Abstract
Preparation of a single cell suspension from solid tissue is vital for a successful flow cytometry experiment. We report a detailed and reproducible method to produce a quality cell suspension from the zebrafish retina. Zebrafish retinas, especially their Müller glia cells, are of particular interest for their inherent regenerative capacity, making them a useful model for regenerative medicine and cell therapy research. Here, we detail a papain-based dissociation that is gentle enough to keep cells intact, but strong enough to disrupt cell-cell and cell-matrix interactions to yield a cell suspension that produces clean and reliable flow cytometric cell sorting results. This procedure consistently results in over 90% viability and three populations of cells based on GFP expression. The dissociation procedure described herein has been optimized for the collection of Müller glia from Tg(apoe:gfp) zebrafish retinas; however, the overall process may be applicable to other cell types in the fish retina, additional flow cytometric techniques, or preparing cell suspensions from similar tissues.
Keywords: dissociation, zebrafish retina, Müller glia, flow cytometric cell sorting, papain
Preparing a single cell suspension from solid tissue is an essential component of any experiment involving flow cytometry. To produce a clean and reliable cell suspension for subsequent labeling and flow cytometric analysis, cell death, damage, and aggregation must be avoided. A major part of this process is the method of dissociation used to harvest intact cells from their extracellular matrix components, which must be optimized for each organism, tissue, and cell type of interest (1). Here, we offer a detailed description on the preparation of a single cell suspension from zebrafish retinas that has been optimized for subsequent flow cytometric cell sorting, used to collect a specific cell type called Müller glia for downstream applications. Zebrafish Müller glia have the inherent ability to give rise to a functional stem cell niche when damaged, making the adult zebrafish retina and especially their Müller glia of interest for regenerative medicine and cell therapy research (2–7). We utilize a transgenic fish expressing GFP under control of the apolipoprotein E (ApoE) promoter, Tg(apoe:gfp), specific for Müller glia in the zebrafish retina (8).
While previous publications have indicated their use of flow cytometric cell sorting within their experimental outline (9–15), to our knowledge there are no reports with sufficient detail to efficiently produce, and reproduce, a quality cell suspension from the zebrafish retina for sorting Müller glia. Here we report an exclusively papain-based dissociation for adult zebrafish retinas for obtaining a single cell suspension and subsequent flow cytometric cell sorting analysis. Papain is a cysteine protease that digests many of the extracellular matrix proteins found in the retina more efficiently and less harshly than other enzymes such as trypsin, collagenase, or dispase (9,11,16,17). Papain has been shown to be the most effective and least damaging proteolytic enzyme for dissociating cells from retinas and other delicate neural tissue in several vertebrate models (17–21).
The vertebrate retina is an extremely complex and specialized tissue, comprised of eight main types of neural and glial cells, each with their own morphologic and phenotypic variations that play unique structural and functional roles (22,23). The cells are arranged in a highly ordered manner, with distinct laminations of cell bodies, processes, and synapses that function together to facilitate the process of phototransduction (24). Histologically, the retina is comprised of three nuclear layers (outer nuclear layer, inner nuclear layer, and ganglion cell layer) and two plexiform layers (inner and outer plexiform layers) in an alternating pattern (25,26). Müller glia are the predominant glia cells in the retina and help maintain retinal homeostasis by providing structural, metabolic, and functional support to the other cells (27,28). They also give rise to stem cells in zebrafish that reconstitute the damaged retina during regeneration but contribute to scar formation in mammals, making them of particular interest for developing regenerative therapies and highlighting the importance of developing tools to study them (6,7,29,30). The size, shape, and proportions of ocular structures differ between zebrafish and humans, adapted to their respective environments; however, their retinas contain the same major cell types, and retinal architecture is highly conserved (31,32). The fish retina therefore serves as an excellent model for studying human retinal biology, with the feasibility of eventual clinical translation.
The noncellular constituents of the retina, comprising the extracellular matrix, are just as diverse and complex as the cells they support. Extracellular matrix is generally comprised of proteins, carbohydrates, proteoglycans, glycoproteins, fibers, and other factors such as enzymes or signaling molecules (33,34). The heterogeneity of the retina and other ocular tissues is reflected in the makeup of extracellular matrix in distinct parts of the retina and its surrounding tissues. Fibronectin, for example, is a common glycoprotein found in many tissues’ extracellular matrices but has not been reported as a significant component of the adult retina or its internal limiting membrane (34). The extracellular matrix not only provides structural integrity but influences a variety of signaling process on a molecular level including retinogenesis, axonal guidance, cell polarization, and angiogenesis (35–37). Because of its heavy involvement in such dynamic and developmentally important processes, it follows that the extracellular matrix makeup changes during development. For example, laminin 5, collagen IV, and fibronectin have been shown to be transiently expressed at certain stages of retinal development in rats (38). Significant extracellular matrix components in the adult retina that need to be digested and separated from their adjacent cells to obtain a cell suspension include laminin, collagens, tenascin-C and –R, and chondroitin sulfate proteoglycans (34,38–41).
