Abstract
Anterior uveitis is the most common anatomic subset of uveitis. We used fluorescence activated cell sorting to characterize T cell cytokine expression in stimulated T cell subsets from patients with HLA B27-associated acute anterior uveitis (AAU) (n=4) compared to healthy controls (n= 14) or subjects with axial spondyloarthritis (n=6). Positive findings among subjects with AAU included a statistically significant increase in stimulated granulocyte-macrophage colony stimulating factor (GM-CSF), IL-17, and IL-22 synthesized by CD8 cells, a trend for stimulated ILC (innate lymphoid cells)-3 cells to synthesize more IL-22 (p=0.07), and stimulated MAIT (mucosa associated innate lymphoid cells)-like cells that express the T cell receptor V alpha 7.2 to express IL-17A, IL-17F, and IL-22 in a greater percentage of cells relative to controls. IL-17F, GM-CSF, and IL-22 represent potentially novel targets in AAU. Our report is arguably the first to implicate IL-17F or ILC-3 and MAIT cells in the pathogenesis of AAU.
Keywords: acute anterior uveitis, spondyloarthritis, cytokine, interleukin, innate lymphoid cell, MAIT cell
INTRODUCTION
Uveitis is a leading cause of acquired blindness (1). The term, uveitis, is used to describe multiple different patterns of inflammation within the eye. Uveitis has distinct subsets and it follows that different forms of inflammation within the eye will differ in terms of pathogenesis. In epidemiologic studies, about 80% of uveitis is anterior (2, 3). Among patients who have acute anterior uveitis in Europe or North America, about 50% express the class I major histocompatibility complex marker, HLA B27 (4). And among those who are HLA B27 positive, approximately 80% have some associated spondyloarthropathy (5). Conversely, if followed over a lifetime, approximately half of all patients with ankylosing spondylitis will have at least one episode of acute anterior uveitis (6).
The immune system is often conceptualized as having two arms, an innate component and an acquired or adaptive component. The latter contributors, primarily T and B lymphocytes, can undergo somatic gene rearrangements to make highly targeted immune responses. But the immune system also includes additional cells that have the light microscopic appearance of lymphocytes, but these lymphoid cells have limited or no ability to make gene rearrangements. These cells are often called innate lymphoid cells. Examples include natural killer cells (NKs), mucosal associated immune T cells (MAITs), and ILCs (innate lymphoid cells) which have been subdivided into ILC-1, 2, and 3 on the basis of the primary cytokines each produces. Natural killer cells express killer immunoglobulin-like receptor (KIR), polymorphic receptors which interact with major histocompatibility complex (MHC) class I. HLA B27 can dimerize on the cell surface and thus activate KIR (7). This has helped lead to a recognition that NK cells could contribute to HLA B27-related diseases such as ankylosing spondylitis (8). Much less is known about MAIT cells or ILC subsets in spondyloarthritis, except for a small number of studies now implicating ILC-3 cells (9, 10). We are unaware of prior studies which have sought to investigate a role for MAIT cells or ILCs in the pathogenesis of acute anterior uveitis.
METHODS;
Subjects:
Subjects with disease in this study had a confirmed diagnosis of recent onset (<7 days) acute anterior uveitis based slit lamp examination, or a confirmed diagnosis of axial spondyloarthritis(AxSpA) diagnosis meeting ASAS criteria (Assessment of Ankylosing Spondylitis) (11). All of the subjects with AAU denied a history of chronic back pain. All of the subjects with AxSpA had a prior history of AAU. Subjects with AAU or AxSpA were HLA B27 positive based on testing performed by a clinical laboratory. For subjects with AxSpa, the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) (12) was 3.0 or greater. All subjects were older than 18 years of age and each had a body mass index of <30. Subjects were excluded for a history of intestinal surgery, for antibiotic use within 3 months of enrollment, for pregnancy, and for an acute intestinal illness within the previous month. Subjects in this study were also enrolled in a study on the microbiome. Several of the inclusion and exclusion criteria such as body mass index, use of antibiotics, and history of intestinal surgery are based on these parameters having a potential effect on the microbiome. Healthy controls were recruited from study advertisements (flyers, OHSU research participation opportunities website, Spondylitis Association of America advertisement), the comprehensive ophthalmology clinic, laboratory staff and their friends and family, and family members of diseased participants. All subjects were provided with informed consent. The study was approved by the OHSU Institutional Review Board. The Study complied with the Declaration of Helsinki and the Code of Ethics of the World Medical Association.
Preparation and stimulation of PBMCs:
Blood samples were collected in a sterile cell preparation tube and centrifuged at 1300g for 20 min. After removing the top plasma layer, the white peripheral blood mononuclear cell (PBMC) layer on top was removed, quantified with Trypan blue, resuspended in freezing media (90% fetal bovine serum (FBS) and 10% dimethylsulfoxide (DMSO)) with 5~10 million cells/ml of freezing media and cryopreserved first in Mr. Frosty freezing container (Thermo Fisher Scientific) at −80 degrees Celsius and then in liquid nitrogen. For stimulation, cryopreserved PBMC were thawed rapidly, washed and resuspended in 5ml of R10 media. PBMCs were quantified with Trypan blue, and seeded into 96-well plates at 1× 106 cells/well and stimulated for 4 hours with phorbol 12-myristate 13-acetate (PMA) (1 μg/ml; Sigma-Aldrich, St Louis, MO, USA), Ionomycin (5 μg/ml; Sigma-Aldrich, St Louis, MO, USA), and brefeldin A (3 μg/ml; Thermo Fisher Scientific, Waltham, MA, USA).
