Abstract
Objective:
International appeals call for interventions to prevent aggression and other behavioral problems in individuals with dementia (IWD). Aggression Prevention Training (APT), based on intervening in 3 contributors to development of aggression (IWD pain, IWD depression and caregiver-IWD relationship problems) aims to reduce incidence of aggression in IWD over 1 year.
Design:
Randomized, controlled trial
Setting:
3 clinics that assess, diagnose and treat dementia
Participants:
228 caregiver-IWD dyads who screened positive for IWD pain, IWD depression or caregiver-IWD relationship problems randomized to APT or Enhanced Usual Primary Care (EU-PC).
Intervention:
APT, a skills-based intervention delivered over 3 months to address pain/depression/caregiver-IWD relationship issues. EU-PC included printed material on dementia and community resources; and 8 brief, weekly support calls.
Measurements:
The primary outcome was incidence of aggression over 1 year, determined by the Cohen Mansfield Agitation Inventory-Aggression Subscale. Secondary outcomes included pain, depression, caregiver-IWD relationship, caregiver burden, positive caregiving, behavior problems and anxiety.
Results:
Aggression incidence and secondary outcomes did not differ between groups. However, in those screening positive for IWD depression or caregiver-IWD relationship problems, those receiving EU-PC had significant increases in depression and significant decreases in quality of the caregiver-IWD relationship, whereas those receiving APT showed no changes in these outcomes over time.
Conclusions:
The cost to patients, family and society of behavioral problems in IWD, along with modest efficacy of most pharmacologic and nonpharmacologic interventions, calls for more study of novel preventive approaches.
Clinical Trial Registration:
Keywords: aggression, dementia, randomized controlled trial
Neuropsychiatric symptoms take a toll on all people facing dementia – individuals with dementia (IWD), family members, caregivers and community. Changes in cognition and personality result in loss and grief, but severe neuropsychiatric symptoms such as aggression can cause physical injury and psychological devastation among those in the lives of IWD.(1) Although it is well-known that most IWD have a clinically significant neuropsychiatric symptom, it is less appreciated that aggression occurs in 40%.(2,3) In a meta-analysis of 48 studies, aggression was the third most common neuropsychiatric symptom, trailing apathy and depression.(3) Verbal and physical aggression appear across care settings and dementia types.(4,5)
Etiologic-driven interventions for aggression are slowly replacing the widespread practice of nonetiologic treatment with antipsychotic medications, given their modest efficacy and substantial morbidity.(6) Genetic, neurobiologic and neuroimaging markers of aggression are understudied and, as yet, have not yielded actionable findings.(7) More evidence exists for pathophysiological and psychological determinants of aggression, including pain, depression and caregiver-IWD relationship quality.(8–11)
In the only nonpharmacological psychosocial intervention to target aggression specifically, 6–8 sessions that taught caregivers to address pain did not decrease 1-year incidence of aggression in Veterans with dementia. (12) Most other psychosocial interventions addressing a variety of neuropsychiatric symptoms lack a conceptual focus for treating the underlying causes of behavior, and none examine prevention. Weaker evidence of interventions to address behavioral and psychological symptoms in dementia supports sensory stimulation and cognitive/emotion-oriented interventions, and stronger evidence supports behavioral-management techniques and music therapy. (13,14) Clearly, evidence for use of nonpharmacologic interventions to treat and prevent aggression is lacking. (15)
Given the severe consequences of aggression and lack of effective pharmacologic and nonpharmacologic interventions, we pursued a preventive approach. Despite international (16) and national (17) recommendations to study prevention in mental health and dementia, no studies to date have examined prevention of aggression or other behavioral problems in IWD. Our prevention strategy is based on intervening in 3 common underlying contributors to the development of aggression (18) – IWD pain, IWD depression and caregiver-IWD relationship problems. We hypothesized that, among care recipient/caregiver dyads with at least 1 of these problems, those treated with 7 to 9 weekly sessions over 3 months with Aggression Prevention Training (APT), a skills-based intervention to address pain/depression/caregiver-IWD relationship issues, would be less likely to develop aggression over 1 year than those who received Enhanced Usual-Primary Care (EU-PC). We also examined between-group differences in improvements over time in overall pain, depression, quality of the caregiver-IWD relationship, caregiver burden, positive caregiving attributes, frequency of behavior problems and anxiety. Given that pain is one of the strongest predictors of development of aggression, (18,19) we then explored whether the effectiveness of APT (relative to EU-PC) on aggression and etiological secondary outcomes (i.e., overall pain, depression and caregiver burden) depended on whether the IWD screened positive for pain.
Methods
This randomized, controlled, single-blind trial received approval from the institutional review boards at Baylor College of Medicine and the Michael E. DeBakey Veterans Affairs Medical Center Research and Development Committee. Patient and caregiver participants provided informed consent and received $25 for each assessment at baseline, 3 (posttreatment), 6, and 12 months.