Disrupting cellular interactions is an essential part of obtaining a retinal cell suspension suitable for flow cytometry. Obtaining intact Müller cells is particularly delicate because of their large size and extensive processes. A balance must therefore be found such that the dissociation is strong enough to disrupt Müller cell contacts with other cells and the surrounding tissue, but gentle enough to preserve the integrity of the Müller glia themselves.
Materials and Methods
Dissection of Adult Zebrafish Retinas for Downstream Dissociation
Wild-type and Tg(apoe:gfp) zebrafish were dark adapted overnight prior to retinal dissection and dissociation, which causes the retinal pigmented epithelium (RPE) and the photoreceptor outer segments to physically move apart, resulting in a clean retina dissection with minimal RPE contamination (42,43). Euthanasia occurred using the IACUC-approved procedure of placing a fish in a divided tank, opposite to the side containing ice, until it is unresponsive to tail pinch. Eyes were enucleated immediately after sacrifice using blunt, curved edge eye dressing forceps and placed in dissection medium, held on ice. Eyes were held on a clean petri dish with Dumont #1 forceps and punctured with a 23 ga needle at the limbus. Iris and lens were dissected away with VANNAS microscissors and Ekhardt or similar forceps (20–25 ga) were used to remove the retina. Retinas were minced and transferred to a collection tube with a minimal volume of dissecting medium and held on ice.
Preparation of a Single Cell Suspension from Adult Zebrafish Retinas
Retinal tissue was dissociated using the Worthington Papain Dissociation System. Briefly, EBSS equilibrated with sterile 95% O2: 5% CO2 was used to reconstitute a vial of papain (Worthington, #LK003176) to give a final working solution of 20 units/ml of papain in 1 mM L-cysteine with 0.5 mM EDTA after the tissue is added and incubated at 37 °C for at least 10 min to fully dissolve and activate the enzyme. DNase I (Worthington, #LK003170) was added to the activated papain for a final concentration of 0.005%. The papain/DNase I solution was then added to the retina samples while maintaining the appropriate working concentrations and triturated with a p1000 pipette (see Supplementary Material).
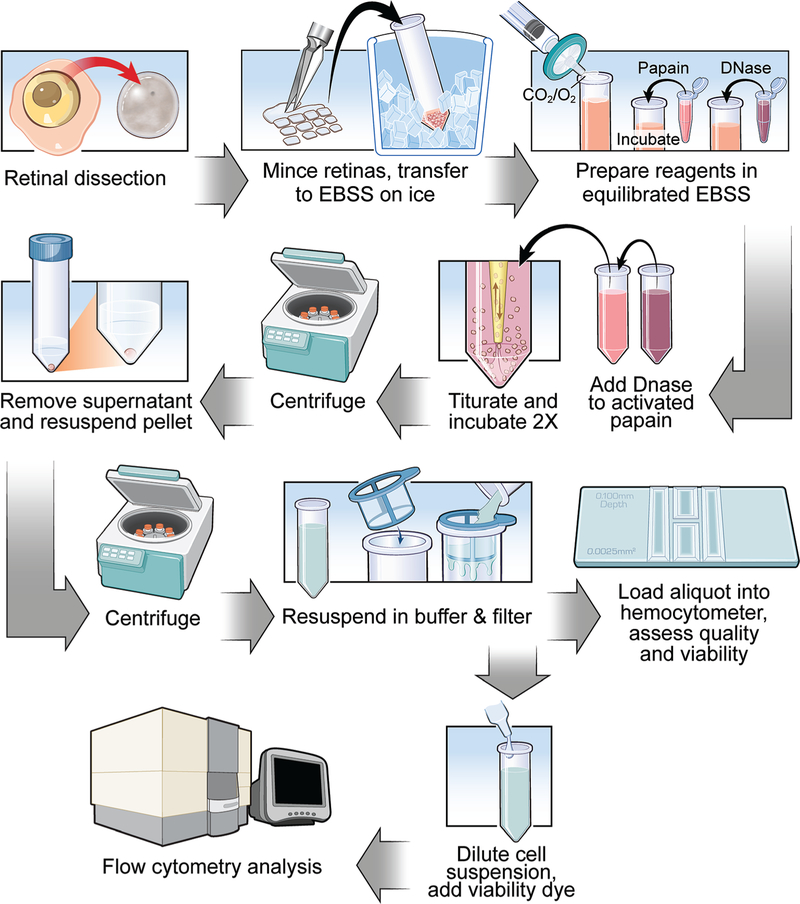
After equilibrating with sterile 95% O2: 5% CO2, the tissue samples were incubated at 37 °C with 160 rpm agitation for 10 min. Trituration, equilibration, and incubation steps were repeated a second time before repeating the trituration for a final time and centrifuging the samples at 850 × g for 5 min at room temperature. The supernatants were removed and pellets resuspended in ovomucoid inhibitor solution to inactivate the papain: 0.95 mg/ml ovomucoid inhibitor (Worthington, #LK003182) and 0.00475% DNase I (Worthington, #LK003170) in equilibrated EBSS. Samples were centrifuged again at 850 × g for 5 min at room temperature, supernatants removed, and resuspended in 550 μl sterile room temperature sorting buffer: 1% FBS, 1 mM EDTA, 25 mM HEPES, pH 7.0–7.4 in 1x PBS (w/o Ca2+, w/o Mg2+). Each cell suspension was then passed through a 30 μm gravity filter. The original tube was rinsed with an additional 550 μl, which was also passed through the filter to result in a final 1 ml suspension, taking into account volume lost in the filter (Fig. 1).
