Abstract
Depression is a common and clinically heterogeneous mental health disorder that is frequently comorbid with other diseases and conditions. Stratification of depression may align sub-diagnoses more closely with their underling aetiology and provide more tractable targets for research and effective treatment. In the current study, we investigated whether genetic data could be used to identify subgroups within people with depression using the UK Biobank. Examination of cross-locus correlations were used to test for evidence of subgroups using genetic data from seven other complex traits and disorders that were genetically correlated with depression and had sufficient power (>0.6) for detection. We found no evidence for subgroups within depression for schizophrenia, bipolar disorder, attention deficit/hyperactivity disorder, autism spectrum disorder, anorexia nervosa, inflammatory bowel disease or obesity. This suggests that for these traits, genetic correlations with depression were driven by pleiotropic genetic variants carried by everyone rather than by a specific subgroup.
Subject terms: Depression, Genetics
Introduction
Depression is a common mental health disorder characterised by persistent feelings of sadness or a loss of interest in day-to-day activities lasting for at least a 2-week period. These feelings can be accompanied by tiredness, changes in appetite, changes in sleep patterns, reduced concentration, feelings of worthlessness or hopelessness and thoughts of self-harm or suicide. Zimmerman et al.1 found that there were 170 different symptom profiles amongst 1566 participants diagnosed with major depressive disorder from the Rhode Island MIDAS project. This variety of different symptom profiles suggest that depression is highly heterogeneous2. Depression is also comorbid with many diseases including cancer3, cardiovascular disease4 and other psychiatric illnesses5. Stratification of depression, to address heterogeneity and comorbidity, may aid in providing valuable aetiological insights and improve treatment efficacy.
Studies aimed at stratifying depression have examined differences between melancholic and atypical depression6, differences between the sexes and recurrence of the disorder7 and used data from other traits, such as neuroticism8 and social contact9 to stratify depression. Twin-based studies10 and genome-wide association studies11,12 have shown depression to be heritable and genetically correlated with a number of other traits and disorders. This shared genetic component could be due to pleiotropic variants shared across all individuals but could also be as a result of a subgroup for the other trait within depression cases. For example, there is a genetic correlation of 0.33 (standard error = 0.03) between depression and bipolar disorder13. If this genetic correlation was due to pleiotropy, then several of the bipolar disorder variants would be carried by most depression cases. However, if this correlation was due to a subgroup, then a greater proportion of the bipolar disorder variants would only be carried by individuals in this subgroup. A subgroup could arise where there is a causal association, a shared molecular pathway, a misclassification between the traits, or an ascertainment bias in the diagnosis of depression.
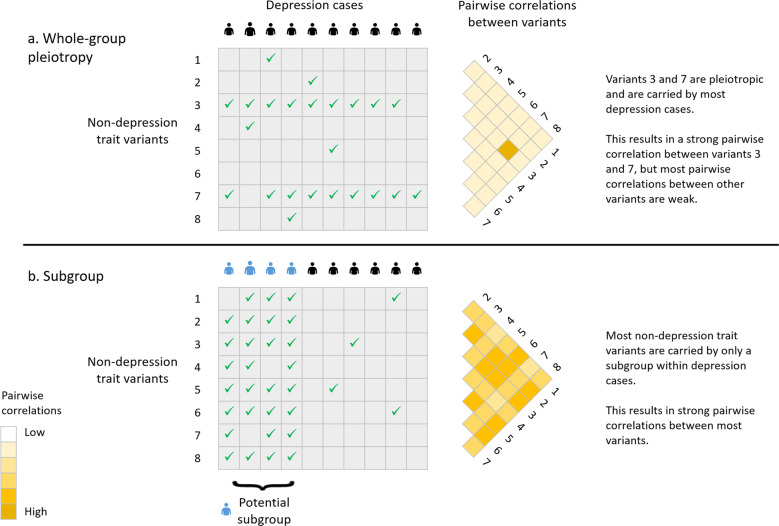
For the current study, BUHMBOX (Breaking Up Heterogeneous Mixture Based On cross(X)-locus correlations)14 was used to determine whether there was evidence of a subgroup within depression that was genetically more similar to other traits. BUHMBOX uses variants associated with a subgroup trait to calculate weighted pairwise correlations of risk allele dosages within depression cases and controls, adjusted for effect size and allele frequency. Where there is a subgroup amongst depression cases that carry a greater proportion of the risk alleles for the non-depression trait, there will be consistent positive pairwise correlations between those variants (as illustrated in Fig. 1). BUHMBOX then calculates a P-value based on the likelihood of the observed pairwise correlations between variants.
