Abstract
Preterm birth (gestational age <37 weeks) has a worldwide prevalence of nearly 11%, and >95% of preterm infants who receive modern neonatal and pediatric care now survive into adulthood. However, improved early survival has been accompanied by long-term increased risks of various chronic disorders, prompting investigations to determine whether preterm birth leads to higher mortality risks in adulthood. A systematic review identified 8 studies with a total of 6,594,424 participants that assessed gestational age at birth in relation to all-cause or cause-specific mortality at any ages ≥18 years. All 6 studies that included persons born in 1967 or later reported positive associations between preterm birth and all-cause mortality in adulthood (attained ages, 18–45 years). Most adjusted relative risks ranged from 1.2 to 1.6 for preterm birth, 1.1 to 1.2 for early term birth (37–38 weeks), and 1.9 to 4.0 for extremely preterm birth (22–27 weeks), compared with full-term birth (variably defined but including 39–41 weeks). These findings appeared independent of sociodemographic, perinatal, and maternal factors (all studies), and unmeasured shared familial factors in co-sibling analyses (assessed in 4 studies). Four of these studies also explored cause-specific mortality and reported associations with multiple causes, including respiratory, cardiovascular, endocrine, and neurological. Two smaller studies based on an earlier cohort born in 1915–1929 found no clear association with all-cause mortality but positive associations with selected cause-specific mortality. The overall evidence indicates that premature birth during the past 50 years is associated with modestly increased mortality in early to mid-adulthood.
INTRODUCTION
In 2014, preterm birth (gestational age <37 completed weeks) had a prevalence of 10.6% worldwide, 9.6% in the US, and 5% to 9% in most European countries.1 Because of treatment advances in the past 50 years,2 over 95% of preterm infants who receive modern neonatal and pediatric care now survive into adulthood.3, 4, 5, 6 However, improved early survival has been accompanied by other long-term health risks. Preterm birth has been linked with increased risks of various comorbidities, including cardiometabolic, respiratory, and neuropsychiatric disorders, that sometimes track from childhood into adulthood or may first manifest in adulthood.3, 4, 5, 6, 7 These sequelae have prompted investigations to determine whether preterm birth leads to increased mortality risks in adulthood. Based on global prevalence and survival data,8, 9 the number of preterm survivors now reaching adulthood exceeds 10 million per year worldwide, and hence even modestly increased mortality in adulthood may have high clinical and public health impacts.
A number of prior studies have established that preterm birth is associated with higher mortality rates in infancy, childhood, and adolescence.9, 10, 11, 12, 13, 14, 15, 16, 17 Fewer studies have explored these associations among adults because of the inherent challenges of following large birth cohorts for several decades needed to assess mortality with sufficient power in adulthood. Linkages of well-established national registries with birth and death records, especially in the Nordic countries, have enabled the largest and most comprehensive investigations to date. The current evidence was assessed by reviewing all studies to date that examined gestational age at birth in relation to mortality in adulthood.
METHODS
A systematic literature review was conducted to identify all studies that examined: (1) gestational age at birth (or any gestational age subgroups) as a predictor, and (2) all-cause or cause-specific mortality at any adult ages (≥18 years) as an outcome. PubMed and CINAHL databases were searched on October 1, 2019, for all study abstracts and titles using the following search terms: (prematurity or (gestation* age) or ((preterm or premature or term) and birth)) and (mortality or death) and (adult*). The search was limited to studies of human beings, without date or language restrictions. After screening the original 1,281 studies found, 30 were chosen for further in-depth evaluation. The bibliographies of selected studies also were searched. Eight studies ultimately met the above inclusion criteria.
The 8 selected studies were reviewed for design and analytic methods, including how gestational age was modeled, attained ages during the follow-up period, and assessment and control for confounding. The reported findings were reviewed for all-cause and cause-specific mortality risk estimates and potential sex-specific differences. In addition, a post hoc analysis based on the largest study to date6 was performed to estimate absolute mortality differences, attributable fraction in the exposed (AFe), and population attributable fraction (PAF) for gestational age subgroups compared with full-term.18
RESULTS
Table 1 summarizes the study designs for the 8 selected studies, which were conducted in only 3 countries (Sweden, Norway, and Australia) and included a total of 6,594,424 unique participants. Each study used an observational retrospective cohort design and Cox proportional hazards regression to compute hazard ratios (HRs) for mortality, with attained age as the Cox model time axis. However, they had other methodologic differences, notably in gestational age ascertainment and modeling, definition of the reference group, and adjustment variables. Because of differences in how gestational age was categorized and modeled, it was not feasible to perform a meta-analysis using the available published data. Five studies assessed both all-cause and cause-specific mortality,5, 6, 19, 20, 21 2 assessed only all-cause mortality,22, 23 and 1 assessed only cause-specific mortality.24 Six studies included persons born in 1967–2015,5, 6, 19, 20, 22, 23 thus overlapping with the modern neonatal care era.2 These studies will be reviewed first, and then the remaining 2 studies based on an earlier cohort born in 1915–1929.21, 24
Table 1.
Characteristics of studies on gestational age at birth and mortality in adulthood.