Figure 1.
Key steps and overall workflow for the dissection, dissociation, and preparation of a single cell suspension from Zebrafish retinas of suitable quality for flow cytometric analysis. Refer to the text for a detailed description of all procedures.
Preparing Samples for Flow Cytometric Cell Sorting
Cell suspensions were counted manually with a Hausser Scientific Bright-Line Improved Neubauer hemacytometer at 10× magnification and initial concentration calculated. The dilution used for counting was mixed 1:1 with 0.4% trypan blue solution for an initial estimate of cell viability. Additional sorting buffer was added to a final concentration of about 4.5 million cells/mL for the ApoE suspenion, or 2 million cells/mL for the unstained WT suspension. An aliquot of 150–300 μl was taken from each of these diluted samples. The ApoE aliquot served as a single color GFP-only control while DRAQ7 DNA dye was added 1:100 (from 0.3 mM original concentration) to the WT suspension for a DRAQ7-only single color control. The remaining ApoE suspension also received DRAQ7 dye at a 1:100 dilution (3 μM final concentration), which is the sample to be sorted, containing both GFP and DRAQ7 signals (Fig. 2).
Figure 2.
Zebrafish retinal cell suspensions suitable for flow cytometric cell sorting. A) Trypan blue staining, showing one dead trypan blue-positive cell (arrow). B) Example Müller glia (GFP+) that has extended its processes after sitting under a coverslip for several minutes. C) Representative fluorescence of cell suspension samples going through the flow cytometer: Three GFP+ Müller glia and two dead, DRAQ7+ cells. Scale bars are 50 μm.
Instrumentation and Gating Strategy
The BD FACSAria II was used with a 100 μm nozzle at20.00 psi for sorting cell suspensions obtained using the methods described herein. The blue laser (488 nm) was used for excitation of the native GFP signal in select cells of the Tg(apoe:gfp) retinal cell suspension, detectable by the 515/20 filter (505LP dichroic). The red laser (640 nm) was used for excitation of DRAQ7 dye, detectable by the 730/45 filter (690LP dichroic). The gating strategy outlined in Figure 3 was applied to separate live, single cells from debris and aggregates and collect each resulting cell population.
Figure 3.
Representative gating strategy for Tg(apoe:gfp) zebrafish retina cell suspensions. A) Cells are separated out from debris based on forward and side scattering. B) Aggregate correction on side scatter. C) Aggregate correction on forward scatter. D) Viable cells selected by exclusion of DRAQ7 dye, which fluoresces at a wavelength of 700 nm. E) Unstained control. F) Single, live cells clustering into three populations based on GFP expression. G) RT-PCR analysis of gfap expression alongside rpl13a (ribosomal protein) housekeeping control gene in each cell population, indicating that the GFP-high population contains Müller glia.
RT-PCR Analysis
GFP-negative, GFP-low, and GFP-high cell populations were sorted directly into TRIzol LS (Invitrogen) and total RNA isolated according to the manufacturer’s protocol. Oligo d(T) primers, random hexamers, and MultiScribe reverse transcriptase (TaqMan Reverse Transcription Reagents, Invitrogen) were used to generate cDNA. PCR reactions used EmeraldAmp GT PCR Master Mix (Takara Bio) and gene-specific primers (gfap F: 5’-GGATGAGATCCAGATGCTGAAGG-3°; gfap R: 5’-CAGATCCTTCCTCTCCGTAGTGG-3′; rpl13a F: 5’-TC TGGAGGACTGTAAGAGGTATGC-3′; rpl13a R: 5’-AG ACGCACAATCTTGAGAGCAG-3′).
Retina Flat-Mount
Retinas for flat-mount were dissected out from dark-adapted Tg(apoe:gfp) fish as described above. The whole retina was transferred to a glass slide and radial cuts made in four equal quadrants using a 15° stab blade to allow the retina to lay flat before mounting with Vectashield.
Microscopy
Flat-mount retinas were imaged with the Leica TCS SP8 confocal laser scanning microscope (Leica Microsystems Europe). Three-dimensional z-stack images were obtained using the 488 nm laser, HyD detector, and HC PL APO CS2 40x/1.30 oil objective taken through the entire thickness of the retina. Images were processed using the 3D Viewer in Leica Application Suite X Software. Tg(apoe:gfp) cell suspensions were imaged with the Zeiss Axio Imager Z1 upright fluorescence microscope (Carl Zeiss Microscopy, LLC). Small droplets (10–20 μl) of the cell suspensions prepared for cell sorting or trypan blue assay were placed on a glass slide and covered with glass coverslip. EGFP (apoe:GFP), Cy5 (DRAQ7), and DIC channels (brightfield) and plan-NEOFLUAR 20x/0.5 objective were used to obtain images, which were processed using ZEN Pro 2.5 imaging software.