Fig. 1. Comparison of the genetic architecture for whole-group pleiotropy and for a subgroup.
Illustration of pairwise correlations between variants for a whole-group pleiotropy, where most depression cases carry a few variants associated with a non-depression trait and b a subgroup within depression cases ( ), where just the subgroup carry many of the non-depression trait variants. A tick indicates a depression case individual is a carrier of that non-depression variant.
), where just the subgroup carry many of the non-depression trait variants. A tick indicates a depression case individual is a carrier of that non-depression variant.
Two definitions of depression were assessed in the UK Biobank15, one based on the Composite International Diagnostic Interview Short Form (CIDI-SF)16 and the other based on a broader help-seeking definition (broad depression)12. Since many traits are genetically correlated with depression13, a power calculation was performed to determine traits with sufficient power to detect a subgroup. Power is determined by the number of depression cases, the size of any subgroup within depression cases, the number of associated variants tested from the subgroup trait and the effect sizes of these variants. We tested sufficiently powered traits for evidence of a subgroup in depression cases using BUHMBOX v0.3814.
Materials and methods
UK Biobank cohort
The UK Biobank is a population-based cohort of 501,726 individuals with imputed genome-wide data for 93,095,623 autosomal genetic variants15. A genetically homogeneous sample of 462,065 individuals was identified using the first two principal components from a 4-means clustering approach. A total of 131,790 individuals were identified as being related up to the third degree (kinship coefficients >0.044) using the KING toolset17 and were removed from the sample. For these related individuals a genomic relationship matrix was calculated to enable the identification of one individual from each related group that could be reinstated. This allowed the reintroduction of 55,745 individuals providing an unrelated sample of 386,020 individuals.
UK Biobank depression phenotypes
Two depression phenotypes were assessed for evidence of subgroups in UK Biobank. Both phenotypes were restricted to only those individuals that had completed the online mental health questionnaire (n = 109,049). The first phenotype analysed was based on the Composite International Diagnostic Interview Short Form (CIDI-SF)18 as used by Davis et al.16 to provide a lifetime instance measure of depression in the UK Biobank. Davis et al.16 provide a more in-depth description of this CIDI-SF phenotype, but in summary cases were defined as having:
At least one core symptom of depression (persistent sadness (Data-Field: 20446) or a loss of interest (Data-Field: 20441)) for most or all days over a two-week period which were present “most of the day” or “all of the day”.
Plus at least another four non-core depressive symptoms with some or a lot of impairment experienced during the worst 2-week period of depression or low mood.
The non-core depressive symptoms that were included in this assessment of the worst episode of depression were: Feelings of tiredness (Data-Field: 20449), Weight change (Data-Field: 20536), Did your sleep change? (Data-Field: 20532), Difficulty concentrating (Data-Field: 20435), Feelings of worthlessness (Data-Field: 20450) and Thoughts of death (Data-Field: 20437). Cases that self-reported another mood disorder were excluded. Controls were determined by not having at least one core symptom of depression or not endorsing at least another four non-core depressive symptoms if at least one core symptom was endorsed. This provided 25,721 CIDI-SF cases and 61,894 controls.