Participants
To be eligible, participants required a documented diagnosis of dementia from 1 of 3 partner clinics (Baylor College of Medicine Geriatric Medicine Associates, Baylor College of Medicine Alzheimer’s Disease and Memory Disorders Center or Kelsey-Seybold Clinics). These clinics were selected because they have appropriate expertise in assessing, diagnosing and treating dementia and have an adequate pool of demographically heterogeneous patients. Patients with International Classification of Diseases, Ninth and Tenth Editions codes for dementia were recruited through direct provider referral and from administrative databases via partial HIPAA waiver; and 2,230 potential participants received telephone screening. Using a screening script, we briefly described the study to the IWD and obtained permission also to interview his/her caregiver. Verbal consent for screening was obtained from both the IWD and caregiver. IWD were included if they were community dwelling, had an unpaid caregiver who had contact with the IWD for at least 2 days and at least 8 hours per week and were willing to participate in the study, and screened positive for at least 1 of 3 risk factors for the development of aggression (pain, depression, caregiver-IWD relationship strain). IWD screened positive for pain if they or their caregiver indicated that the IWD often had physical pain, body aches, and/or discomfort over the past 4 weeks; had at least moderate pain overall over the past several weeks; or had pain that interfered at least some with daily activities.(20) IWD screened positive for depression if they or their caregiver indicated that the IWD often felt downhearted and blue (21) and positive for caregiver-IWD relationship strain if they or their caregiver indicated concerns about tension or strain in their relationship.(22) IWD who were aggressive during the month prior to screening or baseline, based on the Cohen-Mansfield Agitation Inventory (CMAI)—Aggression Subscale,1 were excluded;(23,24) as were those with severe dementia, based on a Functional Assessment Staging Scale (FAST[25]) score > 6; inability to complete the Memory Impairment Screen—Telephone Version (MIS-T[26]); or inability to rate their level of pain, using a pain thermometer.
Randomization
Participants meeting inclusion criteria were assigned randomly after baseline assessment to APT or EU-PC in random blocks of 6 or 8, using a random numbers generator, with assignments placed in concealed envelopes that the research manager (TE) opened in sequential order when participants were randomized.
Measures and Data Collection
Outcome measures were administered by telephone by an independent evaluator blinded to random assignment. The primary outcome was incidence of aggression over 1 year, determined by the caregiver-administered CMAI-Aggression Subscale, comprising 13 aggressive behaviors scored by frequency and disruptiveness. (23,24) For aggression to be present, at least 1 behavior had to be present and disruptive. Secondary outcomes included overall pain, IWD depression, quality of the caregiver-IWD relationship, caregiver burden, positive caregiving attributes, frequency of behavior problems, and anxiety. All measures were completed solely by the caregiver, with the exception of overall pain, which was also completed by the IWD. Overall pain, IWD depression and quality of the caregiver-IWD relationship were considered etiological secondary outcomes. As with prior work, (27) overall pain was assessed with 1 item on the Philadelphia Geriatric Pain Intensity Scale (20) that was answered independently by both the IWD and his/her caregiver. Specifically, IWDs and their caregivers were each asked “Thinking about the past several weeks, please rate how bad your [your loved one’s] pain has been overall” on a 0–5 scale where 0 = “no pain” to 5 = “the pain is almost unbearable.” A pain thermometer was also used as a visual aid, as even individuals with severe dementia can complete the pain thermometer (28). Depression was assessed with the 30-item Geriatric Depression Scale (29) administered to the caregiver) (30). Caregiver burden was assessed by caregiver report on the 22-item Zarit Burden Scale) (31). The quality of the caregiver-IWD relationship was assessed by caregiver report on the 15-item Mutuality Scale, in which respondents rate each item (e.g., “How close do you feel to him/her,” “To what extent do you enjoy the time the two of you spend together”) on a 5 point scale, where 0 = “Not at all” and 5 = “A great deal” (32). Positive caregiving attributes (e.g., “Providing help to my loved one has made me feel more useful,” “Providing help to my loved one has enabled me to appreciate life more”) were assessed by caregiver report on the 9-item Positive Aspects of Caregiving scale, with responses from 1 = “Disagree a Lot” to 5 = “Agree a Lot.” (33) Frequency of behavior problems was assessed with the Revised Memory and Behavior Checklist, (34) and anxiety was assessed with the 5-item Geriatric Anxiety Index – Short Form (35), both administered to the caregiver.
Research assessments of severe levels of pain, aggression and depression symptoms were reported to the participant’s healthcare provider at baseline and at 3, 6 and 12 months.
Interventions
The APT intervention was developed by the investigators with input from an expert panel composed of established intervention researchers in dementia care. The intervention is rooted in the Unmet Needs Model (36) and other currently accepted frameworks for understanding behavioral problems, such as the Antecedent-Behavior- Consequences approach, (37) which underscores the importance of the interaction of individuals and their environment. APT and EU-PC were delivered over a period of up to 3 months by licensed providers with behavioral health expertise, including a masters-level social worker, an advance practice nurse, a registered nurse, as well as trainees in psychology and medicine. In both interventions, participants received a book, Pain Management for Older Adults: A Self-help Guide, (38) and a link to Continuing Medical Education on management of pain in IWD was sent to their healthcare providers. Dyads randomized to APT received 6 to 8 weekly skills training sessions in their home and a telephone wrap-up session, each lasting an average of 45 minutes. APT sessions began immediately following the baseline assessment and had to be completed prior to the 3-month assessment. Four “core” APT sessions covered dementia education, identification and management of pain, improvement of IWD-caregiver communication skills, and behavioral activation through increased pleasant activity planning. During the first session, two to four additional elective sessions were chosen by the IWD and/or caregiver through collaborative goal setting addressing medical treatment for pain and distress, communication problems and challenges, making daily activities more comfortable and enjoyable, and rest and relaxation strategies. The intervention was primarily geared toward the caregiver; but IWD involvement was encouraged, depending on dementia severity and willingness. Sessions included didactics, skill-building, discussion, and role-playing.
