Abstract
Background
How inflammatory cells are recruited into the central nervous system is a topic of interest in a number of neurological injuries. In aneurysmal subarachnoid hemorrhage (SAH), neutrophil accumulation in the central nervous system three days after the hemorrhage is a critical step in the development of Delayed Cerebral Injury (DCI). The mechanism by which neutrophils enter the central nervous system is still unclear.
Methods and Results
To identify human effectors of neutrophil recruitment, cerebrospinal fluid (CSF) samples were taken from a small, selected sample of SAH patients with external ventricular drainage devices (10 patients). Among a battery of CSF cytokines tested three (3) days after SAH, five cytokines were associated with poor 90-day outcome (mRS 3–6). A parallel study in a mouse model of mild SAH showed elevation in three (3) cytokines in the CNS compared to sham. IL-17 and IL-2 were increased in both patients and the mouse model. IL-17 was investigated further because of its known role in neutrophil recruitment. Inhibition of RoRγt, the master transcription factor of IL-17, with the inverse agonist GSK805 suppressed neutrophils entry into the CNS after SAH compared to control. Using an IL-17 reporter mouse, we investigated the source of IL-17 and found that myeloid cells were a common IL-17producing cell type in the meninges after SAH, suggesting an autocrine role for neutrophil recruitment.
Conclusions
Taken together, IL-17 appears to be in important factor in the recruitment of neutrophils into the meninges after SAH and could be an important target for therapies to ameliorate DCI.
Keywords: subarachnoid hemorrhage, neutrophils, IL-17, neuroinflammation, cytokines, Delayed Cerebral Injury
Introduction
Aneurysmal subarachnoid hemorrhage (SAH) has a high incidence of long-term disability. The most common preventable complication is the syndrome of delayed cerebral injury (DCI, also called delayed cerebral ischemia) 1,2,3. DCI includes the delayed onset (typically 4–10 days after the hemorrhage) of acute neurological deficits, confusion, fever, and cerebral vasospasm with long-term deficits in cognition and physical ability 4,5. The delayed onset of DCI provides a unique opportunity to intervene and possibly eliminate its development after SAH.
It is increasingly clear that DCI (vasospasm and neurological deteriorations) does not correlate closely with outcomes in SAH suggesting that there are other factors at play 6. The longstanding theory that DCI leads to ischemia from vasoconstriction has been called into question with the results of trials using vasodilators such as clazosentan and nicardipine 6,7. Inflammatory responses appear to be potent drivers of DCI 8,9. Studies in patients and a murine model of SAH implicate meningeal neutrophils with both DCI and poor outcome 10,11.
Neutrophils enter into the central nervous system (CNS) during aneurysmal subarachnoid hemorrhage (SAH) prior to the development of delayed cerebral injury (DCI) and depletion improves outcome in murine models. Neutrophil trafficking and tissue entry to all tissues (including the meninges) is dependent on the expression of chemoattractants. A number of cytokines and chemokines known to recruit systematic neutrophils to sites of injury have been investigated for their ability to modulate secondary injury after SAH 12,13,14,15. CXCL8 (IL-8), a potent neutrophil chemoattractant, after SAH correlates with the onset of secondary injury (i.e. DCI), however its role in brain injury after SAH is still unknown16. Neutrophil accumulation in the CNS three days after the hemorrhage (SAH) has been shown to be an important step in the development of delayed cerebral injury (DCI) 11,17. Removing or inactivating neutrophils 3 days after SAH prevents the development of DCI suggesting that prevention of neutrophil entry to the CNS is a viable therapeutic target17,10.
Interleukin-17 (IL-17) is a potent neutrophil recruiter that is associated with inflammatory diseases such as rheumatoid arthritis, and neuroinflammatory diseases such as multiple sclerosis (MS) 18,19. Specific inhibition of RORγt (a master regulator of cytokine expression including IL-17) reduces the levels of IL-17, impairs development of Th17 cells (the IL-17 producing lymphocyte), and ameliorates disease progression in a number of diseases including experimental autoimmune encephalomyelitis (EAE, a model of MS) 18. Importantly, disrupting the IL-17 signaling pathway in systemic inflammatory diseases such as rheumatoid arthritis decreases neutrophil influx 19,20. IL-17 is produced in CD4 lymphocytes called T-helper 17 cells (Th17), γδ Tcells and innate lymphoid cells (ILCs), but has been recently described in neutrophils in a number of diseases 21,22,23,24. In SAH, IL-17 production is increased in the patient’s blood, but this does not correlate with clinical outcome suggesting local effects may be important 25.
We have previously shown that development of DCI is dependent on the late accumulation of neutrophils in the CNS10. In this study, we show that products of RORγt (including IL-17) are involved in the recruitment of neutrophils into the meninges.
Materials and Methods
Patients
This study was approved by the Cleveland Clinic Institutional Review Board and was conducted over parts of 2013 and 2014. This prospective, single center, hypothesis-generating study included patients with SAH presenting to the Cleveland Clinic Neurointensive Care unit within the first 24 hours of rupture without co-morbid inflammatory disease, infection, or immunosuppression and with external ventricular drains (EVDs) for treatment of acute hydrocephalus. CSF was collected from consented patients via the burette of the EVD collection system (Medtronic, Inc. Minneapolis, MN) after one hour of drainage on day 3 after the onset of symptoms of hemorrhage. Samples were centrifuged and the supernatant was stored with a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and frozen at −80°C until time for evaluation. Samples were tested with a 36-analyte cytokine/chemokine array (Proteome Profiler Array human cytokine array panel 1 [R&D Systems, Minneapolis, MN]) using the manufacturer’s instructions for use with the Li-Cor Odyssey Clx infrared imaging system (Licor Biosystems, Lincoln, NE). Intensity values were corrected for local membrane effects using a circular perimeter around the sample dot. Relative intensity was defined as corrected sample intensity as a ratio of the negative control dot. Data was tabulated and evaluated using Graphpad Prism 7 software (Graphpad, La Jolla,CA, US).