A second depression phenotype within the UK Biobank cohort was also examined using the broad depression definition from Howard et al.12 with detailed information provided in that paper. In summary, cases had sought help for nerves, anxiety, tension or depression from either a general practitioner or a psychiatrist (Data-Field: 2090 and Data-Field: 2100), whereas controls had not. Cases were supplemented with an additional 132 individuals identified as having a primary or secondary International Classification of Diseases (ICD)-10 diagnosis of a depressive mood disorder from linked hospital admission records (Data-Field: 41202 and Data-Field: 41204). Participants identified with bipolar disorder, schizophrenia or personality disorder and those reporting a prescription for an antipsychotic medication were removed. This provided a total of 36,790 broad depression cases and 70,304 controls. The phenotypic correlation between the CIDI-SF depression phenotype and the broad depression phenotype was 0.61 with the number of cases and controls shared across the two definitions shown in Supplementary Table 1.
Sensitivity analysis
To allow a direct comparison between the two definitions of depression, the main analysis was restricted to those UK Biobank participants that had completed the mental health questionnaire. To examine whether the full UK Biobank sample provided greater power for the detection of subgroups, a sensitivity analysis was conducted using the broad depression phenotype (113,769 cases and 208,811 controls).
Traits examined as subgroups within depression
We selected traits genetically correlated with depression (false discovery rate corrected, q < 0.01) in Howard et al.13 to test as subgroups within depression, which included anthropomorphic, autoimmune, life course, cardiovascular and other psychiatric traits. For each trait, there was a requirement that publicly available summary statistics were available and that the UK Biobank was not included in that study due to potential confounding effects (Supplementary Table 2).
The BUHMBOX power calculation test v0.114 was used to determine whether there was sufficient power to detect a subgroup for each depression correlated trait and to identify the optimum variant selection criterion (P < 5 × 10−8, P < 10−6 or P < 10−4). The power calculation was conducted separately for the CIDI-SF depression phenotype and the broad depression phenotype. Variants from the summary statistics for each subgroup trait were examined in the UK Biobank. Variants that had a call rate <0.99, were out of Hardy–Weinberg equilibrium (P < 10−10), had a hard call threshold <0.25, or had a minor allele frequency <0.01 were excluded. BUHMBOX requires that all variants are available for all individuals and therefore individuals with a call rate <1 were removed. To identify independently segregating variants, clumping was conducted in PLINK v1.90b419 using an r2 value of 0.01 across a 3-Mb window in either CIDI-SF or broad depression control individuals, respectively.
For the power analysis the approach used in Han et al.14 was followed, with 1000 simulated iterations run for each trait, the proportion of individuals in the subgroup was set to the genetic risk score beta coefficient (which represents the upper bound of the heterogeneity proportion) and a nominal subgroup P-value of 0.05 was used. Power analyses were used to identify the optimum variant selection criterion that provided the greatest power for each subgroup trait. Where power was the same across variant selection criteria, the strictest variant selection criterion was selected as the optimum. Variants with P < 10−4 were not publicly available for Squamous Cell Lung Cancer or Lung Cancer and so P < 10−5 was used instead. Only those traits that had a power >0.6 (using the optimum variant selection criterion) were selected to be tested for evidence of a subgroup within depression. A linear regression was used to examine the association between power and the heritability of each subgroup trait and the genetic correlation each subgroup trait shares with depression.
Testing for subgroups within depression
For the traits that had power >0.6, variants meeting the optimum variant selection criterion were extracted from the UK Biobank. The same quality control thresholds and method to identify independently segregating variants as used as previously in the power analysis were applied. BUHMBOX v0.3814 was used to examine shared risk alleles for each subgroup trait within CIDI-SF depression and broad depression. BUHMBOX uses the positive correlations between risk allele dosages in cases to determine whether any sharing of risk alleles is driven by all individuals (whole-group pleiotropy) or by a subset of individuals (Fig. 1). The likelihood of observing such positive correlations are used to determine the subgroup P-values.
Sex, age, genotyping array and the first 20 principal components were fitted as covariates in the subgroup analysis. Bonferroni correction was used to account for the multiple testing of subgroup traits, with P-values <7.14 × 10−3 (0.05/7) or <0.01 (0.05/5) deemed significant for CIDI-SF or broad depression, respectively. No multiple testing correction was applied for the two depression definitions analysed. In the sensitivity analysis, using the full UK Biobank sample, a P-value <8.33 × 10−3 (0.05/6) was deemed significant for broad depression.