Caregivers receiving EU-PC received a booklet from the National Institute on Aging on memory problems, community resources for IWD and caregivers, (39) and 8 brief, weekly calls (average 7 minutes) to query symptom severity, ascertain needs for immediate psychiatric care, and provide minimal support. If caregivers reported pain or other physical or psychological symptoms in the IWD, they were encouraged to address them with their primary care providers.
Statistical Analyses
Study completers were those who completed either the 6- or 12-month assessment or both. We compared differences in demographics and baseline clinical characteristics between study completers and non-completers, using chi-square tests (for categorical variables) and independent samples t-tests (for continuous variables). However, when distributional assumptions were violated, a nonparametric Fishers Exact or Wilcoxon Mann-Whitney U test was conducted. Furthermore, baseline differences between the APT and EU-PC subgroups in demographics and clinical characteristics were evaluated with chi-square tests and independent samples t-tests (or Fishers Exact or Wilcoxon Mann-Whitney U test, where appropriate).
Univariate time-to-event analyses were performed, using discrete-time Cox regression models to evaluate differences between the APT and EU-PC subgroups in the primary outcome of incidence of aggression (yes/no) over time (3, 6 and 12 months). For participants who dropped out, their time until study attrition was used as the exposure period; and they were carried forward as part of the overall incidence count. The effect of treatment group tests the primary hypothesis of interest, as a significant treatment effect indicates a between-group (APT vs EU-PC) difference in time until incidence of aggression.
Differences between APT and EU-PC in change over time (baseline, and 3, 6 and 12 months) for our secondary outcomes of overall pain (as reported by both the IWD and caregiver), IWD depression, caregiver-perceived quality of the caregiver-IWD relationship, caregiver burden, positive caregiving attributes, frequency of behavior problems and anxiety were evaluated using individual linear growth curve models (SAS Proc Mixed, SAS Institute, Inc., Cary, NC) with an autoregressive covariance structure type. Hierarchical linear models account for both various lengths of follow-up for each participant and missing observations not due to attrition. For each of the 8 secondary outcomes, 2 models were conducted. The first (main-effects model) included the main effects of assessment time and treatment group, as well as the respective baseline value for a given outcome. The second (2-way interaction model) included all of the main effects from the first model plus the interaction between treatment group and assessment time. The interaction between treatment group and assessment time was used to evaluate our main hypotheses about secondary outcomes, as a significant interaction indicates that the linear change in the outcome over time varies between APT and EU-PC. Significant interactions between time and treatment group were followed up with simple slopes analyses to examine the effect of time for each APT and EU-PC.
Sample-size calculations were performed, based on the ability to detect a small-to-moderate difference (Cohen’s h = 0.4) in rate of aggression onset (the primary outcome) over a 1-year period between those who received APT and those who received EU-PC, assuming 80% power and a type I error rate of 5%. Given an anticipated rate of aggression onset over a 1-year period of 37% for the control group (18), an effect size of h = 0.4 allows detection of a rate of aggression onset in APT as high as 19%. Given this effect size and potential for up to 10% attrition, our goal was to include 220 total participants so that a minimum of 200 would be available after attrition.
Examination of Pain Screen as a Moderator
Whether the IWD screened positive for pain was examined as a moderator of the relation between treatment group and change over time in aggression and the etiological secondary outcomes of IWD depression and caregiver-perceived quality of the caregiver-IWD relationship. For aggression, pain assessment at screening and its interaction with treatment group were added to the Cox model to examine their effects on the outcome. For depression and quality of the caregiver-IWD relationship, 3 individual linear growth curve models were conducted, using similar models with SAS Proc Mixed, described above. However, the main-effects models also included pain screen, the 2-way interaction models also included the interactions between pain screen and time and pain screen and treatment group, and a 3-way interaction model was conducted that included the interaction between pain screen, time and treatment group.
Results
Figure 1 summarizes participant flow during the study. A total of 228 caregiver-IWD dyads were randomized to APT (n = 114) or EU-PC (n = 114). The total percent of study non-completers was 20.2% (N = 46), similar for APT (n = 25, 21.9%) and EU-PC (n = 21, 18.4%), X2(1) = 0.44, p = 0.51. Relative to non-completers, completers had a higher income (X2(2) = 6.92, p = 0.03), were less likely to be black (X2(3) = 7.81, p = 0.05), had a lower total burden score (t(226) = 2.56, p = 0.01), had a higher MIS-t score (t(225) = −2.46, p = 0.01), and had a lower FAST stage score (t(226) = 2.23, p = 0.02). Completers and non-completers did not significantly differ on any other patient/caregiver demographics or baseline clinical characteristics.
Figure 1.
