Abstract
Background
Alzheimer disease (AD) was once a clinical diagnosis confirmed by postmortem autopsy. Today, with the development of AD biomarkers, laboratory assays to detect AD pathology are able to complement clinical diagnosis in symptomatic individuals with uncertain diagnosis. A variety of commercially available assays are performed as laboratory-developed tests, and many more are in development for both clinical and research purposes.
Content
The role of laboratory medicine in diagnosing and managing AD is expanding; thus, it is important for laboratory professionals and ordering physicians to understand the strengths and limitations of both existing and emerging AD biomarker assays. In this review, we will provide an overview of the diagnosis of AD, discuss existing laboratory assays for AD and their recommended use, and examine the clinical performance of emerging AD biomarkers.
Summary
The field of AD biomarker discovery and assay development is rapidly evolving, with recent studies promising to improve both the diagnosis of symptomatic individuals and enrollment and monitoring of asymptomatic individuals in research studies. However, care must be taken to ensure proper use and interpretation of these assays. For clinical purposes, these assays are meant to aid in diagnosis but are not themselves diagnostic. For individuals without symptoms, AD biomarker tests are still only appropriate for research purposes. Additionally, there are analytical challenges that require careful attention, especially for longitudinal use of AD tests.
Alzheimer disease (AD)3 is a devastating brain disorder that affects patients, their families, and the community. Although progress continues to be made, there is not yet a treatment to stop or slow the progression of the disease. As a result, AD has been the focus of much research, and great strides are being made to improve diagnosis and prediction of the disease. Experts have indicated that diagnostic tools could be the missing link to developing effective treatments (1). The diagnosis of AD was once based entirely on clinical examination and autopsy (2, 3). Now, with the identification of AD-specific biomarkers, including the development of cerebrospinal fluid (CSF) and blood-based clinical assays, the role of laboratory medicine in managing AD is expanding (3–5). The purpose of this review is to familiarize laboratory professionals with the diagnosis of AD, explore the current and future role of laboratory testing in managing the disease, and provide references for individuals interested in learning more about the current research on circulating biomarkers of the disease.
THE PREVALENCE OF AD
AD is the leading cause of dementia and is characterized by progressive memory decline and cognitive impairment including loss of language and behavioral and personality changes (6). Individuals with AD dementia become unable to care for themselves, and the disease is ultimately fatal with an average duration of approximately 10 years. In the US, it is estimated that 1 in 10 individuals over the age of 65 suffers from the disease, with prevalence increasing with age. The estimated prevalence for individuals 65–74 years of age is 3%, 75–84 is 17%, and >85 is 32%, and the incidence of the disease is increasing owing to the aging population (6). The cost to Medicare and Medicaid for individuals with AD is estimated to be $195 billion in 2019, and by 2050, it is estimated to be $770 billion (7). Although progress is being made, treatments have yet to effectively halt the progression of the disease, possibly because AD is diagnosed and treated too late, after irreversible damage has already occurred.
DIAGNOSING AD
AD was first pathologically described by Dr. Alois Alzheimer in 1906, when he conducted an autopsy on one of his patients with dementia, Auguste Deter, who presented 5 years prior with rapidly increasing memory impairment and behavioral changes (8). Dr. Alzheimer described the 2 defining pathological features of AD: amyloid plaques and neurofibrillary tangles.
Over a century after the discovery of the disease, the gold standard for diagnosing definite AD is still a postmortem autopsy identifying neurofibrillary tangles and amyloid plaques in patients who meet the clinical criteria for probable AD (3). Although autopsy is likely to remain the gold standard, significant progress has been made to predict the disease antemortem with diagnoses of probable or possible AD. In 1984, diagnostic guidelines were established by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association working group. Diagnosis relied on clinical and cognitive evaluation for symptoms of dementia with progressively worsening memory loss; deterioration in motor, language, and perceptive functions; and absence of other diseases or disorders that could account for the cognitive decline (2). Sequential CT scans showing progressive brain atrophy were considered supportive to the diagnosis of probable AD. CSF testing was only used to rule out other causes of cognitive decline. Possible AD, which was considered a less confident diagnosis than probable AD, was considered when other causes of cognitive decline were identified that could cause, but were not thought to account for, the patient’s dementia. The 1984 criteria also recommended identifying subtypes of the disease for research purposes, such as early age of onset (i.e., onset <65 years old), familial vs sporadic AD, and the presence of coexisting conditions such as Parkinson disease and trisomy-21 (2).
EVOLVING DIAGNOSTIC CRITERIA
Between 1984 and 2011 substantial progress was made in understanding the progression of AD and the accompanying biological changes. In 2011, the National Institute on Aging and the Alzheimer’s Association redefined the recommendations for diagnosing possible or probable AD (3). The major differences between the old and new guidelines were in recognizing different stages of AD, and the inclusion of AD biomarkers. The 1984 guidelines recognized only the final stage of the disease, whereas the 2011 guidelines recognized 3 stages: Alzheimer’s dementia, mild cognitive impairment (MCI), and preclinical AD. Although MCI diagnosis is intended for both clinical and research use, diagnosis of preclinical AD is currently intended for research use only (3).
To distinguish between these stages, the 2011 guidelines recognized a variety of imaging tests (including amyloid PET, PET glucose uptake, and MRI) and CSF biomarkers that were not available in 1984 (3). Since 2011, additional guidelines have been published on diagnosing AD for research purposes (5). In 2016 an A (β-amyloid)/T(tau)/N (neurodegeneration) classification scheme was proposed to categorize multidomain biomarker findings within individuals. This scheme divides biomarkers into categories and provides dichotomous ratings based on the presence (+) or absence (−) of biomarkers in that category (9). Although this scheme and many of the published guidelines are not yet used for diagnostic purposes, the clinical diagnosis of AD is expected to rapidly evolve. As new AD biomarkers emerge, it is important for both laboratory professionals and ordering physicians to recognize the appropriate use of laboratory tests for AD and current limitations and challenges associated with testing.