Results
Power analyses of potential subgroups traits
To determine whether there was sufficient power (>0.6) to detect a subgroup and identify the optimum variant selection criterion (P < 5 × 10−8, P < 10−6 or P < 10−4) for each trait the BUHMBOX power calculation test v0.114 was used. The genetic risk score beta coefficients, representing an upper bound for heterogeneity proportion, for each trait within either Composite International Diagnostic Interview Short Form (CIDI-SF) depression or broad depression are provided in Supplementary Table 3. The results of the power analysis for detecting a subgroup for 25 available traits within the two depression definitions are provided in Table 1. Five traits had power >0.6 across both the CIDI-SF depression and broad depression definitions: bipolar disorder20, attention deficit/hyperactivity disorder21, autism spectrum disorder22, anorexia nervosa23 and inflammatory bowel disease24. There were two further traits, schizophrenia25 and obesity 326, that had power >0.6 for detection of a subgroup in CIDI-SF depression.
Table 1.
Power analysis for detecting a subgroup for 25 traits within either Composite International Diagnostic Interview Short Form (CIDI-SF) depression or broad depression in the UK Biobank.
| Subgroup trait | PubMed ID | Optimum variant selection criterion | Power | Optimum variant selection criterion | Power |
|---|---|---|---|---|---|
| CIDI-SF depression | Broad depression | ||||
| Neuroticism | 24,828,478 | <10−4 | 0.137 | <10−4 | 0.120 |
| Schizophrenia | 25,056,061 | <10−6 | 0.607 | <10−6 | 0.306 |
| Bipolar disorder | 29,906,448 | <10−4 | 0.912 | <10−4 | 0.727 |
| Attention deficit/hyperactivity disorder | 30,478,444 | <10−4 | 0.912 | <10−4 | 0.992 |
| Autism spectrum disorder | 30,804,558 | <10−4 | 1 | <10−4 | 1 |
| Anorexia nervosa | 28,494,655 | <10−4 | 1 | <10−4 | 1 |
| Triglyceride level | 24,097,068 | <10−4 | 0.183 | <5 × 10−8 | 0.131 |
| Coronary artery disease | 26,343,387 | <10−4 | 0.229 | <5 × 10−8 | 0.071 |
| Crohn’s disease | 26,192,919 | <10−4 | 0.193 | <10−4 | 0.271 |
| Inflammatory bowel disease | 28,067,908 | <10−4 | 0.706 | <10−6 | 0.665 |
| Waist to hip ratio | 25,673,412 | <10−4 | 0.070 | <5 × 10−8 | 0.076 |
| Body fat percentage | 26,833,246 | <10−6 | 0.057 | <10−6 | 0.067 |
| Waist circumference | 25,673,412 | <10−4 | 0.107 | <10−4 | 0.070 |
| Overweight | 23,563,607 | <10−4 | 0.131 | <5 × 10−8 | 0.068 |
| Obesity 1 | 23,563,607 | <10−4 | 0.199 | <10−6 | 0.089 |
| Obesity 3 | 23,563,607 | <10−4 | 0.794 | <10−4 | 0.196 |
| Body mass index | 25,673,413 | <10−4 | 0.101 | <10−4 | 0.073 |
| Age of menarche | 25,231,870 | <10−4 | 0.451 | <5 × 10−8 | 0.081 |
| Age of natural menopause | 26,414,677 | <10−4 | 0.407 | <10−4 | 0.220 |
| Years of schooling | 25,201,988 | <10−4 | 0.105 | <10−4 | 0.089 |
| College completion | 25,201,988 | <10−4 | 0.248 | <10−4 | 0.160 |
| Ever smoked | 20,418,890 | <10−4 | 0.081 | <10−4 | 0.134 |
| Age of smoking initiation | 20,418,890 | <10−4 | 0.061 | <10−4 | 0.062 |
| Squamous cell lung cancera | 28,604,730 | <10−5 | 0.078 | <5 × 10−8 | 0.085 |
| Lung cancera | 28,604,730 | <10−5 | 0.123 | <10−6 | 0.137 |
PubMed identifiers (PubMed ID) for the 25 traits are provided. Bold values indicate that power was > 0.6. The optimum variant selection criterion that maximised power for the subgroup traits are provided.
aVariants with P < 10−4 were not publicly available for Squamous Cell Lung Cancer or Lung Cancer and so variants with P < 10−5 were tested instead.