Antibodies and reagents:
The antibodies used for staining in the T cell panel were Brilliant Violet (BV) 785-labelled anti-CD3 (BioLegend, San Diego, CA, USA), Brilliant Ultraviolet (BUV) 496-labelled anti-CD4 (BD Biosciences, Mountain View, CA, USA), BUV 510-labelled anti-CD8a (BioLegend), Allophycocyanin (APC)-labelled anti-CD45 (Thermo Fisher Scientific), BUV605-labelled anti-TcR Vα7.2 (BioLegend), phycoerythrin (PE)-labelled anti-TCRγδ (BioLegend), Alexa Fluor 700-labelled anti-IFNγ (BioLegend), BV711-labelled anti-IL-17A (BioLegend), Fluorescein isothiocyanate (FITC)-labelled anti-IL-17F (Miltenyl Biotec, Bergisch Gladbach, Germany), phycoerythrin-cyanine 7 (PE-Cy7)-labelled anti-IL-22 (Thermo Fisher Scientific), Peridinin chlorophyll protein-Cy5.5 (PerCP-Cy5.5)-labelled anti-GM-CSF (BioLegend), BUV737-labelled anti-integrin β7 (BD Biosciences, Franklin Lakes, NJ, USA), Peridinin-chlorophyll-protein complex (PerCP)-labelled anti-CD196 (chemokine receptor 6) (CCR6) (R&D Systems, Minneapolis, MN, USA), and PE/Dazzle-labelled anti-CD199(CCR9) (BioLegend).
For the innate lymphoid cell panel, the following antibodies were used: lineage markers ( a cocktail of PE-labelled anti-CD5(BioLegend), PE-labelled anti-CD11b (BioLegend), PE-labelled anti-CD11c (BD Biosciences), PE-labelled anti-CD14 (BioLegend), PE-labelled anti-CD16 (BioLegend), PE-labelled anti-CD19 (BioLegend), PE-labelled anti-CD34 (Thermo Fisher Scientific), PE-labelled anti-CD123 (BioLegend), PE-labelled anti-FcɛR1α (BioLegend)), BV510-labelled anti-CD45 (BioLegend), BUV496-labelled anti-CD4 (BD Biosciences), BV650-labelled anti-CD127(IL-7Rα) (BioLegend), PE/Dazzle labelled anti-CD161 (BioLegend), phycoerythrin-cyanine 7 (PE-Cy5)-labelled anti-CD117(c-kit)(Thermo Fisher Scientific), APC-labelled anti-CD336 (NKp44) (BioLegend), BV605-labelled anti-CD294(CRTH2) (BioLegend), Alexa Fluor 700-labelled anti-IFNγ (BioLegend), BV711-labelled anti-IL-17A (BioLegend), FITC-labelled anti-IL-17F (Miltenyl Biotec), PE-Cy7-labelled anti-IL-22 (Thermo Fisher Scientific), PerCP-Cy5.5-labelled anti-GM-CSF(BioLegend), BUV737-labelled anti-integrin β7 (BD Biosciences), PerCP-labelled anti-CD196(CCR6) (R&D Systems).
Cell sorting:
This study relied on FACS Symphony (BD Bioscience) which allows multi-parametric flow cytometry. We devised a panel to identify immune dysregulation in subjects with AAU or AxSpa using antibodies shown in Table 1 and as described above. We analyzed peripheral blood mononuclear cells (PBMC) for conventional (CD8+ and CD4+ T cells) and non-conventional lymphoid populations (ILC-3s and MAIT cells). ILC-3 cells were CD3+, CD127+, CRTH2-, cKit+, NPK44+. MAIT cells were positive for the T cell receptor, V alpha 7.22. Frequency values were calculated by subtracting unstimulated cell per cents from stimulated values.
Table 1.
Peripheral blood mononuclear cell staining panels for T cells and innate lymphoid cells.
| T cell panel | Innate Lymphoid cell panel |
|
|---|---|---|
| Surface markers | Surface markers | |
| CD45, CD3, CD4, CD8, TCRγδ, TcR Vα7.2 | CD45, CD4, CD127 (IL-7R), CD161, cKIT, NKp44, CRTH2 (PGD2-R) | |
| Effector Cytokines | Effector Cytokines | |
| IFNγ, IL-17A, IL-17F, IL-22 GM-CSF | IFNγ, IL-17A, IL-17F, IL-22 GM-CSF | |
| Gut homing | Gut homing | |
| β7 integrin, CCR6, CCR9 | β7 integrin, CCR6 | |
| Lineage markers(−) | ||
| CD5, CD11b, CD11c, CD14, CD16, CD19, CD34, CD123, FcεR1 |
Statistics:
Data were analyzed using FlowJo v10 and Prism 8 software. Kruskal-Wallis test with Dunn’s multiple comparison used for one-way ANOVA tests. The associations between data and diagnosis groups were analyzed using the zero adjusted gamma regression including age as a confounding factor. The computations were done by the gamlss package (13) for R statistical language (http://www.r-project.org).
