Abstract
Objective:
To describe the treatment of community-acquired pneumonia (CAP) in children under five years in Tanzania.
Methods:
Between January and December 2017, children aged 2–59 months with chest radiography- confirmed CAP were enrolled. The parents were interviewed to collect information on the patients and home-based medication. Clinical information was derived from the patient files. Nasopharyngeal swab and blood samples were collected for isolation of the causative pathogens. Swab samples were analysed by quantitative PCR whereas blood samples were tested using BacT/Alert 3D.
Results:
Overall, 109 children with CAP were included in this analysis. Provision of care to most children was delayed (median = 4.6 days). A quarter (26.6%) were given unprescribed/leftover antibiotics at home. Only one child had positive bacterial culture. Referrals were associated with nasopharyngeal carriage of Streptococcus pneumoniae (p = 0.003) and Haemophilus influenzae (p = 0.004). Of all admitted children, more than a quarter (n = 29) did not need to be hospitalised and inappropriately received injectable instead of oral antibiotics.
Conclusion:
We found high rates of home treatment, particularly with antibiotics. Appropriate health care was delayed for most children because of home treatment. Efforts are needed at the community level to improve awareness of antimicrobial resistance.
Keywords: Home treatment, Community-acquired pneumonia, Children under five years, Delays, Unnecessary hospitalisation
Background
Globally, community-acquired pneumonia (CAP) remains one of the leading causes of death among children below five years of age. Most CAP-related deaths occur in low- and middle-income countries (LMICs). Approximately 70% of these deaths occur in 15 countries, with Tanzania being eighth on the list of countries with high CAP-related deaths (IVAC, 2018). In 2018 alone, pneumonia led to the death of 17,624 children below five years old in Tanzania (IVAC, 2018). The high pneumonia incidence is a drawback in meeting the health sustainable development goals of the United Nations (goal 3) (UN General Assembly, 2015).
Collaborative efforts of different stakeholders like the World Health Organization (WHO), the United Nations Children’s Fund (UNICEF), and the Government of Tanzania have been in place for several years. The country has made significant progress in reducing CAP-related deaths, particularly in the area of CAP prevention, by introducing vaccines with a high coverage (IVAC, 2018). Despite all these efforts, the death rate related to CAP in children aged under five years still remains unacceptably high in Tanzania.
In fact, the measures to prevent and treat CAP in children are available, but reducing morbidity and mortality still remains an enormous challenge (Leung et al., 2016). To stop preventable deaths caused by CAP, correct and timely management is crucial. There have been reports of parents or caregivers delaying seeking appropriate healthcare (Minz et al., 2017; Pajuelo et al., 2018; Umuhoza et al., 2018) and of children not being managed appropriately at healthcare facilities (Lugangira et al., 2017). For example, studies in Uganda and Peru reported that children with cough or other respiratory tract infections were not taken to a healthcare facility in a timely manner (Tuhebwe et al., 2014; Muro et al., 2017; Pajuelo et al., 2018). Parents or caregivers first observe the symptoms for improvement. Officially, dispensing antibiotics without a prescription is not allowed in Tanzania, as in many other countries; however, because of poor monitoring, some parents buy antibiotics from community pharmacies and others use drugs stocked at home to self-treat the child before deciding to seek medical care (Wu and Juurlink, 2014). Often, children visiting the facilities are severely ill, probably because of waiting for improvement, failure of home treatment, and delayed treatment seeking (Pajuelo et al., 2018).
At the health facility, correct diagnosis of CAP followed by appropriate treatment is critical. While the WHO recommends the use of chest radiography in addition to the assessment of clinical features, this is not common in most resource-limited settings (Uwemedimo et al., 2018). In most cases, in practice, syndromic diagnosis is common. For pneumonia, the new WHO guidelines adopted in many resource-limited countries, including Tanzania, recommend home treatment with oral amoxicillin as the first-line treatment. However, in severe cases, patients are supposed to be admitted for close monitoring (WHO, 2013; WHO/UNICEF, 2014; MoHCDGEC, 2018).
Despite all these efforts, CAP continues to be fatal in young children. Delay in appropriate care, self-medication, and inappro- priate hospital management have been linked to poor CAP treatment outcomes. However, the current trends in delays, CAP attributes, self-medication, and antibiotic prescription practices remain unclear in most settings. Thus, this study was designed to examine the CAP management strategies used for treating children aged below five years in Tanzania.
Materials and methods
Clinical study
Between January and December 2017, hospitalised children aged 2–59 months with chest radiography-confirmed CAP were enrolled in this study, as previously described (Ngocho et al., 2019). Children with CAP at three health facilities in Moshi municipality, Kilimanjaro region, i.e. Kilimanjaro Christian Medical Centre, Mawenzi Regional Hospital, and St. Joseph Designated District Hospital, were eligible for participation.
Ten research assistants (1 data manger, 6 nurses, and 3 laboratory technicians) were recruited to assist with data collection. Before starting data collection, the research assistants were trained for one week on the ethical conduct of human research, study aims, and data collection methods. The laboratory technicians were trained on study-specific sample transportation, processing, and storage.