| Country and study | Design | Sample size | Birth years | Latest follow-up year | Attained ages examined (years) | Gestational ages examined (weeks) | Mortality outcomes | Adjustment variables |
|---|---|---|---|---|---|---|---|---|
| Sweden | ||||||||
| Crump (2011)6 | Cohort | 674,820 | 1973–1979 | 2008 | 1–36 | 22–27, 28–33, 34–36, 37–42 (Ref), ≥43, and continuous | All-cause and cause-specific | Sex, birth year, fetal growth, birth order, maternal factors (age, marital status, education), paternal education |
| Crump (2013)19 | Cohort | 679,981 | 1973–1979 | 2008 | 0–36 | <37, 37–38, 39–42 (Ref), ≥43 | All-cause and cause-specific | Sex, birth year, birth order, maternal factors (age, marital status, education), paternal education |
| D’Onofrio (2013)22 | Cohort and co-sibling | 3,300,708 | 1973–2008 | 2009 | 0–36 | 23–27, 28–30, 31–33, 34–36, 37–42 (Ref), and continuous | All-cause | Sex, birth order, maternal and paternal factors (age, education, history of criminal conviction) |
| Crump (2019)5 | Cohort and co-sibling | 4,296,814 | 1973–2015 | 2017 | 0–45 | 22–27, 28–33, 34–36, 37–38, 39–41 (Ref), ≥42, and continuous | All-cause and cause-specific | Sex, birth year, birth order, maternal factors (age, education, smoking) |
| Koupil (2005)24 | Cohort | 11,474 | 1915–1929 | 2001 | 0–87 | 30–35 (Ref), 36–37, 38–39, 40–41, ≥42 | Cause-specific | Sex, birth period, education, individual and maternal marital status and SES |
| Juarez (2016)21 | Cohort and co-sibling | 12,564 | 1915–1929 | 2009 | 0–95 | <37, 37–41 (Ref), ≥42, and continuous | All-cause and cause-specific | Sex, birth year, birth weight, maternal factors (age, parity, marital status, SES) |
| Norway | ||||||||
| Risnes (2016)20 | Cohort and co-sibling | 1,562,647 | 1967–1997 | 2011 | 15–45 | 22–33, 34–36, 37–41 (Ref), ≥42 | All-cause and cause-specific | Sex, birth cohort (10-year groups), multiple birth, maternal factors (age, parity, education) |
| Australia | ||||||||
| Srinivasjois (2017)23 | Cohort | 722,399 | 1980–2010 | 2010 | 0–30 | 24–31, 32–34, 35–36, 37–38, 39–41 (Ref), ≥42 | All-cause | Sex, birth decade, race, maternal factors (age, marital status, parity), mode of delivery, type of labor onset, geographic remoteness, area-level SES |
SES = socioeconomic status, Ref. = reference group
All-Cause Mortality Among Persons Born in 1967 or Later
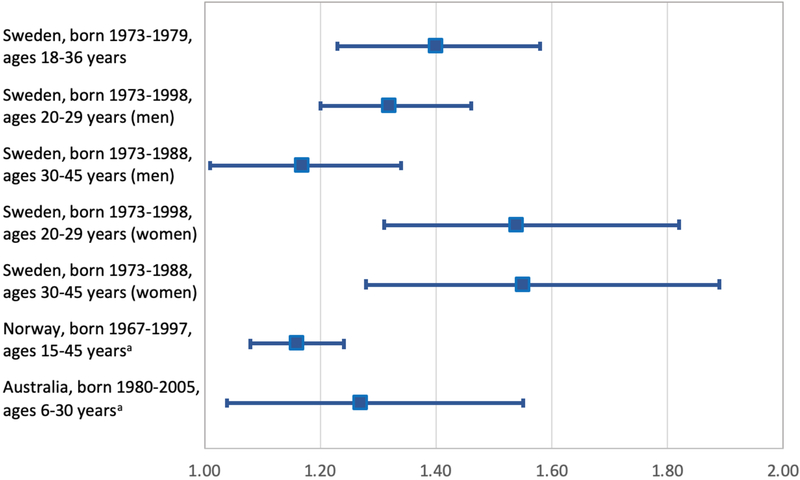
Table 2 reports selected findings for all-cause mortality. The first 3 published studies of gestational age at birth and all-cause mortality in adulthood were based on overlapping cohorts in Sweden.6, 19, 22 The first of these was published in 2011 and included 674,820 persons born as singletons in 1973–1979 who were followed up for mortality through 2008 (maximum age 36 years).6 Among those who survived to age 18 years, each additional week of gestation was associated with a 4% (95% CI, 3% to 6%) lower mortality between ages 18 and 36 years (adjusted HR, 0.96; 95% CI, 0.94–0.97; P<0.001). The adjusted HR for all-cause mortality in adulthood associated with extremely preterm birth (22–27 weeks) was 1.91 (95% CI, 0.62–5.94), with very preterm birth (28–33 weeks) was 1.64 (1.28–2.11), and with late preterm birth (34–36 weeks) was 1.31 (1.13–1.50), compared with full-term birth (37–42 weeks).
Table 2.
Summary of findings for gestational age at birth and all-cause mortality in adulthood.
| Country and study | Age intervals that included adults | Gestational ages examined (weeks) | Reference group (weeks) | Selected all-cause mortality HR (95% CI) |
|---|---|---|---|---|
| Sweden | ||||
| Crump (2011)6 | 18–36 years | 22–27 | 37–42 | 1.91 (0.62–5.94) |
| 28–33 | 1.64 (1.28–2.11) | |||
| 34–36 | 1.31 (1.13–1.50) | |||
| Per additional week | 0.96 (0.94–0.97) | |||
| Crump (2013)19 | 18–36 years | <37 | 39–42 | 1.40 (1.23–1.58) |
| 37–38 | 1.14 (1.05–1.24) | |||
| D’Onofrio (2013)22 | 1–36 years | 23–27 | 37–42 | 2.9 (2.0–4.1)a |
| 28–30 | 2.3 (1.8–2.9)a | |||
| 31–33 | 2.1 (1.8–2.4)a | |||
| 34–36 | 1.6 (1.5–1.7)a | |||
| Crump (2019)5 | 20–29 years | <37 | 39–41 | Men: 1.32 (1.20–1.46); Women: 1.54 (1.31–1.82)a |
| 22–27 | Men: 1.45 (0.69–3.03); Women: 4.00 (1.90–8.41)a | |||
| 28–33 | Men: 1.40 (1.13–1.72); Women: 1.50 (1.04–2.17)a | |||
| 34–36 | Men: 1.30 (1.17–1.46); Women: 1.50 (1.24–1.81)a | |||
| 37–38 | Men: 1.15 (1.08–1.22); Women: 1.16 (1.04–1.30)a | |||
| Per additional week | Men: 0.97 (0.96–0.98); Women: 0.95 (0.93–0.97)a | |||
| 30–45 years | <37 | 39–41 | Men: 1.17 (1.01–1.34); Women: 1.55 (1.28–1.89)a | |
| 22–27 | Men: 1.53 (0.49–4.75); Women: 3.11 (1.00–9.65)a | |||
| 28–33 | Men: 1.15 (0.84–1.57); Women: 2.31 (1.61–3.29)a | |||
| 34–36 | Men: 1.17 (1.00–1.36); Women: 1.35 (1.07–1.70)a | |||
| 37–38 | Men: 1.15 (1.06–1.26); Women: 1.18 (1.04–1.35)a | |||
| Per additional week | Men: 0.98 (0.97–1.00); Women: 0.94 (0.92–0.97)a | |||
| Juarez (2016)21 | 5–29 years | <37 | 37–41 | 0.88 (0.58–1.34)a |
| 30–44 years | <37 | 0.88 (0.52–1.48)a | ||
| 45–59 years | <37 | 1.12 (0.86–1.47)a | ||
| 60–69 years | <37 | 1.19 (0.96–1.48)a | ||
| 70–79 years | <37 | 0.88 (0.73–1.06)a | ||
| ≥80 years | <37 | 1.17 (1.00–1.38)a | ||
| Norway | ||||
| Risnes (2016)20 | 15–45 years | 22–33 | 37–41 | 1.27 (1.09–1.47)a |
| 34–36 | 1.11 (1.02–1.20)a | |||
| Australia | ||||
| Srinivasjois (2017)23 | 6–30 years | 24–31 | 39–41 | 1.3 (0.8–2.3) |
| 32–34 | 1.4 (1.0–2.0) | |||
| 35–36 | 1.1 (0.9–1.4) | |||
| 37–38 | 1.0 (0.9–1.1) |
All HRs were reported to be similar in co-sibling analyses.