RESULTS:
The demographics of the subjects are shown in Table 2. Healthy controls were not HLA typed. The frequency of HLA B27 in Portland, Oregon is approximately 7%, meaning that there was a reasonable likelihood that at least one of the controls was HLA B27+. All of the 6 patients with AxSpa had a prior history of AAU. One of the 6 patients with AxSpa had active AAU at the time of blood draw. Grouping this subject with the AxSpa subjects or with the AAU subjects did not change any of the statistics that are presented below. The patients, especially those with axial spondyloarthropathy, tended to be older than the controls. As a consequence, we used linear regression to exclude age as an important variable contributing to the differences we observed among groups. The groups also differed in usage of biologics with four of the six AxSpa patients receiving a TNF inhibitor. We compared those receiving a biologic to those not receiving a biologic for all the markers discussed below and could find no statistically significant differences, but the numbers studied were small.
Table 2.
Demographics of subjects.
| Demographics | ||||
|---|---|---|---|---|
| Healthy Control | Acute Anterior Uveitis (AAU) |
Axial Spondylarthritis (AxSpA) + Acute Anterior Uveitis (AAU) |
p-value | |
| Sample Type | Blood | Blood | Blood | |
| Subjects (n) | 14 | 4 | 6 | |
| Gender Male (n/%) | 8 (57.1) | 2 (50.0) | 5 (83.3) | 0.46091 |
| HLA-B27 Positive (n/%) | 0 (0); 14 n.d | 4 (100) | 6 (100) | |
| Age (Mean ± SD) | 38.3 ± 10.9 | 39.1 ± 1.4 | 53.5 ± 15.4 | 0.05082 |
| BMI (Mean ± SD) | 24.8 ± 6.7 | 25.6 ± 4.4 | 28.3 ± 4.5 | 0.57552 |
| Biologies (n/%) | 0 (0) | 0 (0) | 4 (66.7) | 0.00071 |
n.d, Not Determined.
Chi-Square
One Way-ANOVA
As shown in Figure 1, stimulated CD8 cells from patients with AAU had a greater increase in the expression of IL-17A, IL-22, or granulocyte-macrophage (GM-CSF) than control cells from healthy individuals or cells from patients with AxSpA. CD8 cells from this patient group also tended to increase IL-17F more than controls but these changes did not reach statistical significance. Because of data which implicate the microbiome of the gut in the pathogenesis of AAU and AxSpa (14), we thought that gut homing receptors might be elevated. The data shown in Figure 2 did not confirm this hypothesis.
Figure 1.

Frequency of cytokine production by stimulated CD8 T cells. HC=healthy controls. AAU-acute anterior uveitis. AxSpA-axial spondyloarthritis. ND-not detected. *p<0.05. ***p<0.001.
Figure 2.
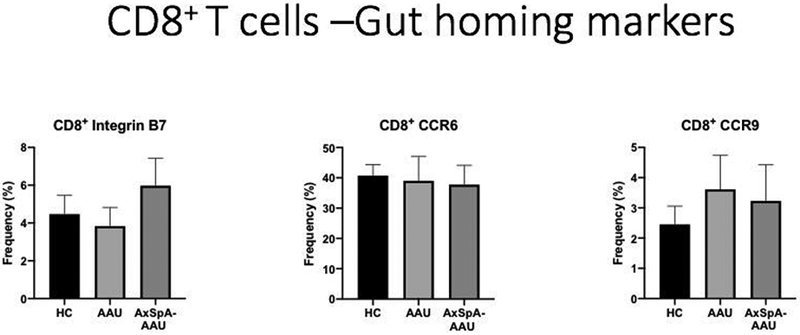
Frequency of gut homing receptors detected on stimulated CD8 lymphocytes. HC=healthy controls. AAU-acute anterior uveitis. AxSpA-axial spondyloarthritis.
As shown in Figure 3, like CD8 cells, stimulated CD4 cells from patients with AAU had similar trends to increased cytokine production frequency compared to CD8 cells, but these changes did not reach statistical significance. As shown in Figure 4, the CCR9 receptor which binds CCL25 (thymus expressed chemokine) and which has been implicated in migration of lymphocytes to the gut (15) was increased in CD4 cells from patients with AAU, although the comparison did not reach statistical significance.
Figure 3.
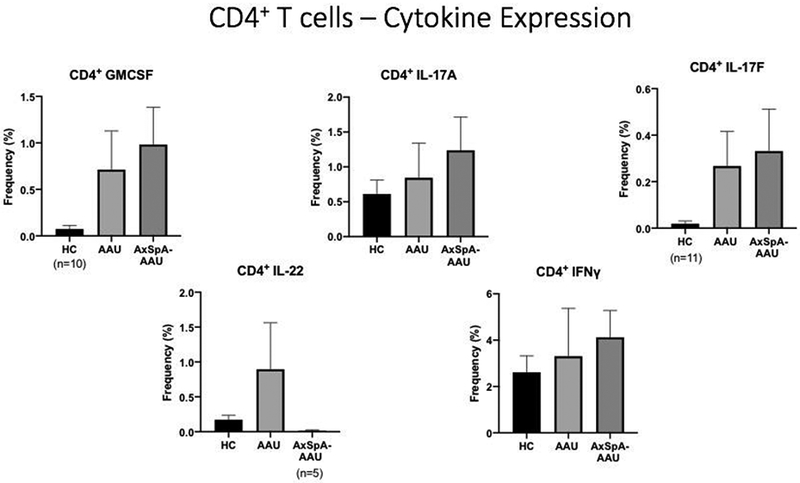
Frequency of cytokine production by stimulated CD4 cells. HC=healthy controls. AAU-acute anterior uveitis. AxSpA-axial spondyloarthritis.
