Abstract
Southern Ocean waters are among the most vulnerable to ocean acidification. The projected increase in the CO2 level will cause changes in carbonate chemistry that are likely to be damaging to organisms inhabiting these waters. A meta‐analysis was undertaken to examine the vulnerability of Antarctic marine biota occupying waters south of 60°S to ocean acidification. This meta‐analysis showed that ocean acidification negatively affects autotrophic organisms, mainly phytoplankton, at CO2 levels above 1,000 μatm and invertebrates above 1,500 μatm, but positively affects bacterial abundance. The sensitivity of phytoplankton to ocean acidification was influenced by the experimental procedure used. Natural, mixed communities were more sensitive than single species in culture and showed a decline in chlorophyll a concentration, productivity, and photosynthetic health, as well as a shift in community composition at CO2 levels above 1,000 μatm. Invertebrates showed reduced fertilization rates and increased occurrence of larval abnormalities, as well as decreased calcification rates and increased shell dissolution with any increase in CO2 level above 1,500 μatm. Assessment of the vulnerability of fish and macroalgae to ocean acidification was limited by the number of studies available. Overall, this analysis indicates that many marine organisms in the Southern Ocean are likely to be susceptible to ocean acidification and thereby likely to change their contribution to ecosystem services in the future. Further studies are required to address the poor spatial coverage, lack of community or ecosystem‐level studies, and the largely unknown potential for organisms to acclimate and/or adapt to the changing conditions.
Keywords: bacteria, climate change, CO2, fish, invertebrates, macroalgae, pH, phytoplankton, Southern Ocean
Antarctic waters are among the most vulnerable in the world to ocean acidification due to their cold temperatures, naturally low levels of calcium carbonate and upwelling that brings deep CO2‐rich waters to the surface. This meta‐analysis demonstrates groups of Antarctic marine biota in waters south of 60°S have a range of tolerances to ocean acidification. Invertebrates and phytoplankton showed negative effects above 500 μatm and 1,000 μatm CO2, respectively, while bacteria appear tolerant to elevated CO2.
1. INTRODUCTION
Much of the anthropogenic atmospheric carbon dioxide (CO2) emissions is estimated to have been absorbed by the world's oceans leading to significant changes in seawater carbonate chemistry and reduced pH (Doney et al., 2012; Frölicher et al., 2015; Gattuso & Hansson, 2011; Gattuso & Lavigne, 2009; Le Quéré et al., 2018; Raven & Falkowski, 1999; Sabine et al., 2004). Polar waters are particularly vulnerable to ocean acidification due to the increased solubility of CO2 at lower temperatures, naturally low levels of calcium carbonate, and a lower buffering capacity (Byrne, Mecking, Feely, & Liu, 2010; Dore, Lukas, Sadler, Church, & Karl, 2009; Ishii et al., 2011; Lovenduski, Gruber, Doney, & Lima, 2007; Orr, Epitalon, & Gattuso, 2014; Orr et al., 2005). High levels of upwelling also occur in polar waters, bringing deep CO2‐rich waters to the surface (Doney, Fabry, Feely, & Kleypas, 2009; Fabry, McClintock, Mathis, & Grebmeier, 2009; Hauri, Friedrich, & Timmermann, 2016; Le Quéré et al., 2007; McNeil & Matear, 2008; Negrete‐García, Lovenduski, Hauri, Krumhardt, & Lauvset, 2019; Orr et al., 2005). In addition, coastal waters have increased levels of terrestrial freshwater input during summer, and lack of photosynthetic driven draw‐down of CO2 during the light‐limited winter months (Gibson & Trull, 1999; Gruber et al., 2012; Kapsenberg, Kelley, Shaw, Martz, & Hofmann, 2015; McNeil, Tagliabue, & Sweeney, 2010; Melzner et al., 2013; Roden, Shadwick, Tilbrook, & Trull, 2013). Thus, organisms living in the Southern Ocean and nearshore Antarctic waters are likely to be exposed to potentially damaging pH and calcium carbonate conditions earlier than elsewhere in the world's oceans.
The pH of Southern Ocean surface waters has already decreased by ≈0.1 pH units and is predicted to decrease by a further ≈0.3 units by 2100 under the IPCC RCP8.5 “business as usual” scenario, equating to surface water CO2 levels >1,000 μatm (Kawaguchi et al., 2013; McNeil & Matear, 2007, 2008). Coupled intercomparison projects (CMIP5) show that low latitude waters could experience a decrease in pH from pre‐industrial levels of 8.17–7.77 by 2100, or 880 to 930 μatm CO2 (Hartin, Bond‐Lamberty, Patel, & Mundra, 2016). Only under the lowest CO2 emissions scenario with stringent mitigation will these changes be avoided (IPCC, 2019). In addition, there is a large natural seasonal variability in pH. During winter, pH is reduced and the concentration of carbonate ions is naturally low in the Southern Ocean and nearshore Antarctic waters. In contrast, during summer, pH and carbonate ion concentrations are higher due to changes in seasonal mixing and biological productivity (Gibson & Trull, 1999; Kapsenberg et al., 2015; McNeil & Matear, 2007; McNeil et al., 2010; Roden et al., 2013). Future predictions of acidification indicate that winter surface waters south of 60°S could reach 1,000 μatm by 2100 when seasonal CO2 cycles are incorporated into model predictions, and areas of the Southern Ocean could experience undersaturation in aragonite by as early as 2030 under the IPCC RCP8.5 (Kawaguchi et al., 2013; McNeil & Matear, 2008; McNeil & Sasse, 2016). Aragonite undersaturation could occur in ≈30% of the Southern Ocean by 2060 and >70% by 2100 (Hauri et al., 2016). The duration of this surface undersaturation is predicted to increase from 1 to 6 months within the next 20 years (Hauri et al., 2016). Surface waters of the Southern Ocean are even predicted to become undersaturated in calcite (the more stable form of calcium carbonate) by 2095, which is several decades earlier than elsewhere in the world's oceans (Hauri et al., 2016; Negrete‐García et al., 2019). The implications of the decrease in the pH and early undersaturation could be significant for many organisms within the Southern Ocean (Wittmann & Pörtner, 2013). Thus, these waters have been suggested to serve as a bellweather for the impacts of ocean acidification globally (Fabry et al., 2009; Negrete‐García et al., 2019).
Meta‐analyses are a useful tool to synthesize the results of multiple studies, to provide meaningful comparisons to determine the overall state of knowledge in an area and highlight areas of uncertainty. A number of meta‐analyses have focused on the global effects of ocean acidification on marine organisms (i.e., Kroeker, Kordas, Crim, & Singh, 2010; Kroeker et al., 2013; Wittmann & Pörtner, 2013). These analyses have found that ocean acidification has a negative effect on the survival, calcification, growth, and reproduction of marine biota but with significant variation in sensitivity among taxonomic groups. All calcifying organisms except crustaceans had larger negative responses than noncalcifying organisms (Kroeker et al., 2010, 2013). Larval and early life stages are also highly sensitive, particularly in mollusks (Kroeker et al., 2013). No meta‐analysis has yet been conducted to specifically examine the effects of ocean acidification on marine organisms in the Southern Ocean and nearshore Antarctic waters, despite the high vulnerability of these regions.
This study presents a comprehensive meta‐analysis on the effect of altered seawater carbonate chemistry on marine organisms south of 60°S. It aims to provide a synthesis of current understanding in the area and highlights knowledge gaps and future research directions. As part of this meta‐analysis, this study investigates whether there are tipping points in the response of marine organisms to ocean acidification and therefore predict at what future CO2 concentration and time organisms inhabiting the Southern Ocean and nearshore Antarctic ecosystems may be significantly affected by ocean acidification.
2. METHODS
2.1. Data selection and suitability criteria
Comprehensive database searches were conducted to compile all English language, peer‐reviewed journal articles and literature reviews that investigated the effect of altered seawater carbonate chemistry on Southern Ocean and/or Antarctic marine organisms. Searches were conducted using the ISI Web of Science [v.5.27.2], Scopus, and ASFA (Aquatic Sciences and Fisheries Abstracts) databases using the search terms given in Table S1 (all searches were completed by the June 2019). The OA‐ICC bibliographic database (https://www.iaea.org/ocean-acidification/) was also searched along with literature cited in bibliographies of all the articles and reviews identified in the literature search. Articles were then screened to only include studies that manipulated the carbonate chemistry to investigate the effects of ocean acidification on marine organisms collected, or experiments conducted south of 60°S. Some studies included a number of species and/or locations, some of which were in the Arctic or north of 60°S. For these, only data from south of 60°S were included in the meta‐analysis and each species was included separately. After this manual screening, 64 papers remained for inclusion in the meta‐analysis. For studies that included more than one experiment or species, each individual experiment/species was included in the analysis. A number of studies investigated the effects of multiple stressors (i.e., ocean acidification and warming). The data associated with the effect of ocean acidification for these studies were included only if all other stressors were at ambient levels. For example, Torstensson, Hedblom, Björk, Chierici, and Wulff (2015) investigated the effect of elevated CO2 and temperature on an Antarctic diatom, and for this study, only the ambient temperature treatments were used. Any studies that did not have a treatment approximate to ambient levels for the costressor were not included in the meta‐analysis, such as those that investigated the effects of elevated CO2 and temperature but included no ambient temperature treatment. Only studies conducted over weeks (up to month) were included in the analysis, and longer experiments studying chronic acclimation and/or adaptation responses of organisms to ocean acidification were excluded (only three papers, Trimborn, Thoms, Petrou, Kranz, & Rost, 2014 and Torstensson et al., 2015 both investigating long‐term effects of elevated CO2 on phytoplankton cultures, and Ericson et al., 2018 investigating the long‐term effect of ocean acidification on Antarctic krill). Screening for experimental procedure used within the studies was not performed due to the limited number of studies available to include in the meta‐analysis.
2.2. CO2 treatment levels and organism responses
The main aim of this study was to determine tolerance of Antarctic marine organisms to increased CO2 and if there were tipping points in their response, therefore analyses were conducted over a range of CO2 treatments above ambient. Ambient was identified as the CO2 treatment reported in the study as being either the “control” or “ambient” (all ambient levels were below ≈500 μatm). The CO2 treatment levels used in studies were separated into the following CO2 categories for the meta‐analysis: pre‐industrial (found in phytoplankton studies only), 500–800, 801–1,000, 1,001–1,500, 1,501–2,000, and >2,000 μatm. If studies reported the level of CO2 in units other than μatm, or if pH was reported, then the CO2 levels were converted into μatm of CO2 using CO2Sys v2.1.xls (Pierrot, Lewis, & Wallace, 2006). The data required for the conversion were reported within all papers except for two, and these were removed from the meta‐analysis as the corresponding author for these papers could not be contacted. If a study did not specify an ambient/control level, then the CO2 treatment closest to the ambient level at time of sample collection was used. Any studies using an ambient CO2 treatment which substantially exceeded 500 μatm were excluded from the meta‐analysis. If a study included multiple treatments in one CO2 category, then the highest treatment was used in the meta‐analysis, except for the pre‐industrial CO2 category in which the lowest was used. Study mean, standard error, and sample size for each experiment/species, biological response (described below), and CO2 category were used in the meta‐analysis. Data reported in graphs were extracted using the software WebPlotDigitizer v1.7.9 (Rohatgi). A number of studies reported biological response data in boxplots with medians and interquartile ranges rather than reporting the mean, standard error, and sample size. These were converted using the method of Luo, Wan, Liu, and Tong (2018a) for the estimation of mean and Luo, Wan, Liu, and Tong (2018b) for estimating the standard error. The equations were as follows:
The final number of studies included in the meta‐analysis was 60 after manual screening and removal of studies that did not meet the above criteria (full list in Document S1).
Data for several taxonomic groups were included in the analysis, including prokaryotes; bacteria, autotrophic eukaryotes; phytoplankton and macroalgae, and heterotrophic eukaryotes; invertebrates and fish (Figures S1–S5). The invertebrates included amphipods (Arthropoda; Crustacea; Amphiphoda), krill (Arthropoda; Crustaceae; Euphausiacea), brachiopods (Brachiopoda), sea stars (Echinodermata; Asteroidea), sea urchins (Echinodermata; Echinoidea), bivalves (Mollusca; Bivalvia), pteropods (Mollusca; Gastropoda; Orthogastropoda; Opisthobranchia; Pteropoda), limpets (Mollusca; Gastropoda; Patellogastropoda), sea snails (Mollusca; Gastropoda; Orthogastropoda; Mesogastropoda; Trochidae), and nemertean worms (Nemertea). The effects of ocean acidification on different biological responses from each taxonomic group were investigated (Table 1). While most studies were conducted on a single species, a number of phytoplankton studies used natural or mixed‐species communities. These multispecies studies were included in the overall meta‐analysis. Further analyses were then conducted separating single species and multispecies phytoplankton studies to compare the difference in responses.
TABLE 1.
Biological responses used for each taxonomic group in the meta‐analysis
| Taxonomic group | Biological response |
|---|---|
| Prokaryotes | |
| Bacteria | Abundance |
| Productivity | |
| Autotrophic eukaryotes | |
| Phytoplankton | Chlorophyll a concentration (Chl a) |
| Net primary productivity (NPP) | |
| Gross primary productivity (GPP) | |
| Effective quantum yield (F v/F m) | |
| Relative electron transport rate (rETR) | |
| Extracellular carbonic anhydrase activity (eCA) | |
| Total fatty acid content (fatty acids) | |
| Particulate organic carbon concentration (POC) | |
| Growth rate a | |
| Macroalgae | Chlorophyll a concentration (Chl a) |
| Effective quantum yield (F v/F m) | |
| Relative electron transport rate (rETR) | |
| Protein content (protein) | |
| Growth rate | |
| Heterotrophic eukaryotes | |
| Invertebrates | Development (fertilization rate or normal development |
| Shell state (adult calcification or dissolution rate) | |
| Growth rate (in adults) | |
| Survival (in adults) | |
| Behavior (adult maximal escape speeds) | |
| Feeding (adult consumption rate or energy absorbed) | |
| Fish | Survival (of embryos) |
| Oxygen (O2) consumption rates (in adults) | |
| Cellular aerobic potential, measured by citrate synthase enzyme activity in adults (CS) | |
| Growth rate (in adults) | |
| Heart rate (in adults) | |
If cell concentration was reported, then growth rate was calculated using (Pearce et al., 2007).