The nurses discussed the study aims with interested parents before asking them to provide written consent for participation. The interviews were conducted in Swahili with the parents or guardians to collect demographic data and clinical characteristics. In addition to demographic characteristics (e.g. age of the child, and sex of the child), data on the following variables were also collected: prior history of medications used, particularly antibiotics, onset of illness, and if the child had medical insurance. Further, data on the diagnosis, investigations conducted, clinical findings (e.g. cyanosis and body temperature), and treatment given were extracted from the children’s clinical files. Next, pneumonia severity was extracted from the files and classified according to the WHO guidelines, as per which children aged 2–59 months with cough and/or difficulty breathing and fast breathing and/or chest in-drawing were considered to have pneumonia while those with severe symptoms (e.g. vomiting, convulsion, unconsciousness, and unable to breastfeed or drink) were considered to have severe pneumonia (WHO/UNICEF, 2014). Further, the guidelines recommend home treatment with amoxicillin for children with pneumonia and hospitalisation for those with severe pneumonia. Anthropometric measurements were recorded; weight was measured using a Seca Digital Baby Scale Model 354 (Seca GmbH & Co. KG, Hamburg, Germany). The weight-for-age Z-score was derived from the WHO child growth standards, and underweight was defined as a Z score below −2 SDs. An HIV test was performed for children with unknown status; serial testing was done with SD Bioline HIV-1/2 3.0, a lateral flow immunochromatographic assay (Sd standard diagnostics, Inc. 65, Borahagal-ro, Giheung-gu, Yongin-si, Gyeonggi-do, Republic of Korea) and Uni-Gold HIV, a lateral flow immunochromatographic assay (Trinity biotech plc, Bray, Co. Wicklow, Ireland), following the Tanzania HIV testing guidelines (MoHCDGEC, 2017).
After conducting the interviews and anthropometric measurements, the nurses collected nasopharyngeal swab samples and blood samples from the participants, following standard microbiological techniques. Swabs were collected using UTM® nasal swab flocked with 3 mL of UTM® medium (cat. No. HCPN305C, Copan, Italy), and blood samples were collected using 2-mL BacT/Alert Pediatric FAN blood culture bottles. Standard recommended antisepsis procedures were used during blood specimen collection. Nasopharyngeal swab samples were immediately transported to the laboratory at Kilimanjaro Clinical Research Institute of Biotechnology and stored at −80 ° C. Later on, these samples were transported with dry ice to the Laboratory of Paediatric Infectious Diseases at Radboud University Medical Center (Radboud UMC) in the Netherlands and were stored at −20 ° C for analysis. The blood samples were analysed at the biotechnology laboratory.
BacT/Alert blood culture for the detection of bacteria in blood samples
At the laboratory, the bottles were inspected for growth, weighed, and then incubated in the BacT/Alert instrument. The automated BacT/Alert instrument monitored them for the presence of bacterial growth. The positive bottles were sub-cultured, and direct smears were prepared, followed by bacteria identification. Samples were declared negative after a five-day incubation period if no growth was observed. Positive samples were sub-cultured in sheep blood agar, MacConkey agar, and chocolate agar; incubated at 37 ° C; and examined for growth followed by biochemical identification of Streptococcus pneumo- niae (S. pneumoniae), Staphylococcus aureus (S. aureus), and Haemophilus influenzae (H. influenzae).
Real-time quantitative PCR (qPCR) for the detection of bacteria and real-time PCR for the detection of respiratory syncytial virus (RSV) and influenza virus A/B
DNA contents of positive controls (S. pneumoniae TIGR4, S. aureus NCTC8178, Moraxella catarrhalis (M. catarrhalis) BBH18, and H. influenzae R2866) were extracted using the Qiagen DNeasy Blood & Tissue kit according to the manufacturer’s instructions (cat. No. 69506, Qiagen German). The DNA contents of positive controls were diluted 10-fold starting at 10 ng/μL. Serial dilution was carried out to quantify the amount of DNA in the clinical samples. Five sets of primers specific to these five bacteria were selected for RT-qPCR. For the identification of S. pneumoniae, the TaqMan assay was performed. For the identification of S. aureus, H. influenzae, Mycoplasma pneumoniae (M. pneumoniae), and M. catarrhalis, the SYBR Green assay was performed. The quantification results were considered positive if the quantification cycle (Cq) values were <36. For detection of RSV, the Diagenode assay was performed using the primer and probe design validated and described by Templeton et al. (2004).
Data analysis
Data processing and analysis were performed in STATA version 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX, StataCorp. LLC). Descriptive analysis was performed to determine the distribution of the participants, CAP management at different levels, and prevalence of bacteria and/or viral isolates. The proportions of various variables were computed and compared using the Chi-squared or Fisher’s exact tests, as applicable. Statistical significance was set at p value <0.05.
Results
Of the 185 children with clinical CAP, 113 (61.1%) had radiography-confirmed CAP, of which 109 had positive findings on both nasopharyngeal culture and blood microbiology tests, who were finally included in this analysis. The median (IQR) age of the participants was 14 months (7–26 months), and the majority (60.6%) were male. About a quarter of the children (20.6%) were underweight. Seventy-four (71.2%) had fever at admission, and the majority (73.4%) were diagnosed with severe pneumonia, and 16% (n = 17) presented with cyanosis as a sign of respiratory distress (Table 1).
Table 1.