Patients were followed during their hospital stay for the development of cerebral vasospasm. A three-tiered strategy to define DCI was employed. Patients in the period of cerebral vasospasm (the 4th through 12th day) with TCD elevations (Mean velocity of >150 cm/s MCA, >130 cm/s ACA, or >90 cm/s basilar) underwent four vessel digital subtraction angiography. In this cohort of patients, all patients who had elevated TCDs above the threshold also showed evidence of cerebral vasospasm on angiography. Patients enrolled in the study were interviewed by telephone 90 days after hemorrhage for determination of modified Rankin Score (mRS). Good outcome on the mRS was considered an mRS 0–2; Poor outcome was an mRS of 3–6. Interviewers who performed the mRS were blinded to laboratory data. The exploratory nature of this analysis did not allow patient number calculation or segregation of patients by demographic or personal characteristics and was conducted over a calendar year.
SAH Model
Young (8–12 weeks old) male C57BL/6 and IL-17 atm1Bcgen mice (Jackson Labs, Bar Harbor, ME) were used. This model of SAH has not been validated in female mice due to the variability in response to intervention (female mice in both SAH and sham groups have highly variable impairment on spatial tasks). For this reason, only male mice were used.
Mice were kept on a 12 hour:12-hour light cycle at room temperature (22–25°C). Food and water were provided ad libitum. All experiments were done with the approval of the University of Virginia Animal Care and Use Committee. To mitigate circadian variation, surgeries were done during morning hours in the same surgical location.
On the day of intervention, mice were exposed to either experimental SAH using a modification of a protocol previously described, or sham surgery 26. Briefly, 8–12-week-old male (C57BL/6 or C57BL/6-IL-17atm1Bcgen) mice were anesthetized using 2% isoflurane and placed under a surgical microscope. Bupivacaine was injected to the site of surgery and a small incision was made in the midline between strap muscles. Muscles in the neck were separated to expose the cisterna magna. A 30-gauge needle was inserted through the dura mater. Its beveled edge was used to transect a conserved subarachnoid vein located just below the dura mater. The animals were held at 30° for 5 minutes to allow the blood to flow cephalad. Animals in the control conditions received a sham operation with all the same procedures omitting the dural puncture and transection of subarachnoid vein. The site of incision was sutured and the animals were placed in a recovery chamber with appropriate analgesia prior to returning to their respective cages.
Littermate animals were randomly assigned to experimental groups for each experiment. C57BL/6-IL-17atm1Bcgen mice were not randomized but littermates were used.
Mouse Cytokine Analyte Array
Mouse cytokine arrays were performed on whole brain homogenates of SAH and sham operated mice three days after hemorrhage. The brains were dissected, homogenized, delipidated and stored in PBS. Samples were centrifuged and the supernatant was stored with a protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and frozen at −80° until time for evaluation. Samples were tested with a 40-analyte mouse cytokine/chemokine array (Proteome Profiler Array mouse cytokine array panel a [R&D Systems, Minneapolis, MN]) using the manufacturer’s instructions with the exception that Li-Cor compatible secondary antibodies (Licor Biosystems, Lincoln, NE) were used to resolve the membranes with a Li-Cor Odyssey Clx infrared imaging system (Licor Biosystems, Lincoln, NE). Intensity values were corrected for local membrane effects using a circular perimeter around the sample dot. Relative intensity was defined as corrected sample intensity as a ratio of the negative control dot. Data was tabulated and evaluated using Graphpad Prism 7 software (Graphpad, La Jolla,CA, US).
GSK 805 Administration
30mg/kg of the RORγt inverse agonist, GSK805 (EMD Millipore, Burlington, MA), was dissolved in 10% DMSO in corn oil to a volume of 0.1mL and administered via a 20-gauge curved gavage needle daily for 14 days as previously described 27. Control animals were given the same dosing volume (0.1 mL) of 10% DMSO in corn oil as a vehicle. On the fourteenth day of the feeding regimen animals were randomized to either SAH or sham surgery. Efficacy of GSK805 to decrease IL-17 was confirmed using an IL-17 reporter mouse. GSK805 was administered daily at the same time in the same room.
Flow cytometry
Flow cytometry was performed on both the brain parenchyma and meninges of SAH and sham operated mice three days after intervention. To generate cell suspensions of brain tissue for flow cytometry, the brain was isolated from the skull and meninges and processed in DMEM media with 10% bovine serum albumin (BSA). Brains were digested using 1mg/ml DNAse and 4 U/ml Papain in Hanks Balanced Salt solution with calcium and magnesium (HBSS). Samples were incubated at 37°C for 45 min, triturated and filtered using a 70 μm cell filter. Cell were then centrifuged and a 40% Percoll solution used to remove all the myelin debris from the cell suspension. Following Percoll separation, the cell suspension was resuspended in DMEM with 10% BSA and kept on ice until staining.
To generate a single cell suspension from the meninges for flow cytometry, the meninges were collected and processed as previously described 28. Briefly, meninges were dissected and processed in DMEM media with 10% bovine serum albumin. Samples were digested using 1 mg/ml DNAse and 1.4 U/ml collagenase in HBSS. Samples were then incubated in a 37°C water bath, triturated and strained through a 70 μm cell filter. Samples were then centrifuged and resuspended in DMEM media with 10% BSA and kept on ice until staining. Once single cell suspensions were obtained, samples were blocked with Fc block and incubated with an antibody cocktail containing anti-CD11b, Ly6G, Ly6C (Life Technologies, Carlsbad, CA, US; 1:200), and CD45 (Biolegend, San Diego, CA, US; 1:200) for 30 minutes at 4°C. To determine cell viability within each suspension, an aliquot of each sample was incubated with the fixable viability dye efluor 506 (ThermoFisher, Waltham, MA, US; 1:1000) for 30 minutes at 4°C. Samples were then analyzed using a Gallios cytometer (Beckman Coulter, Brea, CA, US), and analyzed using FlowJo software (FlowJo, LLC, Ashland, OR, US). Results for total number of neutrophils, percentage of neutrophils (as a percentage of all inflammatory cells), and the ratio of neutrophils (as a percentage) between sham and SAH animals were recorded for both untreated and treated animals. Eight mice in each of the four groups (vehicle Sham, vehicle SAH, RORinh Sham, and RORinh SAH) were used for this part of the study.