CURRENT BIOMARKERS FOR AD
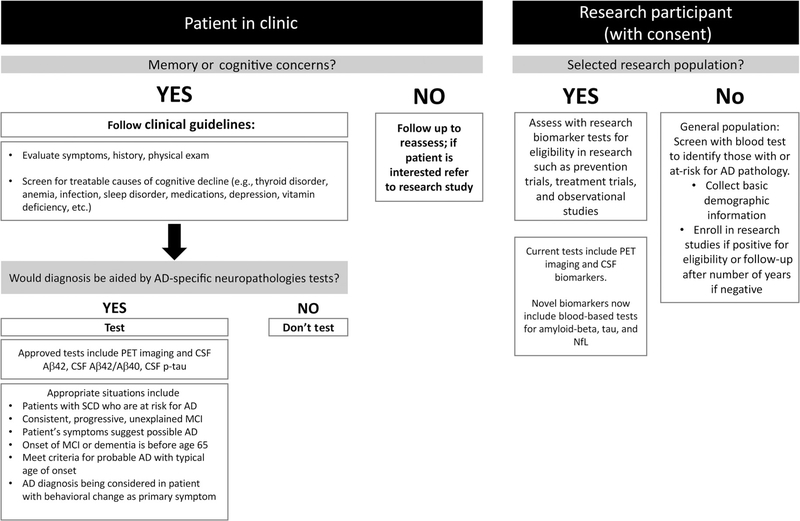
Examples of existing AD biomarkers include CSF markers of amyloid β (Aβ) plaque accumulation including Aβ42 and the Aβ42/Aβ40 ratio, and CSF markers of tau, a major component of neurofibrillary tangles, including phosphorylated tau and truncated tau (a.k.a. total tau) (3, 4). Appropriate clinical use of these biomarkers, suggested by the Alzheimer’s Association workgroup (4), are summarized in Fig. 1.
Fig. 1. Current Utility of AD Biomarkers.
Proposed clinical use (4) and research use of AD biomarkers.
MARKERS OF AMYLOID PLAQUES
Amyloid plaques are formed in the brain from products of amyloid precursor protein (APP), a membrane protein involved in synapse formation and signaling. Secretases cleave APP into hydrophobic Aβ peptides of various lengths. In normal brain physiology, these peptides are cleared into the CSF or are transported into the blood across the blood- brain barrier. However, overproduction or reduction in clearance of amyloid peptides results in formation of the amyloid plaques that are characteristic of AD (10). These plaques primarily contain Aβ peptide 1–42 (Aβ42) and act as a “sink” for the peptide, reducing Aβ42 concentrations in both CSF and blood. Amyloid β 1–40 (Aβ40) is also formed during APP cleavage but is not consistently different between individuals with amyloid plaques and healthy controls. Measuring the ratio of Aβ42 to Aβ40 can help account for interindividual variation in amyloid concentration. Decreased CSF concentrations of Aβ42 or Aβ42/Aβ40 are used to predict the presence of brain amyloid plaques and are included in the National Institute on Aging and the Alzheimer’s Association guidelines for diagnosing preclinical AD and atypical AD (3).
CSF Aβ42 and Aβ40 can be measured by either mass spectrometry or immunoassay. These assays are performed commercially by Athena Diagnostics and Quest, which measure CSF Aβ42 and Aβ40 as laboratory-developed tests. Athena Diagnostics uses an ELISA method, and Quest uses a mass spectrometry assay. Both Roche Diagnostics and Fujirebio have developed immunoassays for Aβ42 and Aβ40. Although not yet commercially available, both manufacturers received breakthrough device designation to accelerate Food and Drug Administration (FDA) clearance for use on their automated platforms (11, 12). Table 1 shows the sensitivity, specificity, and area under the curve (AUC) of these and other assays for predicting positivity by amyloid PET. Of the published studies we reviewed, the specificity of the assays for amyloid plaque detection ranges from 78% to 95%, and the sensitivity ranges from 73% to 96% (Table 1) (13–18).
Table 1.
Current AD biomarkers.a
| Cutoff or median biomarker concentration |
Clinical performance |
|||||||
|---|---|---|---|---|---|---|---|---|
| Biomarker | Analytical method | AD pathology | Healthy control | AUC | Sensitivity | Specificity | Manufacturer | Reference |
| Predicting amyloid plaques | ||||||||
| Ab42, CSF | ELISA | 570 pg/mLb | 1124 pg/mLb | 0.89 | 80% | 85% | Fujirebio INNOTEST | (14,17) |
| Luminex xMAP | NAc | 0.93 | 80% | 89% | Fujirebio INNO-BIA | (14) | ||
| CLEIA | <916 pg/mL | 0.76 | 95% | 51% | Fujirebio Lumipulse | (18) | ||
| ECL | <1098 pg/mL | 0.90 | 73% | 85% | Roche Elecsys | (16) | ||
| ELISA | <507.5 pg/mL | 0.89 | 83% | 83% | Euroimmun | (15) | ||
| ECLIA | <495.9 | 0.74 | 85% | 89% | MSD | (15) | ||
| Simoa | <1742 pg/mL | 0.81 | 73% | 78% | Quanterix | (15) | ||
| SPE + LC-MS/MS | 511 pg/mL | 1152 pg/mL | 0.94d | 93%d | 85%d | Reference Method-C12RMP1 | (21) | |
| Isotope Dilution Mass spectrometry | <1059 pg/mL | 0.85 | 82% | 81% | Reference Method-C11RMP9 | (22,23) | ||
| Ab42/Ab40, CSF | ECLIA | <0.075 | 0.93 | 96% | 82% | Roche Elecsyse | (16) | |
| CLEIA | <0.062 | 0.86 | 88% | 77% | Fujirebio Lumipulsee | (18) | ||
| ELISA | <0.10 | 0.95 | 97% | 88% | Euroimmun | (15) | ||
| ECLIA | <0.09 | 0.98 | 95% | 95% | MSD | (15) | ||
| Simoa | <0.16 | 0.91 | 90% | 90% | Quanterix | (15) | ||
| Mass spectrometry | N/A | N/A | Questf | NA | ||||
| Isotope Dilution Mass spectrometry | <0.085 | 0.95 | 96% | 91% | Reference Method-C11RMP9 | (22,23) | ||
| Tau proteins | ||||||||
| T-tau, CSF | ELISA | 290 pg/mLb | 635 pg/mLb | 0.86 | 80% | 76% | Fujirebio INNOTEST | (14,17) |
| Luminex xMAP | NA | 0.82 | 80% | 65% | Fujirebio INNO-BIA | (14) | ||
| CLEIA | >456 pg/mL | 0.80 | 75% | 83% | Fujirebio Lumipulse | (18) | ||
| ECLIA | >242 pg/mL | 0.81 | 68% | 83% | Roche Elecsys | (16) | ||
| ELISA | >412 pg/mL | 0.88 | 91% | 79% | Euroimmun | (13) | ||
| Mass spectrometry | 29 pmol/L | 17 pmol/L | NA | Merck | (32) | |||
| P-tau, CSF | ELISA | 48 pg/mLb | 79 pg/mLb | 0.83 | 80% | 69% | Fujirebio INNOTEST | (14,17) |
| Luminex xMAP | NA | 0.91 | 80% | 86% | Fujirebio INNO-BIA | (14) | ||
| CLEIA | >63 pg/mL | 0.84 | 80% | 83% | Fujirebio Lumipulse | (18) | ||
| ECLIA | >19.2 pg/mL | 0.84 | 82% | 76% | Roche Elecsys | (16) | ||
| Multibiomarker assays | ||||||||
| P-tau/Ab42, CSF | ELISA | NA | 0.95 | 80% | 94% | Fujirebio INNOTEST | (14,17) | |
| Luminex xMAP | NA | 0.97 | 80% | 98% | Fujirebio INNO-BIA | (14) | ||
| CLEIA | >0.068 | 0.88 | 93% | 89% | Fujirebio Lumipulsee | (18) | ||
| ECLIA | >0.0198 | 0.96 | 92% | 89% | Roche Elecsyse | (16) | ||
| Ab42/tau | ELISA | <1.47 | 0.90 | 91% | 86% | Euroimmun | (13) | |
| Ab42/t-tau and pTau, CSF | ELISA | ATI<1, pTau>61 pg/mL | 0.89g | 88%g | 82%g | Admarkf | (28) | |
Performance, biomarker concentrations, and cutoffs vary based on the gold standard (e.g., PET imaging vs clinical diagnosis), the population tested, and preanalytical variables. These data are compiled from published academic studies and are not for clinical purposes. The numbers listed in this table are meant to provide a general idea of clinical performance, analyte concentrations, and variation between assays. The gold standard for most studies was Pittsburg compound B PET.