HR = hazard ratio
A subsequent study extended these findings by examining early term birth (37–38 weeks) in relation to long-term mortality in nearly the same cohort born in 1973–1979.19 In this study, the adjusted HRs for all-cause mortality at ages 18–36 years associated with preterm (<37 weeks) and early term birth were 1.40 (95% CI, 1.23–1.58) and 1.14 (1.05–1.24), respectively, compared with full-term birth (39–42 weeks).
Another Swedish cohort study included all singletons across additional birth years (1973–2008), which enabled further assessment of mortality particularly early in life in a larger sample size (N=3.3 million).22 This study examined a wide range of psychiatric, educational, and social outcomes in addition to all-cause mortality. However, mortality was assessed only in infancy (age <1 year) and at ages 1–36 years combined, but not in narrower age intervals specifically in adulthood. Adjusted HRs for mortality at ages 1–36 years associated with gestational ages 23–27 weeks, 28–30 weeks, 31–33 weeks, and 34–36 weeks were 2.9 (95% CI, 2.0–4.1), 2.3 (1.8–2.9), 2.1 (1.8–2.4), and 1.6 (1.5–1.7), respectively, compared with full-term birth (37–42 weeks). In addition, this was the first study to include a co-sibling design25, 26 to control for shared familial (genetic and/or environmental) factors. Co-sibling analyses revealed no attenuation of HRs compared with the primary analyses based on the entire population, suggesting that the observed associations were independent of shared familial factors that might predispose to both preterm birth and mortality.
The most recent and largest study of gestational age at birth in relation to mortality in adulthood included all singletons born in Sweden during 1973–2015 (N=4,296,814; maximum attained age 45 years).5 This cohort overlapped with the previously published Swedish studies but extended the birth years and follow-up time examined. Mortality in adulthood was assessed separately at ages 20–29 and 30–45 years. Adjusted HRs for mortality associated with preterm or extremely preterm birth were 1.54 (95% CI, 1.31–1.82) and 4.00 (1.90–8.41) at ages 20–29 years, and 1.55 (1.28–1.89) and 3.11 (1.00–9.65) at ages 30–45 years. In addition, a co-sibling analysis performed in all 3,562,267 individuals with at least one sibling (82.9% of the cohort) showed minimal change in risk estimates, suggesting that the observed associations were not due to shared genetic or environmental factors in families. Sensitivity analyses further showed that exclusion of deaths due to congenital anomalies, other perinatal conditions, or external causes had little effect on the overall findings.
Other studies of gestational age and all-cause mortality have been conducted in Norway and Australia. A Norwegian cohort study of 1,562,647 persons born in 1967–1997 examined mortality through 2011 (ages 15–45 years).20 The reported adjusted HRs for mortality associated with gestational ages 22–33 weeks and 34–36 weeks were 1.27 (95% CI, 1.09–1.47) and 1.11 (1.02–1.20), respectively, compared with full-term birth (37–41 weeks). A co-sibling analysis yielded similar risk estimates compared with the primary analyses, consistent with prior co-sibling analyses in Sweden.5, 22 An earlier Norwegian study of medical and social outcomes of preterm birth published survival curves up to age 36 years for gestational age subgroups, but did not report mortality risk estimates relative to full-term birth.27
An Australian cohort study included 722,399 singletons born in 1980–2010 in Western Australia and examined mortality in the neonatal period (0–28 days), postneonatal period (29–364 days), and ages 1–5 and 6–30 years, but not in narrower age intervals specifically in adulthood.23 Adjusted HRs for mortality at ages 6–30 years associated with gestational ages 24–31 weeks, 32–34 weeks, 35–36 weeks, and 37–38 weeks were 1.3 (95% CI, 0.8–2.3), 1.4 (1.0–2.0) 1.1 (0.9–1.1), and 1.0 (0.9–1.1), respectively, compared with full-term birth (39–41 weeks). Figure 1 summarizes the adjusted HRs for all-cause mortality associated with preterm vs. full-term birth in all cohorts born in 1967 or later.
Figure 1.
Adjusted hazard ratios and 95% CIs for all-cause mortality associated with preterm vs. full-term birth in cohorts born in 1967 or later (total Ns are reported in Table 1).
aPooled estimates from reported preterm subgroups.
All-Cause Mortality Among Persons Born in 1915–1929
Another Swedish study differs from the others by including a much earlier birth cohort from Uppsala, Sweden.21 This study included 12,564 persons born as singletons in 1915–1929 who were followed up for mortality through 2009. The attained age intervals examined were ages 5–29 years and additional 10–15 year increments up to ages ≥80 years. No associations between gestational age and mortality were found, except for a borderline-significant association at ages ≥80 years (preterm vs. full-term: adjusted HR, 1.17; 95% CI, 1.00–1.38). Co-sibling analyses also showed no substantial change in risk estimates compared with the primary analyses.
Sex-Specific Differences
Few studies have explored sex-specific differences in associations between gestational age at birth and adult mortality. In the largest Swedish cohort study, preterm birth was associated with increased mortality in adulthood among both men and women.5 The highest mortality rate across all ages examined (0–45 years) was among preterm-born males, which was significantly higher than among preterm-born females (adjusted HR 1.22, 95% CI 1.17–1.28; P<0.001). A positive additive interaction was found between preterm birth and male sex (i.e., the combined effect of these factors on mortality exceeded the sum of their separate effects; P<0.001), indicating that preterm birth accounted for significantly more deaths among males than females. In adulthood (ages 20–45 years), there was a positive additive interaction between early term (but not preterm) birth and male sex in relation to mortality (P=0.01).
The interaction between gestational age and sex was formally tested and reported in only 2 other studies. No evidence for multiplicative interaction was found in the Norwegian cohort (P=0.3),20 nor in the smaller Swedish cohort born in 1915–1929 (P>0.05).21 Additive interactions are less commonly examined but potentially more informative about public health impact, by indicating in which subgroup preterm birth may account for the most deaths.28, 29
Cause-Specific Mortality
Cause-specific mortality associated with gestational age at birth has been explored only in Swedish and Norwegian cohorts. In the largest Swedish study, gestational age at birth was inversely associated with all major causes of death (including respiratory, cardiovascular, endocrine, neurological, cancer, and external causes) across ages 0–45 years among males and females.5 Mortality from respiratory disorders, congenital anomalies, and other perinatal conditions predominated in infancy and childhood. However, associations with endocrine, cardiovascular, and neurological mortality were observed in young adulthood (ages 20–29 years), and strong associations with endocrine (mostly diabetes) and respiratory mortality extended further into adulthood (adjusted HRs at ages 30–45 years per additional week of gestation, endocrine: 0.85; 95% CI, 0.79–0.92; P<0.001; respiratory: 0.87; 95% CI, 0.80–0.95; P<0.001).