Figure 4.

Frequency of gut homing receptors expressed by stimulated CD4 cells. HC=healthy controls. AAU-acute anterior uveitis. AxSpA-axial spondyloarthritis.
Figure 5 shows the markers used to identify ILC-3 lymphocytes. This figure also shows that ILC-3 cells from patients with AxSpa had an increase in the expression of IL-22 after stimulation, but this was of borderline statistical significance (p=0.07). We also examined cytokine production by ILC-1 and ILC-2 cells, but we did not find differences comparing healthy controls to subjects with either acute anterior uveitis or axial spondyloarthropathy. Figure 6 shows that stimulated lymphocytes that express the T cell receptor, TCR alpha v7.22 from patients with AAU produced increased IL-22, IL-17A, and IL-17F compared to healthy controls and there was also a trend to synthesize increased GM-CSF. The beta 7 integrin dimerizes with the alpha 4 integrin to make a gut homing receptor (15). Surprisingly, as shown in Figure 7, this was reduced among subjects with AAU, but the change did not reach statistical significance.
Figure 5.

The strategy to isolate ILC-3 cells is based on Krabbendam et.al. (35). Differences in the frequency of expression for other cytokines or homing receptors were not detected on ILC3 cells and no differences among the 3 groups were found for ILC1 or ILC2 cells. HC=healthy controls. AAU-acute anterior uveitis. AxSpA-axial spondyloarthritis. SSC-side light scatter. FSC-forward light scatter.
Figure 6.
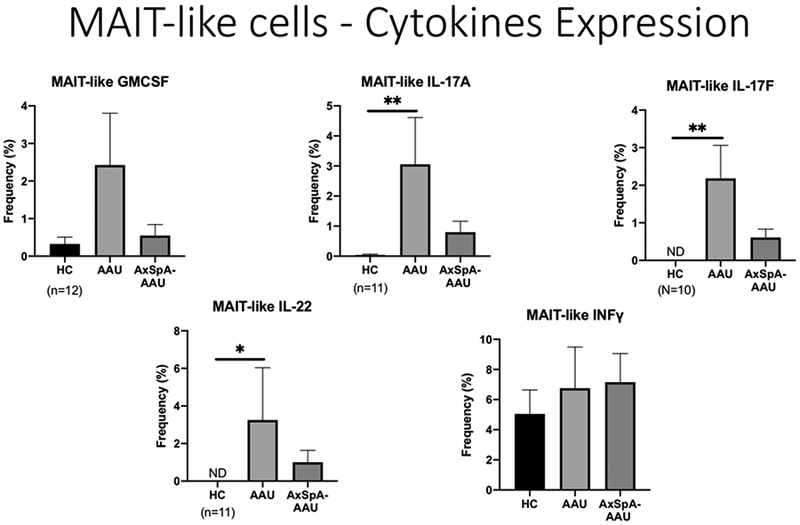
Frequency of cytokine production by stimulated MAIT-like cells which express the TCR V alpha 7.22. HC=healthy controls. AAU-acute anterior uveitis. AxSpA-axial spondyloarthritis. *p<0.05. **p<0.01.
Figure 7.
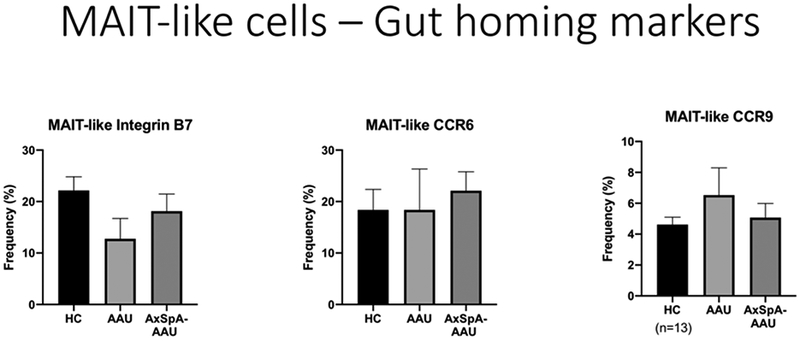
Frequency of gut homing receptor expression by stimulated MAIT-like cells which express the TCR V alpha 7.22. HC=healthy controls. AAU-acute anterior uveitis. AxSpA-axial spondyloarthritis.