Biomarker concentration data from separate study than biomarker performance data.
NA, data not available.
Gold standard was autopsy.
Roche Elecsys and Fujirebio Lumipulse received FDA breakthrough device designation to accelerate the process of applying for FDA clearance for one or more of their AD assays.
Lab-developed test.
Gold standard was clinical diagnosis.
Although performance across assays is comparable, the measured concentrations and optimal cutoffs across the assays vary substantially (19, 20). This variation can be attributed to differences in antibodies, sample processing, and analytical methods. To overcome these challenges, a certified reference material (CRM) and 2 reference methods (Joint Committee for Traceability in Laboratory Medicine database # C12RMP1 and C11RMP9) have recently been developed for measurement in CSF (21). The reference methods use mass spectrometry without antibody enrichment and have high sensitivity and specificity for detecting amyloid plaques (21–23). C12RMP1, which was developed at the University of Pennsylvania, uses solid-phase extraction coupled with LC-MS/MS (21), whereas C11RMP9, which was developed by Roche Diagnostics and the University of Gothenburg, uses isotope dilution mass spectrometry (22, 23). Both assays have a wide linear range (from 100 to 3000 pg/mL and 150–4000 pg/mL, respectively) and acceptable interassay imprecision (2.6%−10% and 6.4%, respectively), making them useful reference methods to set CSF Aβ42 concentrations in CRM. To compare longitudinal and cross-laboratory results, it will be important to use these CRMs to standardize measurements across laboratories.
ISOFORMS OF TAU IN CSF (P-TAU)
Neurofibrillary tangles, one of the pathologies characteristic of AD, contain aggregates of hyper- phosphorylated tau protein. In healthy individuals, tau proteins stabilize microtubules and help give neurons their structure. In AD, tau is phosphor- ylated and dissociates from microtubules, resulting in disassembly of microtubules, aggregation of tau, and impaired communication between neurons (24, 25).
Tau is secreted by neurons into the interstitial fluid of the brain and travels to the CSF and blood, where it is broken down and cleared from the body (25). Increased concentrations of soluble p-tau are associated with the presence of amyloid plaques and neurofibrillary tangles. Most assays measure p- tau181 (phosphorylated threonine 181), but, for research purposes, assays have also been developed to measure p-tau231 (phosphorylated threonine 231) and p-tau199 (phosphorylated serine 199) (17, 26, 27). Depending on the study, the sensitivities and specificities of these assays for detecting amyloid plaques vary substantially, ranging from 80% to 82% and 69% to 86%, respectively (Table 1).
Clinical performance is improved when p-tau is combined with other AD biomarkers, such as Aβ42. This strategy is applied to both assays in development and commercially available assays. For example, a study performed with the Roche Elecsys® assay for CSF p-tau181 reported a sensitivity of 82% and a specificity of 76%. When combined with Aβ42, the ratio of p-tau181 /Aβ42 in CSF increased the sensitivity and specificity to 92% and 89%, respectively (16). Athena Diagnostic’s ADmark® assay measures p-tau181, Aβ42, and t-tau. Clinical performance studies of ADmark used clinical diagnosis instead of PET imaging as the gold standard but reported a similar sensitivity and specificity (28) (Table 1). Mass spectrometry was recently used to identify additional tau phosphorylation sites in CSF of individuals with AD, which may be better able to differentiate between individuals with and without AD, but further investigation is needed (29).
TRUNCATED TAU IN CSF (T-TAU)
Tau is a complex protein with a variety of isoforms and fragments in the central nervous system (30). Barthelemy et al. reported 29 phosphorylation sites on full-length tau, found in brain tissue, and 12 phosphorylation sites on truncated tau, found in CSF (29). When tau is measured, the isoforms detected depend on the antibodies or extraction methods used, which vary between assays (31). What is commonly referred to as “total tau” or “t-tau” in CSF consists almost entirely of n-terminal soluble fragments of tau (truncated tau) in healthy individuals and individuals with AD (29–31). Commercially available immunoassays recognize the mid-domain of tau and do not differentiate between full-length and truncated tau. Therefore, we now use “t-tau” to indicate “truncated tau” in normal and AD CSF. Truncated tau is an actively produced and secreted fragment that is different than the full-length tau released with neuronal cell death. Although increases in CSF p-tau181 concentration are mostly specific to AD, t- tau concentrations are increased in other neurodegenerative diseases. Increased concentrations of t-tau are associated with decreased cognitive function and neurodegeneration, but the exact forms may be different between diseases and are not specific for AD dementia. In addition to immunoassays, mass spectrometry methods to measure t-tau in CSF have been developed (32). In AD, t-tau could represent neurodegeneration, tangles, or amyloid plaques, and future longitudinal studies will be needed to understand these relationships.