A linear regression of subgroup power on the heritability of each subgroup trait and the genetic correlation shared with depression revealed that heritability was positively associated with power (CIDI-SF depression P-value = 5.32 × 10−4; broad depression P-value = 3.48 × 10−4), but genetic correlation with depression was not associated with power (CIDI-SF depression P-value = 0.57; broad depression P-value = 0.21).
The sensitivity analysis, analysing broad depression in the full UK Biobank sample, provided a small increase in power for the majority of subgroups compared to broad depression amongst individuals who had completed the mental health questionnaire. Six traits had power >0.6: bipolar disorder, attention deficit/hyperactivity disorder, autism spectrum disorder, anorexia nervosa, inflammatory bowel disease and schizophrenia (Supplementary Table 4).
Testing for subgroups within depression
BUHMBOX v0.3814 was used to test seven traits for evidence of a subgroup within CIDI-SF depression, five traits within broad depression and six traits in the sensitivity analysis. The results of the subgroup for CIDI-SF and broad depression analyses are provided in Table 2 and the results of the sensitivity analysis are provided in Supplementary Table 5. None of the traits examined provided evidence of a genetic subgroup within depression (P > 0.05) before correction for multiple testing.
Table 2.
Evidence of a subgroup from traits tested within either Composite International Diagnostic Interview Short Form (CIDI-SF) depression or broad depression in the UK Biobank.
| Depression definition | Subgroup trait | Variants | βGRS | Depression cases/controls | Subgroup P-value |
|---|---|---|---|---|---|
| CIDI-SF | Schizophrenia | 180 | 0.077 | 15,311/36,811 | 0.42 |
| Bipolar disorder | 436 | 0.062 | 8140/19,466 | 0.62 | |
| Attention deficit/hyperactivity disorder | 342 | 0.028 | 8522/21,030 | 0.11 | |
| Autism spectrum disorder | 242 | 0.057 | 13,138/31,598 | 0.12 | |
| Anorexia nervosa | 169 | 0.016 | 16,024/38,388 | 0.47 | |
| Inflammatory bowel disease | 954 | 7.37 × 10−3 | 2186/5265 | 0.46 | |
| Obesity 3 | 61 | 0.038 | 22,096/53,312 | 0.55 | |
| Broad | Bipolar disorder | 435 | 0.041 | 11,531/22,186 | 0.60 |
| Attention deficit/hyperactivity disorder | 342 | 0.034 | 12,345/23,844 | 0.07 | |
| Autism spectrum disorder | 242 | 0.051 | 18,802/36,000 | 0.15 | |
| Anorexia nervosa | 169 | 7.87 × 10−3 | 22,946/43,644 | 0.79 | |
| Inflammatory bowel disease | 219 | 8.02 × 10−3 | 22,738/43,355 | 0.64 |
The number of individuals classified as depression cases and depression controls is provided. The number of variants assessed and the genetic risk score beta coefficient (βGRS, representing the upper bound of the heterogeneity proportion) using the optimum variant selection criterion for that trait (as provided in Table 1).
Discussion
Depression is a heterogeneous mental health disorder and is comorbid with many other diseases and illnesses. Over the last few years, valuable progress has been made in understanding the underlying genetic architecture of depression11,13,27. Furthermore, stratifying depression using genetic data remains a key goal within the psychiatric genetics community28 and should lead to improved classification of mental health conditions and more efficacious treatment for patients. Machine learning29,30 and polygenic risk score6,31 approaches offer possible methods for stratification in mental health. In the current study, we used BUHMBOX14 to identify whether traits that were genetically correlated with depression were correlated due to a subgroup, i.e. the correlation was driven by a subset of depressed individuals who had a greater genetic loading for the trait. Evidence of a subgroup within depression may provide future opportunities for stratifying the disease.
