Abstract
Purpose:
To estimate the incidence of medication-free remission of chronic anterior uveitis; to identify predictors thereof.
Design:
Retrospective cohort study
Participants:
Patients diagnosed as having anterior uveitis for greater than 3 months followed at United States tertiary uveitis care facilities
Methods:
Retrospective cohort study based on standardized chart review. Estimation of remission incidence and identification of associated predictors used survival analysis.
Main Outcome Measures:
Incidence of medication-free remission. For the primary analysis, remission was defined as inactive uveitis off treatment at all visits spanning an interval of at least 90 days or—for patients who did not return for follow-up after 90 days—remaining inactive without receiving suppressive medications at all of their last visits. Association of factors potentially predictive of medication-free remission also was studied.
Results:
2795 eyes of 1634 patients with chronic anterior uveitis were followed over 7936 eye-years (4676 person-years). The cumulative medication-free, person-year remission incidence within 5 years was 32.7% [95% Confidence Interval (CI), 30.4% – 35.2%]. Baseline clinical factors predictive of less remission included longer duration of uveitis at presentation [adjusted hazard ratio (aHR), 0.61; 95% CI, 0.44 – 0.83; for 2 – 5 years vs. less than 6 months], bilateral uveitis (aHR, 0.75, 95% CI, 0.59 – 0.96), prior cataract surgery (aHR, 0.70; 95% CI 0.56 – 0.88) and glaucoma surgery (aHR, 0.63; 95% CI, 0.45 – 0.90). Two time-updated characteristics were also predictive of less remission: keratic precipitates (aHR, 0.36; 95% CI 0.21– 0.60) and synechiae (aHR, 0.62; 95% CI, 0.41 – 0.93). Systemic diagnosis with juvenile idiopathic arthritis and spondyloarthropathy also were associated with less remission. Older age at presentation was associated with higher incidence of remission (aHR, 1.29; 95%, CI 1.02 – 1.63, for age ≥ 40 years vs. < 40 years).
Conclusions:
Approximately one-third of chronic anterior uveitis cases remit within five years. Longer duration of uveitis, younger age, bilateral uveitis, prior cataract surgery, glaucoma surgery, presence of keratic precipitates and synechiae, and systemic diagnoses of juvenile idiopathic arthritis and spondyloarthropathy predict less remission; cases with these factors should be managed taking into account the higher probability of a longer disease course.
Uveitis is a major cause of vision loss worldwide.1 Anterior uveitis is the most common anatomic subtype of uveitis, making up the majority of prevalent uveitis cases.2,3 The clinical course of anterior uveitis can be acute, in which the disease persists for less than 3 months, or chronic, in which the disease persists for 3 months or longer.4 Chronic anterior uveitis has a high chance of causing ocular complications such as cataracts, glaucoma, band keratopathy and posterior synechiae.5,6 The goal of treatment is to suppress inflammation and, if possible, achieve remission of the uveitis.7 Initial treatment is almost always with topical corticosteroids. However, corticosteroids themselves can lead to complications, particularly cataract and ocular hypertension, and the risks of these complications increase with greater exposure to corticosteroids.8 Thus in patients with chronic anterior uveitis who do not achieve remission with corticosteroids, often treatment with corticosteroid-sparing systemic therapy is initiated to avoid the risk of further vision loss or injury as a result of the disease itself or long term corticosteroid treatment.
Presently there is limited information to predict whether a patient with chronic uveitis will eventually achieve remission. Identification of factors predictive of remission would be useful to counsel patients accurately regarding their prognosis and to guide clinical management. Identification of modifiable risk factors could provide options to increase chances of remission. Herein, we report the factors associated with medication-free remission of chronic uveitis in a large cohort of eyes of patients managed at tertiary uveitis centers in the United States.
Methods
The design of the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study has been described previously.9 SITE is a retrospective cohort study of patients with ocular inflammatory diseases seen at 5 tertiary referral centers in the United States; the study has been extended to include patients followed at these centers between 1979 and 2010. Only patients with noninfectious uveitis were included in this study, and patients with human immunodeficiency virus (HIV) infection or AIDS were excluded. The project was conducted in accordance with the principles of the Declaration of Helsinki, with approval of the institutional review boards of each institution, each of which granted a waiver of consent that allowed all living and deceased patients to be included.
All patients in the cohort diagnosed as having chronic anterior uveitis were identified. The participating centers used the approach suggested by the Standardization of Uveitis Nomenclature group in classifying cases as having chronic anterior uveitis.4 Patients had to have active inflammation and/or be on active treatment at the baseline visit to be included in the current analysis. Patients with Fuchs’ Heterochromic Iridocyclitis were excluded because of the difficulty in judging activity and remission in this anterior uveitis subtype.
Data Collection
Information on all patients with inflammatory eye disease was obtained from medical records and was entered into computer-based standardized data entry forms by trained reviewers, as described previously.9,10 Briefly, a highly structured chart review was conducted to obtain an extensive panel of data for each patient using a customized database developed specifically for the SITE Study. Data were collected for every date that the patient was seen. Data were collected with quality control checks requiring the reviewer to correct errors in real time to optimize data within the constraints of a retrospective chart review study. The database also included an extensive system of cross-checks within and between forms and similar embedded data quality queries. Data were also inspected for outliers at the time of analysis.