General characteristics of children under five years of age with community-acquired pneumonia (n = 109).
| Variable | n | % |
|---|---|---|
| Age in monthsa (IQR) | 14 (7–26) | |
| Sex | ||
| Male | 66 | 60.6 |
| WAZ score | ||
| Underweight | 22 | 20.6 |
| Fever at admission | ||
| Yes | 74 | 71.2 |
| Cyanosis | ||
| Yes | 17 | 16.0 |
| HIV status | ||
| Positive | 1 | 0.9 |
| Duration of illnessa (IQR) | 4.6 (2–6) | |
| Classification of pneumonia | ||
| Severe pneumonia | 80 | 73.4 |
| Facilities | ||
| KCMC | 46 | 42.2 |
| Mawenzi | 43 | 39.5 |
| St. Joseph | 20 | 18.3 |
WAZ score: weight-for-age Z-score.
Variable expressed as median (IQR).
The majority (n = 70, 64.2%) of the children received treatment at home before visiting the health facility. The common home treatment was the use of antipyretics (n = 33, 30.3%) followed by antibiotics and cough syrups. Twenty-nine (26.6%) children were inappropriately administered unprescribed/leftover antibiotics at home prior to admission. More than a quarter (28.7%) of the children with no health insurance were administered antibiotics at home compared with 19.1% of children with health insurance. The commonly reported antibiotic given at home was amoxicillin (n = 17, 15.6%), followed by ampicillin–cloxacillin (n = 7, 6.4%). Two children were treated with local herbs before visiting a healthcare facility (Figure 1).
Figure 1.
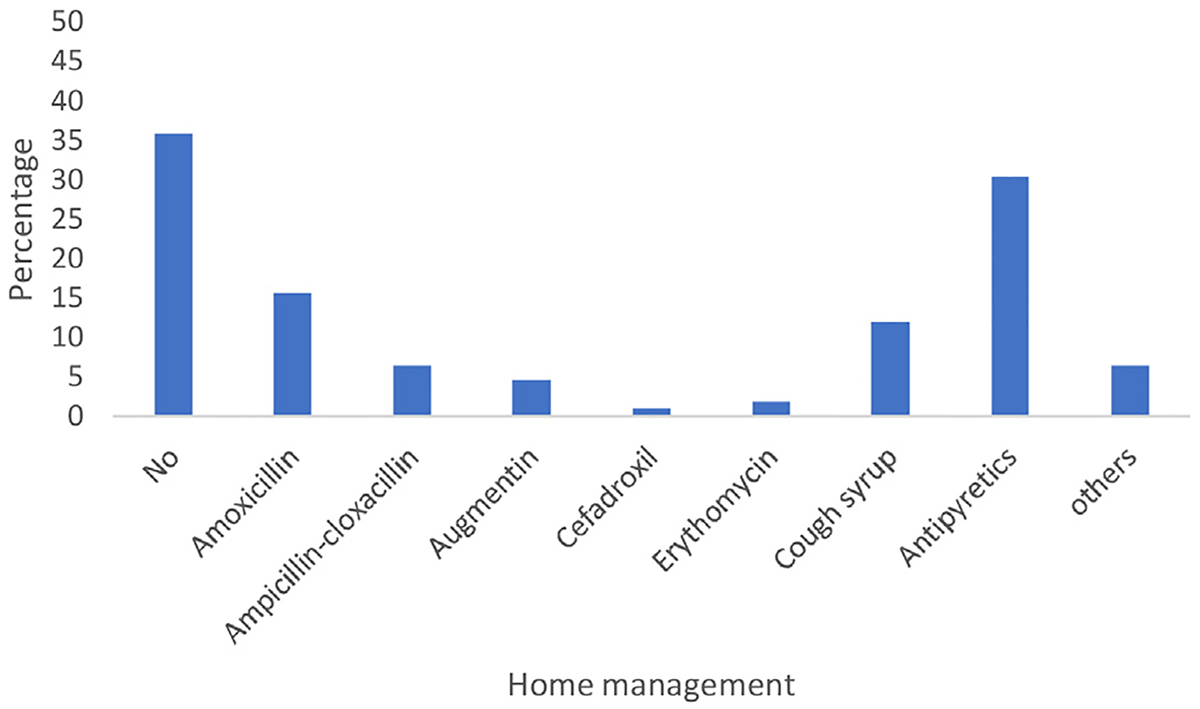
Different types of drugs used for home medication of community-acquired pneumonia before admission (two different classes of antibiotics = 3 children, chlorpheniramine maleate/paracetamol/pseudoephedrine syrup = 1, Lasix = 1, local herbs = 2, loratadine = 1, salbutamol = 1, and sedative = 1).
Only one child had positive blood culture (S. pneumoniae). In the samples of the patients, 79 (69.7%) bacteria and 30 (27.5%) viruses were identified on PCR.