2.3. Data analysis
The data were analyzed following the methods of Kroeker et al. (2010) and Kroeker et al. (2013). For each biological response in each CO2 category, the ln‐transformed response ratio to ocean acidification was calculated for each study (or experiment/species within a study) using the formula:
where and are the mean value for the biological response in the CO2 experimental treatment and the ambient (control) treatment, respectively (Hedges, Gurevitch, & Curtis, 1999). The log response ratio (LnRR) was used due to its robustness to small sample sizes and its ability to detect true effects (Lajeunesse & Forbes, 2003). For all means, an increase in the mean is a positive response; that is, increased growth rate between control and experimental treatments is a positive response. If this was not true, that is, increase in dissolution of shells is a negative response, the mean was made negative. Thus, a positive response ratio represents a positive response of the organisms to ocean acidification, and a negative response ratio represents a negative effect of ocean acidification. The larger the response ratio (either positive or negative), the stronger the response and the effect of ocean acidification.
These response ratios (LnRR) were then weighted by the inverse of the effect size variance (v), calculated by their associated standard error and sample size using:
where and are the standard error for the experimental treatment mean and ambient (control) treatment mean, respectively, and n E and n C are the sample sizes for the experimental mean and ambient (control) mean, respectively (Hedges & Olkin, 1985). As above and are the mean value for the biological response in the CO2 experimental treatment and the ambient (control) treatment, respectively. This weighting ensured that studies with a lower standard error and higher sample size (data with greater certainty) were weighted more heavily in the analysis than those with higher standard error and lower sample size (lower certainty). The meta‐analysis was conducted on both weighted and unweighted response ratios, and there was no significant difference between the results; therefore, like Kroeker et al. (2010) and Kroeker et al. (2013), weighted analyses are presented.
Statistical analyses were conducted using the R v.1.0.136 (R Core Team, 2016) and the add‐on package “Metafor” (Viechtbauer, 2010). A random‐effects model was used to estimate a summary response ratio for each biological response in each CO2 category, weighted by the inverse‐variance weights (v). A random‐effects model was used as it accounts for both random sampling variation in each study and the variation among studies in estimating the effect size. In this model, the true variation in effect size is calculated by the between‐study variance (using the ln‐transformed response ratios, LnRR), with each study weighted by the inverse sum of the individual study variance (v). Restricted maximum‐likelihood estimator (REML) was used to estimate the amount of (residual) heterogeneity (Q), which tests whether the variability in the effect size is larger than would be expected based on sampling variance alone (Brown & Kempton, 1994). Statistical significance of the mean response ratio was based on the 95% confidence interval on the estimated summary response ratio (Viechtbauer, 2010).
Based on the availability of studies, organisms were assigned to functional groups at increasing levels of taxonomic resolution and the summary response ratios of each of these groups were determined for each CO2 category. The groups were:
Prokaryotes (bacteria), eukaryotic autotrophs (phytoplankton and macroalgae), and eukaryotic heterotrophs (invertebrates and fish), with all biological responses combined.
Bacteria, phytoplankton, macroalgae, invertebrates, and fish, with all biological responses combined.
Bacteria, phytoplankton, and invertebrates for each biological response. Due to the lack of studies, macroalgae and fish biological responses were investigated with all CO2 categories combined and for each macroalgal species with all biological responses and CO2 categories combined.
Single species‐ versus community‐level biological responses of phytoplankton.
3. RESULTS
Sixty papers on the effect of altered carbonate chemistry on marine organisms south of 60°S were identified that met all the required selection criteria for inclusion in the meta‐analysis (list of papers included in Document S1). The majority of these studies were conducted in nearshore Antarctic waters, with only four offshore, open Southern Ocean waters studies (Figure 1). It is also apparent that most studies were conducted in areas surrounding the Antarctic Peninsula (particularly King George Island), the Ross Sea and Prydz Bay, East Antarctica with few studies in the oligotrophic waters of the Southern Ocean.
FIGURE 1.
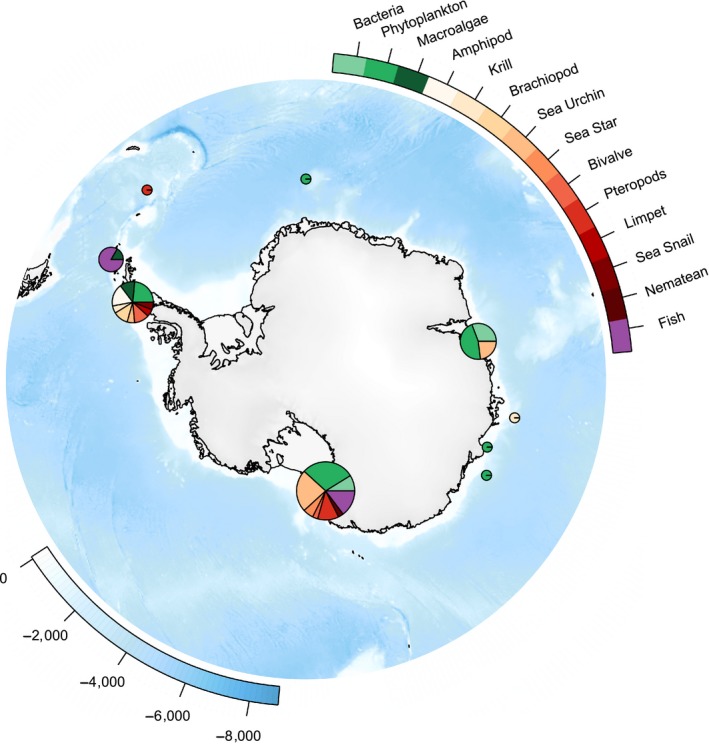
All studies on the effect of ocean acidification on marine organisms south of 60°S included in the meta‐analysis. Pie charts display the relative number of studies per organism at that location. The size of the pie reflects the total number of studies at that location. Figure created using the R package “SOmap” (Maschette, Sumner, & Raymond, 2018)
A total of 377 response ratios were calculated from the 60 studies included in the meta‐analysis (with a number of articles reporting results of multiple experiments and numerous biological responses). Of these response ratios, 40 were for prokaryotes (bacteria) and 337 for eukaryotic organisms. Of the eukaryotic response ratios, 257 were for autotrophic organisms (phytoplankton and macroalgae) and 80 for heterotrophic organisms (invertebrates and fish). Within these groups, there was significant heterogeneity (p < .001), indicating that variability between studies is greater than expected by chance alone (Tables S2–S6). Despite this, there are some clear trends in the response of Antarctic marine organisms to increased CO2 (Figure 2). Autotrophs showed a significant negative response to ocean acidification at CO2 levels >1,000 μatm, whereas in heterotrophs, the response is mainly negative above 1,500 μatm CO2. In contrast, the prokaryotes show an increasingly positive response with increasing CO2 (Figure 2).
FIGURE 2.
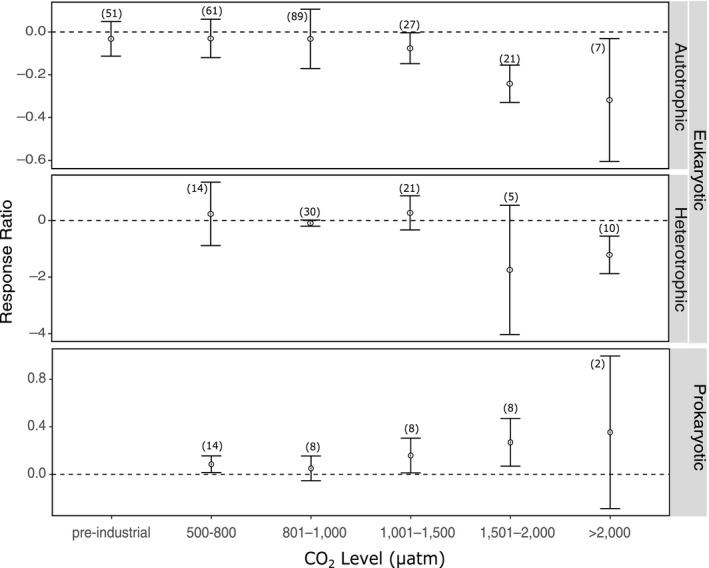
The effect of ocean acidification on heterotrophic and autotrophic eukaryotes, and prokaryotic organisms at different CO2 levels. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category given in brackets. A mean response ratio of zero (hashed line) indicates no effect
The eukaryotic responses were subdivided to determine the effect of ocean acidification on phytoplankton, invertebrates, fish, and macroalgae (Figure 3). Significant heterogeneity was observed in each of these groups (Tables S3–S6). Nevertheless, responses to ocean acidification were significant in all groups. A total of 243 of the 257 autotrophic response ratios were for phytoplankton with only 3 studies conducted on macroalgae (from which 14 response ratios were calculated). For phytoplankton, including all the data and no a priori defined groups, there was significant heterogeneity between studies in their response to increased CO2 (Q = 221,529.7, df = 242, p < .001, Table S3). The response ratios showed an overall negative response to CO2 levels >1,000 μatm (Figure 3) but still significant heterogeneity at all CO2 levels (p < .0001, Table S3). For macroalgae, only 14 response ratios were calculated from three studies, with significant heterogeneity across the mean response of macroalgae both with all response ratios together, as well as when separated by CO2 level (Table S4). Despite this, the response in the 801–1,000 μatm CO2 category was mainly negative but the effect of CO2 in the 1,001–1,500 μatm level was less clear (Figure 3).
FIGURE 3.
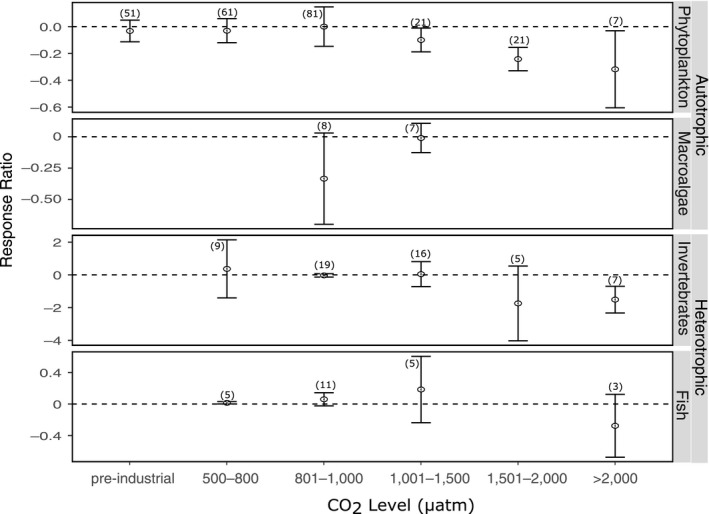
The effect of ocean acidification on phytoplankton, macroalgae, invertebrates, and fish at different CO2 levels. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category given in brackets. A mean response ratio of zero (hashed line) indicates no effect
The response of heterotrophic eukaryotic organisms (invertebrates and fish) to CO2 was negative in CO2 treatments exceeding 1,500 μatm. For invertebrates, when these response ratios were combined, irrespective of the species studied or the response measured, their response to increased CO2 was mainly negative (Figure 3). Significant heterogeneity was observed within each CO2 category (p < .0001 for each CO2 category, Table S5). The response is less clear in fish, where the response ratios are based on a total of 8 studies from which 24 response ratios were calculated.
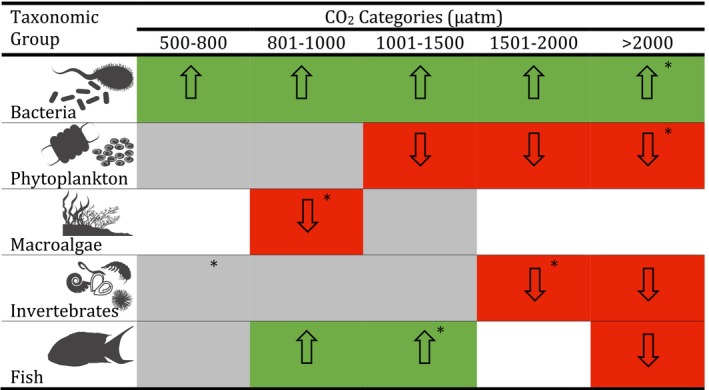
Groups of marine organisms differ greatly in their sensitivity to increased CO2 (Table 2). Bacteria are positively affected irrespective of the CO2 concentration. The few studies of fish also suggest that they are positively affected by CO2 concentrations <1,500 μatm and negatively affected >2,000 μatm, while their responses between 1,500 and 2,000 μatm are currently unknown. In contrast, phytoplankton, macroalgae, and invertebrates are all negatively affected. Invertebrates are the most sensitive to ocean acidification of the taxonomic groups examined, with a negative response to any increase in CO2 concentrations exceeding current levels in Antarctic waters (>500 μatm). Phytoplankton, although less sensitive, were negatively affected at CO2 levels above 1,000 μatm. The few studies on macroalgae provide some indication that they may be negatively affected, but the variation around the response is large and further data are required to gain a more conclusive response.
TABLE 2.
The effect of ocean acidification on bacteria, phytoplankton, macroalgae, invertebrates, and fish at different CO2 levels. Responses are either positive (green), negative (red), no effect (gray), or no data (blank). Responses based on few data points with consequently high variance are marked with *
Few studies have been conducted on the effects of ocean acidification on bacteria in Antarctic and Southern Ocean waters; however, these few provide growing evidence that bacterial communities may also be affected. Four studies met the selection criteria for the meta‐analysis; Maas et al. (2013), Thomson, Davidson, and Maher (2016), Deppeler et al. (2018) and Westwood et al. (2018). These studies reported results that allowed 40 response ratios to be calculated. There is high heterogeneity in the response of bacteria to increased CO2 when these are combined, separated by CO2 level and/or biological response (p < .05, Table S2). Despite the significant heterogeneity, clear, positive response to increased CO2 was found for bacteria (Figure 2), particularly the increase in abundance with increasing CO2 (Figure 4). This trend is less clear for productivity (Figure 4).