Data were collected regarding characteristics potentially predictive of remission, including demographic characteristics of the patients, bilateral versus unilateral uveitis, smoking status, the presence or absence of systemic diseases coexisting with ocular inflammation, and diagnostic and clinical features of anterior uveitis. Clinical findings analyzed as potential predictors included levels of inflammatory activity at presentation and ocular findings including band keratopathy, posterior synechiae, and keratic precipitates.
Main Outcome Measure
The primary objective was to evaluate predictive factors for remission in chronic anterior uveitis cases in a tertiary setting. Data on inflammatory disease activity were collected in a manner approximating the standards established by the Standardization of Uveitis Nomenclature as closely as possible in a retrospective study.4 An expert consensus panel has suggested that remission be defined as at least 3 months of uveitis inactivity absent use of suppressive anti-inflammatory medication.4 Given that the clinical practice at the participating centers did not call for patients to return more than 90 days after initial incidence of remission to verify sustained quiescence, it is likely that many patients did not return within 90 days of the onset of remission, but rather returned much later at the time of a relapse, or not at all if remission was persistent.
Therefore, as a primary analysis, we included eyes that either met the expert consensus definition directly (quiet off treatment at all visits spanning an interval of at least 90 days) or did not return for follow-up after 90 days but had remained inactive without receiving suppressive medications at all of the last visits, as being in remission.11 As a sensitivity analysis, we evaluated remission more conservatively, requiring that eyes meet the expert consensus panel definition directly (quiet off treatment at all visits spanning an interval of at least 90 days), which may be an underestimate of remission. The rationale for the sensitivity analysis was to evaluate whether risk factor associations differed when a very conservative definition of remission was used. Incidence of remission was assessed as the number of events per eye-year of follow-up while at “risk.”
Statistical Analysis
The proportion with remission at specific points were evaluated by calculating the cumulative incidence estimated from the crude Cox regression hazard function, which allowed nonlinear remission rates and confidence intervals consistent with the hazard ratios and accounting for correlation between eyes of the same patient. Plots were created using Kaplan-Meier analysis. Putative predictors of the incidence of remission was evaluated on the basis of hazard ratios and adjusted hazard ratios (aHRs; with 95% confidence intervals [CIs]), which were generated using crude and multivariate Cox proportional hazards models with a robust sandwich estimate to account for correlation between the eyes of individual patients.12 Variables that changed over time were evaluated as time-updated variables. Variables with substantial missing data at baseline (such as smoking) included a missing, or unknown, category. For other variables, patients with missing data at baseline were excluded. Missing values in follow-up were carried forward from previous visits. All statistical analyses were performed with SAS software version 9.3 (SAS Inc., Cary, NC).
Results
2795 eyes of 1634 patients were identified as having chronic anterior uveitis. These were followed up for the incidence of remission over 7935.9 eye-years (4675.7 person-years). The median follow up time was 1.8 eye-years (interquartile range 0.5 – 4.3 eye-years). The median age was 41 years (interquartile range 20 to 54 years). The proportion of males was 35%, and 60% percent of patients were white. At baseline, of the 2795 eyes, 499 (17.9%) eyes were not on medications and were active, 1227 (43.9%) were on medications and active, and 1069 (38.2%) were on medications and inactive. Of the 2296 eyes being treated at baseline, 1015 eyes (44.2%) were being treated with topical drops only, 499 (21.7%) were being treated with systemic medications only, and 782 (34.1%) were being treated with both systemic and ocular medications. Inactivity vs. activity was not based purely on anterior chamber cell but on a global assessment of the chart documentation, intended to capture the overall uveitic activity.
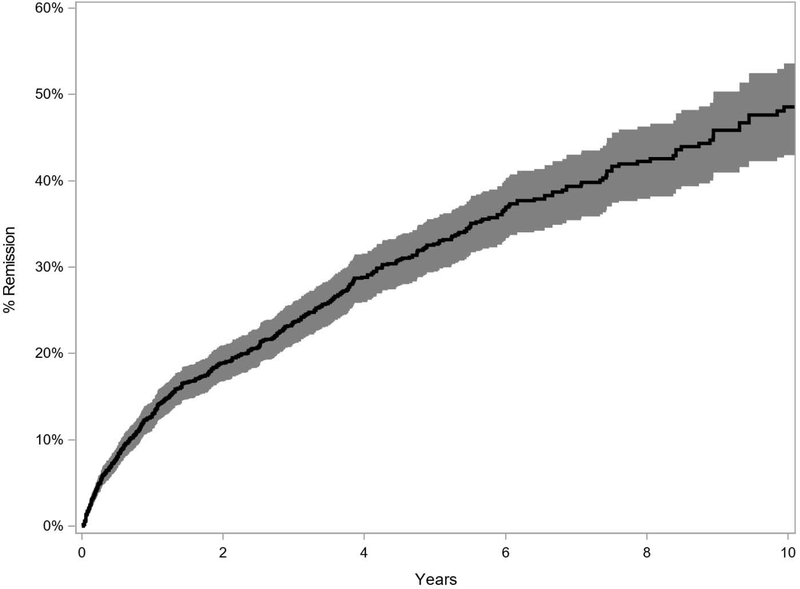
In this tertiary referral population at centers that typically manage severe cases, the Kaplan-Meier estimate of medication-free person-year remission at 5 years was 32.7% (95% CI, 30.4% – 35.2%) (Figure 1) and at 10 years was 48.6% (95% CI, 44.6% – 52.7%). In the more conservative sensitivity analysis, the Kaplan-Meier estimate of person-year remission at 5 years was 24.6% (95% CI, 22.4% – 27.0%) and at 10 years was 36.3% (95% CI, 32.3% – 40.7%) (Supplemental Figure 1, available at http://www.aaojournal.org).