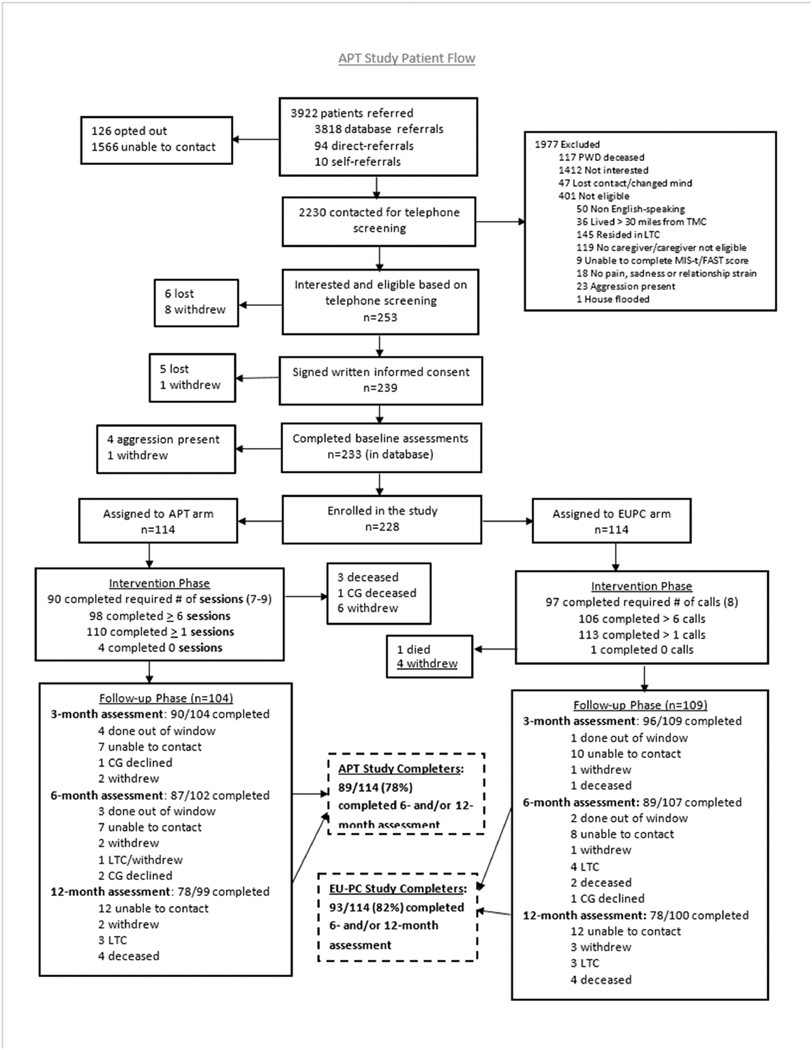
Flow of Participants through the Course of the Study
Sample and Treatment Characteristics
Table 1 presents IWD and caregiver demographics. APT (n = 114) and EU-PC (n = 114) dyads were similar in IWD and caregiver demographics, with the exception that fewer patients in APT had an income at least $50,000 (APT: 19.17% and EU-PC: 26.32%; X2(2) = 6.34, p = 0.04) and fewer patients in APT had previously attended a support group or adult day care (APT: 6.14% and EU-PC: 14.91%; X2(1) = 4.66, p = 0.03). Table 2 presents baseline clinical characteristics for IWDs and their caregivers. Baseline clinical characteristics were similar in both groups. Each screening measure (i.e., pain, depression, and relationship strain) was considered to be endorsed if either the IWD or his/her caregiver responded affirmatively. While 176 (77.19%) of the dyads endorsed the pain screen, 136 (59.65%) endorsed the depression screen, and 120 (52.63%) endorsed the relationship strain screen. Whereas 80 (35.09%) dyads endorsed only 1 screen, 92 (40.35%) endorsed 2, and 56 (24.56%) endorsed all 3.
Table 1.
Baseline Demographics and Clinical Characteristics for IWDs and their Caregivers (N = 228, unless reported otherwise)
| IWD Demographics | Caregiver Demographics | |
|---|---|---|
| Males, N (%) | 131 (57.46) | 51 (22.37) |
| Age (years), mean (SD) | 77.64 (8.97) | 68.17 (11.25) |
| Education, N (%) | ||
| Did not complete HS | 26 (11.40) | 7 (3.07) |
| Completed HS diploma or GED | 45 (19.74) | 41 (17.98) |
| Completed at least some college | 157 (68.86) | 180 (78.95) |
| Race/ethnicity, N (%) | ||
| Non-Hispanic White | 153 (67.11) | 150 (65.79) |
| Black | 48 (21.05) | 46 (20.18) |
| Hispanic | 21 (9.21) | 24 (10.53) |
| Other | 6 (2.63) | 8 (3.51) |
| Annual Net Income (N=193 for IWD and N = 197 for caregivers),N (%) | ||
| <$20,000 | 63 (32.64) | 58 (29.44) |
| $20,000–$49,999 | 93 (48.19) | 77 (39.09) |
| >=$50,000 | 37 (19.17) | 62 (31.47) |
| Number of people living with IWD, mean (SD) | 1.35 (0.98) | N/A |
| IWD Healthcare Use (in the 3-months prior to baseline) | ||
| Any ER visit, N (%) | 42 (18.42) | |
| Any hospitalization, N (%) | 19 (8.33) | N/A |
| Attended senior day care or support group, N % | 24 (10.53) | |
| Current IWD Medication Use | ||
| Any psychotropic medications, N (%) | 102 (44.74) | |
| Any pain medications, N (%) | 68 (29.82) | N/A |
| Any dementia medications, N (%) | 133 (58.33) | |
| MIS-t Total, mean (SD) | 3.88 (2.72) | N/A |
| Referral Source, N (%) | ||
| Kelsey | 161 (70.61) | |
| BCM Geriatrics | 29 (12.72) | N/A |
| BCM Alzheimer’s Center | 38 (16.67) | |
| Lives with IWD, N (%) | N/A | 200 (87.72) |
| Relationship to IWD, N (%) | ||
| Spouse | 157 (68.86) | |
| Other family | N/A | 66 (28.95) |
| Nonfamily | 5 (2.19) | |
Table 2.