FIGURE 4.
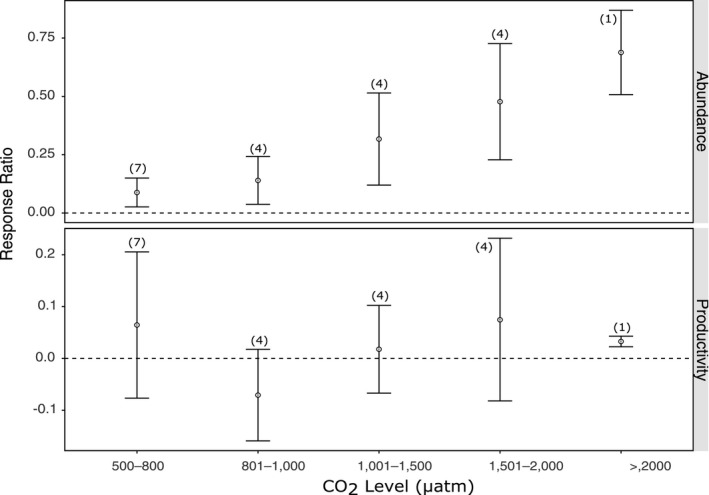
The effect of ocean acidification on the biological responses of bacteria at different CO2 levels. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category in brackets. A mean response ratio of zero (hashed line) indicates no effect
3.1. Phytoplankton
A range of biological responses have been used to quantify the effects of ocean acidification on phytoplankton with the nature and magnitude of the effect differing among biological responses (Figure 5). A negative effect was found on gross primary productivity (GPP), the activity of extracellular carbonic anhydrase (eCA), and the concentration of particulate organic carbon (POC) at CO2 levels above 500 μatm and above 801–1,000 μatm for POC concentration. The concentration of chlorophyll a was mainly positively affected by moderate increases in CO2 (≤1,000 μatm) but above this level the effect was increasingly negative. A clear response could not be discerned for growth rate, F v/F m or rETR with variability in their response to altered CO2 concentrations. Although significant responses were found at a number of CO2 levels, there was still significant heterogeneity at all CO2 levels for both chlorophyll a concentration and growth rates. For example, the response ratio of growth rate at pre‐industrial levels is significantly negative (p = .0417) but there was also significant heterogeneity (Q = 90.511, df = 13, p < .001, Table S3), indicating that there is significant variation in the magnitude that growth rate was reduced in studies. The only biological responses in which the heterogeneity was not significant when separated by CO2 level were gross primary productivity at <1,000 μatm, F v/F m at all levels except 500–800 and 801–1,000 μatm, POC concentration at 1501–2,000 μatm, fatty acid content at 500–800 μatm, and eCA activity at all levels. All other biological responses other CO2 levels had significant heterogeneity (Table S3).
FIGURE 5.
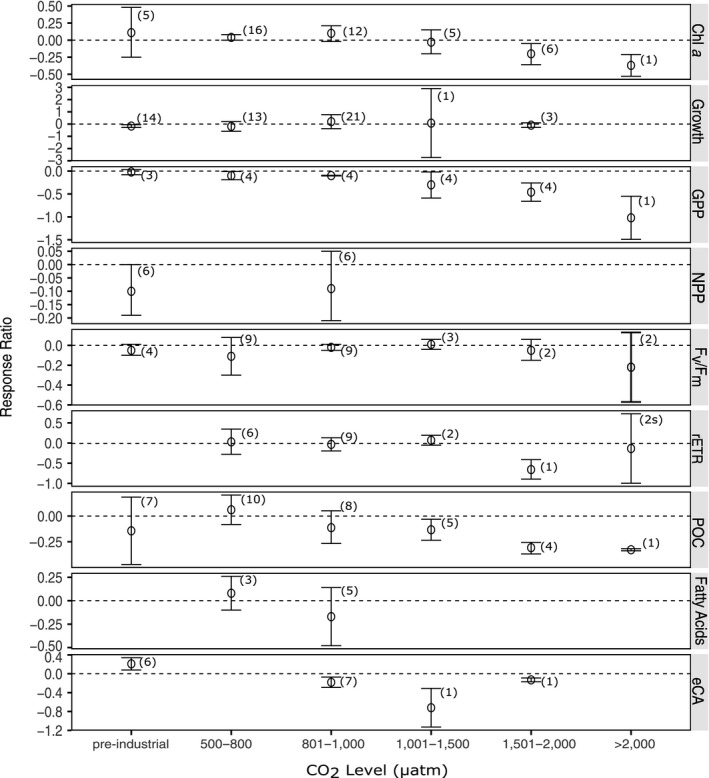
The effect of ocean acidification on the biological responses of phytoplankton to increased CO2 levels. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category in brackets. A mean response ratio of zero (hashed line) indicates no effect. Refer to Table 1 for abbreviation definitions
Responses of phytoplankton were separated depending on the experimental procedure comparing studies on a single species in culture to those performed on natural, mixed communities (Figure 6). Biological responses to ocean acidification differed between the two types of studies, and while often the results were highly variable, a summary table of thresholds levels of CO2 above which negative effects are observed showed that phytoplankton communities are more sensitive to increased CO2 than phytoplankton studied as a single species in a culture (Figure 6 and Table 3). For example, negative effects are observed in phytoplankton community F v/F m and POC while the single‐species studies results did not show a clear response (Table 3). In addition, gross primary productivity of phytoplankton community was negatively affected at CO2 levels above 500 μatm, noting that this biological measurement is not in single‐species, culture studies (Table 3).
FIGURE 6.
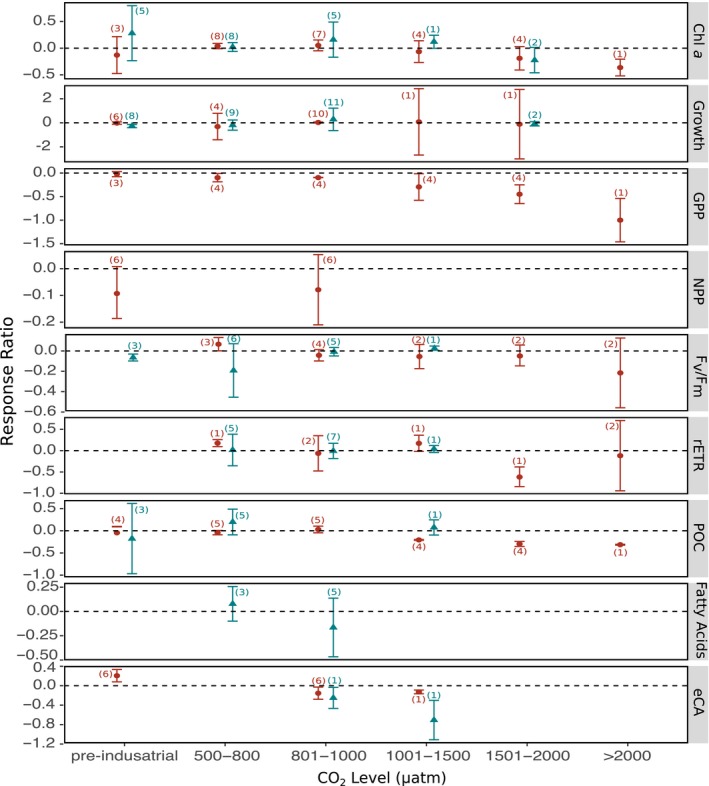
The effect of ocean acidification on the biological responses of phytoplankton to increased CO2 levels, separating studies on single species (shown in blue triangles) from community‐level studies (shown in red circles). Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category in brackets. A mean response ratio of zero (hashed line) indicates no effect. Refer to Table 1 for abbreviation definitions
TABLE 3.
Threshold levels of CO2 (μatm) for a range of biological responses for phytoplankton separating results of studies on single species from those at a community level. Responses are either negative (red), no effect (gray), or uncertain/no data (blank)
| Biological response | Single species | Community |
|---|---|---|
| Chlorophyll a | 1,500 | 1,500 |
| Growth rate | No effect | No effect |
| Gross primary productivity | 500 | |
| Net primary productivity | Few studies | |
| F v/F m | No effect | Highly variable |
| rETR | No effect | Highly variable |
| POC concentration | No effect | 1,000 |
| Fatty acid content | High variable | |
| eCA activity | 800 | 800 |
3.2. Macroalgae
Only three studies have investigated the effect of ocean acidification on macroalgae south of 60°S (Iniguez, Heinrich, Harms, & Gordillo, 2017; Schoenrock et al., 2016; Schram, Schoenrock, McClintock, Amsler, & Angus, 2017) and most of which employed CO2 treatment levels between 800 and 1,000 μatm. Due to the small data set available, all CO2 treatment levels were combined and data compared among biological responses (Figure 7) and species (Figure 8). While the heterogeneity is significant in the response of macroalgae to increased CO2 (Table S4), it appears that rETR is the most sensitive measure in macroalgae to increased CO2 (Figure 7). The concentration of chlorophyll a was also negatively affected by increased CO2, but there is high variance around this mean response. Comparing among species, heterogeneity was again high (Table S4). The crustose algal species, Clathromorphyum obtectulum and Hildenbrandia sp., appear to be more sensitive than fleshy species, Desmarestia anceps and D. menzieseii despite C. obtectulum and Hildenbrandia sp. being investigated at a lower CO2 level (801–1,000 μatm) than D. anceps and D. menzieseii (1,001–1,500 μatm).
FIGURE 7.
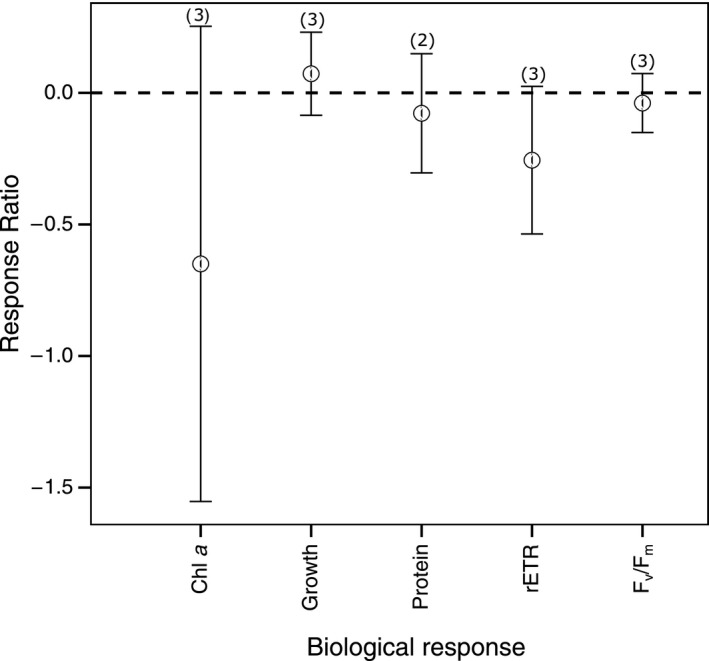
The effect of ocean acidification on the biological responses of macroalgae. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category in brackets. A mean response ratio of zero (hashed line) indicates no effect. Refer to Table 1 for abbreviation definitions
FIGURE 8.
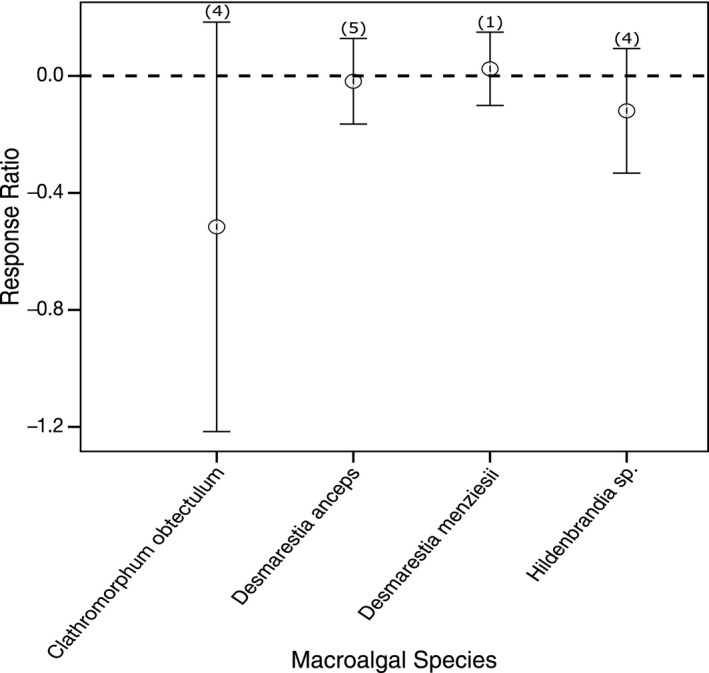
The effect of ocean acidification on species of macroalgae. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category in brackets. A mean response ratio of zero (hashed line) indicates no effect
3.3. Invertebrates
For invertebrates, developmental stages, shell state, and survival of adults were found to be sensitive to increased CO2 (Figure 9). Larval life stages showed reduced normal development and fertilization rate with increasing CO2 >1,500 μatm. In adults, elevated CO2 reduced survival, calcium carbonate production rates, and increased rates of dissolution and shell damage in those species with calcium carbonate shells (Figure 9). Although based on few studies, other measurements such as growth rate, behavior, and feeding appeared to be unaffected by increased CO2 (Figure 9). However, there was significant heterogeneity in most biological responses when separated by CO2 level and with all CO2 levels pooled (Table S5). The only exceptions to this were growth rate and lipid/protein content (Q = 1.496, df = 5, p = .914 and Q = 0.416, df = 1, p = .519, respectively), but the lipid/protein results are derived from a single study only (Schram, Schoenrock, McClintock, Amsler, & Angus, 2016).