Figure 1.
Kaplan Meier Curve of person-year remission (Primary Analysis)
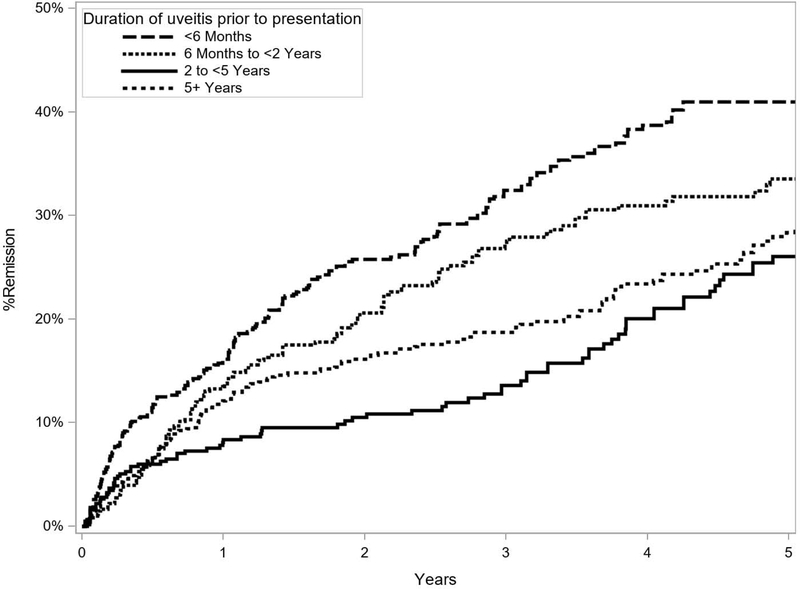
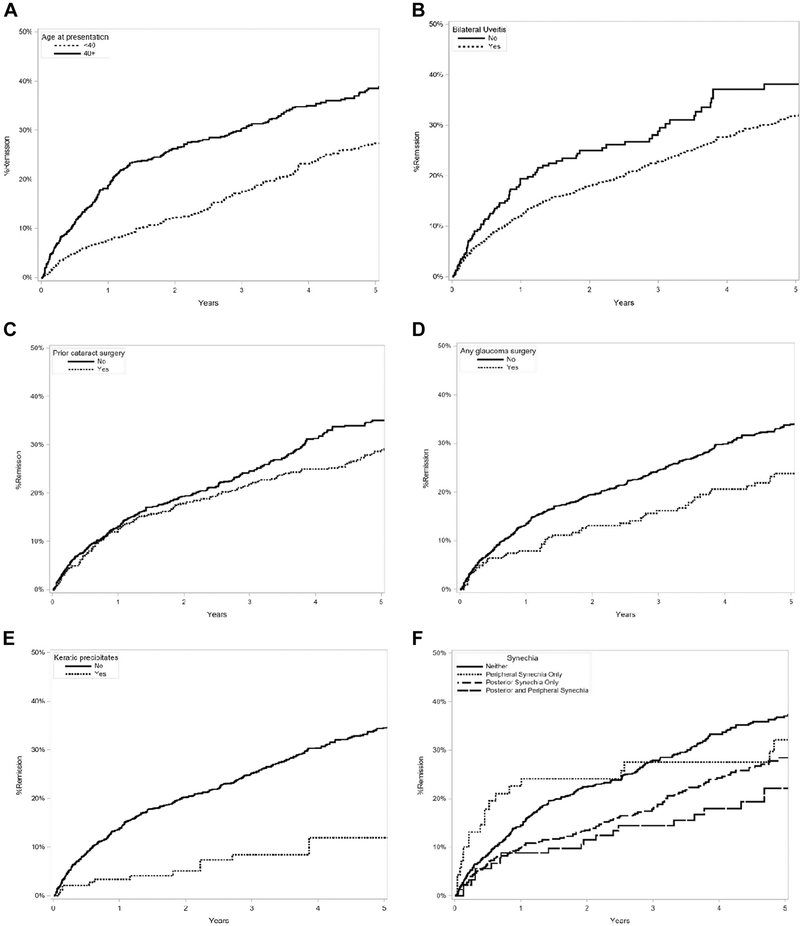
Factors predictive of medication-free remission in the adjusted primary analysis are listed in Table 1 (Supplemental Table 1, available at http://www.aaojournal.org, is a long version of Table 1 that also includes all factors assessed). Factors associated with a lower (less favorable) incidence of remission in the adjusted analysis included a longer duration of uveitis prior to presentation (aHR, 0.61; 95% CI, 0.44–0.83; for 2– 5 years vs. less than 6 months; Figure 2), bilateral uveitis (aHR, 0.75; 95%, CI 0.59 – 0.96; Figure 3), prior cataract surgery (aHR, 0.70; 95% CI, 0.56 – 0.88; Figure 3), history of glaucoma surgery (aHR, 0.63; 95% CI, 0.45 – 0.90; Figure 3), keratic precipitates (aHR, 0.36; 95% CI, 0.21 – 0.60; Figure 3), synechiae (aHR, 0.62; 95% CI, 0.41 – 0.93; for both posterior and peripheral synechiae vs. neither; Figure 3), juvenile idiopathic arthritis (aHR, 0.37; 95% CI, 0.26 – 0.53), and spondyloarthropathy (aHR, 0.35; 95% CI, 0.23 – 0.54). Spondyloarthropathy included three diseases: ankylosing spondylitis, psoriatic arthritis and reactive arthritis. Older age at presentation was associated with higher (more favorable) incidence of remission (aHR, 1.29; 95%, CI 1.02 – 1.63, for age ≥ 40 years vs. < 40 years; Figure 3). In the more conservative sensitivity analysis, associations were similar although statistical power was less with the smaller number of events (Supplemental Table 2, available at http://www.aaojournal.org).