Baseline Clinical Characteristics
| Mean (SD) | |
|---|---|
| FAST | 4.46 (0.94) |
| IWD Overall Pain Severity (IWD-reported) | 1.25 (1.18) |
| IWD Overall Pain Severity (Caregiver-reported) | 1.30 (1.12) |
| GDS | 11.49 (6.36) |
| Mutuality | 2.99 (0.78) |
| Caregiver Burden | 27.39 (15.13) |
| PAC | 24.03 (9.25) |
| RMBC Total Frequency | 7.85 (3.62) |
| GAI | 1.62 (1.79) |
APT = Aggression Prevention Training; EU=PC = Enhanced Usual Care-Primary Care; FAST = Functional Assessment Staging Scale; GDS = Geriatric Depression Scale; PAC = Positive Aspects of Caregiving scale; RMBC = Revised Memory and Behavior Checklist; GAI = Geriatric Anxiety Inventory; IWD = individual with dementia; All measures were caregiver-rated, with the exception of IWD-reported pain severity.
Participants in APT received between 0 and 9 sessions, with an average of 6.67 sessions (SD = 2.22), and those in EU-PC received between 0 and 8 calls, with an average of 7.43 calls (SD = 1.68). All sessions and calls were audiotaped, and a random 10% were reviewed by 2 independent treatment integrity raters (JF [APT group] and SR [EU-PC group]). APT sessions were reviewed for adherence (0 [no adherence] to 8 [optimal adherence]) and competence (0 [no competency] to 8 [excellent competency]). Ratings suggest good adherence (7.24 [SD: 0.92]) and competency (6.81 [SD: 0.95]). EU-PC calls were reviewed for adherence to 6 to 10 specific aspects of the protocol, including symptom assessment, encouragement to contact the primary care physician and crisis management, and the use of other therapeutic techniques beyond supportive listening. Ratings suggested that adherence was excellent (97.77%, SD: 5.56).
Primary Outcome
Incidence of aggression was not different for participants who received APT and those who received EU-PC (n = 29, 29.9% versus n = 23, 23.0%, respectively; X2(1) = 1.21, p = 0.27). Furthermore, contrary to our primary hypothesis, discrete time Cox regression analysis revealed no statistically significant difference between APT and EU-PC in time until incidence of aggression, X2(1) = 1.12, p = 0.29, Hazard Ratio = 1.34 (95% CI = 0.78, 2.32).
Secondary Outcomes
For each secondary outcome, we examined the main effects of each assessment time and treatment group, as well as the interaction between assessment time and treatment group (Table 3). There were significant positive main effects of assessment time for both depression and anxiety, such that (regardless of treatment group) depression and anxiety increased linearly over time. Additionally, there were significant negative main effects of assessment time for both frequency of behavioral problems and the caregiver’s perceptions of the quality of the relationship, such that (regardless of treatment group) these outcomes decreased linearly over time. However, contrary to our hypotheses, linear changes in these secondary outcomes over time did not vary between APT and EU-PC (i.e., there were no interactions between treatment group and assessment time). There was not a significant linear change over time (i.e., no main effect of time) for overall pain (IWD- or caregiver-reported), positive aspects of caregiving, and caregiver burden. Furthermore, there were no differences between treatment groups (i.e., no main effect of treatment group) for any secondary outcome.
Table 3.
Results of Multilevel Models for Eight Separate Secondary Outcomes
| Outcomes | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IWD-reported Overall Pain | Caregiver-reported Overall Pain | GDS | Mutuality | Caregiver Burden | Positive Aspects of Caregiving | RMBC Total Frequency | GAI | |||||||||
| F statistic * | p- value | F statistic * | p- value | F statistic * | p- value | F statistic * | p- value | F statistic * | p- value | F statistic * | p- value | F statistic * | p- value | F statistic * | p-value | |
| Step 1: Main Effects | ||||||||||||||||
| Baseline for each Outcome | 559.0 | <0.001 | 683.3 | <0.001 | 1303.8 | <0.001 | 2078.6 | <0.001 | 2289.08 | <0.001 | 937.47 | <0.001 | 729.87 | <0.001 | 1038.12 | <0.001 |
| Treat ment Group (0= EUPC,1= APT) | 0.05 | 0.83 | 0.84 | 0.36 | 1.65 | 0.20 | 0.01 | 0.97 | 3.28 | 0.07 | 0.79 | 0.38 | 0.14 | 0.71 | 2.63 | 0.11 |
| Assessment Time (0= baseline, 1= 3mo, 2= 6mo, 4= 12mo) | 1.53 | 0.21 | 2.14 | 0.09 | 4.63 | 0.0 03 | 5.43 | 0.0 01 | 2.12 | 0.09 | 0.57 | 0.64 | 5.53 | 0.0 01 | 2.75 | 0.04 |
| Step 2: Interaction** | ||||||||||||||||
| Treatment Group by Assessment Time | 0.21 | 0.89 | 0.56 | 0.64 | 1.48 | 0.22 | 0.38 | 0.77 | 1.59 | 0.19 | 2.43 | 0.06 | 0.33 | 0.80 | 0.83 | 0.48 |
Numerator (num) and denominator (den) degrees of freedom for each effect are as follows: Main effect of Baseline for each outcome and Main effect of Treatment Group: numdf = 1, dendf = 225; Main effect of Assessment Time: numdf = 3, dendf = 513; Interaction between Treatment Group and Assessment Time: numdf = 3, dendf = 510
Interaction models include all main effects from Step 1 (i.e., Baseline for each outcome, treatment group, and assessment time) as well as the treatment group by time interaction effect. GDS = Geriatric Depression Scale; RMBC = Revised Memory and Behavior Checklist, GAI = Geriatric Anxiety Inventory; IWD = individual with dementia; Baseline for each outcome = baseline IWD-reported overall pain, baseline caregiver-reported overall pain, baseline GDS, baseline mutuality, baseline caregiver burden, baseline positive aspects of caregiving, baseline RMBC total frequency, or baseline GAI.