GENETIC MARKERS OF AD
Individuals with dominantly inherited AD (DIAD) have mutations in the genes for presenilin 1, presenilin 2, or APP. Mutations in these genes alter the structure of γ-secretase (presenilin 1, presenilin 2) or structure of Aβ (APP), causing more aggregation-prone forms of Aβ to be generated. Although there is no effective treatment to prevent DIAD, clinical testing for these mutations is available and can be done with clinical genetic counseling guidance and an ordering physician. DIAD is nearly 100% penetrant, and the age of onset is typically in the 30s to 50s with predictable onset by mutation type. DIAD is rare, making up <1% of AD diagnoses, but is a major focus of AD research (33) by informing stages of biomarker changes with the potential to develop effective prevention treatments for DIAD.
The genetic contributions of sporadic AD are largely mediated by APOE4, one of the strongest genetic risk factors of a common disease. The most common APOE alleles are ε2, ε3, and ε4, which encode for apolipoprotein E (ApoE). ApoE2 and ApoE4 differ by only 2 amino acids, but ApoE4 increases the risk of AD by 3-fold for each ε4 allele, whereas ApoE2 lowers AD risk. The prevalence of having one or more APOE ε4 alleles in patients with AD is approximately 50% compared to approximately 25% for the general population (34). Testing for APOE ε4 allele is neither sensitive nor specific for AD. Although there are FDA-approved tests for APOE genotype, including many direct-to- consumer options, this testing is currently discouraged (5).
However, when APOE testing is combined with other biomarkers, it has been shown to improve clinical performance. This strategy is an option in the Athena Diagnostics ADmark® assay (35) and is explored in the recent literature (10, 36, 37). APOE ε4 status can be determined via DNA sequencing or inferred by mass spectrometric analysis of the ApoE protein (38). Although DNA sequencing is the more common approach, analysis by mass spectrometry has the potential for multiplexing and for assessing the effect of ApoE concentration. However, more research needs to be done to determine the utility of this approach.
CLINICAL UTILITY OF EXISTING BIOMARKERS
Although the use of any of these biomarkers was initially recommended only for research purposes (3), more recent reports indicate some of these markers (CSF: Aβ42, Aβ42/β40, p-tau) are appropriate for select clinical situations. For example, a 2018 Alzheimer’s Association working group (4) indicated clinical biomarker testing is appropriate in the setting of a dementia clinic, in patients with early-onset, progressive, or unexplained MCI or with comorbidities that add uncertainty to the diagnosis of AD. Clinical testing is not yet appropriate for asymptomatic individuals and should be performed for research purposes only. The recommended use of these biomarkers is summarized in Fig. 1.
EMERGING BIOMARKERS FOR AD
Efforts to develop effective treatments for AD have been unsuccessful to date. One challenge encountered in therapeutic drug trials has been the lack of a gold standard to accurately diagnose AD, and other challenges include suboptimal target engagement, uncertainty of valid targets, and treating symptomatic individuals too late in the disease when the brain already has irreversible damage (39, 40). A more effective approach may be to prevent AD by developing treatments for at-risk individuals before symptoms begin, similar to the idea of giving statins to individuals who are at risk for heart disease. However, unlike heart disease, there is neither an effective treatment nor an appropriate test to identify individuals without symptoms who at risk for AD. AD pathology such as amyloid plaques and tau tangles can be observed by imaging and CSF assays up to 20 years before symptoms begin (33, 41). However, imaging is expensive, and CSF collection is invasive, making currently available AD tests impractical for enrolling and monitoring large numbers of asymptomatic participants in large therapeutic trials. Blood tests for AD are more appropriate screening tools, but have challenges. Blood is a more complex matrix with a higher background protein content, and concentrations of AD biomarkers are lower in blood than CSF (see approximate biomarker concentrations in Tables 1 and 2). Despite these challenges, many blood-based AD assays are in development. In addition to screening asymptomatic individuals for research purposes, blood- based AD assays have the potential to aid in diagnosing symptomatic individuals and may soon be appropriate for clinical use in some circumstances.
Table 2.
Emerging blood-based AD biomarkers.a
| Emerging blood (plasma) biomarkers | Analytical method | Cutoff or median AD biomarker concentration |
Clinical performanceb |
|||||
|---|---|---|---|---|---|---|---|---|
| AD pathology | Healthy control | AUC | Sensitivity | Specificity | Manufacturer | Reference | ||
| Predicting amyloid plaques | ||||||||
| Aβ42/Aβ40 | ECLIA | Ab42: 23 Ab40:380 | Ab42: 33 Ab40: 482 | 0.77 | 70% | 73% | Roche | (48) |
| IP, LC-MS/MS | <0.1218 | 0.88 | 88% | 76% | C2N Diagnostics | (10,36) | ||
| Aβ40/Aβ42 | IP, MALDI-Mass Spectrometry | 27.656 | 0.89 | 73% | 92% | Shimadzu | (37) | |
| Tau proteins | ||||||||
| T-tau | Simoa | 2.58–5.58 | 3.12–5.37 | c | Quanterix | (61) | ||
| NT1 Simoa Assay | 2.5 | 3.5 | 0.75 | 70% | 78% | Quanterix | (61) | |
| ECLIA | 16.7 | 16.6 | NAd | Roche | (28) | |||
| Neurofilament light chain | ||||||||
| NfL, Plasma | Simoa | 49 | 21 | 0.85 | 84% | 78% | Quanterix | (61) |
| Simoa Homebrew | 49 | 34 | 0.87 | NA | b | (59) | ||
| ECLIA | 44 | 21 | NA | Roche | (48) | |||
| Multibiomarker assays | ||||||||
| Aβ42, Aβ40 + APOE, age | IP, LC-MS/MS | NA | 0.94 | NA | C2N Diagnostics | (10,36) | ||
| IP, MALDI-Mass Spectrometry | 0.52 | 0.89 | 83% | 84% | Shimadzu | (37) | ||
| Aβ42, Aβ40, T-Tau | ECLIA | NA | 0.81 | 89% | 64% | Roche | (48) | |
| Aβ42, Aβ40, T-Tau, NfL, APOE | ECLIA | NA | NA | 73% | 86% | Roche | (48) | |
Performance, biomarker concentrations, and cutoffs vary based on the gold standard (e.g., PET imaging vs clinical diagnosis), the population tested, and preanalytical variables. These data are compiled from published academic studies of the assays and are not meant to be used for clinical purposes. The numbers listed in this table are meant to provide a general idea of clinical performance, analyte concentrations, and variation between assays. All concentrations are in pg/mL. Ratios are unitless.