Table 1.
Factors Predictive of Remission of Chronic Anterior Uveitis in the Adjusted analysis (Hazard Ratios Less than 1 are Unfavorable, Indicating a Lower Incidence of Remission)†
| Remission | Crude | Adjusted* | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | No | Yes | Hazard Ratio (95% CI) | P | Hazard Ratio (95% CI) | P | ||
| Duration of uveitis prior to presentation | <6 Months | 834 (30%) | 593 (71%) | 241 (29%) | Ref | <0.001 | Ref | 0.02 |
| 6 Months to <2 Years | 620 (22%) | 464 (75%) | 156 (25%) | 0.81 (0.63, 1.05) | 0.78 (0.60, 1.03) | |||
| 2 to <5 Years | 512 (18%) | 414 (81%) | 98 (19%) | 0.58 (0.43, 0.78) | 0.61 (0.44, 0.83) | |||
| 5+ Years | 829 (30%) | 662 (80%) | 167 (20%) | 0.63 (0.48, 0.81) | 0.76 (0.58, 1.01) | |||
| Bilateral | No | 379 (14%) | 283 (75%) | 96 (25%) | Ref | 0.008 | Ref | 0.02 |
| Yes | 2416 (86%) | 1850 (77%) | 566 (23%) | 0.73 (0.58, 0.92) | 0.75 (0.59, 0.96) | |||
| Prior cataract surgery | No | 2231 (80%) | 1698 (76%) | 533 (24%) | Ref | 0.03 | Ref | 0.002 |
| Yes | 564 (20%) | 435 (77%) | 129 (23%) | 0.80 (0.65, 0.97) | 0.70 (0.56, 0.88) | |||
| Any glaucoma surgery | No | 2627 (94%) | 1995 (76%) | 632 (24%) | Ref | 0.001 | Ref | 0.01 |
| Yes | 168 (6%) | 138 (82%) | 30 (18%) | 0.59 (0.43, 0.81) | 0.63 (0.45, 0.90) | |||
| Anterior chamber cells at baseline | Quiet | 1153 (43%) | 884 (77%) | 269 (23%) | Ref | <0.001 | Ref | <0.001 |
| 0.5+ | 530 (20%) | 369 (70%) | 161 (30%) | 1.46 (1.17, 1.83) | 1.48 (1.17, 1.87) | |||
| 1+ | 472 (18%) | 377 (80%) | 95 (20%) | 0.89 (0.68, 1.16) | 0.88 (0.66, 1.17) | |||
| ≥2+ | 526 (20%) | 421 (80%) | 105 (20%) | 0.79 (0.61, 1.02) | 0.87 (0.66, 1.15) | |||
| Keratic precipitates | No | 2079 (83%) | 1580 (76%) | 499 (24%) | Ref | <0.001 | Ref | <0.001 |
| Yes | 430 (17%) | 334 (78%) | 96 (22%) | 0.37 (0.22, 0.61) | 0.36 (0.21, 0.60) | |||
| Synechia | Neither | 1940 (72%) | 1459 (75%) | 481 (25%) | Ref | <0.001 | Ref | 0.008 |
| Peripheral Synechia Only | 61 (2%) | 43 (70%) | 18 (30%) | 0.74 (0.42, 1.29) | 0.69 (0.36, 1.32) | |||
| Posterior Synechia Only | 604 (23%) | 488 (81%) | 116 (19%) | 0.67 (0.55, 0.83) | 0.72 (0.58, 0.90) | |||
| Posterior and Peripheral Synechia | 79 (3%) | 66 (84%) | 13 (16%) | 0.56 (0.38, 0.84) | 0.62 (0.41, 0.93) | |||
| Juvenile Idiopathic Arthritis | No | 2192 (78%) | 1602 (73%) | 590 (27%) | Ref | <0.001 | Ref | <0.001 |
| Yes | 603 (22%) | 531 (88%) | 72 (12%) | 0.34 (0.25, 0.47) | 0.37 (0.26, 0.53) | |||
| Spondyloarthropathy | No | 2525 (90%) | 1899 (75%) | 626 (25%) | Ref | <0.001 | Ref | <0.001 |
| Yes | 270 (10%) | 234 (87%) | 36 (13%) | 0.41 (0.27, 0.62) | 0.35 (0.23, 0.54) | |||
| Age at presentation | <40 | 1384 (50%) | 1106 (80%) | 278 (20%) | Ref | <0.001 | Ref | 0.03 |
| 40+ | 1411 (50%) | 1027 (73%) | 384 (27%) | 1.77 (1.46, 2.16) | 1.29 (1.02, 1.63) | |||
Includes pa4ents who did not return for follow-up after 90 days, but had 1 or more follow-up visits before 90 days and had remained inactive without receiving suppressive medications at all of those visits, as being in remission.