Immunohistochemistry
C57B6/J and IL-17 atm1Bcgen mice were transcardially perfused with 4% paraformaldehyde in PBS. Brains and meninges were dissected and processed separately for immunohistochemistry. Brains were post-fixed in 4% paraformaldehyde for 24 hours followed by cryoprotection in a 30% sucrose solution. Brains were then frozen and 30 μm sections collected. Sections containing the hippocampus were selected, rinsed in PBS, and blocked in a 0.3% TritonX with 5% normal goat serum solution. Sections were then incubated with antineutrophil antibody 7/4 (anti-Ly6B) (Abcam Cambridge, MA, US; 1:100), overnight at room temperature. The following day, sections were rinsed and incubated with AlexaFluor conjugated secondary antibody (ThermoFisher Scientific, Waltham, MA, US; 1:500) for 1 hour at room temperature, mounted on slides (ThermoFisher, Waltham, MA, US), and cover-slipped using Vectashield mounting medium with DAPI (Vector laboratories, Burlingame, CA, US). Meninges were removed from the skullcap and kept in PBS until processing. Non-specific binding sites were blocked using PBS containing 1% BSA, 5% normal goat serum, 0.1% Triton-X, 0.2% tween, and Fc block. Meninges were then incubated in a primary antibody cocktail at 4ºC overnight and counterstained with DAPI. Antibodies used include antineutrophil antibody 7/4 (Abcam, Cambridge, MA, US; 1:100 and 1:50), and anti-CD31 antibody (ThermoFisher, Waltham, MA, US: 1:500).
Confocal image acquisition and analysis
All images were obtained on the Olympus FV1200 confocal microscope with Fluoview software. Each image was collected as a Z-stack. Prior to image analysis, all images were deidentified so the reviewers were blinded. and a maximum intensity image was generated by collapsing all stacks using ImageJ/Fiji (NIH, Bethesda, MD, US).
Statistical Analysis
Data was tabulated and evaluated using Graphpad Prism 7 software (Graphpad, La Jolla,CA, US). Student’s T tests were used for comparison of good and poor prognosis in patients, SAH and sham in mice, and vehicle and RORγt inhibitor groups because all were determined to be normally distributed. P values of < or = to 0.05 were considered statistically significant. All data samples were evaluated for outliers using Grubb’s test with an alpha =0.1.
Results
CSF of SAH patients with poor outcome show change in five cytokines at day 3 post hemorrhage
A total of 35 patients with SAH were screened for the study. Twenty-one of the patients were excluded due to lack of an external ventricular drain for CSF diversion, recent infection by patient or family report, or admission Hunt and Hess Grade of 1 (there were no Hunt and Hess Grade 5 patients in this cohort). The remaining 14 patients were consented. Four did not have CSF samples available. The 10 remaining patients with available samples had hospital data and 90-day follow-up collected. Four of the ten patients had a poor outcome by mRS. Three of the four were admitted with Hunt and Hess (H/H) grade 3 and one was H/H grade 2. All four had elevated transcranial Doppler exams (TCDs) and moderate to severe vasospasm reported based on digital subtraction angiography during their ICU stay suggesting cerebral vasospasm. No patients had abrupt changes in physical exam findings that would define DCI. Four of the six patients with good outcomes were H/H grade 3, and two were H/H grade 2. None of the 6 patients with good outcome had increased TCDs. Because of the small sample and the multiple analyte comparison, demographic differences were not compared.
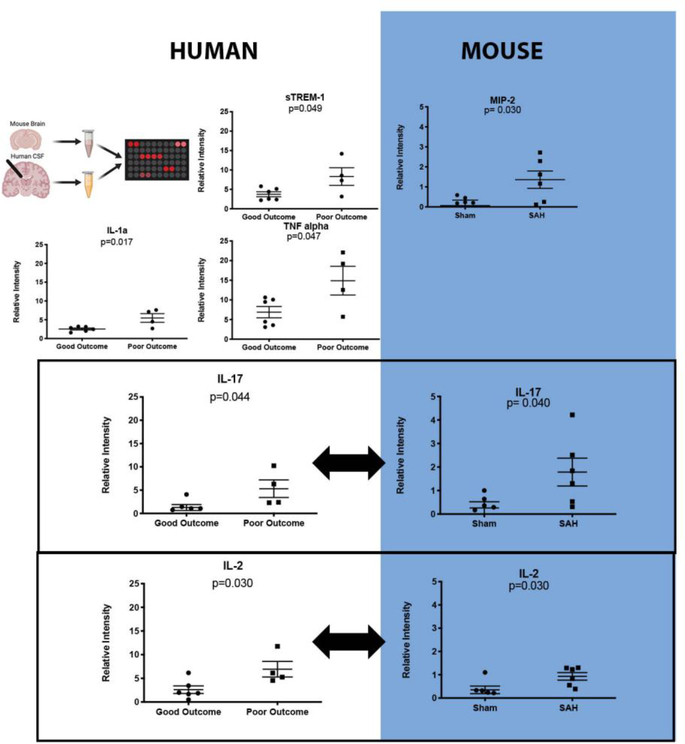
Previous data from our laboratory showed that neutrophils in the CSF peak at three days after SAH and this peak is predictive of the development of DCI 11,17. Cytokine/chemokine evaluation of the samples shows that 31 of the 35 analytes tested did not show a clear pattern of increase or decrease and were not significantly different between groups of patients with good or poor outcomes (mRS 0–2 vs. 3–5) (Table 1). Five cytokines were elevated in patients with poor outcome: IL-1α (2.54 ± 0.3 vs. 5.48 ±1.2 relative units (mean ± SEM), p=0.017), TNFα (6.88 ± 1.4 vs. 14.89 ±3.7 relative units, p=0.047), TREM-1 (3.75 ± 0.6 vs. 8.30 ±2.3 relative units, p=0.049), IL-2 (2.61 ± 0.8 vs. 6.92 ±1.6 relative units, p=0.030), and IL-17 (1.27 ± 0.6 vs. 5.31 ±1.9 relative units, p=0.044) (Table 1 and Figure 1 [Human]). Two more cytokines were greatly increased in aggregate in poor outcome patients: IL-8 (1.66 ± 0.5 vs. 8.83 ±3.9 relative units, p=0.053), and INFγ (1.91 ± 0.5 vs. 7.39 ±2.9 relative units, p=0.054), but the changes were not sufficiently consistent to show statistical significance (mean and standard error information in Supplemental Table 1)
Table 1:
Proteomics array analytes tested in human and mouse experiments. Five analytes from cerebrospinal fluid tested three days after subarachnoid hemorrhage (SAH) were significantly different between patients who had good 90-day outcomes (mRS 1–2) versus those that had poor outcomes (mRS 3–5): IL-1α, IL-2, IL-17, TNF- α, and TREM-1 (Student’s t-test; p≤ 0.05). Two analytes were significantly different in the brains of mice three days after SAH or sham surgery: IL-2 and IL-17 (Student’s t-test; p≤ 0.05). (-) Indicates that the analyte was not present on the array; NS signifies not significantly different. Significantly different analytes are highlighted in grey (See figure 1 for more detail). IL-2 and IL-17 were significantly different in both species.