DISCUSSION:
This investigation has produced a number of novel observations. First we observed an increase in IL-22 production by CD8 cells, and MAIT-like cells in subjects with active, recent acute anterior uveitis. These subjects were HLA B27 positive, but none provided a history of inflammatory, low back arthritis that would be indicative of a spondyloarthropathy. Increased IL-22 has previously been reported in the ileum of patients with spondyloarthropathy (8). ILC3 cells from patients with AAU did not produce increased IL-22, but we did find such an increase among subjects with axial spondyloarthropathy. IL-22 is a cytokine with both beneficial effects such as stimulating the production of anti-microbial peptides like defensins and inflammatory effects such as stimulating the cellular proliferation that can characterize atopic dermatitis (16). IL-22 is especially produced by T cells that synthesize IL-17. IL-22 has been implicated in the uveitis associated with Behcet’s disease (17) and in animal models of uveitis (18-20). Several studies have failed to find increased IL-22 in the aqueous humor of patients with uveitis (21, 22). It could be that IL-22 is more important in the systemic immune response that leads to uveitis as opposed to the local immune response.
Second, we noted increased IL-17A production by CD8 cells from subjects with AAU. An increase in IL-17A and IL-17F synthesis was noted among MAIT-like cells with AAU. IL-17 has been strongly implicated in spondyloarthritis (23), in rodent models of uveitis (24, 25), and in patients with uveitis (26). However, clinical trials to block IL-17 as a treatment for uveitis have yielded inconsistent results (26, 27). An abstract presentation has suggested that blocking IL-17 in patients with spondyloarthritis might reduce the frequency of recurrent AAU (Deodhar, A, presented at EULAR (European League Against Rheumatism), Amsterdam, June, 2018). We believe that this is the first study to implicate IL-17F in the pathogenesis of uveitis. We noted an increase in IL-17F frequency among stimulated MAIT-like cells. IL-17 has several isoforms including predominantly IL-17A and IL-17F. Most clinical trials to date have targeted just IL-17A. A bispecific antibody, bimekizumab, that targets both IL-17A and IL-17F, has shown promising results in a number of clinical trials including one for the treatment of ankylosing spondylitis (28). Our observations support the rationale to use a bispecific antibody.
Third, this is one of the first studies to implicate GM-CSF in the pathogenesis of acute anterior uveitis. At least two monoclonal antibodies to GM-CSF have shown promise in the treatment of rheumatoid arthritis (29, 30). These antibodies have not been studied in uveitis or in spondyloarthropathy to our knowledge.
Fourth, this is the first study to our knowledge that has investigated MAIT-like cells or ILC-3 cells, two important populations of innate lymphoid cells, in acute anterior uveitis. We refer to the cells that we have studied as MAIT-like because they express the T cell receptor, V alpha 7.22 as is typical of MAIT cells. We recognize, however, that some conventional T cells might also express this receptor and thus we cannot say definitively that they are MAIT cells. The data do show, however, that cells expressing this receptor and deriving from subjects with AAU expressed an increased frequency of IL-17A, IL-17F, and GM-CSF after stimulation. MAIT cells have a number of interesting features. They are found predominantly at mucosal surfaces (31). They have a very limited T cell receptor repertoire (31). They appear to respond to derivatives of vitamin B (31). We and others have implicated the intestinal microbiome in the pathogenesis of AAU (32). An increase in circulating MAIT cells could be due to activation in the intestine.
Just as we suspected that MAIT cells might be involved in AAU, we reasoned that ILC-3 cells, because of their importance in the mucosal immune system and because of their production of IL-17, would be activated in AAU patients. Although we found these cells to have increased IL-22 production in axial spondyloarthritis, we could not demonstrate this among patients with AAU. Both AAU and axial spondyloarthritis share many predisposing genes in common, but there also are cytokine related genes that distinguish AAU from axial spondyloarthritis (6). A larger study with longitudinal data should be done to determine if patients with AAU truly have no increased synthesis of IL-22 by ILC-3 cells.
Finally, ours is one of the first studies to analyze gut homing receptors in AAU. The relative specificity of adhesion molecules such as alpha 4 beta 7 integrin has led to the targeting of adhesion molecules to treat diseases such as inflammatory bowel disease. One prior study found an increase in the gut homing molecule CCR6 among lymphocytes from patients with patients with non-infectious uveitis (33). AAU was not studied as a specific entity. Vedolizumab, which blocks alpha 4 beta 7 integrin, has been successful in the treatment of inflammatory bowel disease, but it is less successful in the treatment of extra-intestinal manifestations of IBD (34). Our inability to detect an increase in gut homing receptors is consistent with this clinical observation.
Certainly, our study has limitations. We have performed multiple statistical comparisons and as an exploratory study, we have not corrected for the number of comparisons. The size of our groups is small and the groups differ some in terms of age and medications. As noted above longitudinal observations should be endeavored. Despite these limitations, we believe that this study has produced some highly original observations that are worthy of confirmation and additional pursuit. Our data suggest novel potential targets to treat AAU including MAIT cells, GM-CSF, and IL-17F.
Acknowledgements:
We are grateful to Tammy Martin and Stephen Planck who provided assistance with formatting.
Funding: This study was funded in part by NIH Grant, EY 029266, EY026572, and EY010572. We also acknowledge support from the William and Mary Bauman Family Foundation, the Stan and Madelle Rosenfeld Family Trust, Research to Prevent Blindness, and the Grandmaison Fund for Autoimmunity Research. The funding sources were not involved in the collection or interpretation of data and they did not participate in the writing of this manuscript.