To allow a direct comparison between stricter and broader definitions of depression two phenotypes were examined. For the subgroups examined across both definitions (and using the same variant selection criteria), CIDI-SF depression had greater upper bounds for the heterogeneity proportion for bipolar disorder, autism spectrum disorder and anorexia nervosa, whereas broad depression had a greater upper bound for the heterogeneity proportion for attention deficit/hyperactivity disorder. The heterogeneity upper bound was assessed using genetic risk scores, which suggests that a stricter definition of depression shared a larger genetic component with bipolar disorder, autism spectrum disorder and anorexia nervosa and the broader definition shared a genetic component with attention deficit/hyperactivity disorder. This supports the observations of Cai et al.32 for bipolar disorder, autism spectrum disorder and attention deficit/hyperactivity disorder using genetic correlations (although they did not assess anorexia nervosa). As there were no significant subgroups found within depression, no firm conclusions can be drawn on the effectiveness of using stricter or broader definitions to stratify depression.
The lack of evidence for subgroups within depression for the seven traits examined with BUHMBOX, suggest that the previously reported genetic correlations13 were the result of pleiotropy, i.e. a genetic variant is associated with multiple phenotypes. Pleiotropy can result from either horizontal pleiotropy (where a variant has direct effects on multiple phenotypes) or vertical pleiotropy (where a variant has an effect on a phenotype, then this phenotype influences further traits downstream)33. To assess the presence of vertical pleiotropy a technique known as Mendelian randomisation34 can be used. This technique has been applied previously to depression and the traits examined with BUHMBOX, and no evidence of vertical pleiotropy was found13. This indicates that the genetic correlations between depression and the seven traits examined as subgroups are likely due to horizontal pleiotropy. Gaining a greater understanding of the biological mechanisms associated with pleiotropic variants could be informative for improving our comprehension and treatment for both depression and the correlated traits.
A sensitivity analysis was conducted to investigate whether additional power for detection of subgroups within broad depression could be obtained by analysing the full UK Biobank sample (n = 322,580) compared to the subsample that had completed the mental health questionnaire (n = 109,049). Decreased power was observed for some subgroup traits using the full sample which was due to lower heterogeneity proportions (based on the genetic risk score beta coefficient) and fewer genetic variants available for analysis (as all variants are required to be known and so fewer were available in the full sample). For most subgroup traits greater power was available using the full sample, however most were still underpowered to run the subgroup analysis. Schizophrenia was the only subgroup trait that sufficiently increased in power to exceed the >0.6 threshold, although no evidence of a subgroup was found. The average increase in power using the full sample compared to the mental health questionnaire subsample was only 0.06. However, larger genome-wide association studies of the currently underpowered traits could allow their re-examination as subgroups within depression in the future. The power to detect a subgroup for a trait was also influenced by the trait’s heritability, but not its genetic correlation with depression. Therefore, there is the potential to assess additional highly heritable traits where a feasible subgroup may exist within depression.
The limitations of the current study include selection bias, whereby particular individuals are more likely to participate in population-based cohorts or complete additional assessments, such as the online mental health questionnaire. Participants of the UK Biobank are healthier and from less deprived areas than the general population35 and those that completed the mental health questionnaire had a lower genetic predisposition to severe depression than those who did not36. UK Biobank participants that had either a self-reported or a hospital diagnosis of schizophrenia or bipolar disorder were excluded in the current analysis, which may limit the potential for identifying subgroups for these disorders. Most of the traits that are genetically correlated with depression were not included in the subgroup analysis due to a lack of power (≤0.6). As increasing large genome-wide association studies become available, a greater number of variants will meet the required selection criteria, allowing additional traits to be tested for evidence of a subgroup within depression.
Depression is both polygenic and heterogeneous and stratification of the disorder may lead to improvements in treatment outcomes. We examined 25 traits genetically correlated with depression using individuals that had completed the UK Biobank mental health questionnaire. There were seven traits sufficiently powered to be tested as subgroups within CIDI-SF depression and five traits tested as subgroups within broad depression, although none of these provided evidence for a genetic subgroup within depression. Alternative methodologies for stratification of depression could also be examined (i.e. polygenic risk scores and cluster analysis) along with consideration of other potential stratifiers (i.e. depression severity, depressive symptoms and antidepressant treatment response).