Pain Screen as a Moderator
Discrete time Cox regression revealed that group differences in time until incidence of aggression did not vary, based on whether the IWD screened positive for pain, X2(3) = 3.07, p = 0.38, Hazard Ratio for the interaction between treatment group and pain screen = 0.58 (95% CI = 0.13, 2.64).
There were significant 3-way interactions between pain screen, assessment time and treatment group predicting each IWD depression (F(3, 504) = 3.42, p = 0.02) and caregiver burden (F(3, 502) = 2.89, p = 0.04). Among those who screened positive for pain (n = 176, 77.19%), treatment group and time did not interact to predict any outcome. However, among those who did not screen positive for pain (n = 52, 21.81%), there was a significant interaction between treatment group and time predicting depression (F(3, 111) = 3.33, p = 0.02) and caregiver-perceived quality of the relationship (F(3,111) = 3.04, p = 0.03). Specifically, those receiving EU-PC had significant increases in depression and significant decreases in the quality of the caregiver-IWD relationship (effect of time for depression: F(3, 69) = 3.96, p = 0.01 and effect of time for mutuality: F(3, 69) = 3.60, p = 0.02), whereas those receiving APT showed no changes in these outcomes over time (depression: F(3,42) = 1.21, p = 0.32; mutuality: F(3, 42) = 1.78, p = 0.16).
Discussion
In a sample of persons with dementia at increased risk for aggression, we found no significant differences in aggression incidence between intervention and control groups. Preventing aggression is a high priority, given its physical and emotional toll on caregivers; but there is no indication that APT for Caregivers is effective in preventing aggression. The absence of effect suggests, among other possibilities, that 1) the intervention is not effective in modifying the chosen risk factors of pain, depression, and caregiver-IWD relationship problems; 2) other risk factors warrant intervention beyond the chosen risk factors; 3) aggression occurs at random, regardless of putative risk factors; or 4) measurement issues, including single item screens for depression and relationship strain, as well as assessment largely based on caregiver-report, may have hindered identification of risk factors and increased the likelihood that IWDs were at only minimal risk of developing aggressive behaviors.
APT compared to a control intervention did not have any effects on secondary outcomes, including pain, depression or caregiver perceptions of the relationship. However, in those screening positive for caregiver-IWD relationship problems and/or depression but not pain, those in APT had better outcomes in caregiver-IWD relationship and depression. Perhaps this indicates that depression and caregiver-IWD relationship issues are more amenable to nonpharmacologic interventions than pain. However, interventions for depression or caregiver-IWD relationship to decrease or prevent aggression have not been studied; whereas studies that intervene on pain to decrease aggression have received some attention.
In our own work, a psychosocial skills-based caregiver intervention to better identify and treat pain to prevent aggression was not effective. (12) However, analgesic interventions, largely beneficial in decreasing pain, have not been examined in preventing aggression. (40) Closely partnering with medical providers to facilitate a variety of medical interventions (not just analgesics, but also devices such as transcutaneous electrical nerve stimulation units, physical therapy/restorative exercise programs, massage, acupuncture) might be a fruitful avenue. Similarly, use of antidepressant medication in depressed IWD deserves study, given the compelling link of depression to aggression. (11)
Examining other risk factors as intervention foci might also be fruitful. For example, the APT intervention did not focus on behavioral cause-and-effect skill-building for caregivers beyond specific examples related to pain. The ABC method (antecedent, behavior, consequence) might be an important additional skill for caregivers, as might be a better understanding of restructuring environments to control aggression triggers (e.g., overstimulation due to noise). (41) A related limitation of our study was that effects of the intervention may have been blunted by including patients with any of 3 risk factors, or underpowered for examination of effects on each of the risk factors separately (an analysis that would have been helpful in understanding the lack of APT effect). Future studies should be cautious in addressing more than 1 risk factor or be powered to look at each of them.
Another limitation of this study includes almost exclusive use of caregivers as proxies for measures of patient psychologic and behavioral constructs. Although this is a standard approach in many studies of IWD and was a more pragmatic and cost-effective method, this method may allow triangulation of multiple sources of information that may yield different results. For example, in-home assessments would have allowed direct interview of IWDs, use of observational instruments, use of multiple caregiver informants, and use of instruments that allow ratings based on caregiver and IWD responses and clinical observation during interview (e.g., Rating Anxiety in Dementia, Cornell Scale for Depression in Dementia).(42 ) With increasing availability of technology-enabled assessment of psychologic and behavioral outcomes,(43) these should be considered in future studies.
Prevention strategies are rarely studied in mental health, particularly in older adults and IWD. The cost to patients, family and society of behavioral problems in IWD, along with the modest efficacy of most pharmacologic and nonpharmacologic interventions, calls for much more study of novel preventive approaches. (44)
Highlights.
Can a caregiver-directed skills-based intervention prevent (the development of) aggressive behavior in individuals with dementia?
The caregiver-directed skills-based intervention did not reduce (the development of) aggressive behavior over time compared to a group that received usual care and printed resource materials.
Given the gravity of aggressive behavior and the modest efficacy of available pharmacologic and nonpharmacologic interventions, novel preventive approaches are urgently needed.