In the Norwegian cohort, the earliest gestational ages (<34 weeks) were associated with modestly increased mortality from external causes (adjusted HR, 1.20; 95% CI, 1.01–1.43), which appeared to involve both accidents/violence and suicide, and with non-significantly increased mortality from cancer (1.30; 0.97–1.93; based on only 25 cases) and cardiovascular disease (1.58; 0.94–2.64; 15 cases).20 Other potential causes of death were not reported.
One other study examined cause-specific mortality in 11,474 Swedish men and women born in 1915–1929 who were followed up to a maximum age of 86 years.8 This study reported that gestational age at birth was inversely associated with risk of death from cerebrovascular disease (P for linear trend = 0.03) and specifically occlusive stroke (P=0.02), but not ischemic heart disease (P=0.16). Other potential causes of death were not examined.
Secondary Analyses
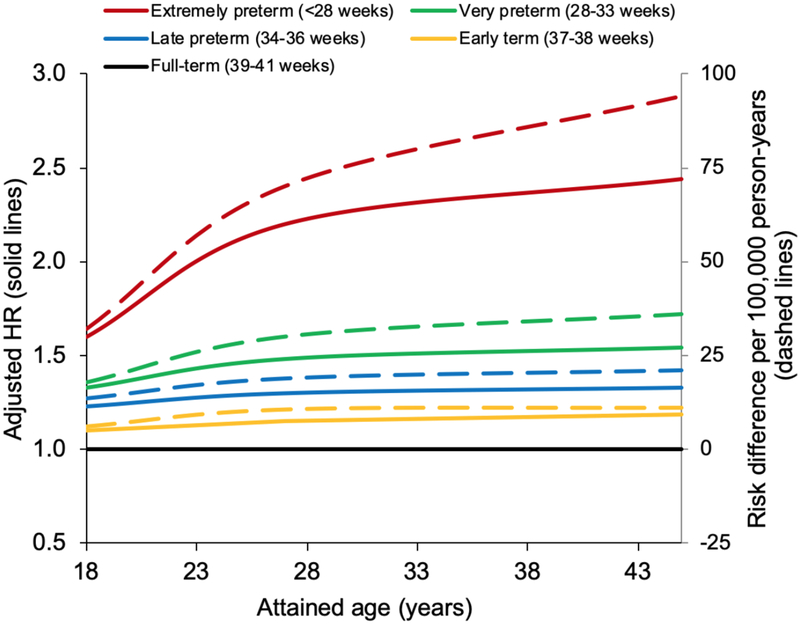
In post hoc analyses, attributable risks were estimated in the largest study to date from Sweden.5 Attributable risks of mortality at ages 18–45 years associated with gestational age at birth were estimated in a subcohort of 2,543,599 persons born in 1973–1997 who were still living in Sweden at age 18 years (Table 3). In this subcohort, each additional week of gestation was associated with a 4% lower risk of death between ages 18 and 45 years (adjusted HR, 0.96; 95% CI, 0.96–0.97). Preterm and extremely preterm birth accounted for an estimated 20.8 (95% CI, 16.4–25.1) and 52.8 (11.2–94.4) additional deaths per 100,000 person-years, respectively, compared with full-term birth (Table 3, risk differences). An estimated 29.7% and 51.8% of deaths at ages 18–45 years among persons born preterm or extremely preterm, and 2.6% and 0.1% of deaths in the entire population, were attributable to preterm or extremely preterm birth, respectively. For early term birth, the attributable fraction in the exposed was 13.9%, and the population attributable fraction was 3.0% (Table 3, AFe and PAF). Figure 2 shows the estimated hazard ratios and absolute risk differences for mortality at ages 18–45 years associated with gestational age subgroups compared with full-term birth.
Table 3.
Associations between gestational age at birth and all-cause mortality at ages 18–45 years, Sweden, 1973–2017.a
| Deaths | Rateb | Unadjusted | Adjustedc | Risk differenced (95% CI) | AFee | PAFf | ||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | P | ||||||
| Preterm (<37 wks) | 1,034 | 69.9 | 1.43 (1.34, 1.53) | 1.34 (1.25, 1.43) | <0.001 | 20.8 (16.4, 25.1) | 29.7% | 2.6% |
| Extremely preterm (22–27 wks) | 23 | 101.9 | 2.14 (1.42, 3.23) | 2.08 (1.38, 3.13) | <0.001 | 52.8 (11.2, 94.4) | 51.8% | 0.1% |
| Very preterm (28–33 wks) | 213 | 74.5 | 1.53 (1.34, 1.75) | 1.40 (1.22, 1.60) | <0.001 | 25.4 (15.4, 35.4) | 34.1% | 0.7% |
| Late preterm (34–36 wks) | 798 | 68.1 | 1.39 (1.30, 1.50) | 1.31 (1.22, 1.41) | <0.001 | 19.0 (14.2, 23.8) | 27.9% | 1.9% |
| Early term (37–38 wks) | 2,941 | 57.0 | 1.17 (1.13, 1.22) | 1.15 (1.10, 1.20) | <0.001 | 7.9 (5.7, 10.2) | 13.9% | 3.0% |
| Full-term (39–41 wks) | 10,805 | 49.1 | Reference | Reference | Reference | |||
| Post-term (≥42 wks) | 1,740 | 53.1 | 1.06 (1.01, 1.11) | 1.04 (0.98, 1.09) | 0.18 | 4.0 (1.4, 6.7) | 7.6% | 1.1% |
| Per additional week (trend) | 0.96 (0.95, 0.97) | 0.96 (0.96, 0.97) | <0.001 | |||||
Crump et al, Lancet Child Adolesc Health 2019; 3(6):408–17.
Mortality rate per 100,000 person-years.
Adjusted for birth year, sex, birth order, maternal age, maternal education, and maternal smoking.
Mortality rate difference per 100,000 person-years.
Attributable fraction among the exposed.
Population attributable fraction.
Figure 2.
Hazard ratios and absolute risk differences for all-cause mortality at ages 18–45 years by gestational age at birth compared with full-term birth, Sweden, 1973–2017.
DISCUSSION
This systematic review indicates that preterm and early term birth were associated with modestly increased mortality in adulthood among persons born in the last ~50 years. Most relative risks associated with preterm or early term birth were in the 1.2–1.6 and 1.1–1.2 ranges, respectively, compared with full-term birth. These associations appeared independent of sociodemographic, perinatal, and maternal factors, as well as unmeasured shared genetic and environmental factors in families that were controlled for using co-sibling analyses. The absolute mortality risks among adults born preterm were overall low, yet nearly one-third of their deaths were estimated to be attributable to preterm birth. These associations were observed among both men and women, but accounted for significantly more deaths among men.5 Based on the overall evidence, premature birth should be considered a chronic condition that is associated with modestly increased mortality risks in early to mid-adulthood.