Results
Müller Glia in Tg(apoe:gfp) Retinas
Tg(apoe:gfp) retinas express GFP under control of the ApoE promoter, specific to Müller glia in zebrafish retinas (8). This specificity is evident in flat-mount images of the retina from Tg(apoe:gfp) fish, as no other retinal cell besides the Müller glia has the morphology exhibited by these GFP+ cells (Fig. 4). Their extensive processes span nearly the entire thickness of the retina, confirming their identity and emphasizing the care that must be taken in gently but thoroughly dissociating these cells.
Figure 4.
Three-dimensional reconstruction images of flat-mounted retina from Tg(apoe:gfp) zebrafish. A) 3-D reconstruction of Tg(apoe: gfp) retina (300 × 300 × 100 μm). B, C) zoomed-in and rotated perspectives of (A). The GFP-positive, elongated processes spanning nearly the entire thickness of the retina exemplify Müller glia morphology, indicating the specificity of the ApoE promoter for Müller glia in the zebrafish retina. All images are oriented with the internal limiting membrane (Müller glia-vitreous junction) at the top and external limiting membrane (Müller glia-photoreceptor junction) at the bottom of the images.
Cell Suspension Counts and Morphology
On average, cell suspensions from 6 to 18-month old zebrafish retinas yield up to 800,000 cells/retina; lower numbers are obtained for younger (smaller) retinas. A trypan blue exclusion assay is also recommended at this stage to quickly assess the viability of cells in suspension. This can easily be done by mixing the dilution used for counting 1:1 with trypan blue solution. Dead or dying cells will take up the trypan blue, while live cells will remain impermeable to the dye and exclude it (44). Counting blue and non-blue cells will therefore give an estimate of cell viability. Cell suspensions that appear to have excessive amounts of debris, cell aggregates, and/or less than 70% viable cells are not recommended for live-cell flow cytometric applications. Figure 2A shows excellent viability, with only 1 cell out of 13 being trypan blue positive, giving an initial estimate of about 92% viability, in agreement with the proportion of DRAQ7 positive cells when analyzed by microscopy (Fig. 2C) or by flow cytometry (Fig. 3D). Our procedure routinely results in overall cell viability of 90–99% (Table 1).
Table 1.
Typical flow cytometric cell sorting results for Tg(apoe: gfp) zebrafish retinal cell suspensions
| CELL VIABILITY | GFP HIGH | GFP LOW | GFP NEGATIVE |
|---|---|---|---|
| 90–99% | 2–4% | 12–17% | 77–83% |
Using a combination of enzyme-based (papain) and mechanical (trituration) disruption of retinal tissue, we are able to produce a cell suspension that yields thorough dissociation, free of clumps and debris (Fig. 2A–C). Moreover, the procedure is gentle enough that the distinctive Müller glia morphology remains intact: when allowing cell suspensions to sit on a slide for several minutes, the Müller glia tend to elongate and extend long processes, characteristic of their in vivo appearance (Fig. 2B; Fig. 4). This also underscores the importance of using the larger nozzle size (100 μm) and corresponding low pressure (20.00 psi) for cell sorting to minimize the potential of shearing their processes during sorting.
Flow Cytometric Cell Sorting
The gating strategy for obtaining Müller glia from a Tg(apoe: gfp) zebrafish retinal cell suspension is outlined in Figure 3. Cells are first separated out from debris (Fig. 3A), followed by aggregate correction on both side scatter (Fig. 3B) and forward scatter (Fig. 3C) to eliminate any groups of multiple cells. Viable single cells are then identified by exclusion of DRAQ7 dye (Fig. 3D). An unstained control is always included and contains 100% GFP negative cells (Fig. 3E), obtained from the WT cell suspension. This gating strategy yields three distinct populations of cells from the ApoE cell suspension based on their GFP expression: GFP-negative, GFP-low, and GFP-high (Fig. 3F). The GFP-high population comprises 2–4% of total sorted cells, while the GFP-low population represents 12–17% of sorted cells, and the majority of cells are in the GFP-negative population, comprising 77–83% of total sorted cells (Table 1).
We have identified the GFPhigh population as Müller glia through RT-PCR analysis of glial fibrillary acidic protein (gfap) expression (Fig. 3G). Gfap, an intermediate filament protein, is a classical marker of zebrafish Müller glia (8,45–47). Gfap expression is only observed in the GFP-high population, but absent in both the GFP-low and GFP-negative populations (Fig. 3G). We have therefore concluded that cells in the GFP-high population are Müller glia. We have hypothesized that the GFP-low population likely represents other retinal cells that have taken up small amounts of GFP given off by Müller glia through extracellular vesicles or other means of intercellular transport. We have also observed that while this small amount of GFP signal in the GFP-low population is detectable by the BDFACSAriaII, it is not visible by confocal microscopy (data not shown). The appropriate distinction between these GFP-high and GFP-low populations and subsequent identification of Müller glia is therefore an important consideration for selecting cells to collect for downstream applications such as RNA-sequencing.
