Abstract
Upregulated expression of P-selectin on activated endothelium and platelets significantly contributes to the initiation and progression of vaso-occlusive crises (VOC), a major cause of morbidity in sickle cell disease (SCD). Crizanlizumab (ADAKVEO®), a humanized monoclonal antibody against P-selectin, primarily inhibits the interaction between leukocytes and P-selectin, and has been shown to decrease the frequency of VOCs in clinical trials. However, the lack of reliable in vitro assays that objectively measure leukocyte adhesion to P-selectin remains a critical barrier to evaluating and improving the therapeutic treatment in SCD. Here, we present a standardized microfluidic biochip whole blood adhesion assay to assess leukocyte adhesion to P-selectin under physiologic flow conditions. Our results demonstrated heterogeneous adhesion by leukocytes to immobilized P-selectin, and dose-dependent inhibition of this adhesion following pre-exposure to Crizanlizumab. Importantly, treatment with Crizanlizumab following adhesion to P-selectin promoted detachment of rolling, but not of firmly adherent leukocytes. Taken together, our results suggest that the microfluidic Biochip system is a promising in vitro assay with which to screen patients, monitor treatment response, and guide current and emerging anti-adhesive therapies in SCD.
Keywords: P-selectin, Microfluidics, Sickle cell disease, Crizanlizumab, SEG101, BioChip, Adhesion
1. Introduction
Sickle cell disease (SCD) is characterized by frequent and unpredictable vaso-occlusive crises (VOC), which lead to acute pain, chronic organ damage, and can contribute to early mortality [1]. Previous studies have revealed a multistep and multicellular paradigm in VOC, which involves activated leukocytes adhering to activated endothelium and circulating sickle erythrocytes, thus hindering blood flow and contributing to VOC propagation [2, 3]. Multiple adhesion receptor/ligand pairs have been identified in these multicellular interactions [4–6]. P-selectin is known to mediate platelet activation, coagulation, and inflammation, and has been found, of all selectins, to be the most essential for initiation and maintenance of the cascade of events triggered by leukocyte adhesion to vascular endothelium during VOC [7, 8]. In vitro and in vivo models showed that inhibition of P-selectin-mediated adhesion pathways significantly reduced leukocyte adhesion to activated endothelium and ameliorated impaired blood flow in SCD [9, 10]. Crizanlizumab is a humanized monoclonal antibody against P-selectin, which, when infused monthly, reduced the annual rate of VOC in patients with SCD in randomized placebo-controlled clinical trials [11, 12]. However, currently, there is no in vitro model for this effect, largely due to the lack of a universally accepted, standardized physiologic flow-based adhesion assay with which to measure leukocyte adhesion to P-selectin.
We have previously reported a microfluidic platform, the SCD Biochip, in which red blood cells from people with homozygous SCD (HbSS) had variable erythrocyte adhesion to the immobilized sub-endothelial protein laminin (LN) in vitro, depending on clinical phenotype, extent of hemolysis, and disease severity [13–15]. Here, we present a standardized microfluidic whole blood adhesion assay using the P-selectin SCD Biochip platform and clinically available whole blood samples to measure leukocyte adhesion to P-selectin under physiologic flow conditions. Such an assay could help visualize cellular adhesion characteristics before and after therapeutic interventions, and may reveal the association between patient-specific adhesion profiles and clinical phenotypes. Indeed, data reported in this study support the inhibitory effect of pre-emptive Crizanlizumab on P-selectin mediated leukocyte adhesion, and of post-treatment Crizanlizumab on leukocyte detachment.