Adjusted for age at presentation, bilateral, duration of uveitis prior to presentation, prior cataract surgery, any glaucoma surgery, anterior chamber cells at baseline, keratic precipitates, synechia, juvenile idiopathic arthritis, spondyloarthropathy
Figure 2.
Kaplan Meier Curve of uveitis remission as a function of duration of uveitis prior to presentation
Figure 3.
Kaplan Meier Curve of uveitis remission as a function of clinical variables (A) age, (B) bilaterality, (C) prior cataract surgery, (D) any glaucoma surgery, (E) keratic precipitates, ( (F) synechiae
Discussion
Our study shows that the person-year remission incidence in chronic anterior uveitis is approximately 33% at 5 years and 49% at 10 years in a tertiary care setting. Remission rates in non-tertiary settings might be higher, because the severe cases have a higher chance of being referred to a tertiary care center. Duration of uveitis, which is indicative of severity of disease, also was associated with decreased chance of remission, and the Kaplan-Meier curves suggest that the incidence of remission is higher in the first several years after uveitis diagnosis. More severe cases of uveitis are more likely to keep following up at tertiary centers over the long term, so the difference in remission rates between the early and later years may be enhanced by the enrichment for severe cases over the long term at tertiary centers.
The primary interest our results provide is with regard to factors predictive of medication-free remission of chronic anterior uveitis, which may provide a basis for counseling patients regarding the likely clinical course of their diseases, as well as providing guidance for clinical management. Greater age at presentation was associated with an increased incidence of remission. A previous SITE study found that age in the younger adult range, when compared with age in the older adult range, was associated with higher risk of relapse among patients with primary remitted anterior uveitis.13 It is not uncommon for more severe cases of uveitis to present earlier in life than less severe cases, which may explain this observation. Bilaterality of the uveitis was also associated with decreased incidence of remission. The previous study of new onset anterior uveitis found that bilaterality of uveitis was associated with decreased rate of remission as well.11 Bilateral disease may reflect a greater degree of immune dysregulation, a greater degree of disease severity, or both, which may be associated with a greater likelihood of persistence of uveitis.
The degree of anterior chamber cell at baseline was predictive of remission. Interestingly, having 0.5+ cell was associated with better chance of achieving remission than having no cell at baseline. The group of patients without cell at baseline may have been enriched for more severe cases which were being treated more aggressively to suppress inflammation, thus achieving no anterior chamber cell, but were still at higher risk for not achieving remission because of their disease severity. Patients with 0.5+ cell at baseline, conversely, may have been patients with milder disease whose physicians were tolerating a minimal level of inflammation while awaiting tertiary evaluation because of their milder disease. The incidence of remission for higher grades of anterior chamber cell (1+ and ≥ 2+) was lower than those without cell, but this did not achieve statistical significance. We acknowledge that there is some difficulty in interpreting these results regarding anterior chamber cell at baseline as patients were at varying stages of disease course and treatment at their baselines.
There are several potential explanations for the association of prior cataract surgery with lower incidence in remission in chronic anterior uveitis. We observed this previously for primary, new-onset anterior uveitis.11 Cataract surgery can lead to persistent ocular inflammation postoperatively due retained lens material, surgical trauma to the eye potentially exposing antigens to the immune system, and/or intraocular-lens associated uveitis (chaffing syndromes).14 Similarly any prior glaucoma surgery also was associated with a lower incidence of remission. Both major types of glaucoma surgery, trabeculectomy and glaucoma tube implantation, can be associated with persistent inflammation. In particular, glaucoma tube implantation can lead to tube-iris touch/chaffing that can cause chronic anterior chamber cellular reaction.15 However, our study design does not allow us to determine if these surgeries truly cause increased inflammation; requirement to undergo cataract and/or glaucoma surgery might be associated with less remission because it is a marker for disease severity. Unfortunately, data on the clinical course before patients came for tertiary care are not available, so we do not have the capacity to accurately and consistently distinguish between patients with a history of uveitis who subsequently underwent eye surgery and were more likely to develop chronic anterior uveitis and patients with no previous uveitis history who underwent surgery which resulted in chronic anterior uveitis.