Of the 109 children enrolled, 29 (26.6%) with non-severe pneumonia not needing admission for injectable antibiotics were unnecessarily hospitalised, and 35 (32.1%) were referred by a lower-level health facility. Of the 35 children who were referred by another facility, 22 (62.9%) were given antibiotics before referral; among them, 1 had co-infection of the urinary tract (UTI), and the rest had conditions like anaemia and malaria not needing antibiotic treatment. Some of the antibiotic combinations given to children were as follows: amoxicillin = 1, ampicillin & gentamicin = 8, ampicillin & ceftriaxone = 1, ampicillin-cloxacillin & gentamicin = 1, ampicillin-cloxacillin, gentamicin & metronidazole = 1, ampicillin–cloxacillin = 2, ceftriaxone = 2, erythromycin & cotri- moxazole = 1, metronidazole & ceftriaxone = 1, benzylpenicillin = 2, and benzylpenicillin & gentamicin = 2. Further, a significant difference was observed in the distribution of referral status with S. pneumoniae (p value = 0.003) and H. influenzae carriage (p value = 0.003) (Table 2).
Table 2.
Distribution of children’s characteristics and the referral status (n = 109).
| Characteristics | Referral status | p-Value | |
|---|---|---|---|
| Yes n(%) | No n(%) | ||
| Age category (months) | 0.749 | ||
| ≤24 months | 10(34.5) | 19(65.5) | |
| >24 months | 25(31.3) | 55(68.7) | |
| Facility | <0.001 | ||
| KCMC | 29(63.0) | 17(37.0) | |
| Mawenzi | 1(2.3) | 42(97.7) | |
| St. Joseph | 5(25.0) | 15(67.9) | |
| Health insurance | 0.749 | ||
| Yes | 28(32.2) | 59(67.8) | |
| No | 6(28.6) | 15(71.4) | |
| WAZ | 0.302 | ||
| Normal | 25(29.4) | 60(70.6) | |
| Under-weight | 9(40.9) | 13(59.1) | |
| Fever on admission | 0.141 | ||
| Yes | 13(43.3) | 17(56.7) | |
| No | 21(28.4) | 23(71.6) | |
| Classification of pneumonia | 0.087 | ||
| Pneumonia | 13(44.8) | 16(55.2) | |
| Severe pneumonia | 22(27.5) | 58(72.5) | |
| S. pneumoniae | 0.003 | ||
| Yes | 7(15.9) | 37(84.1) | |
| No | 28(43.1) | 37(56.9) | |
| H. influenzae | 0.004 | ||
| Yes | 9(18.0) | 41(82.0) | |
| No | 26(44.1) | 33(55.9) | |
| S. aureus | 0.161 | ||
| Yes | 0 | 4(100.0) | |
| No | 35(33.3) | 70(66.7) | |
| M. catarrhalis | 0.072 | ||
| Yes | 12(23.50 | 39(76.5) | |
| No | 23(39.7) | 35(60.3) | |
| RSV | 0.974 | ||
| Yes | 7(31.8) | 15(68.2) | |
| No | 28(32.2) | 59(67.8) | |
RSV: respiratory syncytial virus.
All the children in this cohort were treated with antibiotics, including the 30 (27.5%) with viral CAP. At admission, only 15 (6 cases of pneumonia and 9 of severe pneumonia) children with CAP were prescribed a single antibiotic, while the remaining were prescribed combinations of 2 or 3 three antibiotics (Figure 2). Further, the majority (n = 91, 83.5%) of the children were given gentamicin in combination with other antibiotics, and 16 (14.7%) were either given third-generation cephalosporin alone or in combination with other antibiotics. Overall, the commonly prescribed antibiotics on admission were gentamicin and ampicillin (n = 54, 49.5%), and more were prescribed to children with severe pneumonia (n = 34, 31.2%). The second most commonly prescribed antibiotics were gentamicin, ampicillin, and cloxacillin, usually in combination (ampicillin–cloxacillin) (n = 31, 28.4%). However, antibiotics were usually prescribed for severe pneumonia (n = 29, 26.6%).
Figure 2.
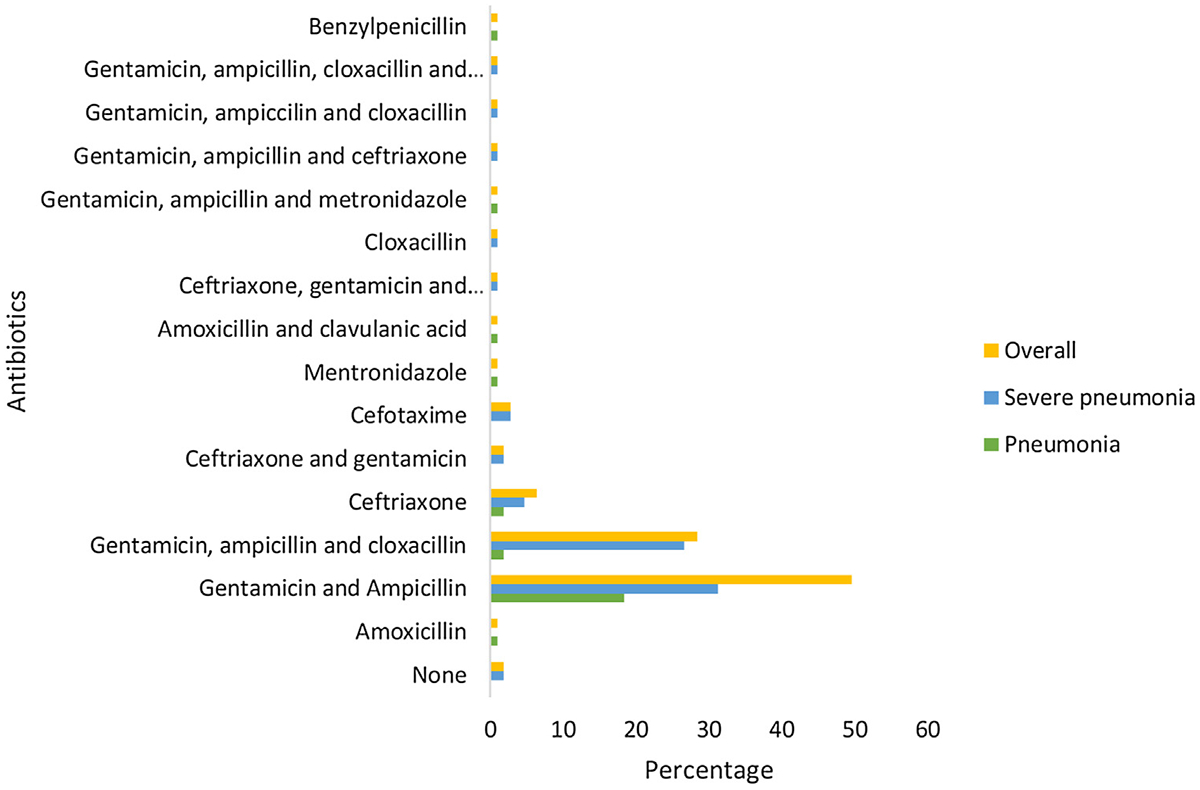
Antibiotics given upon admission.