Funding Acknowledgments:
This work was supported by National Institute of Nursing Research Grant no. 5R)1NR014657 to Mark E. Kunik, MD, MPH, and partly supported by the use of facilities and resources of the Houston VA HSR&D Center for Innovations in Quality, Effectiveness and Safety (CIN13-413) and the South Central Mental Illness Research, Education and Clinical Center.
Footnotes
Aggression was measured on a 7-point Likert scale for frequency and a 5-point Likert scale for disruptiveness. Aggression was considered present if a participant scored a 2 (< once per week) or above on frequency and a 2 (a little) or above on disruptiveness on any of 13 aggressive behaviors.
The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs, the US government or Baylor College of Medicine.
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production processerrors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fauth EB, Gibbons A: Which behavioral and psychological symptoms of dementia are the most problematic? Variability by prevalence, intensity, distress ratings, and associations with caregiver depressive symptoms. Int J Geriatr Psychiatry 2014; 29(3):263–71. [DOI] [PubMed] [Google Scholar]
- 2.Borsje P, Lucassen PLBJ, Wetzels RB, et al. : Neuropsychiatric symptoms and psychotropic drug use in patients with dementia in general practices. Fam Pract 2018; 35(1): 22–28, [DOI] [PubMed] [Google Scholar]
- 3.Zhao QF, Tan L, Wang HF, et al. : The prevalence of neuropsychiatric symptoms in Alzheimer’s disease: Systematic review and meta-analysis. J Affect Disord 2016; 190:264–271. Erratum in: J Affect Disord. 2016; 206:8. [DOI] [PubMed] [Google Scholar]
- 4.Perri R, Monaco M, Fadda L, et al. : Neuropsychological correlates of behavioral symptoms in Alzheimer’s disease, frontal variant of frontotemporal, subcortical vascular, and lewy body dementias: A comparative study. J Alzheimers Dis 2014; 39(3):669–77. [DOI] [PubMed] [Google Scholar]
- 5.Lyketsos CG, Lopez O, Jones B, et al. : Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: Results from the Cardiovascular Health Study. JAMA 2002; 288(12):1475–83. [DOI] [PubMed] [Google Scholar]
- 6.Fazio S, Pace D, Maslow K, et al. : Alzheimer’s Association Dementia Care Practice Recommendations. Gerontologist 2018; 58(suppl_1):S1–S9. [DOI] [PubMed] [Google Scholar]
- 7.Gotovac K, Nikolac Perković M, et al. : Biomarkers of aggression in dementia. Prog Neuropsychopharmacol Biol Psychiatry 2016; 69:125–30. [DOI] [PubMed] [Google Scholar]
- 8.Kolanowski A, Boltz M, Galik E, et al. : Determinants of behavioral and psychological symptoms of dementia: A scoping review of the evidence. Nurs Outlook 2017; 65(5):515–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Husebo BS, Ballard C, Cohen-Mansfield J, et al. : The response of agitated behavior to pain management in persons with dementia. Am J Geriatr Psychiatry 2014; 22(7):708–17. [DOI] [PubMed] [Google Scholar]
- 10.Morgan RO, Sail KR, Snow AL, et al. : Modeling causes of aggressive behavior in patients with dementia. Gerontologist 2013; 53(5):738–47. [DOI] [PubMed] [Google Scholar]
- 11.de Mauleon A, Soto M, Ousset PJ, et al. : Potentially modifiable factors associated withagitation and aggression in Alzheimer’s disease: Results of the ICTUSstudy. Int Psychogeriatr 2019; 31 (10);1509–16 [DOI] [PubMed] [Google Scholar]
- 12.Kunik ME, Snow AL, Wilson N, et al. : Teaching caregivers of persons with dementia to address pain. Am J Geriatr Psychiatry 2017; 25(2):144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abraha I, Rimland JM, Trotta FM, et al. : Systematic review of systematic reviews of nonpharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open 2017; 7(3):e012759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaviola MA, Inder KJ, Dilworth S, et al. : Impact of individualised music listening intervention on persons with dementia: A systematic review of randomised controlled trials. Australas J Ageing 2019. March 26. doi: 10.1111/ajag. [DOI] [PubMed] [Google Scholar]
- 15.Brasure M, Jutkowitz E, Fuchs E, et al. : Nonpharmacologic Interventions for Agitation and Aggression in Dementia [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. March. [PubMed] [Google Scholar]
- 16.British Medical Association. Tackling the causes. Promoting public mental health and investing in prevention; London, 2018. Last accessed October 1, 2019 Available at https://www.bma.org.uk/-/media/files/pdfs/collective%20voice/policy%20research/public%20and%20population%20health/mental%20health/tackling%20the%20causes%20promoting%20public%20mental%20health%20and%20investing%20in%20prevention%20bma%20oct%202018.pdf?la=en [Google Scholar]
- 17.Muñoz RF, Mrazek PJ, Haggerty RJ: Institute of Medicine report on prevention of mental disorders: Summary and commentary. Am Psychol 1996; 51(11): 1116–1122. [DOI] [PubMed] [Google Scholar]
- 18.Kunik ME, Snow AL, Davila JA, et al. : Causes of aggressive behavior in patients with dementia. J Clin Psychiatry 2010; 71(9), 1145–1152. [DOI] [PubMed] [Google Scholar]