Only one Swedish cohort included persons born in earlier years (1915–1929).21, 24 This cohort was the smallest of those reviewed (N=~12,000) but had the unusual opportunity to follow up the participants into older adulthood (maximum ages 80s-90s). The findings differed from those of other studies in that no clear association between preterm birth and all-cause mortality in adulthood was observed (despite a positive association with cerebrovascular disease mortality).21, 24 The null finding for all-cause mortality may potentially be related to survivor bias in this much earlier birth cohort. The most significant breakthroughs in neonatal care, including high-frequency ventilation, antenatal corticosteroids, and surfactant therapy, were introduced in the 1970s-1980s.2 Prior to these advances, preterm infant survival rates were much lower, and the strongest and healthiest infants who survived may be less susceptible to chronic disorders than more recent survivors who are now reaching adulthood in larger numbers.
The current evidence linking premature birth with increased mortality in adulthood is based on large registry-based studies in only 3 countries—Sweden, Norway, and Australia. These countries have birth registries that were established >40 years ago and are linkable with death records, thus enabling several decades of follow-up into adulthood. These registries are extraordinary resources for elucidating long-term health outcomes relevant to the US and many other countries where comparable data are not yet available.30 Population-based studies based on these registries help minimize selection biases that potentially affect convenience sampling of patients. In the Swedish studies reviewed,5, 6, 19, 21, 22, 24 gestational age at birth was based on maternal report of last menstrual period (LMP) before the 1980s, at which time ultrasonographic estimation was gradually adopted.31 Since the early 1990s, all pregnant women in Sweden are offered an ultrasound in the early second trimester, and >96% accept.31, 32 Gestational age was ascertained by LMP in the Norwegian study,20 and by ultrasound or LMP in the Australian study.23 Some evidence has suggested that gestational age is slightly overestimated by LMP compared with ultrasound,31 which may cause a slight conservative bias on risk estimates (i.e., toward the null hypothesis). In the reviewed studies, individual-level data on sociodemographic, perinatal, and maternal factors enabled extensive assessment and control for potential confounders. Family relationship data also have enabled co-sibling designs to control for unmeasured shared familial (genetic and/or environmental) factors. As a result of these rich population-based data, the overall rigor of evidence linking premature birth with increased mortality in early adulthood is strong.
The findings of this review are consistent with prior evidence linking low birth weight with increased long-term mortality. Birth weight has been studied more extensively than gestational age because it is easily measured in the delivery room and more consistently available in birth records, without requiring LMP or ultrasound data. However, birth weight is a composite measure determined by both gestational age and fetal growth rate. The largest meta-analysis to date of birth weight in relation to mortality in adulthood included 22 studies with 36,834 deaths in 394,062 persons, and reported that each additional 1 kg of birth weight was associated with a 6% lower all-cause mortality (adjusted HR, 0.94; 95% CI, 0.92–0.97).33 This finding was driven primarily by an inverse association with cardiovascular mortality (adjusted HR, 0.88; 95% CI, 0.85–0.91). In contrast, birth weight had a modest positive association with cancer mortality, especially among men (adjusted HR, 1.13; 95% CI, 1.07–1.19) compared with women (adjusted HR, 1.04; 95% CI, 0.98–1.10). Although gestational age and fetal growth are correlated and often difficult to disentangle, studies that have examined both factors have reported that low gestational age appeared independently associated with increased mortality in adulthood, with negligible change in risk estimates after adjusting for fetal growth.5, 6, 20
Analyses of cause-specific mortality in the reviewed studies suggested multiple contributing causes, including congenital anomalies; respiratory, cardiovascular, endocrine, and neurological disorders; cancer; and external causes.5, 6, 19, 20, 21, 24 Deaths from congenital anomalies, other perinatal conditions, and respiratory disorders predominated in infancy and early childhood. However, increased mortality from respiratory, cardiovascular, endocrine, and neurological disorders were found to persist into adulthood.5 These diverse findings are consistent with the known biologic effects of preterm birth on fetal and infant development. Preterm birth interrupts normal growth and maturation of all fetal organs, with differential effects depending on the specific gestational age and critical growth periods for different organ systems, thus increasing susceptibility to various disorders later in life.3, 4, 34, 35, 36, 37 Epigenetic changes related to intrauterine and early postnatal environment may also be involved in altering organ structure and metabolism, resulting in early-life programming for chronic disease.38, 39, 40, 41 Iatrogenic factors from intensive care, including suboptimal nutrition and adverse effects of medications or procedures, may further contribute to long-term adverse sequelae.4 Prior studies have linked preterm birth with higher risks of various comorbidities in adulthood, including chronic pulmonary disease,42, 43, 44, 45, 46, 47 hypertension,48, 49, 50, 51, 52 diabetes,53, 54, 55, 56, 57 lipid disorders,58, 59 metabolic syndrome,52, 60 infections,61 thyroid disorders,62, 63, 64 chronic kidney disease,65, 66 ischemic heart disease,67, 68 venous thromboembolism,69 sleep-disordered breathing,70, 71 epilepsy,72, 73 and neurocognitive and psychiatric disorders.74, 75, 76, 77, 78, 79, 80 In studies of comorbidities, individuals born prematurely may potentially be more likely to be diagnosed because of greater contact with the health care system (i.e., detection bias). However, detection bias is most likely to affect relatively asymptomatic conditions early in life, and does not affect mortality reporting. As a result, the overall evidence linking premature birth with increased adult mortality has high validity and suggests multiple important underlying causes.
Most people who were born preterm survive into adulthood without major comorbidities81 and report a high level of function and quality of life.3, 4, 7 However, a significant minority are at increased risk of chronic disorders and premature mortality in early to mid-adulthood. Preterm birth should now be recognized as a chronic condition that requires long-term follow-up for preventive actions, monitoring, and treatment of potential health sequelae across the life course.4, 5 Better awareness of the potential long-term risks is needed to incorporate current findings into clinical practice. Physicians currently seldom seek perinatal histories from adult patients.82, 83, 84 Medical records and initial history taking should routinely include gestational age at birth and other perinatal history to provide essential early-life context for understanding patients’ health.83, 84, 85 Such information can help trigger early preventive actions, patient counseling, and anticipatory screening to reduce long-term risks among patients of all ages who were born prematurely.4, 83, 84, 85
The reviewed studies have certain limitations that also indicate priorities for future research. First, they were limited to only 2 Scandinavian countries (Sweden and Norway) and Australia. Studies are needed in other geographic areas, including low- and middle-income countries, to assess mortality outcomes of preterm birth in other socioeconomic and health care contexts and explore for potential heterogeneity in racial or ethnic subgroups. Second, most registries in the reviewed studies lacked information on types of preterm birth (e.g., spontaneous or medically indicated) during the earliest birth years and hence for individuals with sufficient follow-up into adulthood. Studies with more complete data on preterm birth types and their underlying causes are a high priority to further elucidate mechanisms and improve long-term risk stratification. Third, studies with access to data on lifestyle risk factors later in life (e.g., physical inactivity, poor diet, obesity, smoking, alcohol use) would be useful to assess their potential modifying effects on long-term outcomes in persons born prematurely. Fourth, although co-sibling analyses have provided valuable assessments of shared familial factors, extensions of this approach to involve full and half siblings, adopted siblings, cousins, and potentially twins may be helpful to further examine the relative contributions of genetic and environmental factors. Further development of statistical methods for these designs is also needed. Large cohorts are essential for providing the requisite sample sizes and statistical power for such analyses. Lastly, because of ongoing changes in neonatal and pediatric care, it is unclear to what extent the current findings are generalizable to later birth cohorts. Studies of later cohorts as well as additional follow-up of existing cohorts to older ages will be needed when such data become available.