Discussion
In this protocol, enzymatic and mechanical dissociation are used in conjunction to digest the extracellular matrix, leaving only the cellular components in suspension. This must be executed in a way that also disrupts cell–cell interactions and prevents aggregates without damaging the cells themselves. For example, photoreceptors and other retinal neurons communicate with one another and propagate visual stimuli between synapses via gap junctions, comprised connexin proteins in vertebrates (48–52). Anchoring junctions such as desmosomes and adherens junctions also play an essential role in maintaining the structural organization and therefore functional integrity of the retina (53–55). Müller glia in particular span nearly the entire thickness of the retina and are closely associated with every other major retinal cell type (56,57). A prominent example is the intimate association between Müller glia microvilli and photoreceptor outer segments, playing structural and functional roles in processes such as phototransduction and metabolic homeostasis (58). Müller cell processes also form extensive contacts with neuronal cell bodies, other glia, and blood vessels, providing metabolic support and signaling molecules (57,59).
The Worthington Papain Dissociation System is used here for dissociating adult zebrafish retinal tissue, generating a cell suspension suitable for flow cytometry analysis. We have used a single dissociation kit to successfully dissociate up to 30 total zebrafish retinas; however, it is likely able to accommodate more (up to 0.3–0.4 cm3 tissue). Multiple kits may also be combined for even larger amounts of tissue. If a single kit is used to dissociate multiple samples, however, it is important to maintain consistent volume ratios between samples based on the number of retinas in each to ensure that the correct working concentrations of the enzymes and buffers are used throughout the procedure (see Supporting Information).
We chose to use an exclusively papain-based dissociation because of its ability to dissociate delicate tissues gently and efficiently (17–20). This is in contrast to other previously published methods, which employ harsher enzymes instead of or in addition to papain, such as trypsin (9,11,13), accutase (10), or dispase (12). The only known report of a papain-only dissociation of the zebrafish retina is a recent single-cell RNA sequencing analysis of Müller glia from multiple species, including zebrafish, but does not detail the dissociation or flow cytometric cell sorting procedure (14). Many of these reports are sorting other retinal cells including photoreceptors (9,11,13) and microglia/macrophages (11) from zebrafish retinas. The handful of studies sorting Müller glia employ the Tg(gfap:gfp) transgenic fish (10,12,14,15). To our knowledge, the procedure described herein is the first reported flow cytometric cell sorting of Müller glia from Tg(apoe:gfp) fish. While most of these studies do not report details such as viability, one group does report a comparable overall cell viability to our own of 95% (13). In addition, previous studies have not made a distinction between GFP-high and GFP-low when sorting Müller glia (10,12,14,15). As Müller glia provide metabolic support to the rest of the retina (27,28), it is not unexpected that the GFP expressed by Müller glia under a specific promoter may be given off and subsequently taken up by other retinal cells. This consideration should therefore be taken into account when analyzing such data from previous and future studies.
Procedural Considerations and Troubleshooting
There are several common pitfalls that can occur while preparing a retinal cell suspension and sorting Müller glia from zebrafish retinas. Key steps and common pitfalls are outlined in Table 2, summarizing the most common problems and how to avoid them.
Table 2.
Key steps and common pitfalls in preparing and sorting Müller glia from a zebrafish retinal cell suspension
| KEY STEP | DO | DO NOT |
|---|---|---|
| Retina dissection | Use scissors to mince retinas thoroughly before transferring to collection tube in dissection medium | Place whole retinas in collection tube |
| Papain dissociation | Be sure to add DNase to both the papain solution and inhibitor solution to prevent clumping of exposed DNA from dying cells Use EBSS with a pH indicator |
Invert the tube at any time, as this will cause tissue to stick to sides of tube, decreasing dissociation efficiency and viability Skip the equilibration steps |
| Mechanical dissociation | Triturate the dissociating tissue 5–10 times before, during, and after incubation in papain at 10 min intervals Pipette up and down slowly and gently |
Use a pipette other than p1000 size to triturate the tissue: A smaller size will be too harsh while a larger size will result in an ineffective dissociation Introduce bubbles into solution |
| Centrifugation | Spin samples at 850 × g (use RCF units) | Spin at less than 800 × g, resulting in loss of cells in the supernatant Spin at greater than 900 × g, resulting in an overly compact cell pellet and increase damage to cells |
| Final cell suspension preparation | Warm sorting buffer to room temperature before using in final suspension of cell pellet Pass final cell suspension through a 30 pm gravity filter |
Use cold buffer in final cell suspension, which will result in clumping |
| Preparing samples for flow cytometry | Always prepare an unstained control sample Prepare single color control samples each time a new cell type/marker/fluorophore combination is utilized |
Prepare samples for sorting at greater than 5 million cells/mL, which may cause clumping, reduction in efficiency, and/or decreased yield |
| Cell sorting | Exclude dead cells and debris Collect cells in appropriate buffer, depending on downstream applications |
Use a nozzle size less than 100 pm, as this may cause clogging or exclusion of the large Muller glia cells |
A careful balance of thorough but gentle dissociation must be struck in order to maximize cell separation, while maintaining cellular integrity. Dissected retinas should be minced into approximately equal pieces by making several perpendicular cuts. This increases the surface area of the tissue accessible to the digestive enzymes for the most effective dissociation (1). Using a combination of short incubations in papain and gentle trituration with a sufficiently wide pipette tip in an alternating fashion ensures a more efficient and mild dissociation than one consisting of a single, continuous incubation (9,12,13) or using rigorous trituration with a needle or fire-polished pipette (10,13). Equilibration steps are particularly important to keep the tissue at physiological pH throughout the dissociation, which improves cell viability and prevents clumping, especially as the tissue is first beginning to dissociate. This is not a practice reported by other studies utilizing zebrafish retinal dissociation and may affect the efficiency and quality of resulting cell suspensions.