Supplementary information
Acknowledgements
This research was conducted using the UK Biobank resource, application number 4844. We are grateful to the UK Biobank and all its voluntary participants. The UK Biobank study was conducted under generic approval from the NHS National Research Ethics Service (approval letter dated 17 June 2011, Ref 11/NW/0382). All participants gave full informed written consent. D.M.H is supported by a Sir Henry Wellcome Postdoctoral Fellowship (Reference 213674/Z/18/Z) and a 2018 NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (Ref: 27404). A.M.McI acknowledges support from the Wellcome Trust (Wellcome Trust Strategic Award ‘STratifying Resilience and Depression Longitudinally’ (STRADL) Reference 104036/Z/14/Z and The Sackler Trust. N.R.W. acknowledges NMHRC grants 1078901 and 1087889. C.M.L acknowledges MRC grant MR/N015746/1. S.P.H. acknowledges MRC grant MR/S0151132. This investigation represents independent research part-funded by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Code availability
The R code for BUHMBOX v0.38 and BUHMBOX power calculation test v0.1 are freely available and downloadable from http://software.broadinstitute.org/mpg/buhmbox/.
Conflict of interest
Cathryn Lewis is a member of the Science Advisory Board for Myriad Neuroscience. Andrew McIntosh has received speaker fees from Illumina and Janssen. The authors report no other conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-0848-0).
References
- 1.Zimmerman M, Ellison W, Young D, Chelminski I, Dalrymple K. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Compr. Psychiatry. 2015;56:29–34. doi: 10.1016/j.comppsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Fried EI. Moving forward: how depression heterogeneity hinders progress in treatment and research. Expert Rev. Neurother. 2017;17:423–425. doi: 10.1080/14737175.2017.1307737. [DOI] [PubMed] [Google Scholar]
- 3.Walker J, et al. Prevalence, associations, and adequacy of treatment of major depression in patients with cancer: a cross-sectional analysis of routinely collected clinical data. Lancet Psychiatry. 2014;1:343–350. doi: 10.1016/S2215-0366(14)70313-X. [DOI] [PubMed] [Google Scholar]
- 4.Hare D. L., Toukhsati S. R., Johansson P., Jaarsma T. Depression and cardiovascular disease: a clinical review. Eur. Heart J.35, 1365–1372 (2013). [DOI] [PubMed]
- 5.Avenevoli S, Swendsen J, He J-P, Burstein M, Merikangas K. Major depression in the national comorbidity survey- adolescent supplement: prevalence, correlates, and treatment. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54:37–44.e32. doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milaneschi Y, et al. Polygenic dissection of major depression clinical heterogeneity. Mol. Psychiatry. 2016;21:516–522. doi: 10.1038/mp.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall LS, et al. Genome-wide meta-analyses of stratified depression in Generation Scotland and UK Biobank. Transl. Psychiatry. 2018;8:9. doi: 10.1038/s41398-017-0034-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adams M. J., et al. Genetic stratification of depression by neuroticism: revisiting a diagnostic tradition. Psychol. Med. 1–10 10.1017/S0033291719002629 (2019). [DOI] [PMC free article] [PubMed]
- 9.Sarda A, Munuswamy S, Sarda S, Subramanian V. Using passive smartphone sensing for improved risk stratification of patients with depression and diabetes: cross-sectional observational study. JMIR Mhealth Uhealth. 2019;7:e11041. doi: 10.2196/11041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 11.Wray NR, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat. Genet. 2018;50:668–681. doi: 10.1038/s41588-018-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howard DM, et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018;9:1470. doi: 10.1038/s41467-018-03819-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howard DM, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 2019;22:343–352. doi: 10.1038/s41593-018-0326-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han B, et al. A method to decipher pleiotropy by detecting underlying heterogeneity driven by hidden subgroups applied to autoimmune and neuropsychiatric diseases. Nat. Genet. 2016;48:803–810. doi: 10.1038/ng.