Abbreviations:
- AAU
acute anterior uveitis
- AxSpA
axial spondyloarthritis
- BASDAI
Bath ankylosing spondylitis disease activity index
- CCR
chemokine receptor
- DMSO
dimethylsulfoxide
- EULAR
European League Against Rheumatism
- FACS
fluorescence activated cell sorter
- FBS
fetal bovine serum
- FSC
forward light scatter
- GM-CSF
granulocyte-macrophage colony stimulating factor
- HC
healthy control
- ILC
innate lymphoid cel
- KIR
killer immunoglobulin receptor
- MAIT
mucosal associated immune T cel
- ND
not detected
- NK
natural killer cell
- OHSU
Oregon Health & Science University
- PBMC
peripheral blood mononuclear cell
- SSC
side light scatter
- TCR
T cell receptor
Footnotes
The authors declare no competing interests. Unrelated to this report, Dr. Rosenbaum receives clinical trial support from Pfizer, royalties from UpToDate, and consulting fees from AbbVie, Gilead, Janssen, Novartis, Roche, Celldex, Corvus, Horizon, and UCB.
References
- 1.Nussenblatt RB. The natural history of uveitis. Int Ophthalmol. 1990;14(5–6):303–8. Epub 1990/10/01. PubMed PMID: 2249907. [DOI] [PubMed] [Google Scholar]
- 2.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology. 2004;111(3):491–500; discussion Epub 2004/03/17. doi: 10.1016/j.ophtha.2003.06.014. PubMed PMID: 15019324. [DOI] [PubMed] [Google Scholar]
- 3.Thorne JE, Suhler E, Skup M, Tari S, Macaulay D, Chao J, Ganguli A. Prevalence of Noninfectious Uveitis in the United States: A Claims-Based Analysis. JAMA Ophthalmol. 2016;134(11):1237–45. doi: 10.1001/jamaophthalmol.2016.3229. PubMed PMID: 27608193. [DOI] [PubMed] [Google Scholar]
- 4.Brewerton DA, Webley M, Ward AM. Acute anterior uveitis and the fourteenth chromosome. Advances Inflam Res. 1985;9:225–9. [Google Scholar]
- 5.Juanola X, Loza Santamaria E, Cordero-Coma M, Group SW. Description and Prevalence of Spondyloarthritis in Patients with Anterior Uveitis: The SENTINEL Interdisciplinary Collaborative Project. Ophthalmology. 2016;123(8):1632–6. doi: 10.1016/j.ophtha.2016.03.010. PubMed PMID: 27084561. [DOI] [PubMed] [Google Scholar]
- 6.Robinson PC, Claushuis TA, Cortes A, Martin TM, Evans DM, Leo P, Mukhopadhyay P, Bradbury LA, Cremin K, Harris J, Maksymowych WP, Inman RD, Rahman P, Haroon N, Gensler L, Powell JE, van der Horst-Bruinsma IE, Hewitt AW, Craig JE, Lim LL, Wakefield D, McCluskey P, Voigt V, Fleming P, Spondyloarthritis Research Consortium of Canada A-A-ASCIGoASCWTCCSMD-E, Degli-Esposti M, Pointon JJ, Weisman MH, Wordsworth BP, Reveille JD, Rosenbaum JT, Brown MA. Genetic dissection of acute anterior uveitis reveals similarities and differences in associations observed with ankylosing spondylitis. Arthritis & rheumatology. 2015;67(1):140–51. doi: 10.1002/art.38873. PubMed PMID: 25200001; PMCID: PMC4302162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taurog JD. The role of HLA-B27 in spondyloarthritis. J Rheumatol. 2010;37(12):2606–16. Epub 2010/12/03. doi: 10.3899/jrheum.100889. PubMed PMID: 21123333. [DOI] [PubMed] [Google Scholar]
- 8.Ciccia F, Accardo-Palumbo A, Alessandro R, Rizzo A, Principe S, Peralta S, Raiata F, Giardina A, De Leo G, Triolo G. Interleukin-22 and interleukin-22-producing NKp44+ natural killer cells in subclinical gut inflammation in ankylosing spondylitis. Arthritis Rheum. 2012;64(6):1869–78. Epub 2012/01/04. doi: 10.1002/art.34355. PubMed PMID: 22213179. [DOI] [PubMed] [Google Scholar]
- 9.Ciccia F, Guggino G, Rizzo A, Saieva L, Peralta S, Giardina A, Cannizzaro A, Sireci G, De Leo G, Alessandro R, Triolo G. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann Rheum Dis. 2015;74(9):1739–47. doi: 10.1136/annrheumdis-2014-206323. PubMed PMID: 25902790. [DOI] [PubMed] [Google Scholar]
- 10.Mauro D, Macaluso F, Fasano S, Alessandro R, Ciccia F. ILC3 in Axial Spondyloarthritis: the Gut Angle. Curr Rheumatol Rep. 2019;21(7):37. Epub 2019/06/15. doi: 10.1007/s11926-019-0834-9. PubMed PMID: 31197599. [DOI] [PubMed] [Google Scholar]
- 11.Rudwaleit M, van der Heijde D, Landewe R, Akkoc N, Brandt J, Chou CT, Dougados M, Huang F, Gu J, Kirazli Y, Van den Bosch F, Olivieri I, Roussou E, Scarpato S, Sorensen IJ, Valle-Onate R, Weber U, Wei J, Sieper J. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann Rheum Dis. 2011;70(1):25–31. doi: 10.1136/ard.2010.133645. PubMed PMID: 21109520. [DOI] [PubMed] [Google Scholar]