FIGURE 9.
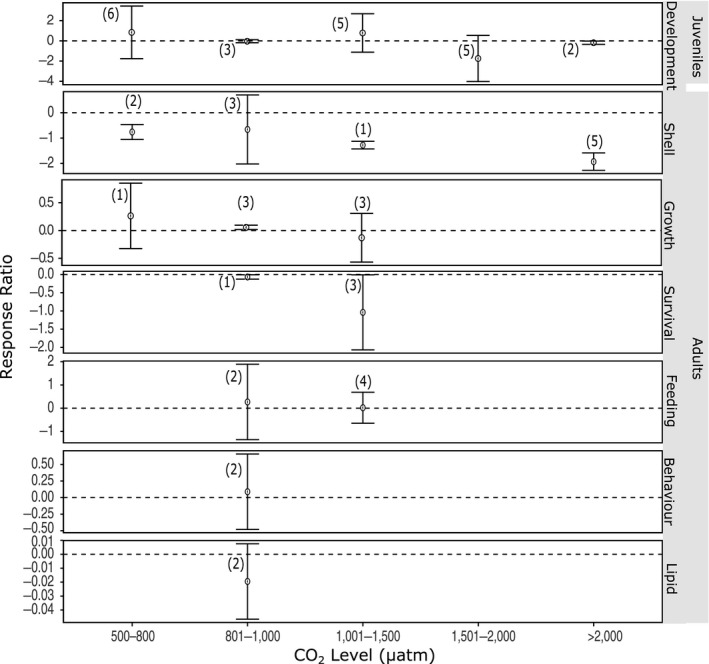
The effect of ocean acidification on the biological responses of invertebrates to increased CO2 levels. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category in level. A mean response ratio of zero (hashed line) indicates no effect. Refer to Table 1 for abbreviation definitions
3.4. Fish
The effect of ocean acidification on Antarctic and Southern Ocean fish has been little studied, with only 8 studies and a total of 24 responses available for this meta‐analysis (Davis et al., 2018; Davis, Miller, Flynn, & Todgham, 2016; Enzor, Hunter, & Place, 2017; Enzor, Zippay, & Place, 2013; Flynn, Bjelde, Miller, & Todgham, 2015; Strobel et al., 2012; Strobel, Graeve, Poertner, & Mark, 2013; Strobel, Leo, Pörtner, & Mark, 2013). While not ideal, due to the lack of data, all the CO2 treatment levels were combined and the results compared among biological responses, namely embryonic survival (development), cellular aerobic potential measured by citrate synthase enzyme activity in adults (CS), growth rate, heart rate, and oxygen (O2) consumption rate (Figure 10). There was no obvious difference in these among CO2 treatments (Figure 10). The cellular aerobic potential (CS) increases slightly under conditions of elevated CO2 while growth rate declines slightly but there was high variance surrounding all the biological responses; therefore, there is no certainty around this response.
FIGURE 10.
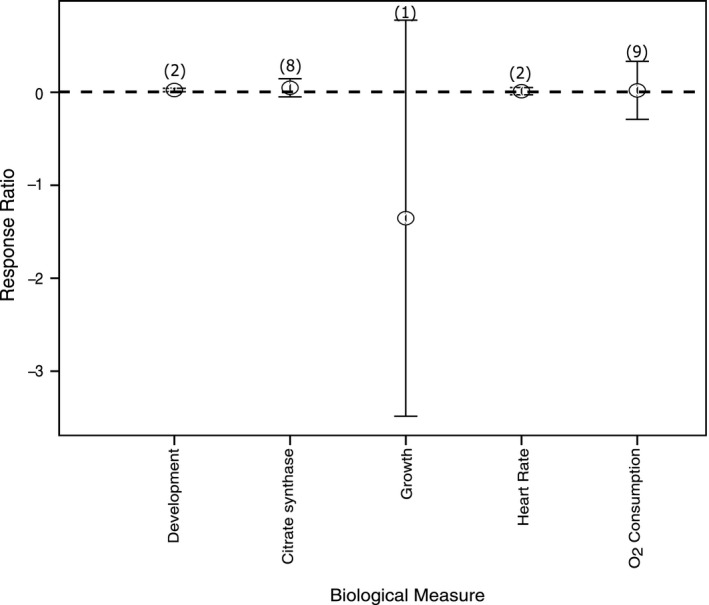
The effect of ocean acidification on the biological responses of fish. Mean response ratios and 95% confidence intervals are shown, with the number of data points in each category in brackets. A mean response ratio of zero (hashed line) indicates no effect. Refer to Table 1 for abbreviation definitions
4. DISCUSSION
This meta‐analysis shows that marine biota south of 60°S are vulnerable to ocean acidification, with only bacteria being positively affected by increases in CO2. Autotrophs, mainly phytoplankton, are negatively affected at CO2 levels above 1,000 μatm with decreases in chlorophyll a, gross primary productivity, and POC, as well as negative impacts on photosynthetic health (F v/F m and rETR) ≥1,500 μatm (Figure 5). Invertebrates are particularly sensitive to increased CO2 with decreased survival, reduced calcification rate, and increased shell dissolution with any increase in CO2 above current levels (Figure 9). Early life invertebrate life stages are also vulnerable to ocean acidification with reduced fertilization rates and increased larval abnormalities found with increased CO2 levels (Figure 9). Few studies have been conducted on the effects of ocean acidification on fish in nearshore Antarctic and Southern Ocean waters; therefore, their response is uncertain. Despite some of the clear trends found in the response of bacteria, phytoplankton, and invertebrates to increased CO2, there is high variability in these trends between studies. This high variation is not surprising as there are relatively few studies investigating the effect of ocean acidification on marine biota in Antarctica (total of 48 studies), and those that have been conducted differed in the organisms studied, the biological responses measured, experimental design, and/or the location in which the studies were performed. Further studies are required to understand the most likely magnitude of response, and the affect this will have on the ecosystem services these marine organisms provide.
4.1. Bacteria
Ocean acidification may cause both direct and indirect effects on the bacterial communities. This meta‐analysis shows that increases in CO2 have an increasingly positive effect on heterotrophic bacterial abundance and productivity in Antarctic waters. Numerous studies in the Arctic have also reported a high resilience of bacteria to ocean acidification with either no effect or an increase in bacterial abundance, growth rate, and productivity with increases in CO2 (Allgaier, Riebesell, Vogt, Thyrhaug, & Grossart, 2008; Baragi, Khandeparker, & Anil, 2015; Grossart, Allgaier, Passow, & Riebesell, 2006; Paulino, Egge, & Larsen, 2007; Wang et al., 2016). Grossart et al. (2006) and Thomson et al. (2016) suggest that these changes in the bacterial community are indirect and driven by CO2‐induced changes in the phytoplankton community, to which they are tightly coupled. Of the four bacterial studies in this meta‐analysis, only Maas et al. (2013) reported biological responses other than abundance and productivity. Maas et al. (2013) found an increase in the activity of some extracellular enzymes as well as a shift in community composition with increased CO2. These trends have also been observed in high‐latitude Northern Hemisphere studies, including the PeECE III and EPOCA mesocosm experiments (Allgaier et al., 2008; Motegi et al., 2013; Paulino et al., 2007; Piontek et al., 2013; Roy et al., 2012; Sperling et al., 2013; Zhang et al., 2013). This suggests that while changes in abundance may be due to indirect CO2 effects, bacteria can still be directly affected by ocean acidification. There is some evidence globally that increases in CO2 can cause shifts in the bacterial community composition, with Bacteriodetes, Polaribacter, and free‐living bacteria are apparently more vulnerable to increased CO2 concentrations than other bacterial groups (Sperling et al., 2013; Zhang et al., 2013). Bacterial communities exposed to elevated CO2 also had a decrease in diversity and become dominated by CO2‐tolerant species (Maas et al., 2013). This suggests that bacterial communities may be quick to adapt to increases in CO2 by changing their community composition. However, many studies have also found no significant change in bacterial community composition with elevated CO2 and therefore, in many communities, bacterial diversity may be resilient to ocean acidification (Lin et al., 2018; Lindh et al., 2013; Oliver, Newbold, Whiteley, & Gast, 2014; Roy et al., 2012; Wang et al., 2016).
4.2. Phytoplankton
There is conflicting evidence in the literature on the sensitivity of noncalcifying phytoplankton to ocean acidification. This meta‐analysis shows that phytoplankton south of 60°S are negatively affected by increases in CO2 at levels above 1,000 μatm, but there is often a positive response below this threshold (Figure 5). Therefore, it is important that the CO2 treatment levels used in studies are taken into account when interpreting potential effects of increased CO2 and comparing effects between studies. The highest CO2 treatment commonly in ocean acidification experiments is approximately 800 μatm. This is below the threshold level of 1,000 μatm identified in the meta‐analysis in this study. Below 1,000 μatm, growth rate, chlorophyll a concentration, and the ratio of F v/F m are either unaffected or significantly increased, but above growth rates, photosynthetic health (F v/F m and rETR), and POC concentration are reduced (Figure 5). This threshold of 1,000 μatm is clearly shown in a series of experiments on nearshore, natural microbial communities in Prydz Bay, East Antarctica, where despite different starting communities and nutrient levels there was a consistent threshold for change in the microbial community structure and function above 1,000 μatm (Davidson et al., 2016; Deppeler et al., 2018; Hancock et al., 2018; Thomson et al., 2016; Westwood et al., 2018).
This meta‐analysis highlights the importance of studying marine organisms as part of a community or ecosystem, demonstrating that single‐species studies often underestimate the effects of ocean acidification as they fail to include complex interactions among species and trophic levels (Figure 6). Single‐species studies also miss potential shifts in the community composition, as commonly found in phytoplankton studies. A number of phytoplankton studies south of 60°S have found a significant shift in community composition; however, there is disagreement on the direction of community change. Some studies found an increase in larger diatoms (Feng et al., 2010; Hoppe et al., 2013; Maas et al., 2013; Tortell et al., 2008), while other studies found that smaller cells were promoted at higher CO2 levels (Davidson et al., 2016; Hancock et al., 2018; Thomson et al., 2016). This may be partially due to regional differences in phytoplankton communities among these studies but again also reflects the CO2 levels used relative to the threshold concentration that elicits community change. Hancock et al. (2018) found that larger diatom species were promoted at intermediate CO2 concentrations (500–1,000 μatm), but above 1,000 μatm, there was a significant decline in larger diatoms and a shift to a community dominated by smaller cells. This observation could explain some of the variability in results of the other studies, where 750–800 μatm is commonly the highest CO2 treatment tested (i.e., Tortell et al., 2008, Feng et al., 2010, Hoppe et al., 2013 and Young, Kranz, Goldman, Tortell, & Morel, 2015). Studies by Tortell et al. (2008), Feng et al. (2010), Hoppe et al. (2013) and Hancock et al. (2018) all show that larger diatoms are promoted by increases in CO2 up to 1,000 μatm. Thus, care must be taken when comparing the effects of ocean acidification among studies with different CO2 treatment levels.
A number of the biological responses of phytoplankton to increased CO2 had a parabolic trend, with positive responses with moderate increases in CO2 but above 1,000 μatm an increasingly negative response (i.e., chlorophyll a concentration and growth rate, Figure 5). A possible explanation for the parabolic responses to increased CO2 could be the interaction of the beneficial effects of CO2 with the inhibitory effects of the coincident rise in H+ ions. Current levels of CO2 in ambient seawater are limiting to phytoplankton growth due to the low affinity of the enzyme ribulose‐1,5‐bisphosphate carboxylase/oxygenase (RuBisCo) for CO2 (Reinfelder, 2011). This enzyme is involved in the first major step of carbon fixation and is present in all eukaryotic phytoplankton. Its half‐saturation concentration for CO2(aq) is, however, substantially higher than present day naturally occurring CO2 levels in seawater (Reinfelder, 2011). To overcome this low affinity of RuBisCo, phytoplankton have developed carbon concentration mechanisms (CCMs) so high rates of photosynthesis can be maintained, but this is an energy‐consuming process (Raven, Cockell, & Rocha, 2008). At elevated CO2, cells possessing efficient CCMs can downregulate these carbon concentrating mechanisms, thereby saving energy while sustaining photosynthetic rate (Rost, Zondervan, & Wolf‐Gladrow, 2008). This is supported by the findings of this meta‐analysis where the activity of extracellular carbonic anhydrase (eCA), an enzyme involved in carbon acquisition and an indicator for CCM activity, is increased at CO2 levels below ambient, but decreased above ambient, reflecting a downregulation of CCMs at increased CO2. If the CCMs are downregulated, then photosynthesis and growth would increase due to the energy saved from not having to operate the CCMs. However, at CO2 levels above 1,000 μatm growth rate, chlorophyll a concentration, primary productivity (NPP and GPP), and photosynthetic health declined. It is likely that the coincident rise in H+ ions with increased CO2 is resulting in the requirement for the phytoplankton to expend energy on proton pumps to maintain intracellular pH (Cyronak, Schulz, & Jokiel, 2015; Deppeler et al., 2018; Gafar, Eyre, & Schulz, 2017; McMinn, Müller, Martin, & Ryan, 2014; Taylor, Brownlee, & Wheeler, 2012). The use of CCMs in phytoplankton varies between species, and some studies have found that diatoms and Phaeocystis are capable of indirect or direct HCO3 ‐ uptake and are not limited by CO2 (Cassar, Laws, Bidigare, & Popp, 2004; Goldman, 1999; Martin & Tortell, 2006; Tortell, DiTullio, Sigman, & Morel, 2002; Tortell & Morel, 2002; Tortell, Reinfelder, & Morel, 1997). For these larger species, the intolerance to increased CO2 is likely to reflect their need to operate proton pumps to combat the rise in H+ concentration.