Two clinical examination findings were associated with a lower incidence of remission: keratic precipitates and synechiae. These factors previously have been associated with greater disease severity.11,16,17 Presence of peripheral anterior synechiae, posterior synechiae alone, and both posterior and peripheral synechiae were associated with a substantially lower incidence of remission. These associations were statistically significant except for peripheral synechiae alone (which were observed infrequently, and hence had less statistical power). Of note, it is likely that peripheral anterior synechiae were under-ascertained because gonioscopy was not uniformly conducted and documented in many of the uveitis practices. Consequently, gonioscopy data was not recorded as part of SITE. If peripheral anterior synechiae were noted in the anterior segment examination, it was recorded as peripheral anterior synechiae being present. If there was no mention of peripheral anterior synechiae, then those patients were recorded as not having peripheral anterior synechiae. It is possible that patients with milder amounts of peripheral anterior synechiae may have been missed with this approach. However, because most cases recorded as not having peripheral anterior synechiae are likely to truly not have peripheral anterior synechiae, it is not likely that the misclassification introduced had any significant effect on the analyses. Because this analysis was time-updated, the rate of remission is decreased no matter when along the disease course the patient develops synechiae. In addition to being markers of severity, it is possible that patients with keratic precipitates and synechiae may have received insufficient treatment for several years. Unfortunately, this study is not designed to determine this as information on clinical course prior to being referred to the tertiary center is lacking, as mentioned above. One direction for future research is to determine if more intensive initial treatment enhances the chances of uveitis remission.
The incidence of remission was also lower in patients with an underlying diagnosis of juvenile idiopathic arthritis or spondyloarthropathy. In our study of acute anterior uveitis remission,11 juvenile idiopathic arthritis was also associated with a similarly lower incidence of remission: 63% lower in the current study and 62% lower in the previous acute anterior uveitis study. There are also several other reports that have found juvenile idiopathic uveitis often have persistent disease.18–21 The diseases included in spondyloarthropathy – ankylosing spondylitis, psoriatic arthritis and reactive arthritis – are also known to be commonly recurrent or chronic.22,23 It is interesting to note that HLA-B27 status was not associated with the incidence of remission, even though HLA-B27 is itself associated with ankylosing spondylitis and psoriatic arthritis. This may be due to the fact that HLA-B27 status was not consistently ordered at these centers, and thus there may be greater misclassification for this variable.
The lower incidence of remission of chronic anterior uveitis in patients afflicted with juvenile idiopathic arthritis or spondyloarthropathy might reflect a more aggressive disease course, or alternately a greater need for systemic therapy for the juvenile idiopathic arthritis or spondyloarthropathy rather than the uveitis itself. Given that the recommended definition of remission4 requires a patient be inactive off anti-inflammatory therapies regardless of why they were given—because the treatment would tend to suppress eye inflammation regardless of the indication for its use—the incidence of remission of anterior uveitis cases associated with both juvenile idiopathic arthritis and spondyloarthropathy might tend to be lower than cases without systemic inflammatory diagnoses in addition to uveitis. In other words, both juvenile idiopathic arthritis and spondyloarthropathy may have had lower rates of remission due to ongoing, long term immunosuppression given for systemic manifestations of disease as opposed to eye disease.
We had hypothesized, but did not find, an association between statin use and rate of remission. Recent reports have suggested a potential benefit of statin use in uveitis patients.24,25 The direction of effect in this study was towards a higher incidence of remission (aHR, 1.17; 95% CI, 0.81 – 1.69) but this was not significant. Neither was use of ACE Inhibitors nor aspirin associated with an altered incidence of remission. Diabetes mellitus also was not found to be associated with a different incidence of remission. Other studies have demonstrated an association between diabetes onset and anterior uveitis onset,26 and have found that the odds of developing anterior uveitis with poor glycemic control to be 2.01 for type 1 diabetes and 1.23 for type 2 diabetes.27 It is possible that diabetes mellitus may be associated with onset of uveitis but does not affect remission rates. Smoking was not associated with a decreased incidence of remission. Other studies have found a higher risk of uveitis with smoking28,29; a prior study from the SITE-1 subset of this database involving all forms of ocular inflammation found a higher incidence of relapse of ocular inflammation among smokers.30 For both diabetes mellitus and smoking, it is also possible that these factors have smaller effects on chronic anterior uveitis remission than the current analyses had power to detect.
The strengths of this study include a large sample size, with consequently increased statistical power, and use of quality control methods to optimize the accuracy of data collection. The main limitation is that the study is retrospective. Incomplete follow-up and missing data likely led to some imprecision in the remission incidence. For example, it is possible that some patients with asymptomatic active chronic uveitis who did not come for follow-up were counted as being in remission, when they were not. Concern with this limitation led us to perform the sensitivity analysis. Most likely, remission might have been associated with less follow-up (because treatment no longer was needed), in which case our primary analysis would be closer to the truth than the sensitivity analysis (in which cases in remission who did not return after remission are counted as censored rather than remitted). Also, on average, more severe cases would be referred for tertiary care and tertiary care physicians may sometimes taper treatment more slowly. Both of these factors could lead to a longer estimated time to remission/lower incidence of remission. Alternatively, remission rates in tertiary care centers may be lower due to pursuit of more aggressive therapies. In either case, the associations observed are supported by pathogenic theories, and in some cases by prior observations, so it is most likely predictive factor results can be qualitatively generalized to tertiary and non-tertiary care settings. Results regarding the absolute incidence of remission likely are more generalizable to tertiary practices; incidence of remission is probably more favorable in non-tertiary settings.