2. Materials and methods
All anonymized blood samples were collected in ethylenediaminetetraacetic acid (EDTA) from individuals with homozygous SCD (HbSS) seen at University Hospital’s Hematology and Oncology Division. Consent were acquired from all individuals on an IRB-approved protocol. Clinical information, including white blood cell count (WBC, 109/L), platelet count (109/L), absolute neutrophil count (ANC, 106/L), reticulocyte count (109/L), lactate dehydrogenase levels (LDH, U/L), ferritin levels (μg/L), hemoglobin A (HbA) %, hemoglobin S (HbS) %, hemoglobin F (HbF) %, and total hemoglobin (g/dF), was obtained from the electronic medical record. The clinical variables of the analyzed individuals are listed in Table 1.
Table 1.
Clinical phenotype of the study population
| Clinical variables | Range | Mean ± SD |
|---|---|---|
| Baseline Leukocyte adhesion to P-selectin | 74 - 4510 | 1428 ± 1460 |
| Age | 19 - 66 | 38 ± 14 |
| Hgb (g/dL) | 5.5 - 10.8 | 8.4 ± 1.5 |
| White Blood Cell Count (109/L) | 3.9 - 20.2 | 11.9 ± 4.3 |
| Platelet Count (109/L) | 101 - 1048 | 398 ± 193 |
| Mean Corpuscular Volume | 68 - 134 | 96 ± 17 |
| Absolute Neutrophil Count (106/L) | 1740 - 13920 | 7145 ± 3102 |
| Absolute Reticulocyte Count (109/L) | 55 - 821 | 365 ± 204 |
| Lactate Dehydrogenase (U/L) | 182 - 1402 | 418 ± 261 |
| Ferritin (μg/L) | 30 - 6820 | 1688 ± 2607 |
| Hemoglobin S (%) | 4.3 - 92.5 | 52.2 ± 23.6 |
| Hemoglobin A (%) | 1.5 - 75.7 | 32.5 ± 24.0 |
| Hemoglobin F (%) | 0.7 - 28.6 | 6.3 ± 9.0 |
| On-Transfusion | 55% of Individuals (12/22) | |
A total of 22 blood samples were obtained from 22 individuals with homozygous HbSS (total N=22, Male=10, Female=12).
SD: Standard deviation.
Microfluidic devices were fabricated using a lamination technique, as previously described [16–18]. Each microfluidic device contained 3 identical microchannels with a height of 50 μm (Fig. 1A), which were designed to recapitulate volume and flow of post-capillary venules. The microchannels were functionalized with recombinant human P-selectin (25 μg/mF) and blocked with 2% bovine serum albumin (BSA) to prevent non-specific adhesion. After tubing assembly, whole blood samples were mixed with Hanks’ buffer modified with calcium and magnesium (1:1 v/v), and 15 pF of the diluted whole blood was injected into the microchannels using New Era NE-300 syringe pump system (Farmingdale, NY) at an approximate shear rate of 1 dyne/cm2. Non-adherent cells were rinsed out by flowing Hanks’ buffer. Phase-contrast images of the microchannels with adherent leukocytes were recorded at 10X with an inverted microscope (Olympus 1X83) and a camera (EXi Blue EXI-BFU-RF-M-14-C), and adherent leukocytes were manually quantified with Adobe Photoshop software (San Jose, CA) in a 32 mm2 area.
Figure 1.