The effect of ocean acidification on sea‐ice algae and in long‐term phytoplankton experiments has not been included in this meta‐analysis as they are difficult to compare to short‐term pelagic phytoplankton studies. Sea‐ice algae experience large seasonal fluctuations in pH and ambient CO2 levels can exceed >1,000 μatm (Coad, McMinn, Nomura, & Martin, 2016; McMinn et al., 2014, 2017; Miller et al., 2011). McMinn (2017) provides a review on the effect of ocean acidification on sea‐ice microbial communities and concludes that sea‐ice brine algal communities tolerate elevated levels of CO2 with negative effects on growth and photosynthesis only at the most extreme CO2 levels, >2,500 μatm. This contrasts with the tolerance of pelagic phytoplankton which were negatively affected at CO2 levels above 1,000 μatm. Also not included in the meta‐analysis are data from long‐term studies, such as those by Trimborn et al. (2014) and Torstensson et al. (2015). These studies aim to investigate the acclimation capacity of phytoplankton to increased CO2, as opposed to examining the impacts of exposure to increased CO2 on key biological responses. Torstensson et al. (2015) found only a small reduction in growth of Nitzschia lecointei after 147 days at 960 μatm (with the experiment run for 194 days in total), although Trimborn et al. (2014) highlight that responses can be species‐specific at 1,000 μatm, which is a similar tolerance level found in this meta‐analysis. Studies on the ability of phytoplankton to acclimate and/or adapt to increased CO2 are rare. The few studies conducted globally suggest that phytoplankton could adapt, partially or totally negating the negative effects of increased CO2 over the longer time scales at which the oceans are actually changing (i.e., Lohbeck, Riebesell, & Reusch, 2012).
4.3. Macroalgae
Very few studies have been conducted on macroalgae in Antarctic waters. From the few studies available, it appears crustose algal species are more vulnerable than noncalcifying fleshy species to ocean acidification, but with species‐specific differences (Figures 7 and 8). However, there is high uncertainty around this response and further studies are needed to accurately predict the response of macroalgae to ocean acidification in Antarctic waters.
Globally, crustose algae have been found to be sensitive to increases in CO2 with decreases in calcification, increased thalli dissolution, and decreased structural integrity (Agostini et al., 2018; Fabricius, Kluibenschedl, Harrington, Noonan, & De'ath, G., 2015; Hofmann & Bischof, 2014; Johnson & Carpenter, 2012; Kuffner, Andersson, Jokiel, Rodgers, & MacKenzie, 2008; McCoy & Kamenos, 2015; Roleda & Hurd, 2012). Ocean acidification reduces biogenic calcification by decreasing the calcium carbonate saturation resulting in reduced calcification and increased dissolution of crustose algal thalli. This reduces the structural integrity and protective function of crustose algae making them susceptible to bioerosion and increased grazing (Johnson & Carpenter, 2012; Koch, Bowes, Ross, & Zhang, 2013). In the west Antarctic Peninsula, the coverage of crustose algae can reach 77% in some subtidal benthic habitats but unfortunately little is known about the role of crustose algae in Antarctic benthic ecology (Amsler, Rowley, Laur, Quetin, & Ross, 1995; Schoenrock et al., 2016). In Arctic analogues, crustose algae provide an important habitat for benthic invertebrates and fish and play a key role in substrate stabilization of subtidal habitats (Chenelot, Jewett, & Hoberg, 2011; Teichert & Freiwald, 2014). Schoenrock et al. (2016) suggest that future ocean acidification conditions could alter algal community structure due to the differences in CO2 tolerance among the species. A change in the algal community structure and a decline in crustose algal integrity could have significant flow‐on effects to the Antarctic benthic ecosystem, particularly benthic communities which are closely associated with or are dependent on macroalgae (Peck, 2018; Schoenrock et al., 2016).
4.4. Invertebrates
This meta‐analysis shows that Antarctic invertebrates are particularly sensitive to ocean acidification with negative effects on calcification, reproduction, and survival. This agrees with the findings of Kroeker et al. (2010, 2013) but globally the sensitivity of invertebrates to elevated CO2 is phylum‐specific, with echinoderms and mollusks less tolerant than crustaceans (Kroeker et al., 2010, 2013). The limited number of studies conducted on Antarctic invertebrates, and the wide range of biological responses measured, prevented phylum‐specific conclusions from being determined in the current meta‐analysis. Despite this, it is clear that invertebrates could be threatened at elevated CO2 concentrations with reduced fertilization rates, increased larval abnormalities, decreased calcification, and increased shell dissolution (Figure 9).
Calcifying organisms are thought to be indicator organisms for ocean acidification as they are at heightened risk from increasing ocean acidification due to the reduction in calcium carbonate saturation state (Fabry et al., 2009; McNeil & Matear, 2008; Orr et al., 2005). Decreases in the saturation state of calcium carbonate due to ocean acidification result in decreased calcification and increased dissolution of calcium carbonate shells, with some invertebrate taxa more vulnerable than others (Andersson, Mackenzie, & Gattuso, 2011). Pteropods, for example, are reportedly particularly vulnerable to ocean acidification due to their weaker aragonite shells (Bednarsek, Tarling, Bakker, Fielding, & Feely, 2014; Gardner, Manno, Bakker, Peck, & Tarling, 2018). In addition, transcriptomic studies suggest that pteropods may not have the plasticity required to adapt to ocean acidification over the timescale in which they will be exposed to undersaturated conditions (Johnson & Hofmann, 2017). For Antarctic echinoderms, distributions show that species living at depth below the current calcite saturation horizon have thinner, more weakly calcified shells than those living above this horizon (Sewell & Hofmann, 2011). This response appears to be species‐specific as some Antarctic echinoderms show no clear trend in calcification with depth or the proportion of highly soluble magnesium‐calcite in their skeletons, suggesting their calcification could depend on the acid–base status of the species (Collard, Ridder, David, Dehairs, & Dubois, 2015; Duquette, Halanych, Angus, & McClintock, 2018). Furthermore, McClintock et al. (2009) showed that the composition of the invertebrate's shell (calcite or aragonite) did not affect the vulnerability of those shells to a decrease in the saturation state of calcium carbonate. Rather, the vulnerability of shells to ocean acidification depended on the strength of the shell matrix (Harper, 2000; McClintock et al., 2009). However, the experiments conducted by McClintock et al. (2009) were conducted on empty shells of Antarctic invertebrates. Cyronak et al. (2015) show that the vulnerability of calcifying organisms to ocean acidification is not due a decline in calcium carbonate saturation driving a decrease in calcification rates, but rather the increase in H+ ions associated with the drop in pH, which makes it difficult for organisms to maintain their pH homeostasis.
Early developmental stages of invertebrates are known to be more sensitive to changes in their environment as their regulatory mechanisms are still developing (Burggren & Warburton, 2005; Kurihara, 2008). This trend in sensitivity is consistent with the current study, which showed negative effects of CO2 concentration on invertebrate development above 1,500 μatm, including decreased rates of fertilization, egg cleavage and hatching, decreased survival of larvae, increased abnormalities, and delayed development of later larval stages (Bylenga, Cummings, & Ryan, 2015, 2017; Byrne et al., 2013; Ericson et al., 2012; Ericson, Lamare, Morley, & Barker, 2010; Foo et al., 2016; Gonzalez‐Bernat, Lamare, & Barker, 2013; Karelitz et al., 2016; Kawaguchi et al., 2010, 2013). This contrasts with biological responses of adults, which only showed a negative response in shell state and survival, although comparatively more studies have focused on the effects of ocean acidification on juveniles than adult invertebrates (Figure 9). All the species included in this meta‐analysis are free spawners with planktonic larvae (Ansell & Harvey, 1997; Nicol, 2006; Pearse & Bosch, 1986; Quetin & Ross, 1984). Waters below 100 m can have CO2 levels considerably higher than at the surface (Kawaguchi et al., 2010, 2013). This means larvae and eggs could experience higher CO2 conditions in mid‐pelagic waters earlier than predicted for surface waters (Kawaguchi et al., 2010). The bivalve L. elliptica may be particularly vulnerable as it uses aragonite to form its shell and spawns during the winter (Ansell & Harvey, 1997) when seawater pH is at its lowest and aragonite is predicted to become seasonally undersaturated by 2030 (Gibson & Trull, 1999; McNeil & Matear, 2008; Roden et al., 2013). What remains unclear is whether marine invertebrates will adapt to reduced pH, and whether individuals that survive the larval phase and grow to adulthood will have stronger, more resilient offspring. Suckling et al. (2015) showed that S. nemayeri grown at 900–1,400 μatm CO2 acclimate both their metabolic and reproductive physiology, and after 2 years, there was no significant difference in urchins exposed to current day and elevated CO2 conditions. Ericson et al. (2018) also showed Antarctic krill, Euphausia superba, have a high resilience to elevated CO2 when exposed over an entire year. These and other studies suggest that some invertebrates may be able to adapt over time to ocean acidification conditions and produce more resilient offspring, but further long‐term studies are needed to fully explore this intergenerational adaptive capacity (Peck, 2018).
As invertebrates play an important role in the Antarctic ecosystem, any decline in their abundance or change in community composition due to ocean acidification could have significant implications for the Antarctic and Southern Ocean ecosystem and the food webs they support. For example, pteropods are a major food source for many zooplankton, fish, and higher predators (Falk‐Petersen, Sargent, Kwasniewski, Gulliksen, & Millar, 2001; Hopkins, 1987) and play a role in the export of carbon to the deep ocean (Manno, Tirelli, Accornero, & Fonda Umani, 2010). Invertebrates such as sea stars, sea urchins, limpets, sea snails, bivalves, and brachiopods are important components in benthic communities as bioturbators, major grazers on macroalgae, and benthic diatoms and play a key role in energy transfer in the Antarctic benthos (McClintock, 1994; Widdicombe, Austen, Kendall, Warwick, & Jones, 2000).
4.5. Fish
Fish appear to tolerate ocean acidification but this is based on only a few studies on the effect of altered carbonate chemistry, highlighting the need for more investigations and on these and other higher trophic level organisms. Global meta‐analyses on the effect of ocean acidification on fish found no effect or only a slight positive effect of increased CO2 on fish growth, but again from limited data (Kroeker et al., 2010, 2013). Some studies suggest that fish may expend more energy under elevated CO2 conditions to maintain osmoregulation particularly when exposed for longer periods of increased CO2 (Flynn et al., 2015; Kidder, Petersen, & Preston, 2006). Studies conducted on the capacity of Antarctic notothenioids have found that some species (Trematomus newnesi and Notothenia rossi) have little ability to physiologically adjust to environmental change (Enzor et al., 2013; Strobel, Graeve, et al., 2013), while others (Pagothenia borchgrevinki, T. bernacchii and T. hansoni) rapidly acclimate (Enzor et al., 2013; Huth & Place, 2016). These may be species‐specific differences in the tolerance of ocean acidification that favor the increasing dominance of some taxa in the future. Unfortunately, the present lack of studies reporting the effects of ocean acidification on fish both in Antarctic and globally means the response of fish is unclear and highlights the need for further investigations.
4.6. Conclusions and future directions
This meta‐analysis highlights the sensitivity of marine organisms in the Southern Ocean and nearshore Antarctic waters to future ocean acidification conditions. The threshold level for negative responses is ≈1,000 μatm for autotrophs and ≈500 μatm for heterotrophs, with only prokaryotes (bacteria) being positively affected by increased levels of CO2. By year 2100, when nearshore Antarctic waters are projected to have CO2 levels between 880 and 1,000 μatm (under the IPCC business as usual scenario, RCP8.5), these waters could experience an increase in the abundance of bacteria, reduction in primary productivity, and phytoplankton communities could be become dominated by smaller cells. Invertebrates could also be threatened with reductions in fertilization rate and increased larval abnormalities occurring at a CO2 concentrations above 1,500 μatm. Adult invertebrates may struggle to both make and maintain their shells due to the negative effects on calcification and increased shell dissolution with undersaturation of aragonite predicted for some areas of the Southern Ocean by 2100 (McNeil & Matear, 2008).
There have been an increasing number of studies focusing on whole ecosystem responses, which take into account natural variability in CO2 levels into the experimental design. Nearshore Antarctic waters, where the majority of the studies included in this meta‐analysis were conducted, have large annual fluctuations in CO2 due to changes in sea‐ice coverage and primary production (Gibson & Trull, 1999; McNeil & Matear, 2008; Roden et al., 2013). Kapsenberg et al. (2015) argue that Antarctic Ocean acidification experiments should incorporate projected pH changes as well as natural temporal pH variability due to the influence these temporal changes could have on organism's ability to adapt to future acidification conditions. Superimposing seasonal pH variability on projected future pH levels has been shown to increase the amplitude of seasonal changes in pH (Kapsenberg et al., 2015). This could be exacerbated further with the reduction in primary productivity found in this meta‐analysis which would further decrease pH and aragonite undersaturation in the future (Kapsenberg et al., 2015). Other than in microbial studies, the majority of ocean acidification studies on Antarctic marine biota have been conducted on a single species in isolation. Such studies are of limited predictive value as they do not include the complex interactions among species and trophic levels that occur in nature. Differences in single‐species versus natural communities are demonstrated by the observed increase in sensitivity of phytoplankton communities to ocean acidification compared to monospecific studies described here. This highlights the need for community‐ and ecosystem‐level studies to better predict the effects of ocean acidification on the nearshore Antarctic and Southern Ocean ecosystem, such as the recent antFOCE experiment (Stark et al., 2018).
Ocean acidification is not occurring in isolation and changing ocean conditions including increasing temperatures are happening over entire ecosystems and longer time periods than most experiments cover. The Southern Ocean and nearshore Antarctic waters are also warming, sea‐ice is predicted to decline, winds are strengthening, upwelling and nutrient availability is changing, mixed layer depths are shallowing, and the ocean fronts are moving southward (Deppeler & Davidson, 2017; Stark, Raymond, Deppeler, & Morrison, 2019). These stressors interact, resulting in additive, synergistic, or antagonistic effects on organisms (Boyd, Brown, Schrum, & Krishnakumar, 2015). To better predict the potential response of organisms and ecosystems to global change, we need to develop an understanding of how organisms will cope when exposed to multiple stressors, plus understand the response of organisms to these stressors over longer time scales, during which organisms have time to acclimate and/or adapt to the changing environment.