| Name | Alternate name | Human | Mouse | Name | Alternate name | Human | Mouse |
|---|---|---|---|---|---|---|---|
| IL-1α | IL-1F1 | p=0.017 | NS | C5/C5a | NS | NS | |
| IL-1β | IL-1F2 | NS | NS | G-CSF | NS | NS | |
| IL-1ra | IL1-F3 | NS | NS | GM-CSF | NS | NS | |
| IL-2 | p=0.030 | p=0.030 | IFN-γ | p=0.054 | NS | ||
| IL-4 | NS | NS | s-ICAM | CD54 | NS | NS | |
| IL-5 | NS | NS | TNF-α | p=0.047 | NS | ||
| IL-6 | NS | NS | TREM-1 | p=0.049 | NS | ||
| IL-10 | NS | NS | IL-3 | - | NS | ||
| IL-12 p70 | NS | NS | IL-7 | - | NS | ||
| IL-13 | NS | NS | IL-17E | NS | - | ||
| IL-16 | NS | NS | IL-32a | NS | - | ||
| IL-17 | p=0.044 | p=0.040 | CCL11 | Eotaxin | - | NS | |
| IL-23 | NS | NS | CCL12 | MCP-5 | - | NS | |
| IL-27 | NS | NS | CCL17 | TARC | - | NS | |
| CCL2 | MCP-1 | NS | NS | CXCL2 | MIP-2 | - | p=0.030 |
| CCL3 | MIP-1α | NS | NS | CXCL9 | MIG | - | NS |
| CCL4 | MIP-1β | NS | NS | CXCL13 | BLC | - | NS |
| CCL5 | RANTES | NS | NS | CD40L | NS | - | |
| CXCL1/IL-8 | * | P=0.053 | NS | MIF | NS | - | |
| CXCL10 | IP-10 | NS | NS | M-CSF | - | NS | |
| CXCL11 | I-TAC | NS | NS | SerpinE1 | PAI-1 | NS | - |
| CXCL12 | SDF-1 | NS | NS | TIMP-1 | - | NS |
CXCL1 in mice and CXCL8/IL-8 in humans serve homologous functions although they are not orthologs. Individual mean and standard error values are presented in Supplemental Table 1.
Figure 1: Cytokine analysis of patients with subarachnoid hemorrhage (SAH) and a mouse model of SAH show overlapping cytokines.
Schematic of experimental plan. Cytokine analysis of Day 3 CSF of patients with SAH dichotomized between patients who had a good outcome (defined by mRS 0–2) versus poor outcome (mRS 3–5) shows that five cytokines, TNFα, sTREM-1, IL-1α, IL-17 and IL-2, discriminate patients who go on to have good versus poor outcome (above dotted line). Similarly, cytokine analysis of brain homogenates 3 days after SAH shows that three cytokines are significantly different between mice with SAH versus sham, MIP-2, IL-17, and IL-2 (below dotted line). IL-17 and IL-2 are similarly increased in both human SAH and mouse SAH (Box). Student’s T test p≤ 0.05 defined statistical significance.
Brain/meninges homogenates of mice with SAH show change in three cytokines on day 3 after hemorrhage
To determine whether SAH leads to changes in cytokine expression in mice, we tested our previously validated model of mild SAH with a similar cytokine array panel. Due to inaccessibility of CSF in mice, the cytokine array was performed on brain homogenates. Cytokine/chemokine evaluation of the homogenates shows that 37 of the 40 analytes did not have significant differences between sham and SAH (Table 1). Three cytokines were elevated in SAH mice compared to sham: MIP-2 (0.067 ± 0.3 vs. 1.36 ±0.4 relative units (mean ± SEM), p=0.030), IL-2 (0.35 ± 0.2 vs. 0.93 ±0.2 relative units, p=0.030), and IL-17 (0.26 ± 0.3 vs. 1.79 ±0.6 relative units, p=0.040) (Figure 1 [Mice]).
Two cytokines are elevated in both human SAH and a murine model of SAH
Only IL-2 and IL-17 were significantly elevated in both the human condition and the mouse model of SAH. IL-2 predominant modulates T cell function, and there is currently no evidence of IL-2’s effect on neutrophil recruitment or activation. We therefore focused on IL 17, a cytokine implicated in neutrophil recruitment and activation in a number of inflammatory diseases (Figure 1).