Centrifugation conditions are another important consideration during the final steps of dissociation. While 850 × g is on the high end of typical centrifugation speeds for live cells, zebrafish retinal cells are particularly buoyant, and we have found that using lower speeds results in significant loss of cells in the supernatant. Spinning at room temperature helps ameliorate clumping during resuspension.
Adding a viability dye such as DRAQ7 to the final cell suspension is an essential step and should not be excluded. In addition to being more accurate and quantitative than trypan blue, it ensures that no dead or dying (DRAQ7+) cells are included in the sorted population(s) of interest at the time of collection. Single color and unstained control samples must also be prepared to appropriately set the gates for each unique experiment.
Applications
After sorting, these cells may be used for various downstream applications, and the collection buffer should be chosen accordingly. For example, if analysis on live cells will be performed, complete cell culture media should be used and collected at room temperature, while molecular analysis on nucleic acids or proteins may call for sorting directly into the appropriate lysis buffer at the protocol-indicated temperature. The methods described herein for obtaining a cell suspension from zebrafish retina, and sorting a cell suspension for isolating Müller glia, serve as valuable tools for regenerative ophthalmology and zebrafish retinal cell biology. It should be noted that this procedure is specifically optimized for collecting Müller glia from Tg(apoe:gfp) zebrafish retinas; however, the overall process outlined herein may be more broadly applicable to other cell types within the zebrafish retina, additional flow cytometric techniques, and/or preparation of cell suspensions from similarly delicate and heterogenic neural tissues to offer novel cell-specific insights.
Supplementary Material
ACKOWLEDGEMENTS
We would like to thank the Lerner Research Institute Flow Cytometry Core for their assistance and expertise, including Eric Schultz, Joseph Gerow, and Jena Korecky, under the direction of Kewal Asosingh. We also thank Dave Shumick of the Cleveland Clinic Center for Medical Art and Photography for his illustration work in Figure 1.
FUNDING
This work is funded by NEI1K08EY023608 (AY) and Research to Prevent Blindness Center Award unrestricted grant to Cleveland Clinic Lerner College of Medicine. KA is supported by NIH grant 5T32EY024236-04.
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- 1.Reichard A, Asosingh K. Best practices for preparing a single cell suspension from solid tissues for flow cytometry. Cytometry A 2019;95:219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenkowski JR, Raymond PA. Müller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Retin Eye Res 2014;40:94–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron DA. Cellular proliferation and neurogenesis in the injured retina of adult zebrafish. Vis Neurosci 2000;17:789–797. [DOI] [PubMed] [Google Scholar]
- 4.Stenkamp DL. Neurogenesis in the fish retina. Int Rev Cytol 2007;259:173–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thummel R, Kassen SC, Enright JM, Nelson CM, Montgomery JE, Hyde DR. Characterization of Müller glia and neuronal progenitors during adult zebrafish retinal regeneration. Exp Eye Res 2008;87:433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldman D. Muller glial cell reprogramming and retina regeneration. Nat Rev Neurosci 2014;15:431–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Müller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development 2013;140:4510–4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol 2006;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun C, Galicia C, Stenkamp DL. Transcripts within rod photoreceptors of the Zebrafish retina. BMC Genomics 2018;19:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sifuentes CJ, Kim J-W, Swaroop A, Raymond PA. Rapid, dynamic activation of Müller glial stem cell responses in Zebrafish. Invest Ophthalmol Vis Sci 2016;57: 5148–5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun C, Mitchell DM, Stenkamp DL. Isolation of photoreceptors from mature, developing, and regenerated zebrafish retinas, and of microglia/macrophages from regenerating zebrafish retinas. Exp Eye Res 2018;177:130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. PNAS 2009;106:9310–9315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glaviano A, Smith AJ, Blanco A, McLoughlin S, Cederlund ML, Heffernan T, Sapetto-Rebow B, Alvarez Y, Yin J, Kennedy BN. A method for isolation of cone photoreceptors from adult zebrafish retinae. BMC Neurosci 2016;17:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoang T, Wang J, Boyd P, Wang F, Santiago C, Jiang L, Lahne M, Todd LJ, Saez C, Yoo S, et al. Cross-species transcriptomic and epigenomic analysis reveals key regulators of injury response and neuronal regeneration in vertebrate retinas. bioRxiv 2019;717876. [Google Scholar]