This meta‐analysis shows that ocean acidification can detrimentally affect marine organisms south of 60°S and the ecosystem services they provide. There is currently poor spatial coverage, with most studies conducted in nearshore Antarctic waters surrounding the Antarctic Peninsula, the Ross Sea and Prydz Bay, East Antarctica where many Antarctic bases are located. While this lack of coverage and focus around the location of Antarctic bases is understandable given the logistical constraints of conducting Antarctic fieldwork and experiments, this limitation does need to be recognized when assessing the vulnerability of Antarctic marine organisms to ocean acidification. There is an overall lack of data available on the effect of ocean acidification on Antarctic marine organisms. Consequently, estimates of the effect of elevated CO2 in this meta‐analysis are highly variable, with most studies examining differing organisms and biological responses at different locations to all other studies. Outside of microbial studies, no studies have been conducted on natural communities or entire ecosystems (although see Stark et al., 2018), which could have quite different responses and sensitivities to monospecific studies. Better predictions of the future state of the Southern Ocean and nearshore Antarctic ecosystems require more studies that incorporate entire communities over timescales that allow organisms to acclimate and potentially adapt, to a range of CO2 and multistressor scenarios.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Alyce Meredith Hancock: Conceptualization (lead); Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Project administration (lead); Software (lead); Validation (lead); Visualization (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Catherine King: Conceptualization (supporting); Data curation (supporting); Investigation (supporting); Project administration (supporting); Supervision (supporting); Writing‐review & editing (supporting). Jonathan Stark: Conceptualization (supporting); Data curation (supporting); Funding acquisition (equal); Investigation (supporting); Supervision (supporting); Writing‐review & editing (supporting). Andrew McMinn: Data curation (supporting); Funding acquisition (equal); Investigation (supporting); Supervision (supporting); Writing‐review & editing (supporting). Andrew Davison: Conceptualization (supporting); Data curation (supporting); Funding acquisition (equal); Investigation (supporting); Supervision (supporting); Writing‐review & editing (supporting).
Supporting information
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Australian Government Department of Environment and Energy as part of Australian Antarctic Science project 4026 and 4127 at the Australian Antarctic Division, as well as Australian Research Council's Special Research Initiative for Antarctic Gateway Partnership (Project ID SR140300001) and the Australian Governments Cooperative Research Centres Program through the Antarctic Climate and Ecosystems Cooperative Research Centre (ACE CRC). We thank Dale Maschette for developing Figure 1.
Hancock AM, King CK, Stark JS, McMinn A, Davidson AT. Effects of ocean acidification on Antarctic marine organisms: A meta‐analysis. Ecol Evol. 2020;10:4495–4514. 10.1002/ece3.6205
DATA AVAILABILITY STATEMENT
Raw data used in the meta‐analysis are available via the Australian Antarctic Division Data Centre, https://doi.org/10.26179/5e4f475eb6fbd.
REFERENCES
- Agostini, S. , Harvey, B. P. , Wada, S. , Kon, K. , Milazzo, M. , Inaba, K. , & Hall‐Spencer, J. M. (2018). Ocean acidification drives community shifts towards simplified non‐calcified habitats in a subtropical‐temperate transition zone. Scientific Reports, 8(1), 11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgaier, M. , Riebesell, U. , Vogt, M. , Thyrhaug, R. , & Grossart, H.‐P. (2008). Coupling of heterotrophic bacteria to phytoplankton bloom development at different pCO2 levels: A mesocosm study. Biogeosciences, 5(1), 317–359. [Google Scholar]
- Amsler, C. D. , Rowley, R. J. , Laur, D. R. , Quetin, L. B. , & Ross, R. M. (1995). Vertical distribution of Antarctic Peninsular macroalgae: Cover, biomass and species composition. Phycologia, 34(5), 424–430. [Google Scholar]
- Andersson, A. , Mackenzie, F. , & Gattuso, J.‐P. (2011). Effects of ocean acidification on benthic processes, organisms, and ecosystems In Gattuso J.‐P., & Hansson L. (Eds.), Ocean acidification (pp. 122–150). New York, NY: Oxford University Press. [Google Scholar]
- Ansell, A. D. , & Harvey, R. (1997). Protected larval development in the Antarctic bivalve Laternula elliptica (King and Broderip) (Anomalodesmata: Laternulidae). Journal of Molluscan Studies, 63(2), 285–286. [Google Scholar]
- Baragi, L. V. , Khandeparker, L. , & Anil, A. C. (2015). Influence of elevated temperature and pCO2 on the marine periphytic diatom Navicula distans and its associated organisms in culture. Hydrobiologia, 762(1), 127–142. [Google Scholar]
- Bednarsek, N. , Tarling, G. A. , Bakker, D. C. E. , Fielding, S. , & Feely, R. A. (2014). Dissolution dominating calcification process in polar pteropods close to the point of aragonite undersaturation. PLoS ONE, 9(10), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd, P. W. , Brown, C. J. , Schrum, C. , & Krishnakumar, P. K. (2015). Modes of interactions between environmental drivers and marine biota. Frontiers in Marine Science, 2, 1–7. [Google Scholar]
- Brown, H. , & Kempton, R. (1994). The application of REML in clinical trials. Statistics in Medicine, 13(16), 1601–1617. [DOI] [PubMed] [Google Scholar]
- Burggren, W. , & Warburton, S. (2005). Comparative development physiology: An interdisciplinary convergence. Annual Review of Physiology, 67(1), 203–223. [DOI] [PubMed] [Google Scholar]
- Bylenga, C. H. , Cummings, V. J. , & Ryan, K. G. (2015). Fertilisation and larval development in an Antarctic bivalve, Laternula elliptica, under reduced pH and elevated temperatures. Marine Ecology Progress Series, 536, 187–201. [Google Scholar]
- Bylenga, C. H. , Cummings, V. J. , & Ryan, K. G. (2017). High resolution microscopy reveals significant impacts of ocean acidification and warming on larval shell development in Laternula elliptica . PLoS ONE, 12(4), e0175706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, M. , Ho, M. A. , Koleits, L. , Price, C. , King, C. K. , Virtue, P. , … Lamarek, M. (2013). Vulnerability of the calcifying larval stage of the Antarctic sea urchin Sterechinus neumayeri to near‐future ocean acidification and warming. Global Change Biology, 19(7), 2264–2275. [DOI] [PubMed] [Google Scholar]
- Byrne, R. H. , Mecking, S. , Feely, R. A. , & Liu, X. (2010). Direct observations of basin‐wide acidification of the North Pacific Ocean. Geophysical Research Letters, 37(2), 1–5. [Google Scholar]
- Cassar, N. , Laws, E. A. , Bidigare, R. R. , & Popp, B. N. (2004). Bicarbonate uptake by Southern Ocean phytoplankton. Global Biogeochemical Cycles, 18(2). 10.1029/2003GB002116 [DOI] [Google Scholar]
- Chenelot, H. , Jewett, S. C. , & Hoberg, M. K. (2011). Macrobenthos of the nearshore Aleutian Archipelago, with emphasis on invertebrates associated with Clathromorphum nereostratum (Rhodophyta, Corallinaceae). Marine Biodiversity, 41(3), 413–424. [Google Scholar]
- Coad, T. , McMinn, A. , Nomura, D. , & Martin, A. (2016). Effect of elevated CO2 concentration on microalgal communities in Antarctic pack ice. Deep‐Sea Research Part II, 131, 160–169. [Google Scholar]
- Collard, M. , De Ridder, C. , David, B. , Dehairs, F. , & Dubois, P. (2015). Could the acid‐base status of Antarctic sea urchins indicate a better‐than‐expected resilience to near‐future ocean acidification? Global Change Biology, 21(2), 605–617. [DOI] [PubMed] [Google Scholar]
- Cyronak, T. , Schulz, K. G. , & Jokiel, P. L. (2015). The omega myth: What really drives lower calcification rates in an acidifying ocean. ICES Journal of Marine Science, 73(3), 558–562. [Google Scholar]
- Davidson, A. T. , McKinlay, J. , Westwood, K. , Thomson, P. G. , van den Enden, R. , de Salas, M. , … Berry, K. (2016). Enhanced CO2 concentrations change the structure of Antarctic marine microbial communities. Marine Ecology Progress Series, 552, 93–113. [Google Scholar]
- Davis, B. E. , Flynn, E. E. , Miller, N. A. , Nelson, F. A. , Fangue, N. A. , & Todgham, A. E. (2018). Antarctic emerald rockcod have the capacity to compensate for warming when uncoupled from CO2‐acidification. Global Change Biology, 24(2), e655–e670. [DOI] [PubMed] [Google Scholar]
- Davis, B. E. , Miller, N. A. , Flynn, E. E. , & Todgham, A. E. (2016). Juvenile Antarctic rockcod (Trematomus bernacchii) are physiologically robust to CO2‐acidified seawater. Journal of Experimental Biology, 219(8), 1203–1213. [DOI] [PubMed] [Google Scholar]
- Deppeler, S. L. , & Davidson, A. T. (2017). Southern ocean phytoplankton in a changing climate. Frontiers in Marine Science, 4, 1–28. [Google Scholar]
- Deppeler, S. , Petrou, K. , Schulz, K. G. , Westwood, K. , Pearce, I. , McKinlay, J. , & Davidson, A. (2018). Ocean acidification of a coastal Antarctic marine microbial community reveals a critical threshold for CO2 tolerance in phytoplankton productivity. Biogeosciences, 15(1), 209. [Google Scholar]
- Doney, S. C. , Fabry, V. J. , Feely, R. A. , & Kleypas, J. A. (2009). Ocean acidification: The other CO2 problem. Annual Review of Marine Science, 1, 169–192. [DOI] [PubMed] [Google Scholar]
- Doney, S. C. , Ruckelshaus, M. , Emmett Duffy, J. , Barry, J. P. , Chan, F. , English, C. A. , … Talley, L. D. (2012). Climate change impacts on marine ecosystems. Annual Review of Marine Science, 4(1), 11–37. [DOI] [PubMed] [Google Scholar]
- Dore, J. E. , Lukas, R. , Sadler, D. W. , Church, M. J. , & Karl, D. M. (2009). Physical and biogeochemical modulation of ocean acidification in the central North Pacific. Proceedings of the National Academy of Sciences of the United States of America, 106(30), 12235–12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette, A. , Halanych, K. M. , Angus, R. A. , & McClintock, J. B. (2018). Inter and intraspecific comparisons of the skeletal Mg/Ca ratios of high latitude Antarctic echinoderms. Antarctic Science, 30(3), 160–169. [Google Scholar]
- Enzor, L. A. , Hunter, E. M. , & Place, S. P. (2017). The effects of elevated temperature and ocean acidification on the metabolic pathways of notothenioid fish. Conservation Physiology, 5(1), cox019 10.1093/conphys/cox019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzor, L. A. , Zippay, M. L. , & Place, S. P. (2013). High latitude fish in a high CO2 world: Synergistic effects of elevated temperature and carbon dioxide on the metabolic rates of Antarctic notothenioids. Comparative Biochemistry and Physiology, Part A, 164(1), 154–161. [DOI] [PubMed] [Google Scholar]
- Ericson, J. A. , Hellessey, N. , Kawaguchi, S. , Nicol, S. , Nichols, P. D. , Hoem, N. , & Virtue, P. (2018). Adult Antarctic krill proves resilient in a simulated high CO2 ocean. Communications Biology, 1(1), 190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson, J. A. , Ho, M. A. , Miskelly, A. , King, C. K. , Virtue, P. , Tilbrook, B. , & Byrne, M. (2012). Combined effects of two ocean change stressors, warming and acidification, on fertilization and early development of the Antarctic echinoid Sterechinus neumayeri . Polar Biology, 35(7), 1027–1034. [Google Scholar]
- Ericson, J. A. , Lamare, M. D. , Morley, S. A. , & Barker, M. F. (2010). The response of two ecologically important Antarctic invertebrates (Sterechinus neumayeri and Parborlasia corrugatus) to reduced seawater pH: Effects on fertilisation and embryonic development. Marine Biology, 157(12), 2689–2702. [Google Scholar]
- Fabricius, K. E. , Kluibenschedl, A. , Harrington, L. , Noonan, S. , & De'ath, G. (2015). In situ changes of tropical crustose coralline algae along carbon dioxide gradients. Scientific Reports, 5(1), 9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry, V. J. , McClintock, J. B. , Mathis, J. T. , & Grebmeier, J. M. (2009). Ocean acidification at high latitudes: The Bellweather. Oceanography, 22(4), 160–171. [Google Scholar]
- Falk‐Petersen, S. , Sargent, J. R. , Kwasniewski, S. , Gulliksen, B. , & Millar, R.‐M. (2001). Lipids and fatty acids in Clione limacina and Limacina helicina in Svalbard waters of the Arctic Ocean: Trophic implications. Polar Biology, 24, 163–170. [Google Scholar]
- Feng, Y. , Hare, C. E. , Rose, J. M. , Handy, S. M. , DiTullio, G. R. , Lee, P. A. , … Hutchins, D. A. (2010). Interactive effects of iron, irradiance and CO2 on Ross Sea phytoplankton. Deep‐Sea Research Part I Oceanographic Research Papers, 57(3), 368–383. [Google Scholar]