Inhibition of IL-17 production decreases neutrophil entry into the meninges
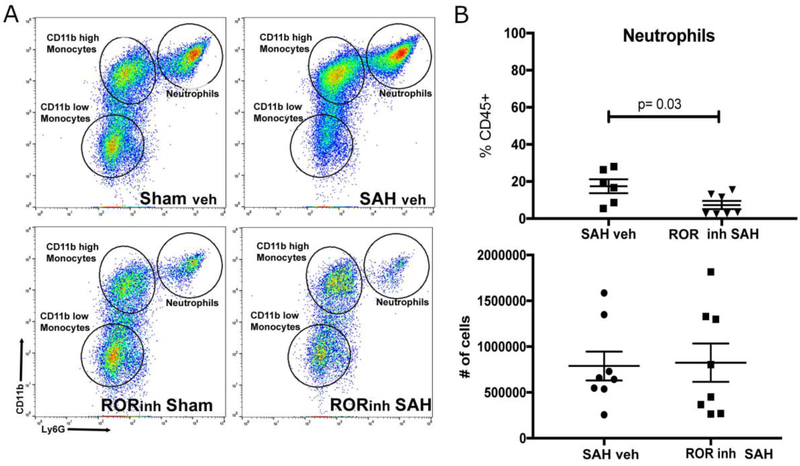
To investigate the role of IL-17 in the recruitment of neutrophils, we employed a reverse agonist of RORγt (GSK805, MilliporeSigma, Burlington, MA), the regulator of IL-17 production. In a small control experiment to confirm our previous findings, C57BL/6 mice with subarachnoid hemorrhage, myeloid cells in general and neutrophils in particular were more abundant in the meninges than the brain (Supplemental Figure 1A). When GSK805 was administered to mice prior to intervention, the downregulation of RORγt decreased neutrophils (as a percentage of all inflammatory cells) in the meninges (1.40 ratio of sham/SAH vehicle ± 0.2 vs. 0.62 ratio of sham/SAH GSK805 ± 0.2, p=0.028) (Figure 2). Due to inter-animal variability (likely due to intravascular neutrophil accumulation), we did not see significant differences between the absolute number of neutrophils in the meninges (Figure 2). Consistent with our previous data, there was a decrease in monocytes in the brain (not sufficient samples to test for significance) but no changes in brain neutrophils or microglia, or in meningeal activated (CD11bhigh) and less activated (CD11blow) monocytes (Supplemental Figure 1B) 10.
Figure 2: Flow cytometric analysis of meninges of mice three days after SAH with the RORγt reverse agonist GSK805 (ROR inh) or vehicle.
A) Shows representative images of single cell suspensions of meninges. Samples were gated on singlet, live/dead, and CD45+ and the image shows a flow plot with CD11b staining on Y axis and Ly6G staining on X axis. The images show that there are two predominant myeloid cell types after SAH: monocytes (CD45+Ly6ChighLy6GintCD11bhigh/low), and neutrophils (CD45+Ly6ClowLy6GhighCD11bhigh). Monocytes can be classified as either CD11bhigh or CD11blow. B) There is a significant difference between the percentage of neutrophils in the vehicle treatment SAH animal and the GSK 805 (ROR inh) SAH animals (p=0.03) suggesting an effect of GSK805 on neutrophil entry. There were no differences between the CD11blow or CD11high monocyte groups (see Supplemental Figure 1B). The absolute number of neutrophils in the meninges of both groups was not different, likely due to retained inflammatory cells adherent to the walls of blood vessels. To investigate this, we proceeded to immunohistochemical evaluation (Figure 3).
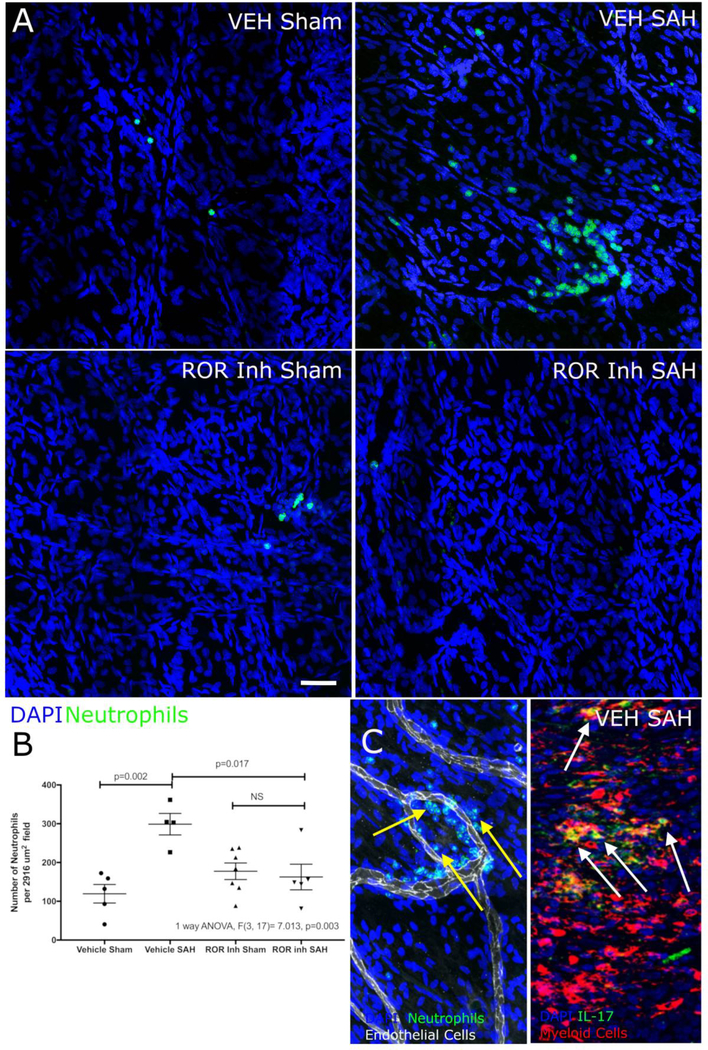
Immunohistochemistry of the fibrous meninges (the meninges that does not include venous sinuses) confirmed the decreased neutrophils in SAH mice after GSK805 administration (Figure 3A and B). One question raised by the flow cytometry data in Figure 2 is whether neutrophils enter the meninges proper or adhere to the vessel wall. To test this, we used an anti-CD31 antibody to delineate the blood vessels and found that the neutrophils in SAH mice are entering the meninges (Figure 3C). Also, in an IL-17 reporter strain of mice, we found that much of the IL-17 producing cells in SAH mice colocalized with the neutrophil/myeloid cell marker Lys6B (Figure 3c). From this data, we can conclude that a large proportion of IL-17 positive cells co-localize with markers for myeloid cells (Figure 3c). This suggests that IL-17 production by neutrophils may contributes to the recruitment of more neutrophils into the meninges after SAH.