One child with severe pneumonia died before the nasopharyngeal swabs or blood samples could be collected; the death was likely caused by delayed treatment. The child was referred by the health centre after three days of persistent symptoms; at the health centre, the first dose of antibiotics by injection was not given and no home treatment was reported.
Discussion
In this study, we found that children were delayed (median = 4.6 days) in receiving appropriate care. The majority (64.2%) of the children received home treatment and a quarter (26.6%) were given unprescribed/leftover antibiotics at home. The children were commonly given amoxicillin (15.6%); 2 were given herbal medicines before admission. Twenty-nine (26.6%) with non-severe pneumonia not needing admission for injectable antibiotics were unnecessarily hospitalised. The common isolates from the nasopharyngeal samples were M. catarrhalis (46.8%), H. influenzae (45.9%), S. pneumoniae (40.4%), and RSV (20.2%). In all, 33% of the children were referred by a lower-level healthcare facility; referrals were associated with nasopharyngeal carriage of S. pneumoniae (p = 0.003) and H. influenzae (p = 0.004). The majority of the children were given more than one antibiotic, mostly gentamicin in combination with other antibiotics.
In this population, the majority of the caregivers tried home medication before visiting a hospital; at the onset of illness, children were given antipyretic drugs, cough syrups, and antibiotics, resulting in delayed appropriate care. Similar to this finding, several previous studies, particularly in LMICs, have reported high levels of home self-medication. A study in India reported that 80.7% of children with CAP were given home-based treatment (Minz et al., 2017) and one study in Nigeria found that 75.2% of the caregivers initiated home-based treatment (Ukwaja et al., 2012). Home-based treatment of children is among the most important reasons for the delay in seeking healthcare, which was also observed in this study. The median duration of symptoms before seeking healthcare was 4.6 days (2–6 days), similar to the delays reported previously (Minz et al., 2017). In addition to home remedies, there are several other reasons for the delay in seeking appropriate health care, such as caregivers’ lack of knowledge on the signs and symptoms of CAP, perception of the disease not being severe enough to seek healthcare, and financial challenges (Minz et al., 2017; Pajuelo et al., 2018). Children succumb easily to diseases when appropriate care is delayed, leading to complications. Such delays may be attributed to the initiation of home treatment and possibly to the caregivers waiting for the symptoms to improve. More efforts are needed to educate the parents and caregivers on the importance of seeking appropriate medical care within 24 h of the onset of respiratory symptoms and fever.
Home-based treatment with antibiotics for CAP in children presenting with cough was high (26.6%) in this population. The practice of antibiotic self-medication is a known risk factor for the development of antibiotic resistance (Horumpende et al., 2018a). Antibiotic self-treatment was undertaken because of the lack of knowledge on the consequences of self-medication including antibiotic resistance (Grigoryan et al., 2007, 2008) and lack of health insurance (Saradamma et al., 2000). In this study, more than a quarter (28.7%) of the children with no health insurance were given antibiotics at home compared to 19.1% of the children with health insurance. In most cases, these antibiotics are left-overs from past prescriptions or are obtained from private drug retailers without a prescription (Källander et al., 2005; Ukwaja et al., 2012; Mboya et al., 2018). In line with this, one study in Macedonia found that 71.4% of the people in the community kept antibiotics in their homes to use in cases of illness (Ivanovska et al., 2013). The irrational use of antibiotics is linked to easy access due to the lack of appropriate drug regulatory mechanisms in most countries (Ayukekbong et al., 2017). The caregivers use their experience of previous pneumonia episodes or ask advice from friends and relatives to administer self-medication to their children. They first try medications previously prescribed by healthcare providers in a previous similar episode before taking the child to the hospital (Pajuelo et al., 2018). This behaviour leads to delayed diagnosis and treatment of the child. Other effects of antibiotic self-medication include incorrect choice of antibiotics and unclear dosage as well as duration of treatment. All these factors predispose the patient to polypharmacy drug interactions, drug toxicity, and increased risk of mortality due to treatment failure and development of antibiotic resistance (Bennadi, 2013). The patients presenting at the healthcare facility are most likely to be experiencing the severe form of the disease with poor progress as a consequence of home-based antibiotic treatment failure. Similar to this finding, several previous studies, particularly from LMICs with easy access to antibiotics, have reported high levels of home self-medication and use of antibiotics for cough or acute respiratory tract infections (Källander et al., 2005).