In summary, this review of 8 published studies found strong evidence for an association between preterm or early term birth and modestly increased mortality in adulthood among persons born in the last ~50 years. Multiple underlying causes are likely, including respiratory, cardiovascular, endocrine, and neurological disorders, consistent with the known effects of premature birth on the development of multiple organ systems. Premature birth should be recognized as a chronic condition that requires long-term follow-up to facilitate preventive actions, timely detection, and treatment of adverse health sequelae in adulthood. A high priority for future research is to elucidate protective factors that promote long-term resilience and well-being in persons born prematurely, especially those born at the earliest gestational ages.
ACKNOWLEDGMENTS
Funding:
This work was supported by the National Heart, Lung, and Blood Institute at the National Institutes of Health (R01 HL139536). The funding agency had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the writing of the manuscript or the decision to submit it for publication.
Footnotes
Conflicts of Interest:
We declare that we have no conflicts of interest.
REFERENCES
- 1.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019, 7(1): e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manley BJ, Doyle LW, Davies MW, Davis PG. Fifty years in neonatology. J Paediatr Child Health 2015, 51(1): 118–121. [DOI] [PubMed] [Google Scholar]
- 3.Raju TNK, Pemberton VL, Saigal S, Blaisdell CJ, Moxey-Mims M, Buist S, et al. Long-Term Healthcare Outcomes of Preterm Birth: An Executive Summary of a Conference Sponsored by the National Institutes of Health. J Pediatr 2017, 181: 309–318 e301. [DOI] [PubMed] [Google Scholar]
- 4.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr 2017, 106(9): 1409–1437. [DOI] [PubMed] [Google Scholar]
- 5.Crump C, Sundquist J, Winkleby MA, Sundquist K. Gestational age at birth and mortality from infancy into mid-adulthood: a national cohort study. Lancet Child Adolesc Health 2019, 3(6): 408–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crump C, Sundquist K, Sundquist J, Winkleby MA. Gestational age at birth and mortality in young adulthood. JAMA 2011, 306(11): 1233–1240. [DOI] [PubMed] [Google Scholar]
- 7.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371(9608): 261–269. [DOI] [PubMed] [Google Scholar]
- 8.Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller AB, et al. Born too soon: the global epidemiology of 15 million preterm births. Reprod Health 2013, 10 Suppl 1: S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz J, Lee AC, Kozuki N, Lawn JE, Cousens S, Blencowe H, et al. Mortality risk in preterm and small-for-gestational-age infants in low-income and middle-income countries: a pooled country analysis. Lancet 2013, 382(9890): 417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group E, Fellman V, Hellstrom-Westas L, Norman M, Westgren M, Kallen K, et al. One-year survival of extremely preterm infants after active perinatal care in Sweden. JAMA 2009, 301(21): 2225–2233. [DOI] [PubMed] [Google Scholar]
- 11.Ancel PY, Goffinet F, Group E-W, Kuhn P, Langer B, Matis J, et al. Survival and morbidity of preterm children born at 22 through 34 weeks’ gestation in France in 2011: results of the EPIPAGE-2 cohort study. JAMA Pediatr 2015, 169(3): 230–238. [DOI] [PubMed] [Google Scholar]
- 12.Swamy GK, Ostbye T, Skjaerven R. Association of preterm birth with long-term survival, reproduction, and next-generation preterm birth. JAMA 2008, 299(12): 1429–1436. [DOI] [PubMed] [Google Scholar]
- 13.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in Care Practices, Morbidity, and Mortality of Extremely Preterm Neonates, 1993–2012. JAMA 2015, 314(10): 1039–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patel RM, Kandefer S, Walsh MC, Bell EF, Carlo WA, Laptook AR, et al. Causes and timing of death in extremely premature infants from 2000 through 2011. N Engl J Med 2015, 372(4): 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Jesus LC, Pappas A, Shankaran S, Kendrick D, Das A, Higgins RD, et al. Risk factors for post-neonatal intensive care unit discharge mortality among extremely low birth weight infants. J Pediatr 2012, 161(1): 70–74 e71–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews TJ, MacDorman MF, Thoma ME. Infant Mortality Statistics From the 2013 Period Linked Birth/Infant Death Data Set. Natl Vital Stat Rep 2015, 64(9): 1–30. [PubMed] [Google Scholar]
- 17.Watkins WJ, Kotecha SJ, Kotecha S. All-Cause Mortality of Low Birthweight Infants in Infancy, Childhood, and Adolescence: Population Study of England and Wales. PLoS Med 2016, 13(5): e1002018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions (3rd ed.). Wiley: New York, 2003. [Google Scholar]
- 19.Crump C, Sundquist K, Winkleby MA, Sundquist J. Early-term birth (37–38 weeks) and mortality in young adulthood. Epidemiology 2013, 24(2): 270–276. [DOI] [PubMed] [Google Scholar]
- 20.Risnes KR, Pape K, Bjorngaard JH, Moster D, Bracken MB, Romundstad PR. Premature Adult Death in Individuals Born Preterm: A Sibling Comparison in a Prospective Nationwide Follow-Up Study. PLoS One 2016, 11(11): e0165051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Juarez S, Goodman A, De Stavola B, Koupil I. Birth characteristics and all-cause mortality: a sibling analysis using the Uppsala birth cohort multigenerational study. J Dev Orig Health Dis 2016, 7(4): 374–383. [DOI] [PubMed] [Google Scholar]
- 22.D’Onofrio BM, Class QA, Rickert ME, Larsson H, Langstrom N, Lichtenstein P. Preterm birth and mortality and morbidity: a population-based quasi-experimental study. JAMA Psychiatry 2013, 70(11): 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srinivasjois R, Nembhard W, Wong K, Bourke J, Pereira G, Leonard H. Risk of Mortality into Adulthood According to Gestational Age at Birth. J Pediatr 2017, 190: 185–191 e181. [DOI] [PubMed] [Google Scholar]