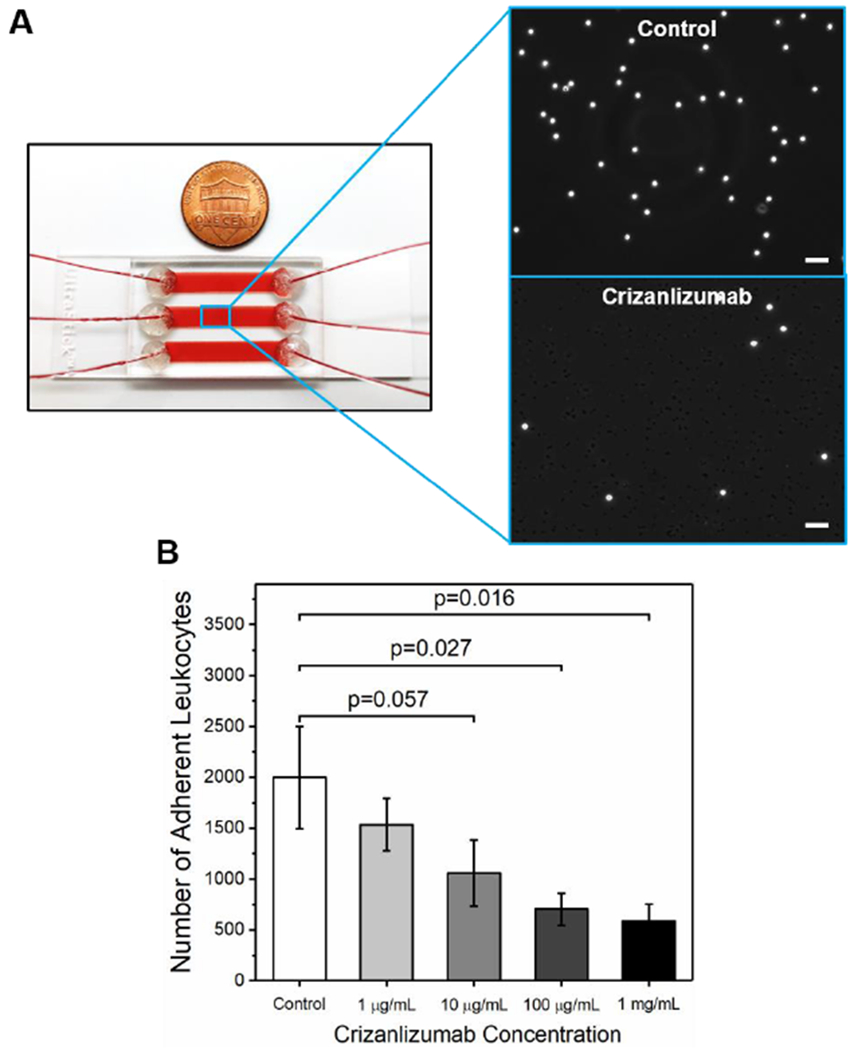
Microfluidic whole blood assay of leukocyte adhesion to P-selectin and the inhibitory effect Crizanlizumab pre-treatment. (A) An assembled SCD Biochip platform consisting of 3 parallel microchannels with blood flow is shown. Insets: High-resolution phase-contrast microscope images of adherent leukocytes and reduction of leukocyte adhesion under Crizanlizumab pre-treatment are shown. Scale bars represent a length of 50 μm. (B) Crizanlizumab pre-treatment significantly reduced leukocyte adhesion to P-selectin in a dose-dependent manner. Horizontal lines between individual groups represent a statistically significant difference based on a paired t-test. Error bars represent the standard error of the mean.
Crizanlizumab (ADAKVEO®, SEG101) stock solution (10 mg/mF) was donated by Novartis (Basel, Switzerland). For the inhibition experiments, P-selectin immobilized-microchannels were pre-treated with Hanks buffer as vehicle control, or Crizanlizumab with graded concentrations of 1, 10, 100 μg/mL, or 1 mg/mL at 37°C for 30 minutes. Thereafter, the adhesion assay was performed. Human IgG2 (Sigma Aldrich, St. Louis, MO), at 100 μg/mL, was included as the isotype control for Crizanlizumab. For the detachment experiments, modified microchannels with individual inlets for separately injecting blood and drug-containing buffer were designed to allow transition from washing buffer to Crizanlizumab during rinsing. Two synchronous pumps connected to these inlets were programmed such that Crizanlizumab at 10 μg/mL was injected into microchannels after Hanks buffer without a disruption in shear rate. The programmed flow rates of the two synchronous pumps over the course of the experiment are shown in Figure 2A, which insured a constant shear rate in the microchannels. Cell behaviors were also continuously recorded (Supplementary Video 1). Phase contrast images of the adherent leukocytes were collected during Hanks’s buffer rinse and Crizanlizumab flow for comparison.