Penicillin, particularly amoxicillin, was the most commonly used antibiotic (15.6%) for self-medication at home without an established diagnosis. Our study findings are in agreement with those of other studies conducted in other parts of the world (Grigoryan et al., 2006; Alhomoud et al., 2017). The reason for self-medication with penicillin-based medications is that they are cheap and readily available over the counter from community drug outlets (Horumpende et al., 2018b; Mboya et al., 2018). Amoxicillin self-medication is a major factor contributing to the rapidly increasing burden of drug resistance to this group of antibiotics (Harbarth and Samore, 2005). Of particular concern is the growing evidence of unacceptably high resistance rates to penicillin, particularly amoxicillin and ampicillin, in most LMICs like Tanzania (Festo et al., 2011; Ayukekbong et al., 2017; Kumburu et al., 2017). This makes it challenging for providers to treat bacterial infections in LMICs, where drug susceptibility testing facilities are not readily available and other classes of antibiotics are either not available in some places or not affordable. The observed irrational use is a serious setback in the fight against antimicrobial resistance.
More than a quarter of the children not needing in-patient care, in violation of the revised WHO 2014 Pneumonia Treatment Guidelines (WHO, 2014), were admitted to the hospital. The guidelines on the management of childhood illnesses (WHO, 2013), the Tanzanian Standard Treatment Guidelines, and the Essential Medicines List for Children and Adolescents recommend outpatient treatment of non-severe pneumonia (pneumonia) (MoHCDGEC, 2018). Moreover, besides unnecessary admission, the antibiotics prescribed did not follow any of the aforementioned guidelines. While oral amoxicillin is the recommended antibiotic for pneumonia, many children were treated with gentamicin and ampicillin. In addition to ampicillin and gentamicin, some children were given a third antibiotic, such as ceftriaxone, cloxacillin, or metronidazole. This inappropriate and excessive antibiotic consumption is common in healthcare facilities, in particular, in those without diagnostic facilities (Ayukekbong et al., 2017). This practice is unnecessary for hospitalised children since clinical progress can be monitored and decisions can be made on a case-by-case basis. Most of the recommendations advocate the addition of another class of antibiotics after 48–72 h if there is no improvement (MoHCDGEC, 2018). Clinicians should follow the available guidelines to avoid inappropriate and excessive antimicrobial prescriptions, which is a known risk factor for antimicrobial resistance (Cusini et al., 2010).
The majority of the children carried CAP-causing bacteria, such as H. influenzae or S. pneumoniae but not M. pneumoniae. Furthermore, RSV was isolated from about a quarter of the children, similar to the findings of the PERCH study describing a significant association between the detection of viruses (mainly RSV) in the nasopharynx and diagnosis of pneumonia (Pneumonia Etiology Research for Child Health (PERCH) Study Group, 2019). All the children in our study were treated with antibiotics, although some had viral CAP. Viral infection only requires supportive care (oxygen supply and/or mechanical ventilation) since no treatment is available, and these types of infections are self-limiting. Clinicians should consider a delay in prescribing antibiotics and consider a simple biomarker-based test, such as C-reactive protein and procalcitonin, to distinguish between viral and bacterial infections, especially in non-severe cases (Do et al., 2016). In addition, a rapid viral test can guide physicians in deciding whether or not to initiate antibiotic treatment (Llor and Bjerrum, 2014). Though the diagnostic values of these biomarkers and viral rapid tests have their limitations (Harris et al., 2011), these tests could help reduce the irrational use of antibiotics.
The findings should be interpreted with some caution because more than a quarter of the children with CAP, including those with the severe form, had not undergone diagnostic chest radiography and were not enrolled in our study. While the use of chest radiography to diagnose pneumonia remains debatable (Harris et al., 2011; 2017), several guidelines recommend chest radiography-based diagnosis for children with severe pneumonia (Harris et al., 2011; WHO, 2013; 2017). In this setting, providers at lower levels were reluctant to order chest radiography even when it was available. This limits the generalizability of the findings since it is not clear how the decision to recommend chest radiography was made. Also, home antibiotic use was reported by the caregivers, who could have underestimated the use because of social desirability bias.
In conclusion, we found high rates of home medication for children with CAP, resulting in a delay in appropriate health care. Treating young children with a cough or other respiratory tract symptoms at home with antibiotics is still a common practice in Tanzania. Paediatric CAP patients not requiring hospitalisation were, in violation of the WHO guidelines, still admitted. In addition, prescription guidelines were not followed, leading to inappropriate and excessive use of antibiotics, which could be a prelude to the development of antimicrobial resistance. More efforts are needed to reduce non-prescription and unnecessary and inappropriate use of antibiotics by improving the awareness and understanding of antimicrobial resistance both at the community level and in healthcare settings through effective communication, education, and training. Additionally, to reduce unnecessary hospitalisation of children with pneumonia, providers should be trained on how to implement the revised WHO guidelines.