Figure 3: Immunohistochemistry of meningeal neutrophils after SAH in IL-17 reporter mice with and without RORγt inhibition.
A and B) Mice with SAH showed more staining for neutrophils (green) than sham mice (consistent with flow cytometric data). Inhibition of RORγt (ROR inh) showed fewer neutrophils (green) in the SAH group than the vehicle treated mice. White bar represents 50υ μm. C) To answer the question about whether the neutrophils were in the parenchyma of the meninges or within the lumen of blood vessels, we co-stained meninges with anti-CD31(vascular endothelial cells) and neutrophils. This showed that the large majority of neutrophils reside outside the blood vessel lumen (yellow arrows). Additionally, we stained IL-17 reporter mice with a myeloid cell antibody and found a large number of IL-17 producing cells (green) that colocalized with myeloid cells (red) (white arrows and yellow stained cells) suggesting that neutrophils may be source of IL-17 after SAH allowing for autocrine recruitment of more neutrophils.
Discussion
The understanding that delayed cerebral injury (DCI) after SAH is dependent on neutrophil infiltration of the CNS leads to a host of important unanswered questions. Paramount among these is how neutrophils get into the CNS. This work implicates the cytokine IL-17 as a driver for neutrophil recruitment. It also suggests that neutrophils themselves may be an important source of IL-17 as part of an autocrine recruitment pathway. Traditionally, neutrophils are thought to originate from the bone marrow and infiltrate into peripheral tissues. Two recent works suggest that neutrophils may reside in peripheral tissues, the spleen and the meninges but their significance is unclear 29,30
In this work, we corroborate cytokine expression in human SAH and a mouse SAH model. This approach allows the comparison of inflammatory mediators that are conserved across species. Cytokine studies, both in SAH and in other diseases in rodents have not always led to successful interventions in patients. By looking for common cytokine changes between the two species, we are more likely to focus on conserved pathways that are critical to delayed brain inflammation. Because IL-17 is more closely associated with neutrophil recruitment and activation than IL-2, it was reasonable to focus on IL-17. Others have found similar inflammatory cytokine profiles in serum after SAH although IL-17 was not identified as a significant marker suggesting CSF detection may result from a local phenomenon16,31.
Neutrophil infiltration in the CNS has been implicated in neuronal loss, neurological deficits, and deficits in neuronal function 32,33,34. Indeed, similar to our results, others have demonstrated that neutrophil infiltration into the CNS after mild traumatic brain injury leads to neuronal damage 35,34. In our model of SAH, we have shown that neutrophil infiltration is critical to the development of DCI and that neutrophils depletion prevents DCI 10.
The next logical question is to determine how neutrophils are recruited into the meninges. Our data suggests that in both patients and our animal model, IL-17 is a likely mediator of neutrophil recruitment after SAH. IL-17 is known to attract neutrophils into sites of inflammation such as the joints in rheumatoid arthritis and in multiple sclerosis 19,18,36,37. The data presented in this work suggests that IL-17 is also important for the recruitment of neutrophils into the CNS.
A second question that presents itself is the origin of IL-17 in the CNS. There is evidence that neutrophils themselves can produce IL-17 in some conditions21,38,39. Our imaging of neutrophils/myeloid cells in the meninges of IL-17 green florescent protein (GFP) reporter mice, show many neutrophils co-localized with GFP (i.e. IL-17) suggesting that neutrophils are a source of this cytokine in the meninges after SAH. Based on these results, we hypothesize that either neutrophils enter the subarachnoid space during the initial hemorrhage or are present in the meninges prior to the hemorrhage, and release IL-17, which in turn leads to the recruitment of more neutrophils.
It is also possible that IL-17 acts solely as a neutrophil activation factor. Though IL-17 release is known to affect neutrophil activity, it is unknown how its release in the subarachnoid space leads to recruitment of more neutrophils into the meningeal space40. It is also possible that, that after SAH, IL-17 leads to activation and release of oxidative factors into the meninges thereby creating more damage to the tissue and release of neutrophil activating danger associated proteins (DAMPs) which then leads the recruitment of more neutrophils. Both of these possibilities remain to be tested.
Interestingly, in our model of SAH, very few neutrophils enter the brain parenchyma; the more prominent infiltration is into the meninges. Recruitment of neutrophils (which are produced in the bone marrow and recruited from the blood) may be the best point at which to stop entry where the difficulty of crossing the blood-brain barrier is obviated. Although neutrophils reside in peripheral tissues (including the meninges), there is no evidence that in the meninges they can divide 30,29. Our previous data shows accumulation of neutrophils in the CSF in humans and the central nervous system in mice suggesting a strong contribution of peripheral neutrophils which would need to be recruited into the meninges to act 11,17.
There a number of limitations of this study. First, the human cohort presented here is small and precludes the evaluation of comorbidities and risk factors. Because this cohort was followed intensively and with serial tests, it served the function of finding putative inflammatory pathways important in SAH. Second, the mouse model of SAH is validated in male mice but not female mice. Human SAH, and indeed DCI, occur more frequently in women than men. We believe that the information learned from this validated model offers insight that would not be possible if we use mice of both sexes. In the future, developing validated, reproducible models for female mice in mild SAH will be critical to develop a complete picture of RORγt function in SAH. Third, inhibition of GSK805 has effects on cytokine release other than for IL-17. RORγt exhibits major control over IL-22 and minor control of IL26, IL23R and CCR6 release from inflammatory cells. Because our cytokine data suggests IL-17 is elevated in both mice and men, we hypothesize that RORγt inhibition works through IL-17 production. Other RORγt need to be investigated.