No known manufacturer.
Higher NfL concentrations in AD than in controls but not recommended as a standalone assay.
NA, data not available.
A variety of blood-based biomarkers for early detection of AD are being developed by both academia and industry (42, 43). We limited our review to blood-based AD biomarkers that have been replicated, are specifically linked with AD pathophysiology, and that we believe are closest to being ready for clinical practice.
A BLOOD TEST TO DETECT AND PREDICT AMYLOID PLAQUE FORMATION
Assays are being validated to quantify Aβ42 and Aβ40 in blood to identify amyloid plaques in individuals without symptoms for research purposes, and for diagnosing individuals with symptoms. The difference in Aβ42 concentration between amyloid-positive and amyloid-negative individuals is smaller in blood than in CSF. Additionally, blood is a more complex matrix with lower concentrations of Aβ42 and Aβ40, making analysis more challenging (44).
A multitude of prior studies using ELISA found no difference between Aβ42 concentrations in the plasma of individuals with AD and healthy controls (45, 46). In 2017, the first highly specific plasma Aβ test for AD amyloid plaques was reported with a sensitive and precise mass spectrometry method (10). Similar to CSF, this report demonstrated reduced Aβ42/Aβ40 in plasma, with a correlation coefficient between CSF and plasma of approximately 0.7 (10). Since the initial reports, multiple groups have replicated these findings of decreased plasma Aβ42/Aβ40 ratio in the presence of amyloid plaques determined by using amyloid PET imaging or CSF assays (36, 37, 47, 48). Studies measuring plasma Aβ42 and Aβ40 by immunoprecipitation-mass spectrometry reported higher AUCs of approximately 0.88 for detecting amyloid plaques than studies using immunoassays, which reported AUCs of approximately 0.77 (10, 36, 37) (Table 2).
A recent study demonstrated that plasma Aβ42/ Aβ40 can predict the conversion from amyloid-negative PET scans to amyloid-positive PET scans, years before plaques are visible by PET (36). This finding suggests that plasma Aβ may be more sensitive at detecting the development of amyloid plaques than the currently approved amyloid PET scans. However, further research is needed to replicate these results, ideally with prospective longitudinal studies.
Although assays measuring plasma Aβ42 and Aβ40 hold promise, especially for enrollment and monitoring in clinical trials, larger studies are needed to assess clinical performance, analytical performance, and preanalytical variables across platforms (49). Different analytical methods produce different Aβ42 concentrations and Aβ42/ Aβ40 ratios, pointing to the need for a CRM and reference methods, similar to that which is available for CSF Aβ42 (50–52). These methods will be especially important if research participants or patients are followed longitudinally. Additionally, before these assays are performed clinically, care must be taken to ensure appropriate use (4).
TAU ISOFORMS IN BLOOD
Concentrations of both t-tau and p-tau are approximately 100 times lower in blood than in CSF (Table 1, Table 2), making analysis in blood challenging. It is unclear if the multiple p-tau isoforms identified in CSF (29, 30) are also present in blood. A small study (n = 35) measured plasma p-tau181 and was able to discriminate between AD and control with a sensitivity of 60% and a specificity of 86% (53). Further work with proteolytic fragments of tau suggest that AD-specific tau may be quantifiable in plasma (31). These initial promising studies suggest that plasma tau measurements may be able to accurately inform on tau pathophysiology and provide additional diagnostic and prognostic information on patients’ clinical status.
Similar to analysis in CSF, t-tau on its own is not specific for AD. The plasma Quanterix Simoa t-tau assay had an area under the ROC of 0.60 when compared to amyloid PET, with better performance of blood p-tau181 at 0.80 (54). When combined with measurement of Aβ42 and Aβ40, t-tau improves test sensitivity for amyloid plaques (55).
NEUROFILAMENT LIGHT CHAIN
Neurofilaments are a major component of the neuronal cytoskeleton and are composed of neurofilament heavy chain, neurofilament medium chain, and neurofilament light chain (NfL). When neurons die, this protein is released into CSF and blood and is a marker of neurodegeneration (56). Multiple studies evaluated neurofilament proteins in the CSF and blood of individuals with MCI, AD dementia, non-AD dementia, and healthy controls (57–59). NfL is not specific for AD, but increased concentrations in individuals with AD are associated with increased memory problems. When measured alongside other AD biomarkers, NfL may improve performance of screening tests and may be useful for long-term longitudinal disease monitoring.
To date, most methods for measuring NfL have relied on immunoassays, and although not yet FDA approved, automated immunoassays have been developed to measure NfL. On its own, NfL is neither sensitive nor specific for diagnosing AD, but its elevation coincides with onset and progression of brain lesions, MCI, and AD, and other neurodegenerative diseases (60). Quanterix Simoa offers an NfL assay, currently for research use only (61), and Roche has also developed an assay to measure NfL that when combined with plasma Aβ42 and Aβ40 increases the sensitivity of amyloid plaque detection (using CSF Aβ42/Aβ40 as the gold standard) from 75% to 86% but decreases the specificity from 72% to 70% (48).
NfL undergoes multiple posttranslational modifications (62), which are not identified with existing immunoassays. Analysis by mass spectrometry may lead to discovery of important disease- specific posttranslational modifications; however, quantitative mass spectrometry assays for NfL are not yet available.
CONSIDERATIONS FOR PRESENT AND FUTURE CLINICAL USE
Biomarker tests of AD, including FDA-approved amyloid PET scans and commercially performed CSF assays, are currently used in the clinical diagnosis of symptomatic patients. Although these tests detect AD pathology, they are not diagnostic of disease by themselves. It is important to interpret these tests within the clinical context. For example, approximately one-third of older, cognitively normal people have amyloid plaques in their brain (63). Amyloid plaques by themselves do not guarantee that a patient’s cognitive symptoms are due to AD. In the context of a clinical presentation consistent with AD, detection of amyloid plaques, tau tangles, and neurodegeneration can provide high confidence that AD is the cause of disease. In contrast, a negative test result of amyloid plaques strongly indicates a non-AD cause and appropriately directs the search for other diagnoses. Future AD clinical tests will combine and compare abnormalities in multiple key pathologic proteins, such as amyloid-β, tau, and NfL to help ensure diagnosis and stage the disease. This approach promises to optimize the accurate diagnosis of patients and lead to better clinical management of prognosis and treatment. Table 3 illustrates the theoretical effect improving AUC can have on test performance. With hypothetical data to generate AUCs of 0.85 and 0.95, and choosing a cutoff that balances sensitivity and specificity, the 0.1 increase in AUC reduces the false-positive rate from 23% to 12%. If a cutoff is set in the hypothetical data to optimize sensitivity (e.g., to be used as a screening test), the false-negative rate would be reduced from 18% to 2% (Table 3).