- 12.Calin A, Dijkmans B, Emery P, Hakala M, Kalden J, Leirisalo M, Mola EM, Salvarani C, Sanmarti R, Sany J, Sibilia J, Sieper J, Van der Linden S, Veys E, Appel M, Pedersen R, Fatenejad S. Assessments of disease activity and functionality by enbrel-treated ankylosing spondylitis patients in a multicenter, placebo-controlled trial. Arthritis Rheum. 2003;48(9):S172. [Google Scholar]
- 13.Rigby RA, Stasinopoulos DM. Generalized additive models for location, scale, and shape. J R Stat Soc, C (Applied Statistics). 2005;54:507–54. [Google Scholar]
- 14.Rosenbaum JT, Asquith M. The microbiome and HLA-B27-associated acute anterior uveitis. Nature reviews Rheumatology. 2018. Epub 2018/10/12. doi: 10.1038/s41584-018-0097-2. PubMed PMID: 30301938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzutsev A, Hogg A, Sui Y, Solaymani-Mohammadi S, Yu H, Frey B, Wang Y, Berzofsky JA. Differential T cell homing to colon vs. small intestine is imprinted by local CD11c(+) APCs that determine homing receptors. J Leukoc Biol. 2017;102(6):1381–8. Epub 2017/09/28. doi: 10.1189/jlb.1A1116-463RR. PubMed PMID: 28951425; PMCID: PMC5669635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouyang W, O’Garra A. IL-10 Family Cytokines IL-10 and IL-22: from Basic Science to Clinical Translation. Immunity. 2019;50(4):871–91. Epub 2019/04/18. doi: 10.1016/j.immuni.2019.03.020. PubMed PMID: 30995504. [DOI] [PubMed] [Google Scholar]
- 17.Sugita S, Kawazoe Y, Imai A, Kawaguchi T, Horie S, Keino H, Takahashi M, Mochizuki M. Role of IL-22- and TNF-alpha-producing Th22 cells in uveitis patients with Behcet’s disease. J Immunol. 2013;190(11):5799–808. Epub 2013/05/01. doi: 10.4049/jimmunol.1202677. PubMed PMID: 23630362; PMCID: PMC3659956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y, Kim TW, Park YS, Jeong EM, Lee DS, Kim IG, Chung H, Hwang YI, Lee WJ, Yu HG, Kang JS. The Role of Interleukin-22 and Its Receptor in the Development and Pathogenesis of Experimental Autoimmune Uveitis. PLoS One. 2016;11(5):e0154904. Epub 2016/05/12. doi: 10.1371/journal.pone.0154904. PubMed PMID: 27166675; PMCID: PMC4864334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhuyan ZA, Asanoma M, Iwata A, Ishifune C, Maekawa Y, Shimada M, Yasutomo K. Abrogation of Rbpj attenuates experimental autoimmune uveoretinitis by inhibiting IL-22-producing CD4+ T cells. PLoS One. 2014;9(2):e89266. Epub 2014/03/04. doi: 10.1371/journal.pone.0089266. PubMed PMID: 24586644; PMCID: PMC3938452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugita S, Kawazoe Y, Imai A, Usui Y, Takahashi M, Mochizuki M. Suppression of IL-22-producing T helper 22 cells by RPE cells via PD-L1/PD-1 interactions. Invest Ophthalmol Vis Sci. 2013;54(10):6926–33. Epub 2013/09/26. doi: 10.1167/iovs.13-12703. PubMed PMID: 24065812. [DOI] [PubMed] [Google Scholar]
- 21.Chen W, Zhao B, Jiang R, Zhang R, Wang Y, Wu H, Gordon L, Chen L. Cytokine Expression Profile in Aqueous Humor and Sera of Patients with Acute Anterior Uveitis. Curr Mol Med. 2015;15(6):543–9. Epub 2015/08/05. PubMed PMID: 26238370. [DOI] [PubMed] [Google Scholar]
- 22.Abu El-Asrar AM, Berghmans N, Al-Obeidan SA, Gikandi PW, Opdenakker G, Van Damme J, Struyf S. Expression of interleukin (IL)-10 family cytokines in aqueous humour of patients with specific endogenous uveitic entities: elevated levels of IL-19 in human leucocyte antigen-B27-associated uveitis. Acta Ophthalmol. 2019;97(5):e780–e4. Epub 2019/02/15. doi: 10.1111/aos.14039. PubMed PMID: 30761764. [DOI] [PubMed] [Google Scholar]
- 23.Baeten D, Baraliakos X, Braun J, Sieper J, Emery P, van der Heijde D, McInnes I, van Laar JM, Landewe R, Wordsworth P, Wollenhaupt J, Kellner H, Paramarta J, Wei J, Brachat A, Bek S, Laurent D, Li Y, Wang YA, Bertolino AP, Gsteiger S, Wright AM, Hueber W. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet. 2013;382(9906):1705–13. doi: 10.1016/S0140-6736(13)61134-4. PubMed PMID: 24035250. [DOI] [PubMed] [Google Scholar]