As our understanding of DCI as an inflammatory disease improves, the search for targets of therapy can be refined. IL-17 may be a target to prevent neutrophils from getting to the meninges and prevent DCI. More work is necessary to determine if inhibition of RORγt improves mouse spatial memory function (the hallmark of DCI in the mouse model). In addition, direct inhibition of IL-17 needs to be investigated, as it may be a better option as a therapeutic agent than preventing transcription of the IL-17 gene. Finally, if inhibition of IL-17 is to become an important therapeutic target, it will have to be administered after SAH not before as in this study. Therefore, the timing of inhibition and rapidity of effect needs to be addressed.
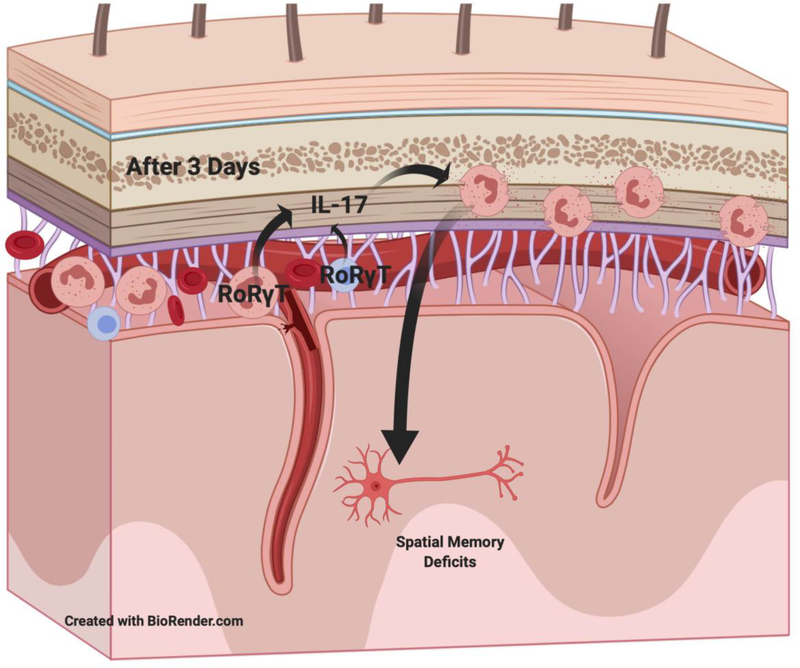
This study offers a possible important piece of a model by which neutrophils affect the development of DCI after SAH by implicating IL-17 (Figure 4). It reinforces the finding that neutrophils enter the CNS into the meninges more than the brain. And it identifies neutrophils as a possible source for the cytokines to recruit more neutrophils. Further investigation into the how this cytokine interacts with blood neutrophils will be critical for our understanding.
Figure 4: Model of possible IL-17 role in the development of delayed cerebral injury (DCI) after subarachnoid hemorrhage.
The data in this paper supports a model of neutrophils production of IL-17 that leads to recruitment of neutrophils into the meninges that is an important step in the development of DCI.
Supplementary Material
Acknowledgements
This work was funded by The Aneurysm and AVM Foundation (JP and JG), NIH 1RO1NS0749971 and K08 NS 051350 (JP).
Abbreviations
- BSA
Bovine Serum Albumin
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- DMEM
Dulbecco’s modified eagle medium
- DCI
Delayed Cerebral Injury
- DMSO
Dimethylsulfoxide
- EVD
External ventricular drain
- GFP
Green fluorescent protein
- IL-17
Intraleukin-17
- mRS
Modified Rankin Scale
- MS
Multiple sclerosis
- PBS
Phosphate buffered saline
- RoRγt
RAR-related orphan receptor gamma t
- SAH
Subarachnoid hemorrhage
- TNFα
Tumor necrosis factor
Footnotes
The patient work was completed at the Cleveland Clinic under the supervision of JJP and JAG.
The animal work was completed at the University of Virginia under the supervision of JJP.
References
- 1.Suarez JI. Diagnosis and Management of Subarachnoid Hemorrhage. Continuum (Minneap Minn). 2015;21(5 Neurocritical Care):1263–1287. [DOI] [PubMed] [Google Scholar]
- 2.Diringer MN, Bleck TP, Claude Hemphill J, 3rd, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society’s Multidisciplinary Consensus Conference. Neurocrit Care. 2011;15(2):211–240. [DOI] [PubMed] [Google Scholar]
- 3.Suarez JI, Tarr RW, Selman WR. Aneurysmal subarachnoid hemorrhage. N Engl J Med. 2006;354(4):387–396. [DOI] [PubMed] [Google Scholar]
- 4.Al-Khindi T, Macdonald RL, Schweizer TA. Cognitive and functional outcome after aneurysmal subarachnoid hemorrhage. Stroke. 2010;41(8):e519–536. [DOI] [PubMed] [Google Scholar]
- 5.Wong GK, Lam S, Ngai K, et al. Evaluation of cognitive impairment by the Montreal cognitive assessment in patients with aneurysmal subarachnoid haemorrhage: prevalence, risk factors and correlations with 3 month outcomes. J Neurol Neurosurg Psychiatry. 2012;83(11):1112–1117. [DOI] [PubMed] [Google Scholar]
- 6.Macdonald RL, Higashida RT, Keller E, et al. Randomised trial of clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid hemorrhage undergoing surgical clipping (CONSCIOUS-2). Acta Neurochir Suppl. 2013;115:27–31. [DOI] [PubMed] [Google Scholar]
- 7.Stuart D, Christian R, Uschmann H, Palokas M. Effectiveness of intrathecal nicardipine on cerebral vasospasm in non-traumatic subarachnoid hemorrhage: a systematic review. JBI Database System Rev Implement Rep. 2018;16(10):2013–2026. [DOI] [PubMed] [Google Scholar]
- 8.Dhar R, Diringer MN. The burden of the systemic inflammatory response predicts vasospasm and outcome after subarachnoid hemorrhage. Neurocrit Care. 2008;8(3):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Provencio JJ, Vora N. Subarachnoid hemorrhage and inflammation: bench to bedside and back. Semin Neurol. 2005;25(4):435–444. [DOI] [PubMed] [Google Scholar]
- 10.Provencio JJ, Swank V, Lu H, et al. Neutrophil depletion after subarachnoid hemorrhage improves memory via NMDA receptors. Brain Behav Immun. 2016;54:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provencio JJ, Fu X, Siu A, Rasmussen PA, Hazen SL, Ransohoff RM. CSF neutrophils are implicated in the development of vasospasm in subarachnoid hemorrhage. Neurocrit Care. 2010;12(2):244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaetani P, Tartara F, Pignatti P, Tancioni F, Rodriguez y Baena R, De Benedetti F. Cisternal CSF levels of cytokines after subarachnoid hemorrhage. Neurol Res. 1998;20(4):337–342. [DOI] [PubMed] [Google Scholar]
- 13.Hirashima Y, Nakamura S, Endo S, Kuwayama N, Naruse Y, Takaku A. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem Res. 1997;22(10):1249–1255. [DOI] [PubMed] [Google Scholar]