Acknowledgements
The authors would like to acknowledge the children who participated in this study and the parents for consent. Furthermore, the authors would like to thank the staff at all healthcare facilities and the authorities for their support.
Funding
This research was supported by the Fogarty International Centre of the National Institutes of Health under Award Number D43TW010138 and partly by the German Academic Exchange Service (DAAD). The funding bodies had no role in the design of the study; collection, analysis, and interpretation of the data; or in writing the manuscript.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Ethical consideration
The study was approved by the Kilimanjaro Christian Medical University College Research Ethics Review Committee (CREC) under certificate number 948 and the Tanzanian National Health Research Ethics Committee under certificate NIMR/HQ/R.8a/Vol. IX/2422. The participants’ parents or legal guardians were fully informed of the risks and benefits of participating in the study and understood that participation was voluntary. They were clearly made aware that non-participation would not affect treatment whatsoever. Before enrolment, the parents or legal guardians of the eligible children signed a written consent form.
References
- Alhomoud F, Aljamea Z, Almahasnah R, Alkhalifah K, Basalelah L, Alhomoud FK. Self-medication and self-prescription with antibiotics in the Middle East—do they really happen? A systematic review of the prevalence, possible reasons, and outcomes. Int J Infect Dis 2017;57(April):3–12. [DOI] [PubMed] [Google Scholar]
- Ayukekbong JA, Ntemgwa M, Atabe AN. The threat of antimicrobial resistance in developing countries: causes and control strategies. Antimicrob Resist Infect Control 2017;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennadi D Self-medication: a current challenge. J Basic Clin Pharm 2013;5 (December (1)):19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusini A, Rampini SK, Bansal V, Ledergerber B, Kuster SP, Ruef C, et al. Different patterns of inappropriate antimicrobial use in surgical and medical units at a tertiary care hospital in Switzerland: a prevalence survey. PLoS One 2010;5 (November (11))e14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do NTT, Ta NTD, Tran NTH, Than HM, Vu BTN, Hoang LB, et al. Point-of-care C-reactive protein testing to reduce inappropriate use of antibiotics for non-severe acute respiratory infections in Vietnamese primary health care: a randomised controlled trial. Lancet Glob Health 2016;4(9):e633–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festo E, Kidenya BR, Hokororo A, Mshana SE. Predictors of urinary tract infection among febrile children attending at Bugando Medical Centre Northwestern, Tanzania. Arch Clin Microbiol 2011;2(5). [Google Scholar]
- Grigoryan L, Burgerhof JGM, Degener JE, Deschepper R, Lundborg CS, Monnet DL, et al. Attitudes, beliefs and knowledge concerning antibiotic use and self-medication: a comparative European study. Pharmacoepidemiol Drug Saf 2007;16(November (11)):1234–43. [DOI] [PubMed] [Google Scholar]
- Grigoryan L, Burgerhof JGM, Degener JE, Deschepper R, Lundborg CS, Monnet DL, et al. Determinants of self-medication with antibiotics in Europe: the impact of beliefs, country wealth and the healthcare system. J Antimicrob Chemother 2008;61(May (5)):1172–9. [DOI] [PubMed] [Google Scholar]
- Grigoryan L, Haaijer-Ruskamp FM, Burgerhof JGM, Mechtler R, Deschepper R, Tambic-Andrasevic A, et al. Self-medication with antimicrobial drugs in Europe. Emerg Infect Dis 2006;12(March (3)):452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbarth S, Samore MH. Antimicrobial resistance determinants and future control. Emerg Infect Dis 2005;11(June (11)):794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Clark J, Coote N, Fletcher P, Harnden A, McKean M, et al. British Thoracic Society guidelines for the management of community acquired pneumonia in children: update 2011. Thorax 2011;66 Suppl 2(October (Suppl. 2)) ii1–23. [DOI] [PubMed] [Google Scholar]
- Horumpende PG, Said SH, Mazuguni FS, Antony ML, Kumburu HH, Sonda TB, et al. Prevalence, determinants and knowledge of antibacterial self-medication: a cross sectional study in North-eastern Tanzania Singer AC, editor. PLoS One 2018a;13(October (10))e0206623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horumpende PG, Sonda TB, van Zwetselaar M, Antony ML, Tenu FF, Mwanziva CE, et al. Prescription and non-prescription antibiotic dispensing practices in part I and part II pharmacies in Moshi Municipality, Kilimanjaro Region in Tanzania: a simulated clients approach. PLoS One 2018b;13(11)e0207465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVAC. Pneumonia and diarrhea progress report 2018. 2018.