- 15.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol 2010;12:1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussain RZ, Miller-Little WA, Doelger R, Cutter GR, Loof N, Cravens PD, Stüve O. Defining standard enzymatic dissociation methods for individual brains and spinal cords in EAE. Neurol Neuroimmunol Neuroinflamm 2018;5:e437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huettner JE, Baughman RW. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci 1986;6:3044–3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bader CR, MacLeish PR, Schwartz EA. Responses to light of solitary rod photoreceptors isolated from tiger salamander retina. Proc Natl Acad Sci U S A 1978;75: 3507–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townes-Anderson E, MacLeish PR, Raviola E. Rod cells dissociated from mature salamander retina: Ultrastructure and uptake of horseradish peroxidase. J Cell Biol 1985;100:175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam DMK. Biosynthesis of acetylcholine in turtle photoreceptors. Proc Natl Acad Sci U S A 1972;69:1987–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anon. PDS.pdf. Available at: http://www.worthington-biochem.com/PDS/PDS.pdf. Accessed November 30, 2018.
- 22.Hoon M, Okawa H, Santina LD, Wong ROL. Functional architecture of the retina: Development and disease. Prog Retin Eye Res 2014;42:44–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Masri RA, Percival KA, Koizumi A, Martin PR, Grünert U. Survey of retinal ganglion cell morphology in marmoset. J Comp Neurol 2016;0:236–258. [DOI] [PubMed] [Google Scholar]
- 24.Seung HS, Sümbül U. Neuronal cell types and connectivity: Lessons from the retina. Neuron 2014;83:1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kolb H, Nelson R, Ahnelt P, Cuenca N. Chapter 1 cellular organization of the vertebrate retina. Progress in Brain Research. Vol 131 Concepts and Challenges in Retinal Biology (Progress in Brain Research). The Netherlands: Elsevier, 2001; p. 3–26. [DOI] [PubMed] [Google Scholar]
- 26.Wu SM. Synaptic organization of the vertebrate retina: General principles and species-specific variations: The Friedenwald lecture. Invest Ophthalmol Vis Sci 2010; 51:1264–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bringmann A, Pannicke T, Grosche J, Francke M, Wiedemann P, Skatchkov SN, Osborne NN, Reichenbach A. Müller cells in the healthy and diseased retina. Prog Retin Eye Res 2006;25:397–424. [DOI] [PubMed] [Google Scholar]
- 28.Sorrentino FS, Allkabes M, Salsini G, Bonifazzi C, Perri P. The importance of glial cells in the homeostasis of the retinal microenvironment and their pivotal role in the course of diabetic retinopathy. Life Sci 2016;162:54–59. [DOI] [PubMed] [Google Scholar]
- 29.Hippert C, Graca AB, Barber AC, West EL, Smith AJ, Ali RR, Pearson RA. Müller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS One 2015;10:e0120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasha SPBS, Münch R, Schäfer P, Oertel P, Sykes AM, Zhu Y, Karl MO. Retinal cell death dependent reactive proliferative gliosis in the mouse retina. Sci Rep 2017;7: 9517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Avanesov A, Malicki J. Analysis of the retina in the Zebrafish model. Methods Cell Biol 2010;100:153–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gestri G, Link BA, Neuhauss SC. The visual system of Zebrafish and its use to model human ocular diseases. Dev Neurobiol 2012;72:302–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Ubaidi MR, Naash MI, Conley SM. A perspective on the role of the extracellular matrix in progressive retinal degenerative disorders. Invest Ophthalmol Vis Sci 2013;54:8119–8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reinhard J, Joachim SC, Faissner A. Extracellular matrix remodeling during retinal development. Exp Eye Res 2015;133:132–140. [DOI] [PubMed] [Google Scholar]
- 35.Bishop PN. The role of extracellular matrix in retinal vascular development and preretinal neovascularization. Exp Eye Res 2015;133:30–36. [DOI] [PubMed] [Google Scholar]
- 36.Vecino E, Heller JP, Veiga-Crespo P, Martin KR, Fawcett JW. Influence of extracellular matrix components on the expression of integrins and regeneration of adult retinal ganglion cells. PLoS One 2015;10:e0125250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryan CD, Chien C-B, Kwan KM. Loss of laminin alpha 1 results in multiple structural defects and divergent effects on adhesion during vertebrate optic cup morpho-genesis. Dev Biol 2016;416:324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor L, Arnér K, Engelsberg K, Ghosh F. Scaffolding the retina: The interstitial extracellular matrix during rat retinal development. Int J Dev Neurosci 2015;42: 46–58. [DOI] [PubMed] [Google Scholar]