- 14.Zeiler FA, Thelin EP, Czosnyka M, Hutchinson PJ, Menon DK, Helmy A. Cerebrospinal Fluid and Microdialysis Cytokines in Aneurysmal Subarachnoid Hemorrhage: A Scoping Systematic Review. Front Neurol. 2017;8:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polin RS, Bavbek M, Shaffrey ME, et al. Detection of soluble E-selectin, ICAM-1, VCAM-1, and L-selectin in the cerebrospinal fluid of patients after subarachnoid hemorrhage. J Neurosurg. 1998;89(4):559–567. [DOI] [PubMed] [Google Scholar]
- 16.Savarraj JPJ, Parsha K, Hergenroeder GW, et al. Systematic model of peripheral inflammation after subarachnoid hemorrhage. Neurology. 2017;88(16):1535–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Provencio JJ, Altay T, Smithason S, Moore SK, Ransohoff RM. Depletion of Ly6G/C(+) cells ameliorates delayed cerebral vasospasm in subarachnoid hemorrhage. J Neuroimmunol. 2011;232(1–2):94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang R, Tian A, Wang J, Shen X, Qi G, Tang Y. miR26a modulates Th17/T reg balance in the EAE model of multiple sclerosis by targeting IL6. Neuromolecular Med. 2015;17(1):24–34. [DOI] [PubMed] [Google Scholar]
- 19.Wang B, Tang Y, Sun X, et al. Increased IL-6 expression on THP-1 by IL-34 stimulation upregulated rheumatoid arthritis Th17 cells. Clin Rheumatol. 2017. [DOI] [PubMed] [Google Scholar]
- 20.Shoda H, Nagafuchi Y, Tsuchida Y, et al. Increased serum concentrations of IL-1 beta, IL-21 and Th17 cells in overweight patients with rheumatoid arthritis. Arthritis Res Ther. 2017;19(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170(4):2106–2112. [DOI] [PubMed] [Google Scholar]
- 22.Li L, Huang L, Vergis AL, et al. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120(1):331–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Katayama M, Ohmura K, Yukawa N, et al. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One. 2013;8(5):e62231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187(1):490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaudhry SR, Guresir E, Vatter H, et al. Aneurysmal subarachnoid hemorrhage lead to systemic upregulation of IL-23/IL-17 inflammatory axis. Cytokine. 2017;97:96–103. [DOI] [PubMed] [Google Scholar]
- 26.Altay T, Smithason S, Volokh N, Rasmussen PA, Ransohoff RM, Provencio JJ. A novel method for subarachnoid hemorrhage to induce vasospasm in mice. J Neurosci Methods. 2009;183(2):136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao S, Yosef N, Yang J, et al. Small-molecule RORgammat antagonists inhibit T helper 17 cell transcriptional network by divergent mechanisms. Immunity. 2014;40(4):477–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gadani SP, Smirnov I, Smith AT, Overall CC, Kipnis J. Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J Exp Med. 2017;214(2):285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mrdjen D, Pavlovic A, Hartmann FJ, et al. High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease. Immunity. 2018;48(3):599. [DOI] [PubMed] [Google Scholar]
- 30.Deniset JF, Surewaard BG, Lee WY, Kubes P. Splenic Ly6G(high) mature and Ly6G(int) immature neutrophils contribute to eradication of S. pneumoniae. J Exp Med. 2017;214(5):1333–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savarraj J, Parsha K, Hergenroeder G, et al. Early Brain Injury Associated with Systemic Inflammation After Subarachnoid Hemorrhage. Neurocrit Care. 2018;28(2):203–211. [DOI] [PubMed] [Google Scholar]
- 32.Ji KA, Eu MY, Kang SH, Gwag BJ, Jou I, Joe EH. Differential neutrophil infiltration contributes to regional differences in brain inflammation in the substantia nigra pars compacta and cortex. Glia. 2008;56(10):1039–1047. [DOI] [PubMed] [Google Scholar]
- 33.Barone FC, Hillegass LM, Price WJ, et al. Polymorphonuclear leukocyte infiltration into cerebral focal ischemic tissue: myeloperoxidase activity assay and histologic verification. J Neurosci Res. 1991;29(3):336–345. [DOI] [PubMed] [Google Scholar]
- 34.Roth TL, Nayak D, Atanasijevic T, Koretsky AP, Latour LL, McGavern DB. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505(7482):223228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Corps KN, Roth TL, McGavern DB. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72(3):355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sarkar S, Fox DA. Targeting IL-17 and Th17 cells in rheumatoid arthritis. Rheum Dis Clin North Am. 2010;36(2):345–366. [DOI] [PubMed] [Google Scholar]
- 37.Kostic M, Dzopalic T, Zivanovic S, et al. IL-17 and glutamate excitotoxicity in the pathogenesis of multiple sclerosis. Scand J Immunol. 2014;79(3):181–186. [DOI] [PubMed] [Google Scholar]
- 38.Hu S, He W, Du X, et al. IL-17 Production of Neutrophils Enhances Antibacteria Ability but Promotes Arthritis Development During Mycobacterium tuberculosis Infection . EBioMedicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522(7556):345–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor PR, Roy S, Leal SM Jr., et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.