There are other limitations with the design of this retrospective study. The degree of adherence to therapy is unknown. In patients who have associated systemic illnesses, we are unable to consistently and accurately discern whether the indication for initiation of systemic medications was the systemic illness or the uveitis. We are unable to conduct a straightforward assessment of the effect of immunosuppressive therapy on remission because receiving the therapy itself leads to the case being classified as not in remission, and most cases are treated for a protracted period of time before tapering is attempted to see if remission has occurred. This indication-for-treatment bias is particularly a problem in a study of anterior uveitis, a condition that often can be managed with topical therapies, wherein immunosuppression would be used for the most severe cases. In addition, any assessment of treatment is difficult using the remission definition at least 3 months of uveitis inactivity absent use of suppressive anti-inflammatory medication,4 because of there is a type of immortal-person-time bias since treatment is part of the outcome and always is going to be negatively associated with remission by definition. There are likely some patients who required sustained immunosuppression for their systemic immune-mediated disease and thus would not be able to achieve this study’s definition of remission, even if their uveitis was in remission. In this retrospective study looking at clinic notes, it is difficult to accurately parse out the reason for continuation of treatment in such cases. Thus, as mentioned above for juvenile idiopathic arthritis and spondyloarthropathy, patients with associated immune-mediated diseases may be less likely to meet criteria for remission because of the need for ongoing treatment of their systemic disease. We have limited power to address the related question of what proportion of patients who develop chronic uveitis while on immunosuppression for systemic disease and require treatment with topical steroid drops are subsequently able to discontinue drops while maintaining their baseline level of immunosuppression. Prospective studies designed to evaluate these issues are important for a comprehensive understanding of disease remission in uveitis. A final point is that remission may not be permanent in some cases. Discussion of these remission estimates with patients must include the caveat that remitted chronic anterior uveitis has a risk of relapse; the incidence of future relapses is not captured by the current analyses.
In summary, our study of cases of chronic anterior uveitis receiving tertiary uveitis care suggests that the incidence of remission over time is substantial, with about half of cases in remission by ten years. We identified several factors associated with a less favorable incidence of medication free remission, including longer duration of uveitis, younger age, bilateral uveitis, cataract surgery, glaucoma surgery, concurrent diagnoses of juvenile idiopathic arthritis or spondyloarthropathy and presence of keratic precipitates and synechiae at any point in the disease course. Patients presenting with these risk factors (perhaps markers of greater disease severity) are probably at higher risk of persistent inflammation, particularly those with more than one of these factors. Reciprocally, patients lacking these factors would be more likely to experience remission. Patients with these potential risk factors for non-remission in uveitis should be educated and managed taking into account the higher probability of a chronic course, which in turn may indicate closer monitoring and/or longer-term treatment of these cases prior to tapering attempts.
Supplementary Material
Among 2795 eyes with chronic anterior uveitis, medication-free remission occurred in approximately 33% within 5 years; clinical markers of increased disease severity, and juvenile idiopathic arthritis and spondyloarthropathy diagnoses were associated with less remission.
Acknowledgments
Financial Support: Primary support from NIH grant R21 EY026717 (Dr. Kempen); NIH University of Pennsylvania Core Grant for Vision Research 2P30EYEY001583 (Bethesda, MD); Massachusetts Eye and Ear Global Surgery Program (Boston, MA); Sight for Souls (Philadelphia, PA), and Research to Prevent Blindness (New York, NY). The funding organizations had no role in the design or conduction of this research.
Conflicts of Interest:
James Rosenbaum: Abbvie (Consultant); Gilead (Consultant); Janssen (Consultant); Eyevensys (Consultant); UpToDate (Consultant); Pfizer (Financial Support); Novartis (Consultant); Roche (Consultant); Alcon Research Institute (Financial Support)
Grace Levy-Clarke: Abbvie (Consultant, Lecture Fees); Allergan (Grant Support); Mallinckrodt (Consultant, Grant Support); Sanofi (Grant Support; Lecture Fees)
Eric Suhler: Eyevensys (Consultant); Santen (Consultant); EyeGate (Consultant, Financial Support); Abbvie (Consultant, Financial Support); Clearside (Consultant, Financial Support); EyePoint (Consultant, Financial Support)
Jennifer Thorne: Abbvie (Consultant
John Kempen: Clearside (Consultant); Santen (Consultant); Gilead (Consultant)
Footnotes
Meeting Presentation: This paper was presented at the Association for Research in Vision and Ophthalmology Annual Meeting, Vancouver, Canada, 2019.