Table 3.
Decreased false-positive and false-negative rates with increased AUC.a
| AUC | Sensitivity | Specificity | False positive rate, % | False negative rate, % |
|---|---|---|---|---|
| 0.8 | 82% | 75% | 25% | 18% |
| 77% | 77% | 23% | 23% | |
| 70% | 85% | 15% | 30% | |
| 0.85 | 82% | 78% | 22% | 18% |
| 78% | 78% | 22% | 22% | |
| 72% | 86% | 14% | 28% | |
| 0.9 | 94% | 72% | 28% | 6% |
| 88% | 78% | 22% | 12% | |
| 86% | 84% | 16% | 14% | |
| 0.95 | 98% | 74% | 26% | 2% |
| 88% | 88% | 12% | 12% | |
| 79% | 94% | 6% | 21% |
For any AUC, there will be a variety of sensitivities and specificities that depend on the shape of the ROC curve. The values in this table are based on hypothetical data and are meant to illustrate the large improvements in false-positive and false-negative rates that could be observed from small improvements in AUC. Based on this hypothetical data, the difference between a test with an AUC of 0.85 and 0.95 can improve the false-positive and false-negative rate from 22% to 12% for balanced sensitivity and specificity. When used as a screening test (i.e., when the cutoff is moved to improve sensitivity at the expense of specificity), the numbers of false negatives can improve from 18% to 2%.
Blood-based AD biomarker assays are currently in development, and a subset of these assays that we believe are closest to being clinically available are summarized in Table 2. Although blood-based assays may aid in the diagnosis of symptomatic individuals, in the near future, they may also be used to enroll and monitor asymptomatic individuals in observational and therapeutic research trials. However, diagnostic testing is not yet appropriate for clinical use in individuals without symptoms, until effective prevention approaches are developed.
With all AD assays, alterations in preanalytical and analytical handling can cause substantial differences in results. These differences may be magnified in plasma where the matrix is more complex, concentrations are lower, and gaps between “healthy” and “disease” states are smaller. To enable plasma assays to be ready for commercial or laboratory-developed test clinical use, preanalytical and analytical factors will need to be well validated. In addition, use of these tests for longitudinal testing introduces additional considerations of stability and reference standards. Currently, measured concentrations or ratios are not translatable across platforms, and each laboratory performing testing needs to validate reference intervals specific to their assay to advise the ordering physician on how to interpret the results. Once commercially available, CRM and reference methods may be able to help address interassay variability.
Diagnostic AD tests developed over the past 15 years have profoundly changed our understanding and ability to track, monitor, and diagnose AD. Reliable and clinically feasible blood tests are now in development and likely to transform AD research and clinical practice even further. The Alzheimer’s Association Global Biomarkers Standardization Consortium and the International Federation of Clinical Chemistry Working Group for CSF proteins are making progress to standardize these biomarkers. Consensus on gold standards, development of CRMs and reference methods for all AD biomarkers (currently used and emerging) will enable more rapid and efficient research and clinical trials for AD prevention and treatment studies. Moving these biomarkers from research testing to clinical diagnostic testing will require further, properly designed clinical studies to evaluate the clinical diagnostic utility and to compare the utility of emerging assays to existing gold standards (such as FDA-approved amyloid PET). Collaboration between academic researchers, clinical chemists, industry leaders, and the FDA will be necessary to move these and any future AD biomarkers toward the clinic.
IMPACT STATEMENT.
Laboratory professionals will increasingly be involved in laboratory testing for diagnosing and managing Alzheimer disease (AD). In this review, we will familiarize laboratory professionals with the diagnosis of AD, explore the current and future role of laboratory testing in managing the disease, and provide references for individuals interested in learning more about the current research on biomarkers of AD.
Acknowledgments
The authors acknowledge Dr. Yan Li for contributing her AUC analysis of false-positive and false-negative rates, and Drs. Ann Gronowski and Henrik Zetterberg for their helpful feedback in preparation of this review.
R.J. Bateman, financial support, administrative support.
Research Funding: R.J. Bateman, NIH, Alzheimer’s Association, BrightFocus Foundation, Rainwater Foundation Tau Consortium, Association for Frontotemporal Degeneration, The Cure Alzheimer’s Foundation, The Tau SILK Consortium (Abbvie, Biogen, and Eli Lilly and Co.), Anonymous Foundation.
Footnotes
Authors’ Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form.
Nonstandard abbreviations: AD, Alzheimer disease; CSF, cerebrospinal fluid; MCI, mild cognitive impairment; Aβ, amyloid beta; Aβ 42, amyloid beta peptide with amino acids 1 to 42; Aβ 40, amyloid beta peptide with amino acids 1 to 40; p-tau, phosphorylated tau; t-tau, total-tau or truncated tau; APP, amyloid precursor protein; FDA, Food and Drug Administration; AUC, area under curve; CRM, certified reference material; DIAD, dominantly inherited Alzheimer disease; ApoE, apolipoprotein E; NfL, neurofilament light chain
Human genes: APOE, apolipoprotein E.
Employment or Leadership: None declared. Consultant or Advisory Role: R.J. Bateman, C2N, Pfizer, Johnson & Johnson, Hoffman La Roche/Genentech, AC Immune. Stock Ownership: R.J. Bateman, C2N. Honoraria: M.M. Budelier, MSACL Annual Conference, ACLPS Annual Meeting; R.J. Bateman, Johnson & Johnson. Expert Testimony: None declared. Patents: M.M. Budelier, WU: T-018941; R.J. Bateman, 7,892,845, Patent Application 62/515,294.
REFERENCES
- 1.Alzheimer’s Association. Earlier diagnosis. https://www.alz.org/alzheimers-dementia/research_progress/earlier-diagnosis (Accessed June 2019).
- 2.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34: 939–44. [DOI] [PubMed] [Google Scholar]
- 3.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011;7:263–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaw LM, Arias J, Blennow K, Galasko D, Molinuevo JL, Salloway S, et al. Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement 2018;14:1505–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol 2014;13:614–29. [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 2019;15:321–87. [Google Scholar]
- 7.Alzheimer’s Association. 2019. Alzheimer’s disease facts and figures. https://www.alz.org/media/documents/alzheimers-facts-and-figures-2019-r.pdf (Accessed June 2019).
