chondrocyte proliferation and differentiation, Development 128(22) (2001) 4523–34. [DOI] [PubMed] [Google Scholar]
- [38].Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM, PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps, Development 129(12) (2002) 2977–86. [DOI] [PubMed] [Google Scholar]
- [39].Mak KK, Kronenberg HM, Chuang PT, Mackem S, Yang Y, Indian hedgehog signals independently of PTHrP to promote chondrocyte hypertrophy, Development 135(11) (2008) 1947–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P, SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse, Dev Biol 183(1) (1997) 108–21. [DOI] [PubMed] [Google Scholar]
- [41].Wright E, Hargrave MR, Christiansen J, Cooper L, Kun J, Evans T, Gangadharan U, Greenfield A, Koopman P, The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos, Nat Genet 9(1) (1995) 15–20. [DOI] [PubMed] [Google Scholar]
- [42].Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B, The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6, Genes Dev 16(21) (2002) 2813–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, de Crombrugghe B, Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors, Proc Natl Acad Sci U S A 102(41) (2005) 14665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Martin JF, Olson EN, Identification of a prx1 limb enhancer, Genesis 26(4) (2000) 225–9. [PubMed] [Google Scholar]
- [45].Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ, Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer, Genesis 33(2) (2002) 77–80. [DOI] [PubMed] [Google Scholar]
- [46].Matsushita Y, Nagata M, Welch J, Wong S, Ono W, Ono N, Notch effector Hes1 marks an early perichondrial population of skeletal progenitor cells at the onset of endochondral bone development, bioRxiv (2020). [Google Scholar]
- [47].Knoblich JA, Mechanisms of asymmetric stem cell division, Cell 132(4) (2008) 583–97. [DOI] [PubMed] [Google Scholar]
- [48].Morrison SJ, Kimble J, Asymmetric and symmetric stem-cell divisions in development and cancer, Nature 441(7097) (2006) 1068–74. [DOI] [PubMed] [Google Scholar]
- [49].Mizuhashi K, Nagata M, Matsushita Y, Ono W, Ono N, Growth Plate Borderline Chondrocytes Behave as Transient Mesenchymal Precursor Cells, J Bone Miner Res 34(8) (2019) 1387–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Hartmann C, Tabin CJ, Dual roles of Wnt signaling during chondrogenesis in the chicken limb, Development 127(14) (2000) 3141–59. [DOI] [PubMed] [Google Scholar]
- [51].Hill TP, Später D, Taketo MM, Birchmeier W, Hartmann C, Canonical Wnt/beta-catenin signaling prevents osteoblasts from differentiating into chondrocytes, Dev Cell 8(5) (2005) 727–38. [DOI] [PubMed] [Google Scholar]
- [52].Guo X, Day TF, Jiang X, Garrett-Beal L, Topol L, Yang Y, Wnt/beta-catenin signaling is sufficient and necessary for synovial joint formation, Genes Dev 18(19) (2004) 2404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Usami Y, Gunawardena AT, Francois NB, Otsuru S, Takano H, Hirose K, Matsuoka M, Suzuki A, Huang J, Qin L, Iwamoto M, Yang W, Toyosawa S, Enomoto-Iwamoto M, Possible Contribution of Wnt-responsive Chondroprogenitors to The Postnatal Murine Growth Plate, J Bone Miner Res (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, Ma’ayan A, Frenette PS, Differential cytokine contributions of perivascular haematopoietic stem cell niches, Nat Cell Biol 19(3) (2017) 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ, Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow, Cell Stem Cell 15(2) (2014) 154–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T, The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche, Immunity 33(3) (2010) 387–99. [DOI] [PubMed] [Google Scholar]
- [57].Matsushita Y, Nagata M, Kozloff KM, Welch JD, Mizuhashi K, Tokavanich N, Hallett SA, Link DC, Nagasawa T, Ono W, Ono N, A Wnt-mediated transformation of the bone marrow stromal cell identity orchestrates skeletal regeneration, Nat Commun 11(1) (2020) 332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Shi Y, He G, Lee WC, McKenzie JA, Silva MJ, Long F, Gli1 identifies osteogenic progenitors for bone formation and fracture repair, Nat Commun 8(1) (2017) 2043. [DOI] [PMC free article] [PubMed] [Google Scholar]