- 24.Koupil I, Leon DA, Lithell HO. Length of gestation is associated with mortality from cerebrovascular disease. J Epidemiol Community Health 2005, 59(6): 473–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donovan SJ, Susser E. Commentary: Advent of sibling designs. Int J Epidemiol 2011, 40(2): 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Onofrio BM, Lahey BB, Turkheimer E, Lichtenstein P. Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. Am J Public Health 2013, 103 Suppl 1: S46–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moster D, Lie RT, Markestad T. Long-term medical and social consequences of preterm birth. N Engl J Med 2008, 359(3): 262–273. [DOI] [PubMed] [Google Scholar]
- 28.Knol MJ, Egger M, Scott P, Geerlings MI, Vandenbroucke JP. When one depends on the other: reporting of interaction in case-control and cohort studies. Epidemiology 2009, 20(2): 161–166. [DOI] [PubMed] [Google Scholar]
- 29.Greenland S Interactions in epidemiology: relevance, identification, and estimation. Epidemiology 2009, 20(1): 14–17. [DOI] [PubMed] [Google Scholar]
- 30.Crump C, Sundquist K, Winkleby MA. Transnational research partnerships: leveraging big data to enhance US health. J Epidemiol Community Health 2015, 69(11): 1029–1030. [DOI] [PubMed] [Google Scholar]
- 31.Hogberg U, Larsson N. Early dating by ultrasound and perinatal outcome. A cohort study. Acta Obstet Gynecol Scand 1997, 76(10): 907–912. [DOI] [PubMed] [Google Scholar]
- 32.Swedish Agency for Health Technology Assessment and Assessment of Social Services. Routine ultrasound examination during pregnancy. 1998. [cited October 10, 2019] Available from: https://www.sbu.se/en/publications/sbu-assesses/routine-ultrasound-examination-during-pregnancy/ [Google Scholar]
- 33.Risnes KR, Vatten LJ, Baker JL, Jameson K, Sovio U, Kajantie E, et al. Birthweight and mortality in adulthood: a systematic review and meta-analysis. Int J Epidemiol 2011, 40(3): 647–661. [DOI] [PubMed] [Google Scholar]
- 34.Ingelfinger JR, Nuyt AM. Impact of fetal programming, birth weight, and infant feeding on later hypertension. J Clin Hypertens (Greenwich) 2012, 14(6): 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abitbol CL, Rodriguez MM. The long-term renal and cardiovascular consequences of prematurity. Nat Rev Nephrol 2012, 8(5): 265–274. [DOI] [PubMed] [Google Scholar]
- 36.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007, 357(19): 1946–1955. [DOI] [PubMed] [Google Scholar]
- 37.Urs R, Kotecha S, Hall GL, Simpson SJ. Persistent and progressive long-term lung disease in survivors of preterm birth. Paediatr Respir Rev 2018, 28: 87–94. [DOI] [PubMed] [Google Scholar]
- 38.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998, 95(2): 115–128. [PubMed] [Google Scholar]
- 39.Barker DJ. The origins of the developmental origins theory. J Intern Med 2007, 261(5): 412–417. [DOI] [PubMed] [Google Scholar]
- 40.Gillman MW, Barker D, Bier D, Cagampang F, Challis J, Fall C, et al. Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD). Pediatr Res 2007, 61(5 Pt 1): 625–629. [DOI] [PubMed] [Google Scholar]
- 41.Matthews SG, McGowan PO. Developmental programming of the HPA axis and related behaviours: epigenetic mechanisms. J Endocrinol 2019, 242(1): T69–T79. [DOI] [PubMed] [Google Scholar]
- 42.Bolton CE, Bush A, Hurst JR, Kotecha S, McGarvey L. Lung consequences in adults born prematurely. Thorax 2015, 70(6): 574–580. [DOI] [PubMed] [Google Scholar]
- 43.Vollsaeter M, Clemm HH, Satrell E, Eide GE, Roksund OD, Markestad T, et al. Adult respiratory outcomes of extreme preterm birth. A regional cohort study. Ann Am Thorac Soc 2015, 12(3): 313–322. [DOI] [PubMed] [Google Scholar]
- 44.Clemm HH, Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Exercise capacity after extremely preterm birth. Development from adolescence to adulthood. Ann Am Thorac Soc 2014, 11(4): 537–545. [DOI] [PubMed] [Google Scholar]
- 45.Vollsaeter M, Roksund OD, Eide GE, Markestad T, Halvorsen T. Lung function after preterm birth: development from mid-childhood to adulthood. Thorax 2013, 68(8): 767–776. [DOI] [PubMed] [Google Scholar]
- 46.Crump C, Winkleby MA, Sundquist J, Sundquist K. Risk of asthma in young adults who were born preterm: a Swedish national cohort study. Pediatrics 2011, 127(4): e913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saarenpaa HK, Tikanmaki M, Sipola-Leppanen M, Hovi P, Wehkalampi K, Siltanen M, et al. Lung Function in Very Low Birth Weight Adults. Pediatrics 2015, 136(4): 642–650. [DOI] [PubMed] [Google Scholar]
- 48.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 2012, 59(2): 226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of hypertension among young adults who were born preterm: a Swedish national study of 636,000 births. Am J Epidemiol 2011, 173(7): 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansson S, Iliadou A, Bergvall N, Tuvemo T, Norman M, Cnattingius S. Risk of high blood pressure among young men increases with the degree of immaturity at birth. Circulation 2005, 112(22): 3430–3436. [DOI] [PubMed] [Google Scholar]
- 51.Hovi P, Vohr B, Ment LR, Doyle LW, McGarvey L, Morrison KM, et al. Blood Pressure in Young Adults Born at Very Low Birth Weight: Adults Born Preterm International Collaboration. Hypertension 2016, 68(4): 880–887. [DOI] [PubMed] [Google Scholar]
- 52.Parkinson JR, Hyde MJ, Gale C, Santhakumaran S, Modi N. Preterm birth and the metabolic syndrome in adult life: a systematic review and meta-analysis. Pediatrics 2013, 131(4): e1240–1263. [DOI] [PubMed] [Google Scholar]
- 53.Crump C, Sundquist J, Sundquist K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. Diabetologia (in press). [DOI] [PMC free article] [PubMed]
- 54.Kajantie E, Strang-Karlsson S, Hovi P, Wehkalampi K, Lahti J, Kaseva N, et al. Insulin sensitivity and secretory response in adults born preterm: the Helsinki Study of Very Low Birth Weight Adults. J Clin Endocrinol Metab 2015, 100(1): 244–250. [DOI] [PubMed] [Google Scholar]
- 55.Mathai S, Cutfield WS, Derraik JG, Dalziel SR, Harding JE, Robinson E, et al. Insulin sensitivity and beta-cell function in adults born preterm and their children. Diabetes 2012, 61(10): 2479–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crump C, Winkleby MA, Sundquist K, Sundquist J. Risk of diabetes among young adults born preterm in Sweden. Diabetes Care 2011, 34(5): 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofman PL, Regan F, Jackson WE, Jefferies C, Knight DB, Robinson EM, et al. Premature birth and later insulin resistance. N Engl J Med 2004, 351(21): 2179–2186. [DOI] [PubMed] [Google Scholar]