- 8.Maurer K, Volk S, Gerbaldo H. Auguste D and Alzheimer’s disease. Lancet 1997;349:1546–9. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Feldman HH, Frisoni GB, et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 2016;87:539–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ovod V, Ramsey KN, Mawuenyega KG, Bollinger JG, Hicks T, Schneider T, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement 2017;13:841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujirebio Diagnostics. Fujirebio Diagnostics receives FDA breakthrough device designation for Lumipulse® G B- amyloid ratio (1–42/1–40) quantitative in vitro diagnostic test. https://www.fujirebio-us.com/media-and-events/news/2019/fujirebio-diagnostics-receives-fdabreakthrough-device-designation (Accessed June 2019).
- 12.Roche Diagnostics. FDA grants Breakthrough Device Designation for Roche’s Elecsys cerebrospinal fluid (CSF) assays to support the improved diagnosis of Alzheimer’s disease. https://www.roche.com/dam/jcr:4cae6bb1-7b58-492d-91c9-994afcdd29dd/de/20180720-MR_DIA_AAIC_en.pdf (Accessed June 2019).
- 13.Chiasserini D, Biscetti L, Farotti L, Eusebi P, Salvadori N, Lisetti V, et al. Performance evaluation of an automated ELISA system for Alzheimer’s disease detection in clinical routine. J Alzheimers Dis 2016;54:55–67. [DOI] [PubMed] [Google Scholar]
- 14.Fagan AM, Shaw LM, Xiong C, Vanderstichele H, Mintun MA, Trojanowski JQ, et al. Comparison of analytical platforms for cerebrospinal fluid measures of beta-amyloid 1–42, total tau, and p-tau181 for identifying Alzheimer disease amyloid plaque pathology. Arch Neurol 2011;68:1137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janelidze S, Zetterberg H, Mattsson N, Palmqvist S, Vanderstichele H, Lindberg O, et al. CSF Aβ42/Aβ40 and Aβ42/Aβ38 ratios: better diagnostic markers of Alzheimer disease. Ann Clin Transl Neurol 2016;3: 154–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla- Utermann R, Quan M, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimers Dement 2018;14:1460–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Willemse EAJ, van Maurik IS, Tijms BM, Bouwman FH, Franke A, Hubeek I, et al. Diagnostic performance of Elecsys immunoassays for cerebrospinal fluid Alzheimer’s disease biomarkers in a nonacademic, multicenter memory clinic cohort: the ABIDE project. Alzheimers Dement (Amst) 2018;10:563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alcolea D, Pegueroles J, Munoz L, Camacho V, Lopez-Mora D, Fernandez-Leon A, et al. Agreement of amyloid PET and CSF biomarkers for Alzheimer’s disease on Lumipulse. Ann Clin Transl Neurol 2019;6:1815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw LM, Hansson O, Manuilova E, Masters CL, Doecke JD, Li QX, et al. Method comparison study of the Elecsys(R) beta-Amyloid (1–42) CSF assay versus comparator assays and LC-MS/MS. Clin Biochem 2019; 72:7–14. [DOI] [PubMed] [Google Scholar]
- 20.Bjerke M, Portelius E, Minthon L, Wallin A, Anckarsater H, Anckarsater R, et al. Confounding factors influencing amyloid beta concentration in cerebrospinal fluid. Int J Alzheimers Dis 2010;2010:986310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korecka M, Waligorska T, Figurski M, Toledo JB, Arnold SE, Grossman M, et al. Qualification of a surrogate matrix- based absolute quantification method for amyloid- beta(4)(2) in human cerebrospinal fluid using 2D UPLC- tandem mass spectrometry. J Alzheimers Dis 2014;41: 441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leinenbach A, Pannee J, Dulffer T, Huber A, Bittner T, Andreasson U, et al. Mass spectrometry-based candidate reference measurement procedure for quantification of amyloid-beta in cerebrospinal fluid. Clin Chem 2014;60:987–94. [DOI] [PubMed] [Google Scholar]
- 23.Pannee J, Portelius E, Minthon L, Gobom J, Andreasson U, Zetterberg H, et al. Reference measurement procedure for CSF amyloid beta (Aβ)1–42 and the CSF Aβ1–42/Aβ1– 40 ratio—a cross-validation study against amyloid PET. J Neurochemistry 2016;139:651–8. [DOI] [PubMed] [Google Scholar]
- 24.Schaffer C, Sarad N, DeCrumpe A, Goswami D, Herrmann S, Morales J, et al. Biomarkers in the diagnosis and prognosis of Alzheimer’s disease. J Lab Autom 2015;20: 589–600. [DOI] [PubMed] [Google Scholar]
- 25.Zetterberg H Review: tau in biofluids—relation to pathology, imaging and clinical features. Neuropathol Appl Neurobiol 2017;43:194–9. [DOI] [PubMed] [Google Scholar]
- 26.Lifke V, Kollmorgen G, Manuilova E, Oelschlaegel T, Hillringhaus L, Widmann M, et al. Elecsys® Total-Tau and Phospho-Tau (181P) CSF assays: analytical performance of the novel, fully automated immunoassays for quantification of tau proteins in human cerebrospinal fluid. Clin Biochem 2019;72:30–8. [DOI] [PubMed] [Google Scholar]
- 27.Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, et al. Measurement of Phosphorylated Tau Epitopes in the Differential Diagnosis of Alzheimer Disease. Arch Gen Psychiatry 2004;61:95–102. [DOI] [PubMed] [Google Scholar]
- 28.Tariciotti L, Casadei M, Honig LS, Teich AF, McKhann Ii GM, Tosto G, and Mayeux R. Clinical experience with cerebrospinal fluid abeta42, total and phosphorylated tau in the evaluation of 1,016 individuals for suspected dementia. J Alzheimers Dis 2018;65:1417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barthelemy NR, Mallipeddi N, Moiseyev P, Sato C, Bateman RJ. Tau phosphorylation rates measured by mass spectrometry differ in the intracellular brain vs. extracellular cerebrospinal fluid compartments and are differentially affected by Alzheimer’s disease. Front Aging Neurosci 2019;11:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato C, Barthelemy NR, Mawuenyega KG, Patterson BW, Gordon BA, Jockel-Balsarotti J, et al. Tau kinetics in neurons and the human central nervous system. Neuron 2018;97:1284–98.e1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Mengel D, Keshavan A, Rissman RA, Billinton A, Perkinton M, et al. Learnings about the complexity of extracellular tau aid development of a blood-based screen for Alzheimer’s disease. Alzheimers Dement 2019;15:487–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McAvoy T, Lassman ME, Spellman DS, Ke Z, Howell BJ, Wong O, et al. Quantification of tau in cerebrospinal fluid by immunoaffinity enrichment and tandem mass spectrometry. Clin Chem 2014;60:683–9. [DOI] [PubMed] [Google Scholar]
- 33.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N Engl J Med 2012;367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farrer LA, Cupples LA, Haines LJ, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. JAMA 1997;278:1349–56. [PubMed] [Google Scholar]
- 35.Athena Diagnostics. ADmark Alzheimer’s evaluation. https://www.athenadiagnostics.com/view-full-catalog/a/admark-reg;-alzheimer-s-evaluation (Accessed June 2019).