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bycroft C, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562:203–209. doi: 10.1038/s41586-018-0579-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis KAS, et al. Mental health in UK Biobank – development, implementation and results from an online questionnaire completed by 157 366 participants: a reanalysis. BJPsych Open. 2020;6:e18. doi: 10.1192/bjo.2019.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manichaikul A, et al. Robust relationship inference in genome-wide association studies. Bioinformatics. 2010;26:2867–2873. doi: 10.1093/bioinformatics/btq559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen H-U. The World Health Organization Composite International Diagnostic Interview short-form (CIDI-SF) Int. J. Methods Psychiatr. Res. 1998;7:171–185. doi: 10.1002/mpr.47. [DOI] [Google Scholar]
- 19.Chang CC, et al. Second-generation PLINK: rising to the challenge of larger and richer datasets. GigaScience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruderfer DM, et al. Genomic dissection of bipolar disorder and schizophrenia, Including 28 subphenotypes. Cell. 2018;173:1705–1715.e1716. doi: 10.1016/j.cell.2018.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demontis D, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat. Genet. 2019;51:63–75. doi: 10.1038/s41588-018-0269-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grove J, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat. Genet. 2019;51:431–444. doi: 10.1038/s41588-019-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duncan L, et al. Significant locus and metabolic genetic correlations revealed in genome-wide association study of Anorexia Nervosa. Am. J. Psychiatry. 2017;174:850–858. doi: 10.1176/appi.ajp.2017.16121402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Lange KM, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017;49:256. doi: 10.1038/ng.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–427. doi: 10.1038/nature13595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berndt SI, et al. Genome-wide meta-analysis identifies 11 new loci for anthropometric traits and provides insights into genetic architecture. Nat. Genet. 2013;45:501–512. doi: 10.1038/ng.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hyde CL, et al. Identification of 15 genetic loci associated with risk of major depression in individuals of European descent. Nat. Genet. 2016;48:1031–1036. doi: 10.1038/ng.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIntosh AM, Sullivan PF, Lewis CM. Uncovering the genetic architecture of major depression. Neuron. 2019;102:91–103. doi: 10.1016/j.neuron.2019.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chekroud AM, et al. Cross-trial prediction of treatment outcome in depression: a machine learning approach. Lancet Psychiatry. 2016;3:243–250. doi: 10.1016/S2215-0366(15)00471-X. [DOI] [PubMed] [Google Scholar]
- 30.Trakadis YJ, Sardaar S, Chen A, Fulginiti V, Krishnan A. Machine learning in schizophrenia genomics, a case-control study using 5,090 exomes. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2019;180:103–112. doi: 10.1002/ajmg.b.32638. [DOI] [PubMed] [Google Scholar]
- 31.Wigmore E. M., et al. Genome-wide association study of antidepressant treatment resistance in a population-based cohort using health service prescription data and meta-analysis with GENDEP. Pharmacogenomics J.10.1038/s41397-019-0067-3 2019. [DOI] [PMC free article] [PubMed]
- 32.Cai N, et al. Minimal phenotyping yields genome-wide association signals of low specificity for major depression. Nat. Genet. 2020;52:437–447. doi: 10.1038/s41588-020-0594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyler AL, Asselbergs FW, Williams SM, Moore JH. Shadows of complexity: what biological networks reveal about epistasis and pleiotropy. BioEssays. 2009;31:220–227. doi: 10.1002/bies.200800022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014;23:R89–R98. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fry A, et al. Comparison of sociodemographic and health-related characteristics of UK Biobank articipants With those of the general population. Am. J. Epidemiol. 2017;186:1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams M. J., et al. Factors associated with sharing e-mail information and mental health survey participation in large population cohorts. Int. J. Epidemiol.10.1093/ije/dyz134 (2019). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The R code for BUHMBOX v0.38 and BUHMBOX power calculation test v0.1 are freely available and downloadable from http://software.broadinstitute.org/mpg/buhmbox/.