Figure 2.
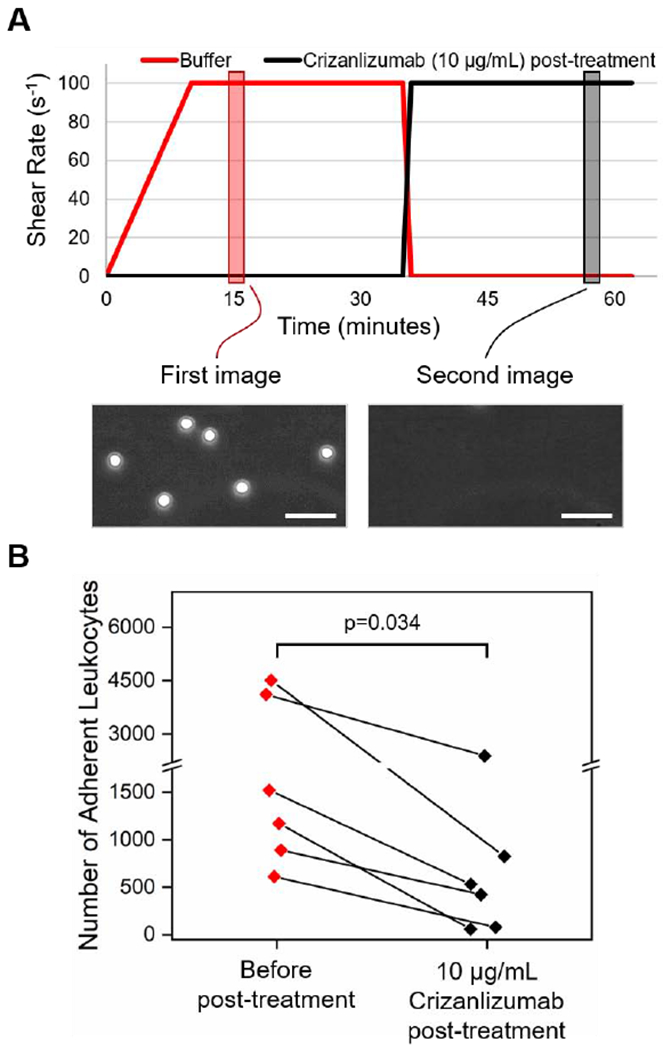
Crizanlizumab post-treatment promotes the detachment of adherent leukocytes to P-selectin. (A) Shown are the flow rates of the two programmed synchronous pumps adopted in the detachment experiments over time. Two images were recorded at two different time points to compare the effect of Crizanlizumab post-treatment. (B) Crizanlizumab post-treatment led to the detachment of adherent leukocytes on P-selectin. Horizontal line between individual groups represents a statistically significant difference based on a paired t-test (p<0.05). Scale bars represent a length of 50 μm.
Leukocyte adhesion data were reported as mean ± standard error of the mean (SEM). Clinical variables of the study population were reported as mean ± standard deviation (SD). Statistical analyses were performed with Minitab (Minitab Inc., State College, PA). A test for normality was initially performed on relevant variables. A paired t-test was performed to compare paired groups before and after Crizanlizumab treatment. Statistical significance was determined based on a p-value less than 0.05 (p<0.05).
3. Results
In this study, we examined the effect of Crizanlizumab on leukocyte adhesion to P-selectin in two distinct ways. First, the P-selectin immobilized microchannels were pre-treated with Crizanlizumab at varying concentrations prior to the adhesion experiments to analyze the utility of the drug in preventing leukocyte adhesion. In the second approach, the adhesion assay was performed first, followed by treatment with Crizanlizumab at a fixed concentration to assess whether it would reverse already-established adhesive interactions between leukocytes and P-selectin.