- 36.Schindler SE, Bollinger JG, Ovod V, Mawuenyega KG, Li Y, Gordon BA, et al. High precision plasma amyloid-β 42/40 predicts current and future brain amyloidosis. Neurology 2019;93:e1647–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakamura A, Kaneko N, Villemagne VL, Kato T, Doecke J, Dore V, et al. High performance plasma amyloid-beta biomarkers for Alzheimer’s disease. Nature 2018;554: 249–54. [DOI] [PubMed] [Google Scholar]
- 38.Baker-Nigh AT, Mawuenyega KG, Bollinger JG, Ovod V, Kasten T, Franklin EE, et al. Human central nervous system (CNS) ApoE isoforms are increased by age, differentially altered by amyloidosis, and relative amounts reversed in the CNS compared with plasma. J Biol Chem 2016;291:27204–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suzuki K, Iwata A, Iwatsubo T. The past, present, and future of disease-modifying therapies for Alzheimer’s disease. Proc Jpn Acad Ser B Phys Biol Sci 2017;93: 757–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bateman RJ, Aisen P, De Stropper B, Fox NC, Lemere CA, Ringman JM, et al. Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res Ther 2011;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McDade E, Wang G, Gordon BA, Hassenstab J, Benzinger TLS, Buckles V, et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 2018;91:e1295–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blennow K A review of fluid biomarkers for Alzheimer’s disease: moving from CSF to blood. Neurol Ther 2017;6: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molinuevo JL, Ayton S, Batrla R, Bednar MM, Bittner T, Cummings J, et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol 2018;136:821–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zetterberg H, Burnham SC. Blood-based molecular biomarkers for Alzheimer’s disease. Mol Brain 2019; 12:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen P, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid proteins 1–40 and 1–42 in Alzheimer disease. Arch Neurol 2000;57:100–5. [DOI] [PubMed] [Google Scholar]
- 46.Song F, Poljak A, Valenzuela M, Mayeux R, Smythe GA, Sachdev PS. Meta-analysis of plasma amyloid-beta levels in Alzheimer’s disease. J Alzheimers Dis 2011;26:365–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fandos N, Perez-Grijalba V, Pesini P, Olmos S, Bossa M, Villemagne VL, et al. Plasma amyloid beta 42/40 ratios as biomarkers for amyloid beta cerebral deposition in cognitively normal individuals. Alzheimers Dement (Amst) 2017;8:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Palmqvist S, Janelidze S, Stomrud E, Zetterberg H, Karl J, Zink K, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related beta-amyloid status. [Epub ahead of print] JAMA Neurol June 24, 2019. as doi: 10.1001/jamaneurol.2019.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bateman RJ, Blennow K, Doody R, Hendrix S, Lovestone S, Salloway S, et al. Plasma biomarkers of AD emerging as essential tools for drug development: an EU/US CTAD Task Force report. J Prev Alzheimers Dis 2019;6:169–73. [DOI] [PubMed] [Google Scholar]
- 50.Hampel H, O’Bryant SE, Molinuevo JL, Zetterberg H, Masters CL, Lista S, et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol 2018;14:639–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hansson O, Mikulskis A, Fagan AM, Teunissen C, Zetterberg H, Vanderstichele H, et al. The impact of preanalytical variables on measuring cerebrospinal fluid biomarkers for Alzheimer’s disease diagnosis: a review. Alzheimer’s Dement 2018;10:1313–33. [DOI] [PubMed] [Google Scholar]
- 52.Blennow K, Zetterberg H. The past and the future of Alzheimer’s disease fluid biomarkers. J Alzheimers Dis 2018;62:1125–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tatebe H, Kasai T, Ohmichi T, Kishi Y, Kakeya T, Waragai M, et al. Quantification of plasma phosphorylated tau to use as a biomarker for brain Alzheimer pathology: pilot case-control studies including patients with Alzheimer’s disease and down syndrome. Mol Neurodegener 2017; 12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mielke MM, Hagen CE, Xu J, Chai X, Vemuri P, Lowe VJ, et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement 2018;14:989–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mattson N, Zetterberg H, Janelidze S, Insel PS, Andreasson U, Stomrud E, et al. Plasma tau in Alzheimer disease. Neurology 2016;87:1827–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan A, Rao MV, Veeranna, Nixon RA. Neurofilaments and neurofilament proteins in health and disease. Cold Spring Harb Perspect Biol 2017;9:a018309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mielke MM, Syrjanen JA, Blennow K, Zetterberg H, Vemuri P, Skoog I, et al. Plasma and CSF neurofilament light: relation to longitudinal neuroimaging and cognitive measures. Neurology 2019;93:e252–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olsson B, Portelius E, Cullen NC, Sandelius A, Zetterberg H, Andreasson U, et al. Association of cerebrospinal fluid neurofilament light protein levels with cognition in patients with dementia, motor neuron disease, and movement disorders. JAMA Neurol 2019;76:318–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mattsson N, Andreasson U, Zetterberg H, Blennow K. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol 2017;74:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin YS, Lee WJ, Wang SJ, Fuh JL. Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci Rep 2018;8:17368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lewczuk P, Ermann N, Andreasson U, Schultheis C, Podhorna J, Spitzer P, et al. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimers Res Ther 2018;10:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dale JM, Garcia ML. Neurofilament phosphorylation during development and disease: which came first, the phosphorylation or the accumulation? J Amino Acids 2012;2012:382107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jansen WJ, Ossenkopple R, Knol DL, Timjms BM, Scheltens P, Verhey FRJ, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a metaanalysis. JAMA 2015;313:1924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]