- 19.Habiger TF, Flo E, Achterberg WP, et al. : The interactive relationship between pain, psychosis, and agitation in people with dementia: Results from a cluster-randomised clinical trial. Behav Neurol 2016; 7036415. Epub 2016 May 9. doi: 10.1155/2016/7036415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmelee PA, Smith B, Katz IR. Pain complaints and cognitive status among elderly institution residents. J Am Geriatr Soc 1993. ;41:517–522. [DOI] [PubMed] [Google Scholar]
- 21.Martinez de la Iglesia J, Onis Vilches MC, Duenas Herrero R, et al. : Abbreviating the brief. Approach to ultra-short versions of the Yesavage questionnaire for the diagnosis of depression. Aten Primaria 2005; 35(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bass DM, Judge KS, Snow AL et al. Negative caregiving effects among caregivers of veterans with dementia. Am J Geriatr Psychiatry 2012; 20(3):239–247. [DOI] [PubMed] [Google Scholar]
- 23.Cohen-Mansfield J Agitated behaviors in the elderly II. Preliminary results in the cognitively deteriorated. J Am Geriatr Soc 1986;34:722–727. [DOI] [PubMed] [Google Scholar]
- 24.Shahar K, Snow AL, Souchek J, et al. : Cut point definition of agitation. Clin Gerontol 2004; 27:15–23. [Google Scholar]
- 25.Reisberg B, Sclan SG, Franssen E, et al. : Dementia staging in chronic care populations. Alzheimer Dis Assoc Disord 1994; 1:S188–S205. [PubMed] [Google Scholar]
- 26.Lipton RB, Katz MJ, Kuslansky G, et al. : Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc 2003; 51(10):1382–90. [DOI] [PubMed] [Google Scholar]
- 27.Snow AL, Dani R, Souchek J, et al. : Comorbid psychological symptoms and quality of life in patients with dementia. Am J Geriatr Psychiatry 2005;13(5):393–401. [DOI] [PubMed] [Google Scholar]
- 28.Roland M, Morris R: A study of the natural history of low-back pain, part II: Development of guidelines for trials of treatment in primary care. Spine 1983;8:145–50. [DOI] [PubMed] [Google Scholar]
- 29.Yesavage JA, Brink TL, Rose TL, et al. : Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 1982-1983; 17(1):37–49. [DOI] [PubMed] [Google Scholar]
- 30.Burke WJ, Houston MJ, Boust SJ, et al. : Use of the Geriatric Depression Scale in dementia of the Alzheimer type. J Am Geriatr Soc 1989; 37(9):856–860. [DOI] [PubMed] [Google Scholar]
- 31.Zarit SH, Anthony CR, Boutselis M: Interventions with care givers of dementia patients: Comparison of two approaches. Psychol Aging 1987; 2:225–232. [DOI] [PubMed] [Google Scholar]
- 32.Archbold PG, Stewart BJ. Family Caregiving Inventory. Portland, OR: Department of Family Nursing, School of Nursing, Oregon Health Sciences University; 1986. [Google Scholar]
- 33.Tarlow BJ, Wisniewski SR, Belle SH, et al. : Positive aspects of caregiving, contributions of the REACH project to the development of a new measure for Alzheimer’s caregiving. Res Aging 2004; 26:429–453. [Google Scholar]
- 34.Teri L, Truzx P, Uomoto RGJ, et al. : Assessment of behavioral problems in dementia: The revised Memory and Behavioral Problems Checklist. Psychol Aging 1992; 7:630–631. [DOI] [PubMed] [Google Scholar]
- 35.Byrne GJ, Pachana NA: Development and validation of a short form of the Geriatric Anxiety Inventory–the GAI-SF. Intern Psychogeriatr 2011; 23(1):125–131. [DOI] [PubMed] [Google Scholar]
- 36.Cohen-Mansfield J. Theoretical framework for behavioral problems in dementia. Alzheimer’s Care Q 2000;1:8–21. [Google Scholar]
- 37.Beck CR, Vogelpohl TS, Rasin JH. Effects of behavioral interventions on disruptive behavior and affect in demented nursing home residents. Nurs Res 2002;51:219–28. [DOI] [PubMed] [Google Scholar]
- 38.Hadjistavo-Poulos T, Hadjistavo-Poulos HD (eds). Pain management for older adults: A self-help guide. 1st ed Seattle: International Association for the Study of Pain; 2008. [Google Scholar]
- 39.National Institute on Aging. Understanding Memory Loss. What To Do When You Have Trouble Remembering. NIH Publication No. 10–5442. National Institutes of Health and National Institute. US Department of Health and Human Services; Silver Spring, MD, September 2010. [Google Scholar]
- 40.Tampi RR, Hassell C, Joshi P, et al. : Analgesics in the management of behavioral and psychological symptoms of dementia: A perspective review. Drugs Context 2017; 6:212508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teri L, Logsdon RG: Assessment and management of behavioral disturbances in Alzheimer’s disease. Compr Ther 2000; 26(3), 169–175. [DOI] [PubMed] [Google Scholar]
- 42.Alexopoulos GS, Abrams RC, Young RC, et al. : Cornell Scale for Depression in Dementia. Biol Psychiatry 1988; 23(3):271–84. [DOI] [PubMed] [Google Scholar]
- 43.Teipel S, König A, Hoey J, et al. : Use of nonintrusive sensor-based information and communication technology for real-world evidence for clinical trials in dementia Alzheimers Dement 2018; 14(9):1216–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gitlin LN, Kales HC, Marx K, et al. : A randomized trial of a web-based platform to help families manage dementia-related behavioral symptoms: The WECare AdvisorTM. Contemp Clin Trials 2017; 62:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]