- 24.Kezic JM, Glant TT, Rosenbaum JT, Rosenzweig HL. Neutralization of IL-17 ameliorates uveitis but damages photoreceptors in a murine model of spondyloarthritis. Arthritis Res Ther. 2012;14(1):R18. Epub 2012/01/25. doi: ar3697 [pii] 10.1186/ar3697. PubMed PMID: 22269151; PMCID: PMC3392808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaufmann U, Diedrichs-Mohring M, Wildner G. Dynamics of intraocular IFN-gamma, IL-17 and IL-10-producing cell populations during relapsing and monophasic rat experimental autoimmune uveitis. PLoS One. 2012;7(11):e49008. doi: 10.1371/journal.pone.0049008. PubMed PMID: 23155443; PMCID: 3498374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letko E, Yeh S, Foster CS, Pleyer U, Brigell M, Grosskreutz CL, Group AAS. Efficacy and safety of intravenous secukinumab in noninfectious uveitis requiring steroid-sparing immunosuppressive therapy. Ophthalmology. 2015;122(5):939–48. doi: 10.1016/j.ophtha.2014.12.033. PubMed PMID: 25638011. [DOI] [PubMed] [Google Scholar]
- 27.Dick AD, Tugal-Tutkun I, Foster S, Zierhut M, Melissa Liew SH, Bezlyak V, Androudi S. Secukinumab in the treatment of noninfectious uveitis: results of three randomized, controlled clinical trials. Ophthalmology. 2013;120(4):777–87. Epub 2013/01/08. doi: 10.1016/j.ophtha.2012.09.040. PubMed PMID: 23290985. [DOI] [PubMed] [Google Scholar]
- 28.Reis J, Vender R, Torres T. Bimekizumab: The First Dual Inhibitor of Interleukin (IL)-17A and IL-17F for the Treatment of Psoriatic Disease and Ankylosing Spondylitis. BioDrugs. 2019. Epub 2019/06/07. doi: 10.1007/s40259-019-00361-6. PubMed PMID: 31172372. [DOI] [PubMed] [Google Scholar]
- 29.Taylor PC, Saurigny D, Vencovsky J, Takeuchi T, Nakamura T, Matsievskaia G, Hunt B, Wagner T, Souberbielle B, Group NS. Efficacy and safety of namilumab, a human monoclonal antibody against granulocyte-macrophage colony-stimulating factor (GM-CSF) ligand in patients with rheumatoid arthritis (RA) with either an inadequate response to background methotrexate therapy or an inadequate response or intolerance to an anti-TNF (tumour necrosis factor) biologic therapy: a randomized, controlled trial. Arthritis Res Ther. 2019;21(1):101. Epub 2019/04/20. doi: 10.1186/s13075-019-1879-x. PubMed PMID: 30999929; PMCID: PMC6471864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crotti C, Biggioggero M, Becciolini A, Agape E, Favalli EG. Mavrilimumab: a unique insight and update on the current status in the treatment of rheumatoid arthritis. Expert opinion on investigational drugs. 2019;28(7):573–81. Epub 2019/06/19. doi: 10.1080/13543784.2019.1631795. PubMed PMID: 31208237. [DOI] [PubMed] [Google Scholar]
- 31.Meermeier EW, Harriff MJ, Karamooz E, Lewinsohn DM. MAIT cells and microbial immunity. Immunol Cell Biol. 2018;96(6):607–17. Epub 2018/02/17. doi: 10.1111/imcb.12022. PubMed PMID: 29451704; PMCID: PMC6045460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenbaum JT, Dick AD. The Eyes Have it: A Rheumatologist’s View of Uveitis. Arthritis & rheumatology. 2018;70(10):1533–43. Epub 2018/05/24. doi: 10.1002/art.40568. PubMed PMID: 29790291; PMCID: PMC6160350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verhagen FH, Hiddingh S, Rijken R, Pandit A, Leijten E, Olde Nordkamp M, Ten Dam-van Loon NH, Nierkens S, Imhof SM, de Boer JH, Radstake T, Kuiper JJW. High-Dimensional Profiling Reveals Heterogeneity of the Th17 Subset and Its Association With Systemic Immunomodulatory Treatment in Non-infectious Uveitis. Front Immunol. 2018;9:2519. Epub 2018/11/16. doi: 10.3389/fimmu.2018.02519. PubMed PMID: 30429855; PMCID: PMC6220365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chateau T, Bonovas S, Le Berre C, Mathieu N, Danese S, Peyrin-Biroulet L. Vedolizumab Treatment in Extra-Intestinal Manifestations in Inflammatory Bowel Disease: A Systematic Review. Journal of Crohn’s & colitis. 2019. Epub 2019/05/12. doi: 10.1093/ecco-jcc/jjz095. PubMed PMID: 31076751. [DOI] [PubMed] [Google Scholar]
- 35.Krabbendam L, Nagasawa M, Spits H, Bal SM. Isolation of Human Innate Lymphoid Cells. Curr Protoc Immunol. 2018:e55. Epub 2018/06/30. doi: 10.1002/cpim.55. PubMed PMID: 29957859. [DOI] [PubMed] [Google Scholar]