- Ivanovska V, Zdravkovska M, Bosevska G, Angelovska B. Antibiotics for upper respiratory infections: public knowledge, beliefs and self-medication in the Republic of Macedonia. Pril (Makedonska Akad na Nauk i Umet Oddelenie za Med Nauk) 2013;34(2):59–70. [PubMed] [Google Scholar]
- Källander K, Nsungwa-Sabiiti J, Balyeku A, Pariyo G, Tomson G, Peterson S. Home and community management of acute respiratory infections in children in eight Ugandan districts. Ann Trop Paediatr 2005;25(December (4)):283–91. [DOI] [PubMed] [Google Scholar]
- Kumburu HH, Sonda T, Mmbaga BT, Alifrangis M, Lund O, Kibiki G, et al. Patterns of infections, aetiological agents and antimicrobial resistance at a tertiary care hospital in northern Tanzania. Trop Med Int Health 2017;22(April (4)):454–64. [DOI] [PubMed] [Google Scholar]
- Leung DT, Chisti MJ, Pavia AT. Prevention and control of childhood pneumonia and diarrhea. Pediatr Clin North Am 2016;63(February (1)):67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llor C, Bjerrum L. Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 2014;5(December (6)):229–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugangira K, Kazaura M, Kalokola F. Morbidity and mortality of children aged 2–59 months admitted in the Tanzania Lake Zone’s public hospitals: a cross-sectional study. BMC Res Notes 2017;10(October (1)):502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mboya EA, Sanga LA, Ngocho JS. Irrational use of antibiotics in the Moshi Municipality Northern Tanzania: a cross sectional study. Pan Afr Med J 2018;31:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minz A, Agarwal M, Singh JV, Singh VK. Care seeking for childhood pneumonia by rural and poor urban communities in Lucknow: a community-based cross-sectional study. J Fam Med Prim Care 2017;6(2):211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoHCDGEC. National guidelines for the management of HIV and AIDS. 6th ed 2017. Dar es Salaam. [Google Scholar]
- MoHCDGEC. Standard treatment guidelines and essential medicines list for children and adolescents.1sted Dares Salaam: The United Republic of Tanzania Ministry of Health, Community Development, Gender, Elderly and Children; 2018. [Google Scholar]
- Muro F, Meta J, Renju J, Mushi A, Mbakilwa H, Olomi R, et al. “It is good to take her early to the doctor” — mothers’ understanding of childhood pneumonia symptoms and health care seeking in Kilimanjaro region, Tanzania. BMC Int Health Hum Rights 2017;17(December (1)):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngocho JS, de Jonge MI, Minja L, Olomi GA, Mahande MJ, Msuya SE, et al. Modifiable risk factors for community-acquired pneumonia in children under 5 years of age in resource-poor settings: a case-control study. Trop Med Int Health 2019;24 (April (4)):484–92. [DOI] [PubMed] [Google Scholar]
- Pajuelo MJ, Anticona Huaynate C, Correa M, Mayta Malpartida H, Ramal Asayag C, Seminario JR, et al. Delays in seeking and receiving health care services for pneumonia in children under five in the Peruvian Amazon: a mixed-methods study on caregivers’ perceptions. BMC Health Serv Res 2018;18(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pneumonia Etiology Research for Child Health (PERCH) Study Group. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: the PERCH multi-country case-control study. Lancet (London, England) 2019;394(June (10200)):757–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saradamma RD, Higginbotham N, Nichter M. Social factors influencing the acquisition of antibiotics without prescription in Kerala State, south India. Soc Sci Med 2000;50(March (6)):891–903. [DOI] [PubMed] [Google Scholar]
- Templeton KE, Scheltinga SA, Beersma MFC, Kroes ACM, Claas ECJ. Rapid and sensitive method using multiplex real-time PCR for diagnosis of infections by influenza A and influenza B viruses, respiratory syncytial virus, and para-influenza viruses 1, 2, 3, and 4. J Clin Microbiol 2004;42(April (4)):1564–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuhebwe D, Tumushabe E, Leontsini E, Wanyenze RK. Pneumonia among children under five in Uganda: symptom recognition and actions taken by caretakers. Afr Health Sci 2014;14(December (4)):993–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukwaja KN, Talabi AA, Aina OB. Pre-hospital care seeking behaviour for childhood acute respiratory infections in south-western Nigeria. Int Health 2012;4 (December (4)):289–94. [DOI] [PubMed] [Google Scholar]
- Umuhoza C, Karambizi AC, Tuyisenge L, Cartledge P. Caregiver delay in seeking healthcare during the acute phase of pediatric illness, Kigali, Rwanda. Pan Afr Med J 2018;30:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN General Assembly. Sustainable development goals — the solar impulse foundation. 2015.
- Uwemedimo OT, Lewis TP, Essien EA, Chan GJ, Nsona H, Kruk ME, et al. Distribution and determinants of pneumonia diagnosis using Integrated Management of Childhood Illness guidelines: a nationally representative study in Malawi. BMJ Glob Health 2018;3(March (2))e000506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO/UNICEF. Revised WHO classification and treatment of pneumonia in children at health facilities Revised WHO classification and treatment of pneumonia in children at health facilities: evidence summaries. World Health Organization; 2014. [PubMed] [Google Scholar]
- WHO. Pocket book of hospital care for children Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. 2nd ed World Health Organization; 2013. [PubMed] [Google Scholar]
- WHO. Revised WHO classification and treatment of pneumonia in children at health facilities: evidence summaries. 2014. [PubMed] [Google Scholar]
- Wu PE, Juurlink DN. Unused prescription drugs should not be treated like leftovers. CMAJ 2014;186(August (11)):815–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