- 39.Hunter DD, Brunken WJ. Beta 2 laminins modulate neuronal phenotype in the rat retina. Mol Cell Neurosci 1997;10:7–15. [DOI] [PubMed] [Google Scholar]
- 40.McAdams BD, McLoon SC. Expression of chondroitin sulfate and keratan sulfate proteoglycans in the path of growing retinal axons in the developing chick. J Comp Neurol 1995;352:594–606. [DOI] [PubMed] [Google Scholar]
- 41.Perez RG, Halfter W. Tenascin in the developing Chick visual system: Distribution and potential role as a modulator of retinal axon growth. Dev Biol 1993;156: 278–292. [DOI] [PubMed] [Google Scholar]
- 42.Hodel C, Neuhauss SCF, Biehlmaier O. Time course and development of light adaptation processes in the outer zebrafish retina. Anat Rec A Discov Mol Cell Evol Biol 2006;288A:653–662. [DOI] [PubMed] [Google Scholar]
- 43.Ali MA. Retinomotor response: Characteristics and mechanisms. Vision Res 1971; 11:1225–1288. [DOI] [PubMed] [Google Scholar]
- 44.Tennant JR. Evaluation of the TRYPAN blue technique for determination of cell viability. Transplantation 1964;2:685–694. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-De Luna RI, Ku RY, Aruck AM, Santiago F, Viczian AS, San Mauro D, Zuber ME. Müller glia reactivity follows retinal injury despite the absence of the glial fibrillary acidic protein gene in Xenopus. Dev Biol 2017;426:219–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J Neurosci 2007;27:7028–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vihtelic TS, Hyde DR. Light-induced rod and cone cell death and regeneration in the adult albino zebrafish (Danio rerio) retina. J Neurobiol 2000;44:289–307. [DOI] [PubMed] [Google Scholar]
- 48.Raviola E, Gilula NB. Gap junctions between photoreceptor cells in the vertebrate retina. PNAS 1973;70:1677–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodenough DA, Paul DL. Gap junctions. Cold Spring Harb Perspect Biol 2009;1: a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy K, Kumar S, Bloomfield SA. Gap junctional coupling between retinal amacrine and ganglion cells underlies coherent activity integral to global object perception. Proc Natl Acad Sci U S A 2017;114:E10484–E10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Klaassen LJ, de Graaff W, van Asselt JB, Klooster J, Kamermans M. Specific connectivity between photoreceptors and horizontal cells in the zebrafish retina. J Neurophysiol 2016;116:2799–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kántor O, Varga A, Nitschke R, Naumann A, Énzsöly A, Lukáts Á, Szabó A, Németh J, Völgyi B. Bipolar cell gap junctions serve major signaling pathways in the human retina. Brain Struct Funct 2017;222:2603–2624. [DOI] [PubMed] [Google Scholar]
- 53.Kong Y, Naggert JK, Nishina PM. The impact of Adherens and tight junctions on physiological function and pathological changes in the retina In: Ash JD, Anderson RE, LaVail MM, Bowes Rickman C, Hollyfield JG, Grimm C, editors. Retinal Degenerative Diseases. Advances in Experimental Medicine and Biology. New York, New York: Springer International Publishing, 2018; p. 545–551. [DOI] [PubMed] [Google Scholar]
- 54.Alves CH, Pellissier LP, Wijnholds J. The CRB1 and adherens junction complex proteins in retinal development and maintenance. Prog Retin Eye Res 2014;40:35–52. [DOI] [PubMed] [Google Scholar]
- 55.Tolun G, Vijayasarathy C, Huang R, Zeng Y, Li Y, Steven AC, Sieving PA, Heymann JB. Paired octamer rings of retinoschisin suggest a junctional model for cell-cell adhesion in the retina. Proc Natl Acad Sci U S A 2016;113:5287–5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reichenbach A, Schneider H, Leibnitz L, Reichelt W, Schaaf P, Schümann R. The structure of rabbit retinal Müller (glial) cells is adapted to the surrounding retinal layers. Anat Embryol 1989;180:71–79. [DOI] [PubMed] [Google Scholar]
- 57.Newman E, Reichenbach A. The Müller cell: A functional element of the retina. Trends Neurosci 1996;19:307–312. [DOI] [PubMed] [Google Scholar]
- 58.Garlipp MA, Gonzalez-Fernandez F. Cone outer segment and Müller microvilli peri-cellular matrices provide binding domains for interphotoreceptor retinoid-binding protein (IRBP). Exp Eye Res 2013;113:192–202. [DOI] [PubMed] [Google Scholar]
- 59.Skytt DM, Toft-Kehler AK, Brændstrup CT, Cejvanovic S, Gurubaran IS, Bergersen LH, Kolko M. Glia-neuron interactions in the retina can be studied in cocultures of Müller cells and retinal ganglion cells. Biomed Res Int 2016; 2016:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.