These assays revealed that leukocytes from individuals with HbSS SCD displayed significant and heterogeneous adhesion to immobilized P-selectin under physiologic flow conditions in the absence of Crizanlizumab treatment (N=8). Crizanlizumab pre-treatment of P-selectin functionalized microchannels led to a dose-dependent reduction in leukocyte adhesion. Leukocyte adhesion was significantly lower in the microchannels pre-treated with Crizanlizumab at concentrations of 100 μg/mL or 1 mg/mL, compared to non-Crizanlizumab controls (Fig. 1B, 100 μg/mL: 703 ± 161, range: 131-1474, p=0.027; 1 mg/mL: 587 ± 168, range:81-1403, p=0.016; control: 1998 ± 503, range: 4256-526, paired t-test) while the significance was borderline for the concentration of 10 μg/mL (Fig. 1B, 1057 ± 325, range: 195-2948, p=0.057, paired t-test). Leukocyte adhesion in the 1 μg/mL Crizanlizumab treated microchannels was not significantly different compared to the non-treated microchannels (Fig. 1B, 1535 ± 258, range: 257-2732, p>0.05, paired t-test). Furthermore, the isotype control did not show an inhibitory effect comparable with Crizanlizumab (2290 ± 849 vs 596 ± 221, N=3) at 100 μg/mL. Of note, these assays were performed using blood samples from on-transfusion individuals. We also assessed blood samples from nontransfusion individuals (on-hydroxyurea or supportive care) using the P-selectin microchannels with 1 mg/mL Crizanlizumab pre-treatment, similar inhibitory effect was observed (1 mg/mL Crizanlizumab vs control: 179 ± 79 vs 327 ± 101, range: 39-691 vs 74-742, N=8, p=0.063, paired t-test).
Our results also demonstrated that post-treatment of leukocytes with Crizanlizumab, after the leukocytes adhered to the immobilized P-selectin, resulted in detachment of rolling leukocytes whereas firmly attached cells remained adherent. As shown in Figure 2B, there was a significant decrease in leukocyte adhesion before and after introducing Crizanlizumab into the microchannels (719 ± 354 vs 2138 ± 700, range: 613-4510 vs 58-2388, N=6 (4 out of which were on-transfusion), p=0.034, paired t-test). These findings support the role of Crizanlizumab in preventing VOC, by blocking leukocyte adhesions; however, Crizanlizumab may also mitigate a VOC due to its ability to reverse some, but not all, established aberrant adhesive interactions.
4. Conclusions
Previous studies have shown that Crizanlizumab significantly reduces the frequency of pain crises in SCD and decreases the annual rate of hospitalized days [11]. In this study, using the P-selectin SCD Biochip, we showed that under physiologic flow conditions, Crizanlizumab significantly ameliorates leukocyte adhesion to immobilized P-selectin. Importantly, Crizanlizumab applied after adhesion, impacted rolling leukocytes, although firmly-adherent leukocytes were unresponsive. This may, at least in part, explain the in vivo heterogeneity of Crizanlizumab reported previously.
Our results suggest that the in vitro microfluidic whole blood adhesion assay, the SCD Biochip, has the potential to enhance our understanding of the underlying mechanisms necessary to develop targeted therapeutic strategies during drug development and in clinical trials. We are presently exploring whether these assays can predict individual patient response to targeted therapies.
Supplementary Material
Acknowledgements
The authors acknowledge with gratitude the contributions of patients and clinicians at Seidman Cancer Center (University Hospitals, Cleveland), and .Novartis AG (Basel, Switzerland) for donating Crizanlizumab for in vitro studies described in this manuscript.
Funding source
This work was supported by the Clinical and Translational Science Collaborative of Cleveland, UL1TR002548 from the National Center for Advancing Translational Sciences component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, Case-Coulter Translational Research Partnership Program, National Heart, Lung, and Blood Institute R01HL133574 and OT2HL152643, National Science Foundation CAREER Award 1552782, and NIH T32HL134622 grants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
YM, UG, EK, JAL, and UAG are inventors of intellectual property (PCT/US2018/022888 filed on March 16, 2018 and provisional patent application 62/928,109 filed on November 1, 2019) on the microfluidic BioChip technology presented in this manuscript. Competing interests of Case Western Reserve University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict of Interest Management Plan.