- Flynn, E. E. , Bjelde, B. E. , Miller, N. A. , & Todgham, A. E. (2015). Ocean acidification exerts negative effects during warming conditions in a developing Antarctic fish. Conservation Physiology, 3(1), cov033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foo, S. A. , Sparks, K. M. , Uthicke, S. , Karelitz, S. , Barker, M. , Byrne, M. , & Lamare, M. (2016). Contributions of genetic and environmental variance in early development of the Antarctic sea urchin Sterechinus neumayeri in response to increased ocean temperature and acidification. Marine Biology, 163(6). 10.1007/s00227-016-2903-1 [DOI] [Google Scholar]
- Frölicher, T. L. , Sarmiento, J. L. , Paynter, D. J. , Dunne, J. P. , Krasting, J. P. , & Winton, M. (2015). Dominance of the Southern Ocean in anthropogenic carbon and heat uptake in CMIP5 models. Journal of Climate, 28(2), 862–886. [Google Scholar]
- Gafar, N. A. , Eyre, B. D. , & Schulz, K. G. (2017). A conceptual model for projecting coccolithophorid growth, calcification and photosynthetic carbon fixation rates in response to global ocean change. Frontiers in Marine Science, 4, 433. [Google Scholar]
- Gardner, J. , Manno, C. , Bakker, D. C. E. , Peck, V. L. , & Tarling, G. A. (2018). Southern Ocean pteropods at risk from ocean warming and acidification. Marine Biology, 165(1), 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattuso, J.‐P. , & Hansson, L. (Eds.) (2011). Ocean acidification. Oxford, UK: Oxford University Press. [Google Scholar]
- Gattuso, J.‐P. , & Lavigne, H. (2009). Approaches and software tools to investigate the impact of ocean acidification. Biogeosciences, 6(2), 4413–4439. [Google Scholar]
- Gibson, J. A. E. , & Trull, T. W. (1999). Annual cycle of fCO2 under sea‐ice and in open water in Prydz Bay, East Antarctica. Marine Chemistry, 66(187–200), 42. [Google Scholar]
- Goldman, J. C. (1999). Inorganic carbon availability and the growth of large marine diatoms. Marine Ecology Progress Series, 180, 81–91. [Google Scholar]
- Gonzalez‐Bernat, M. J. , Lamare, M. , & Barker, M. (2013). Effects of reduced seawater pH on fertilisation, embryogenesis and larval development in the Antarctic seastar Odontaster validus . Polar Biology, 36(2), 235–247. [Google Scholar]
- Grossart, H.‐P. , Allgaier, M. , Passow, U. , & Riebesell, U. (2006). Testing the effect of CO2 concentration on the dynamics of marine heterotrophic bacterioplankton. Limnology & Oceanography, 51(1), 1–11. [Google Scholar]
- Gruber, N. , Hauri, C. , Lachkar, Z. , Loher, D. , Frolicher, T. L. , & Plattner, G.‐K. (2012). Rapid progression of ocean acidification in the California Current System. Science, 337(6091), 220–223. [DOI] [PubMed] [Google Scholar]
- Hancock, A. M. , Davidson, A. T. , McKinlay, J. , McMinn, A. , Schulz, K. G. , & van den Enden, R. L. (2018). Ocean acidification changes the structure of an Antarctic coastal protistan community. Biogeosciences, 15(8), 2393–2410. [Google Scholar]
- Harper, E. M. (2000). Are calcitic layers an effective adaptation against shell dissolution in the Bivalvia? Journal of Zoology, 251(2), 179–186. [Google Scholar]
- Hartin, C. A. , Bond‐Lamberty, B. , Patel, P. , & Mundra, A. (2016). Ocean acidification over the next three centuries using a simple global climate carbon‐cycle model: Projections and sensitivities. Biogeosciences, 13(15), 4329–4342. [Google Scholar]
- Hauri, C. , Friedrich, T. , & Timmermann, A. (2016). Abrupt onset and prolongation of aragonite undersaturation events in the Southern Ocean. Nature Climate Change, 6(2), 172–176. [Google Scholar]
- Hedges, L. V. , Gurevitch, J. , & Curtis, P. S. (1999). The meta‐analysis of response ratios in experimental ecology. Ecology, 80(4), 1150–1156. [Google Scholar]
- Hedges, L. V. , & Olkin, I. (1985). Statistical methods for meta‐analysis. New York, NY: Academic Press. [Google Scholar]
- Hofmann, L. C. , & Bischof, K. (2014). Ocean acidification effects on calcifying macroalgae. Aquatic Biology, 22, 261–279. [Google Scholar]
- Hopkins, T. (1987). Midwater food web in McMurdo Sound, Ross Sea, Antarctica. Marine Biology, 96(1), 93–106. [Google Scholar]
- Hoppe, C. J. M. , Hassler, C. S. , Payne, C. D. , Tortell, P. D. , Rost, B. , & Trimborn, S. (2013). Iron limitation modulates ocean acidification effects on Southern Ocean phytoplankton communities. PLoS ONE, 8(11), e79890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huth, T. J. , & Place, S. P. (2016). RNA‐seq reveals a diminished acclimation response to the combined effects of ocean acidification and elevated seawater temperature in Pagothenia borchgrevinki . Marine Genomics, 28, 87–97. [DOI] [PubMed] [Google Scholar]
- Iniguez, C. , Heinrich, S. , Harms, L. , & Gordillo, F. J. L. (2017). Increased temperature and CO2 alleviate photoinhibition in Desmarestia anceps: From transcriptomics to carbon utilization. Journal of Experimental Botany, 68(14), 3971–3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (2019). IPCC special report on the ocean and cryosphere in a changing climate. Geneva, Switzerland: IPCC. [Google Scholar]
- Ishii, M. , Kosugi, N. , Sasano, D. , Saito, S. , Midorikawa, T. , & Inoue, H. Y. (2011). Ocean acidification off the south coast of Japan: A result from time series observations of CO2 parameters from 1994 to 2008. Journal of Geophysical Research: Oceans, 116(6), 1–9. [Google Scholar]
- Johnson, K. M. , & Hofmann, G. E. (2017). Transcriptomic response of the Antarctic pteropod Limacina helicina antarctica to ocean acidification. BMC Genomics, 18(1), 812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. D. , & Carpenter, R. C. (2012). Ocean acidification and warming decrease calcification in the crustose coralline alga Hydrolithon onkodes and increase susceptibility to grazing. Journal of Experimental Marine Biology and Ecology, 434–435, 94–101. [Google Scholar]
- Kapsenberg, L. , Kelley, A. L. , Shaw, E. C. , Martz, T. R. , & Hofmann, G. E. (2015). Nearshore Antarctic pH variability has implications for the design of ocean acidification experiments. Scientific Reports, 5(1). 10.1038/srep09638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karelitz, S. E. , Uthicke, S. , Foo, S. A. , Barker, M. F. , Byrne, M. , Pecorino, D. , & Lamare, M. D. (2016). Ocean acidification has little effect on developmental thermal windows of echinoderms from Antarctica to the tropics. Global Change Biology, 23(2), 657–672. [DOI] [PubMed] [Google Scholar]
- Kawaguchi, S. , Ishida, A. , King, R. , Raymond, B. , Waller, N. , Constable, A. , … Ishimatsu, A. (2013). Risk maps for Antarctic krill under projected Southern Ocean acidification. Nature Climate Change, 3(9), 843–847. [Google Scholar]
- Kawaguchi, S. , Kurihara, H. , King, R. , Hale, L. , Berli, T. , Robinson, J. P. , … Ishimatsu, A. (2010). Will krill fare well under Southern Ocean acidification? Biology Letters, 7(2), 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidder, G. W. , Petersen, C. W. , & Preston, R. L. (2006). Energetics of osmoregulation: I. Oxygen consumption by Fundulus heteroclitus . Journal of Experimental Zoology, 305, 309–317. [DOI] [PubMed] [Google Scholar]
- Koch, M. , Bowes, G. , Ross, C. , & Zhang, X.‐H. (2013). Climate change and ocean acidification effects on seagrasses and marine macroalgae. Global Change Biology, 19(1), 103–132. [DOI] [PubMed] [Google Scholar]
- Kroeker, K. J. , Kordas, R. L. , Crim, R. , Hendriks, I. E. , Ramajo, L. , Singh, G. S. , … Gattuso, J. P. (2013). Impacts of ocean acidification on marine organisms: Quantifying sensitivities and interaction with warming. Global Change Biology, 19(6), 1884–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroeker, K. J. , Kordas, R. L. , Crim, R. N. , & Singh, G. G. (2010). Meta‐analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters, 13(11), 1419–1434. [DOI] [PubMed] [Google Scholar]
- Kuffner, I. B. , Andersson, A. J. , Jokiel, P. L. , Rodgers, K. S. , & MacKenzie, F. T. (2008). Decreased abundance of crustose coralline algae due to ocean acidification. Nature Geoscience, 1(2), 114–117. [Google Scholar]
- Kurihara, H. (2008). Effects of CO2‐driven ocean acidification on the early developmental stages of invertebrates. Marine Ecology Progress Series, 373, 275–284. [Google Scholar]
- Lajeunesse, M. J. , & Forbes, M. R. (2003). Variable reporting and quantitative reviews: A comparison of three meta‐analytical techniques. Ecology Letters, 6(5), 448–454. [Google Scholar]
- Le Quéré, C. , Andrew, R. , Friedlingstein, P. , Sitch, S. , Hauck, J. , Pongratz, J. , … Zheng, B. (2018). Global Carbon Budget 2018. Earth System Science Data, 10(4), 2141–2194. [Google Scholar]
- Le Quéré, C. , Rödenbeck, C. , Buitenhuis, E. T. , Conway, T. J. , Langenfelds, R. , Gomez, A. , … Heimann, M. (2007). Saturation of the Southern Ocean CO2 sink due to recent climate change. Science, 316(5832), 1735–1738. [DOI] [PubMed] [Google Scholar]
- Lin, X. , Huang, R. , Li, Y. , Li, F. , Wu, Y. , Hutchins, D. A. , … Gao, K. (2018). Inter‐active network configuration maintains bacterioplankton community structure under elevated CO2 in a eutrophic coastal mesocosm experiment. Biogeosciences, 15(2), 551–565. [Google Scholar]
- Lindh, M. V. , Riemann, L. , Baltar, F. , Romero‐Oliva, C. , Salomon, P. S. , Granéli, E. , & Pinhassi, J. (2013). Consequences of increased temperature and acidification on bacterioplankton community composition during a mesocosm spring bloom in the Baltic Sea. Environmental Microbiology Reports, 5(2), 252–262. [DOI] [PubMed] [Google Scholar]
- Lohbeck, K. T. , Riebesell, U. , & Reusch, T. B. H. (2012). Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geoscience, 5, 346–351. [Google Scholar]
- Lovenduski, N. S. , Gruber, N. , Doney, S. C. , & Lima, I. D. (2007). Enhanced CO2 out‐gassing in the Southern Ocean from a positive phase of the Southern Annular Mode. Global Biogeochemical Cycles, 21(2). 10.1029/2006GB002900 [DOI] [Google Scholar]
- Luo, D. , Wan, X. , Liu, J. , & Tong, T. (2018a). Optimally estimating the sample mean from the sample size, median, mid‐range, and/or mid‐quartile range. Statistical Methods in Medical Research, 27(6), 1785–1805. [DOI] [PubMed] [Google Scholar]
- Luo, D. , Wan, X. , Liu, J. , & Tong, T. (2018b). Testing normality using the summary statistics with application to meta‐analysis. arXiv, arXiv preprint: 1801.09456. [Google Scholar]
- Maas, E. W. , Law, C. S. , Hall, J. A. , Pickmere, S. , Currie, K. I. , Chang, F. H. , … Caird, D. (2013). Effect of ocean acidification on bacterial abundance, activity and diversity in the Ross Sea, Antarctica. Aquatic Microbial Ecology, 70(1), 1–15. [Google Scholar]
- Manno, C. , Tirelli, V. , Accornero, A. , & Fonda Umani, S. (2010). Importance of the contribution of Limacina helicina faecal pellets to the carbon pump in Terra Nova Bay (Antarctica). Journal of Plankton Research, 32(2), 145–152. [Google Scholar]
- Martin, C. L. , & Tortell, P. D. (2006). Bicarbonate transport and extracellular carbonic anhydrase activity in Bering Sea phytoplankton assemblages: Results from isotope disequilibrium experiments. Limnology and Oceanography, 51(5), 2111–2121. [Google Scholar]
- Maschette, D. , Sumner, M. , & Raymond, B. (2018). SOmap: Southern Ocean maps. R package version 0.1.3.9000. Retrieved from https://github.com/AustralianAntarcticDivision/SOmap [Google Scholar]
- McClintock, J. B. (1994). Trophic biology of Antarctic shallow‐water echinoderms. Marine Ecology Progress Series, 111, 191–202. [Google Scholar]
- McClintock, J. B. , Angus, R. A. , McDonald, M. R. , Amsler, C. D. , Catledge, S. A. , & Vohra, Y. K. (2009). Rapid dissolution of shells of weakly calcified Antarctic benthic macroorganisms indicates high vulnerability to ocean acidification. Antarctic Science, 21(5), 449–456. [Google Scholar]
- McCoy, S. J. , & Kamenos, N. A. (2015). Coralline algae (rhodophyta) in a changing world: Integrating ecological, physiological, and geochemical responses to global change. Journal of Psychology, 51(1), 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn, A. (2017). Ice acidification: A review of the effects of ocean acidification on sea ice microbial communities. Biogeosciences, 14, 3927–3935. [Google Scholar]
- McMinn, A. , Müller, M. N. , Martin, A. , & Ryan, K. G. (2014). The response of Antarctic sea ice algae to changes in pH and CO2 . PLoS ONE, 9(1), e86984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMinn, A. , Müller, M. N. , Martin, A. , Ugalde, S. C. , Lee, S. , Castrisios, K. , & Ryan, K. G. (2017). Effects of CO2 concentration on a late summer surface sea ice community. Marine Biology, 164(4), 87. [Google Scholar]
- McNeil, B. I. , & Matear, R. J. (2007). Climate change feedbacks on future oceanic acidification. Tellus, Series B: Chemical and Physical Meteorology, 59(2), 191–198. [Google Scholar]