This article contains additional online-only material. The following should appear online-only: Supplemental Tables 1 and 2 and Supplemental Figure 1.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Suttorp-Schulten MS, Rothova A. The possible impact of uveitis in blindness: a literature survey. Br J Ophthalmol 1996;80:844–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gritz DC, Wong IG. Incidence and prevalence of uveitis in Northern California; the Northern California Epidemiology of Uveitis Study. Ophthalmology 2004;111:491–500. [DOI] [PubMed] [Google Scholar]
- 3.Acharya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol 2013;131:1405–12. [DOI] [PubMed] [Google Scholar]
- 4.Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durrani OM, Meads CA, Murray PI. Uveitis: a potentially blinding disease. Ophthalmologica 2004;218:223–36. [DOI] [PubMed] [Google Scholar]
- 6.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, Kijlstra A. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kruh J, Foster CS. The philosophy of treatment of uveitis: past, present and future. Dev Ophthalmol 2012;51:1–6. [DOI] [PubMed] [Google Scholar]
- 8.Thorne JE, Woreta FA, Dunn JP, Jabs DA. Risk of cataract development among children with juvenile idiopathic arthritis-related uveitis treated with topical corticosteroids. Ophthalmology 2010;117:1436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempen JH, Daniel E, Gangaputra S, et al. Methods for identifying long-term adverse effects of treatment in patients with eye diseases: the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study. Ophthalmic Epidemiol 2008;15:47–55. [DOI] [PubMed] [Google Scholar]
- 10.Kempen JH, Daniel E, Dunn JP, et al. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study. Bmj 2009;339:b2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Artornsombudh P, Pistilli M, Foster CS, et al. Factors predictive of remission of new-onset anterior uveitis. Ophthalmology 2014;121:778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang KY, Zeger SL. Regression analysis for correlated data. Annu Rev Public Health 1993;14:43–68. [DOI] [PubMed] [Google Scholar]
- 13.Grunwald L, Newcomb CW, Daniel E, et al. Risk of relapse in primary acute anterior uveitis. Ophthalmology 2011;118:1911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Llop SM, Papaliodis GN. Cataract Surgery Complications in Uveitis Patients: A Review Article. Semin Ophthalmol 2018;33:64–9. [DOI] [PubMed] [Google Scholar]
- 15.Chow A, Burkemper B, Varma R, Rodger DC, Rao N, Richter GM. Comparison of surgical outcomes of trabeculectomy, Ahmed shunt, and Baerveldt shunt in uveitic glaucoma. J Ophthalmic Inflamm Infect 2018;8:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woreta F, Thorne JE, Jabs DA, Kedhar SR, Dunn JP. Risk factors for ocular complications and poor visual acuity at presentation among patients with uveitis associated with juvenile idiopathic arthritis. Am J Ophthalmol 2007;143:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AlBloushi AF, Alfawaz AM, Al-Dahmash SA, et al. Incidence, Risk Factors and Surgical Outcomes of Cataract among Patients with Uveitis in a University Referral Hospital in Riyadh, Saudi Arabia. Ocul Immunol Inflamm 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Kotaniemi K, Arkela-Kautiainen M, Haapasaari J, Leirisalo-Repo M. Uveitis in young adults with juvenile idiopathic arthritis: a clinical evaluation of 123 patients. Ann Rheum Dis 2005;64:871–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassi A, Corona F, Casellato A, Carnelli V, Bardare M. Prevalence and outcome of juvenile idiopathic arthritis-associated uveitis and relation to articular disease. J Rheumatol 2007;34:1139–45. [PubMed] [Google Scholar]
- 20.Kalinina Ayuso V, van de Winkel EL, Rothova A, de Boer JH. Relapse rate of uveitis post-methotrexate treatment in juvenile idiopathic arthritis. Am J Ophthalmol 2011;151:217–22. [DOI] [PubMed] [Google Scholar]
- 21.Horton S, Jones AP, Guly CM, et al. Adalimumab in juvenile-idiopathic arthritis-associated uveitis (JIA-U): 5-year follow-up of the Bristol participants of the SYCAMORE trial. Am J Ophthalmol 2019. [DOI] [PubMed] [Google Scholar]
- 22.Murray PI, Rauz S. The eye and inflammatory rheumatic diseases: The eye and rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis. Best Pract Res Clin Rheumatol 2016;30:802–25. [DOI] [PubMed] [Google Scholar]
- 23.Sharma SM, Jackson D. Uveitis and spondyloarthropathies. Best Pract Res Clin Rheumatol 2017;31:846–62. [DOI] [PubMed] [Google Scholar]
- 24.Borkar DS, Tham VM, Shen E, et al. Association between statin use and uveitis: results from the Pacific Ocular Inflammation study. Am J Ophthalmol 2015;159:707–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirinsky IV, Biryukova AA, Shirinsky VS. Simvastatin as an Adjunct to Conventional Therapy of Non-infectious Uveitis: A Randomized, Open-Label Pilot Study. Curr Eye Res 2017;42:1713–8. [DOI] [PubMed] [Google Scholar]
- 26.Watanabe T, Keino H, Nakayama K, Taki W, Echizen N, Okada AA. Clinical features of patients with diabetic anterior uveitis. Br J Ophthalmol 2019;103:78–82. [DOI] [PubMed] [Google Scholar]
- 27.Ansari AS, de Lusignan S, Hinton W, Munro N, Taylor S, McGovern A. Glycemic control is an important modifiable risk factor for uveitis in patients with diabetes: A retrospective cohort study establishing clinical risk and ophthalmic disease burden. J Diabetes Complications 2018;32:602–8. [DOI] [PubMed] [Google Scholar]
- 28.Yuen BG, Tham VM, Browne EN, et al. Association between Smoking and Uveitis: Results from the Pacific Ocular Inflammation Study. Ophthalmology 2015;122:1257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin P, Loh AR, Margolis TP, Acharya NR. Cigarette smoking as a risk factor for uveitis. Ophthalmology 2010;117:585–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galor A, Feuer W, Kempen JH, et al. Adverse effects of smoking on patients with ocular inflammation. Br J Ophthalmol 2010;94:848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.