References
- 1.Rees DC, Williams TN, and Gladwin MT, Sickle-cell disease. Lancet, 2010. 376(9757): p. 2018–31. [DOI] [PubMed] [Google Scholar]
- 2.Frenette PS, Sickle cell vaso-occlusion: multistep and multicellular paradigm. Curr Opin Hematol, 2002. 9(2): p. 101–6. [DOI] [PubMed] [Google Scholar]
- 3.Turhan A, et al. , Primary role for adherent leukocytes in sickle cell vascular occlusion: a new paradigm. Proc Natl Acad Sci U S A, 2002. 99(5): p. 3047–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsui NM, et al. , P-selectin mediates the adhesion of sickle erythrocytes to the endothelium. Blood, 2001. 98(6): p. 1955–1962. [DOI] [PubMed] [Google Scholar]
- 5.Setty BNY and Stuart MJ, Vascular cell adhesion molecule-1 is involved in mediating Hypoxia-induced sickle red blood cell adherence to endothelium: Potential role in sickle cell disease. Blood, 1996. 88(6): p. 2311–2320. [PubMed] [Google Scholar]
- 6.Kaul DK, Finnegan E, and Barabino GA, Sickle red cell-endothelium interactions. Microcirculation, 2009. 16(1): p. 97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence MB and Springer TA, Leukocytes Roll on a Selectin at Physiological Flow-Rates - Distinction from and Prerequisite for Adhesion through Integrins. Cell, 1991. 65(5): p. 859–873. [DOI] [PubMed] [Google Scholar]
- 8.Robinson SD, et al. , Multiple, targeted deficiencies in selectins reveal a predominant role for P-selectin in leukocyte recruitment. Proc Natl Acad Sci U S A, 1999. 96(20): p. 11452–11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mayadas TN, et al. , Leukocyte Rolling and Extravasation Are Severely Compromised in P-Selectin-Deficient Mice. Cell, 1993. 74(3): p. 541–554. [DOI] [PubMed] [Google Scholar]
- 10.Manwani D and Frenette PS, Vaso-occlusion in sickle cell disease: pathophysiology and novel targeted therapies. Blood, 2013. 122(24): p. 3892–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ataga KI, et al. , Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. New England Journal of Medicine, 2017. 376(5): p. 429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kutlar A, et al. , Effect of crizanlizumab on pain crises in subgroups of patients with sickle cell disease: A SUSTAIN study analysis. Am J Hematol, 2019. 94(1): p. 55–61. [DOI] [PubMed] [Google Scholar]
- 13.Alapan Y, et al. , Sickle cell disease biochip: a functional red blood cell adhesion assay for monitoring sickle cell disease. Transl Res, 2016. 173: p. 74–91 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim M, et al. , Hypoxia-enhanced adhesion of red blood cells in microscale flow. Microcirculation, 2017. 24(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Man YC, et al. , Heterogeneous Hypoxia-Mediated Neutrophil and Red Blood Cell Adhesion to E-Selectin in Microscale Flow. Blood, 2018. 132. [Google Scholar]
- 16.Alapan Y, Little JA, and Gurkan UA, Heterogeneous red blood cell adhesion and deformability in sickle cell disease. Sci Rep, 2014. 4: p. 7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kucukal E, et al. , Red blood cell adhesion to heme-activated endothelial cells reflects clinical phenotype in sickle cell disease. Am J Hematol, 2018. 93(8): p. 1050–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akkaya B, et al. , Mercury leads to abnormal red blood cell adhesion to laminin mediated by membrane sulfatides. Biochim Biophys Acta Biomembr, 2019. 1861(6): p. 1162–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