- 58.Crump C, Sundquist J, Sundquist K. Association of preterm birth with lipid disorders in early adulthood: A Swedish cohort study. PLoS Med 2019, 16(10): e1002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hovi P, Kajantie E, Soininen P, Kangas AJ, Jarvenpaa AL, Andersson S, et al. Lipoprotein subclass profiles in young adults born preterm at very low birth weight. Lipids Health Dis 2013, 12: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Markopoulou P, Papanikolaou E, Analytis A, Zoumakis E, Siahanidou T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J Pediatr 2019, 210: 69–80 e65. [DOI] [PubMed] [Google Scholar]
- 61.Crump C, Sundquist K, Sundquist J. Prematurity and mortality in childhood and early adulthood (reply). JAMA 2012, 307: 32–33. [DOI] [PubMed] [Google Scholar]
- 62.Crump C, Winkleby MA, Sundquist J, Sundquist K. Preterm birth and risk of medically treated hypothyroidism in young adulthood. Clin Endocrinol (Oxf) 2011, 75(2): 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Phillips DI, Osmond C, Baird J, Huckle A, Rees-Smith B. Is birthweight associated with thyroid autoimmunity? A study in twins. Thyroid 2002, 12(5): 377–380. [DOI] [PubMed] [Google Scholar]
- 64.Kajantie E, Phillips DI, Osmond C, Barker DJ, Forsen T, Eriksson JG. Spontaneous hypothyroidism in adult women is predicted by small body size at birth and during childhood. J Clin Endocrinol Metab 2006, 91(12): 4953–4956. [DOI] [PubMed] [Google Scholar]
- 65.Crump C, Sundquist J, Winkleby MA, Sundquist K. Preterm birth and risk of chronic kidney disease from childhood into mid-adulthood: national cohort study. BMJ 2019, 365: l1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ruggajo P, Skrunes R, Svarstad E, Skjaerven R, Reisaether AV, Vikse BE. Familial Factors, Low Birth Weight, and Development of ESRD: A Nationwide Registry Study. Am J Kidney Dis 2016, 67(4): 601–608. [DOI] [PubMed] [Google Scholar]
- 67.Crump C, Howell EA, Stroustrup A, McLaughlin MA, Sundquist J, Sundquist K. Association of Preterm Birth With Risk of Ischemic Heart Disease in Adulthood. JAMA Pediatr 2019, doi: 10.1001/jamapediatrics.2019.1327 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zoller B, Sundquist J, Sundquist K, Crump C. Perinatal risk factors for premature ischaemic heart disease in a Swedish national cohort. BMJ Open 2015, 5(6): e007308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zoller B, Li X, Sundquist J, Sundquist K, Crump C. Gestational age and risk of venous thromboembolism from birth through young adulthood. Pediatrics 2014, 134(2): e473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Crump C, Friberg D, Li X, Sundquist J, Sundquist K. Preterm birth and risk of sleep-disordered breathing from childhood into mid-adulthood. Int J Epidemiol 2019, pii: dyz075. doi: 10.1093/ije/dyz075 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paavonen EJ, Strang-Karlsson S, Raikkonen K, Heinonen K, Pesonen AK, Hovi P, et al. Very low birth weight increases risk for sleep-disordered breathing in young adulthood: the Helsinki Study of Very Low Birth Weight Adults. Pediatrics 2007, 120(4): 778–784. [DOI] [PubMed] [Google Scholar]
- 72.Crump C, Sundquist K, Winkleby MA, Sundquist J. Preterm birth and risk of epilepsy in Swedish adults. Neurology 2011, 77(14): 1376–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li W, Peng A, Deng S, Lai W, Qiu X, Zhang L, et al. Do premature and postterm birth increase the risk of epilepsy? An updated meta-analysis. Epilepsy Behav 2019, 97: 83–91. [DOI] [PubMed] [Google Scholar]
- 74.Nosarti C, Reichenberg A, Murray RM, Cnattingius S, Lambe MP, Yin L, et al. Preterm birth and psychiatric disorders in young adult life. Archives of general psychiatry 2012, 69(6): E1–8. [DOI] [PubMed] [Google Scholar]
- 75.Crump C, Winkleby MA, Sundquist K, Sundquist J. Preterm birth and psychiatric medication prescription in young adulthood: a Swedish national cohort study. Int J Epidemiol 2010, 39(6): 1522–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Monfils Gustafsson W, Josefsson A, Ekholm Selling K, Sydsjo G. Preterm birth or foetal growth impairment and psychiatric hospitalization in adolescence and early adulthood in a Swedish population-based birth cohort. Acta psychiatrica Scandinavica 2009, 119(1): 54–61. [DOI] [PubMed] [Google Scholar]
- 77.Hack M, Youngstrom EA, Cartar L, Schluchter M, Taylor HG, Flannery D, et al. Behavioral outcomes and evidence of psychopathology among very low birth weight infants at age 20 years. Pediatrics 2004, 114(4): 932–940. [DOI] [PubMed] [Google Scholar]
- 78.Lund LK, Vik T, Skranes J, Brubakk AM, Indredavik MS. Psychiatric morbidity in two low birth weight groups assessed by diagnostic interview in young adulthood. Acta Paediatr 2011, 100(4): 598–604. [DOI] [PubMed] [Google Scholar]
- 79.Burnett AC, Anderson PJ, Cheong J, Doyle LW, Davey CG, Wood SJ. Prevalence of psychiatric diagnoses in preterm and full-term children, adolescents and young adults: a meta-analysis. Psychological medicine 2011, 41(12): 2463–2474. [DOI] [PubMed] [Google Scholar]
- 80.de Jong M, Verhoeven M, van Baar AL. School outcome, cognitive functioning, and behaviour problems in moderate and late preterm children and adults: a review. Semin Fetal Neonatal Med 2012, 17(3): 163–169. [DOI] [PubMed] [Google Scholar]
- 81.Crump C, Winkleby MA, Sundquist J, Sundquist K. Prevalence of Survival Without Major Comorbidities Among Adults Born Prematurely. JAMA 2019, 322(16): 1580–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bolton CE, Bush A, Hurst JR, Kotecha S, McGarvey L, Stocks J, et al. Are early life factors considered when managing respiratory disease? A British Thoracic Society survey of current practice. Thorax 2012, 67(12): 1110. [DOI] [PubMed] [Google Scholar]
- 83.Crump C Medical history taking in adults should include questions about preterm birth. BMJ 2014, 349: g4860. [DOI] [PubMed] [Google Scholar]
- 84.Crump C Birth history is forever: implications for family medicine. J Am Board Fam Med 2015, 28(1): 121–123. [DOI] [PubMed] [Google Scholar]
- 85.Crump C, Sundquist K, Sundquist J. Adult outcomes of preterm birth. Prev Med 2016, 91: 400–401. [DOI] [PubMed] [Google Scholar]