- McNeil, B. I. , & Matear, R. J. (2008). Southern Ocean acidification: A tipping point at 450ppm atmospheric CO2 . Proceedings of the National Academy of Sciences of the United States of America, 105(48), 18860–18864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil, B. I. , & Sasse, T. P. (2016). Future ocean hypercapnia driven by anthropogenic amplification of the natural CO2 cycle. Nature, 529(7586), 383. [DOI] [PubMed] [Google Scholar]
- McNeil, B. I. , Tagliabue, A. , & Sweeney, C. (2010). A multi‐decadal delay in the onset of corrosive ‘acidified’ waters in the Ross Sea of Antarctica due to strong air‐sea CO2 disequilibrium. Geophysical Research Letters, 37(19), L19607. [Google Scholar]
- Melzner, F. , Thomsen, J. , Koeve, W. , Oschlies, A. , Gutowska, M. A. , Bange, H. W. , … Körtzinger, A. (2013). Future ocean acidification will be amplified by hypoxia in coastal habitats. Marine Biology, 160(8), 1875–1888. [Google Scholar]
- Miller, L. A. , Papakyriakou, T. N. , Collins, R. E. , Deming, J. W. , Ehn, J. K. , MacDonald, R. W. , … Sutherland, N. (2011). Carbon dynamics in sea ice: A winter flux time series. Journal of Geophysical Research: Oceans, 116(2), 1–20. [Google Scholar]
- Motegi, C. , Tanaka, T. , Piontek, J. , Brussaard, C. P. D. , Gattuso, J. P. , & Weinbauer, M. G. (2013). Effect of CO2 enrichment on bacterial metabolism in an Arctic fjord. Biogeosciences, 10(5), 3285–3296. [Google Scholar]
- Negrete‐García, G. , Lovenduski, N. S. , Hauri, C. , Krumhardt, K. M. , & Lauvset, S. K. (2019). Sudden emergence of a shallow aragonite saturation horizon in the Southern Ocean. Nature Climate Change, 9(4), 313–317. [Google Scholar]
- Nicol, S. (2006). Krill, currents, and sea ice: Euphausia superba and its changing environment. AIBS Bulletin, 56(2), 111–120. [Google Scholar]
- Oliver, A. E. , Newbold, L. K. , Whiteley, A. S. , & van der Gast, C. J. (2014). Marine bacterial communities are resistant to elevated carbon dioxide levels. Environmental Microbiology Reports, 6(6), 574–582. [DOI] [PubMed] [Google Scholar]
- Orr, J. C. , Epitalon, J.‐M. , & Gattuso, J.‐P. (2014). Comparison of seven packages that compute ocean carbonate chemistry. Biogeosciences, 11(4), 5327–5397. [Google Scholar]
- Orr, J. C. , Fabry, V. J. , Aumont, O. , Bopp, L. , Doney, S. C. , Feely, R. A. , … Yool, A. (2005). Anthropogenic ocean acidification over the twenty‐first century and its impact on calcifying organisms. Nature, 437(7059), 681–686. [DOI] [PubMed] [Google Scholar]
- Paulino, A. I. , Egge, J. K. , & Larsen, A. (2007). Effects of increased atmospheric CO2 on small and intermediate sized osmotrophs during a nutrient induced phytoplankton bloom. Biogeosciences, 4(6), 4173–4195. [Google Scholar]
- Pearce, I. , Davidson, A. T. , Bell, E. M. , & Wright, S. (2007). Seasonal changes in the concentration and metabolic activity of bacteria and viruses at an Antarctic coastal site. Aquatic Microbial Ecology, 47(1), 11–23. [Google Scholar]
- Pearse, J. S. , & Bosch, I. (1986). Are the feeding larvae of the commonest Antarctic asteroid really demersal? Bulletin of Marine Science, 39(2), 477–484. [Google Scholar]
- Peck, L. S. (2018). Antarctic marine biodiversity: Adaptations, environments and responses to change. Oceanography and Marine Biology: An Annual Review, 56, 105–236. [Google Scholar]
- Pierrot, D. , Lewis, E. , & Wallace, D. (2006). MS Excel program developed for CO2 system calculations. Oak Ridge, TN: Carbon Dioxide Information Analysis Center, ORNL, U.S. Department of Energy. [Google Scholar]
- Piontek, J. , Borchard, C. , Sperling, M. , Schulz, K. G. , Riebesell, U. , & Engel, A. (2013). Response of bacterioplankton activity in an Arctic fjord system to elevated pCO2: Results from a mesocosm perturbation study. Biogeosciences, 10(1), 297–314. [Google Scholar]
- Quetin, L. B. , & Ross, R. M. (1984). Depth distribution of developing Euphausia superba embryos, predicted from sinking rates. Marine Biology, 79(1), 47–53. [Google Scholar]
- R Core Team (2016). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Raven, J. A. , Cockell, C. S. , & De La Rocha, C. L. (2008). The evolution of inorganic carbon concentrating mechanisms in photosynthesis. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 363(1504), 2641–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raven, J. , & Falkowski, P. (1999). Oceanic sinks for atmospheric CO2 . Plant, Cell and Environment, 22(6), 741–755. [Google Scholar]
- Reinfelder, J. R. (2011). Carbon concentrating mechanisms in eukaryotic marine phytoplankton. Annual Review of Marine Science, 3(1), 291–315. [DOI] [PubMed] [Google Scholar]
- Roden, N. P. , Shadwick, E. H. , Tilbrook, B. , & Trull, T. W. (2013). Annual cycle of carbonate chemistry and decadal change in coastal Prydz Bay, East Antarctica. Marine Chemistry, 155, 135–147. [Google Scholar]
- Rohatgi, A. Web plot digitizer. Retrieved from https://automeris.io/WebPlotDigitizer [Google Scholar]
- Roleda, M. Y. , & Hurd, C. L. (2012). Seaweed responses to ocean acidification In Wiencke C., & Bischof K. (Eds.), Seaweed biology (pp. 407–431). New York, NY: Springer. [Google Scholar]
- Rost, B. , Zondervan, I. , & Wolf‐Gladrow, D. (2008). Sensitivity of phytoplankton to future changes in ocean carbonate chemistry: Current knowledge, contradictions and research directions. Marine Ecology Progress Series, 373, 227–237. [Google Scholar]
- Roy, A.‐S. , Gibbons, S. M. , Schunck, H. , Owens, S. , Caporaso, J. G. , Sperling, M. , … Gilbert, J. A. (2012). Ocean acidification shows negligible impacts on high‐latitude bacterial community structure in coastal pelagic mesocosms. Biogeosciences, 9(9), 13319–13349. [Google Scholar]
- Sabine, C. L. , Feely, R. A. , Gruber, N. , Key, R. M. , Lee, K. , Bullister, J. L. , … Rios, A. F. (2004). The oceanic sink for anthropogenic CO2 . Science, 305(5682), 367–371. [DOI] [PubMed] [Google Scholar]
- Schoenrock, K. M. , Schram, J. B. , Amsler, C. D. , McClintock, J. B. , Angus, R. A. , & Vohra, Y. K. (2016). Climate change confers a potential advantage to fleshy Antarctic crustose macroalgae over calcified species. Journal of Experimental Marine Biology and Ecology, 474, 58–66. [Google Scholar]
- Schram, J. B. , Schoenrock, K. M. , McClintock, J. B. , Amsler, C. D. , & Angus, R. A. (2016). Testing Antarctic resilience: The effects of elevated seawater temperature and decreased pH on two gastropod species. ICES Journal of Marine Science, 73(3), 739–752. [Google Scholar]
- Schram, J. B. , Schoenrock, K. M. , McClintock, J. B. , Amsler, C. D. , & Angus, R. A. (2017). Ocean warming and acidification alter Antarctic macroalgal biochemical composition but not amphipod grazer feeding preferences. Marine Ecology Progress Series, 581, 45–56. [Google Scholar]
- Sewell, M. A. , & Hofmann, G. E. (2011). Antarctic echinoids and climate change: A major impact on the brooding forms. Global Change Biology, 17(2), 734–744. [Google Scholar]
- Sperling, M. , Piontek, J. , Gerdts, G. , Wichels, A. , Schunck, H. , Roy, A. S. , … Engel, A. (2013). Effect of elevated CO2 on the dynamics of particle‐attached and free‐living bacterioplankton communities in an Arctic fjord. Biogeosciences, 10(1), 181–191. [Google Scholar]
- Stark, J. S. , Raymond, T. , Deppeler, S. L. , & Morrison, A. K. (2019). Antarctic seas In Sheppard C. (Ed.), World seas: An environmental evaluation (pp. 1–44). Cambridge, MA: Academic Press. [Google Scholar]
- Stark, J. S. , Roden, N. P. , Johnstone, G. J. , Milnes, M. , Black, J. G. , Whiteside, S. , … Roberts, D. (2018). Carbonate chemistry of an in‐situ free‐ocean CO2 enrichment experiment (antFOCE) in comparison to short term variation in Antarctic coastal waters. Scientific Reports, 8(1), 2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel, A. , Bennecke, S. , Leo, E. , Mintenbeck, K. , Pörtner, H. O. , & Mark, F. C. (2012). Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and pCO2 . Frontiers in Zoology, 9, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel, A. , Graeve, M. , Poertner, H. O. , & Mark, F. C. (2013). Mitochondrial acclimation capacities to ocean warming and acidification are limited in the Antarctic Nototheniid Fish, Notothenia rossii and Lepidonotothen squamifrons . PLoS ONE, 8(7), e68865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel, A. , Leo, E. , Pörtner, H. O. , & Mark, F. C. (2013). Elevated temperature and pCO2 shift metabolic pathways in differentially oxidative tissues of Notothenia rossii . Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 166(1), 48–57. [DOI] [PubMed] [Google Scholar]
- Suckling, C. C. , Clark, M. S. , Richard, J. , Morley, S. A. , Thorne, M. A. , Harper, E. M. , & Peck, L. S. (2015). Adult acclimation to combined temperature and pH stressors significantly enhances reproductive outcomes compared to short‐term exposures. Journal of Animal Ecology, 84(3), 773–784. [DOI] [PubMed] [Google Scholar]
- Taylor, A. R. , Brownlee, C. , & Wheeler, G. L. (2012). Proton channels in algae: Reasons to be excited. Trends Plant Science, 17(11), 675–684. [DOI] [PubMed] [Google Scholar]
- Teichert, S. , & Freiwald, A. (2014). Polar coralline algal CaCO3− production rates correspond to intensity and duration of the solar radiation. Biogeosciences, 11(3), 833–842. [Google Scholar]
- Thomson, P. G. , Davidson, A. T. , & Maher, L. (2016). Increasing CO2 changes community composition of pico‐and nano‐sized protists and prokaryotes at a coastal Antarctic site. Marine Ecology Progress Series, 554, 51–69. [Google Scholar]
- Torstensson, A. , Hedblom, M. , Björk, M. M. , Chierici, M. , & Wulff, A. (2015). Long‐term acclimation to elevated pCO2 alters carbon metabolism and reduces growth in the Antarctic diatom Nitzschia lecointei . Proceedings of the Royal Society B: Biological Sciences, 282(1815), 20151513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortell, P. D. , DiTullio, G. R. , Sigman, D. M. , & Morel, F. M. M. (2002). CO2 effects on taxonomic composition and nutrient utilization in an Equatorial Pacific phytoplankton assemblage. Marine Ecology Progress Series, 236, 37–43. [Google Scholar]
- Tortell, P. D. , & Morel, F. M. (2002). Sources of inorganic carbon for phytoplankton in the eastern Subtropical and Equatorial Pacific Ocean. Limnology and Oceanography, 47(4), 1012–1022. [Google Scholar]
- Tortell, P. D. , Payne, C. D. , Li, Y. , Trimborn, S. , Rost, B. , Smith, W. O. , … DiTullio, G. R. (2008). CO2 sensitivity of Southern Ocean phytoplankton. Geophysical Research Letters, 35(4), 1–5. [Google Scholar]
- Tortell, P. D. , Reinfelder, J. R. , & Morel, F. M. (1997). Active uptake of bicarbonate by diatoms. Nature, 390(6657), 243.9384376 [Google Scholar]
- Trimborn, S. , Thoms, S. , Petrou, K. , Kranz, S. A. , & Rost, B. (2014). Photophysiological responses of Southern Ocean phytoplankton to changes in CO2 concentrations: Short‐term versus acclimation effects. Journal of Experimental Marine Biology and Ecology, 451, 44–54. [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta‐analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. [Google Scholar]
- Wang, Y. , Zhang, R. , Zheng, Q. , Deng, Y. , Van Nostrand, J. D. , Zhou, J. , & Jiao, N. (2016). Bacterioplankton community resilience to ocean acidification: Evidence from microbial network analysis. ICES Journal of Marine Science, 73(3), 865–875. [Google Scholar]
- Westwood, K. , Thomson, P. , van den Enden, R. , Maher, L. , Wright, S. , & Davidson, A. (2018). Ocean acidification impacts primary and bacterial production in Antarctic coastal waters during austral summer. Journal of Experimental Marine Biology and Ecology, 498, 46–60. [Google Scholar]
- Widdicombe, S. , Austen, M. , Kendall, M. , Warwick, R. , & Jones, M. (2000). Bioturbation as a mechanism for setting and maintaining levels of diversity in subtidal macrobenthic communities. Hydrobiologia, 440(1), 369–377. [Google Scholar]
- Wittmann, A. C. , & Pörtner, H.‐O. (2013). Sensitivities of extant animal taxa to ocean acidification. Nature Climate Change, 3(11), 995. [Google Scholar]
- Young, J. N. , Kranz, S. A. , Goldman, J. A. L. , Tortell, P. D. , & Morel, F. M. M. (2015). Antarctic phytoplankton down‐regulate their carbon‐concentrating mechanisms under high CO2 with no change in growth rates. Marine Ecology Progress Series, 532, 13–28. [Google Scholar]
- Zhang, R. , Xia, X. , Lau, S. C. K. , Motegi, C. , Weinbauer, M. G. , & Jiao, N. (2013). Response of bacterioplankton community structure to an artificial gradient of pCO2 in the Arctic Ocean. Biogeosciences, 10(6), 3679–3689. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Data Availability Statement
Raw data used in the meta‐analysis are available via the Australian Antarctic Division Data Centre, https://doi.org/10.26179/5e4f475eb6fbd.


