Abstract
Purpose
To evaluate reduction in intraocular pressure (IOP) and medications for open-angle glaucoma (OAG) patients 12 months post-ab-interno circumferential viscodilation (VISCO360, Sight Sciences, Menlo Park, CA) in conjunction with cataract surgery.
Setting
Surgical center (New York, United States).
Design
Retrospective study of all OAG patients treated with 360-degree ab-interno viscodilation with cataract surgery by a single surgeon (NR) having 12 months of follow-up. Eyes were stratified by baseline IOP. Group 1: ≥18 mmHg (n=111). Group 2: <18 mmHg (n=69).
Methods
IOP was measured using Goldmann applanation tonometry. Medications, the number of medication-free eyes in each group at 12 months, and adverse events (AE) are reported. Analysis includes descriptive statistics and t-tests evaluating change from baseline.
Results
Groups 1 and 2 had mean baseline IOP of 22 and 14.3 mmHg. Medication use was 0.9 and 1.1 for Groups 1 and 2. At 12 months IOP for Group 1 was reduced 22% to 17.2 mmHg (p<0.0001) on 1.0 medications (p=0.7). IOP for Group 2 was similar to baseline (15.4 mmHg) but with a reduction in medications to 0.6 (p<0.05). The proportion medication free at 12 months was 32% and 47% for Groups 1 and 2 versus 34% and 26% at baseline, respectively. There were few AE (hyphema 1.7%, IOP elevation >10 mmHg >30 days post-op 1.1%, mild inflammation <1%) and no secondary surgical interventions excepting a single paracentesis, one-day postoperative.
Discussion
Treatment goals for the two groups differed. Pressure reduction (Group 1) or medication reduction (Group 2). Viscodilation achieved significant IOP reduction in Group 1 and medication reduction in Group 2 with many patients (both groups) medication free at 12 months. AE were infrequent and transient.
Conclusion
Circumferential ab-interno viscodilation can be combined with cataract surgery and provide an IOP lowering and medication reduction benefit sustained for at least 12 months, for many patients with OAG.
Keywords: viscodilation, MIGS, open-angle glaucoma, glaucoma surgery, VISCO360, canaloplasty, OMNI
Introduction
Circumferential ab-interno canaloplasty is a minimally invasive glaucoma procedure that traces its origins to ab externo nonpenetrating procedures like sinusotomy and viscocanalostomy,1,2 and more recently, to canaloplasty ab externo.3 In contrast to these earlier techniques, canaloplasty ab interno does not require conjunctival dissection or a scleral flap. Unlike circumferential ab-interno trabeculotomy (e.g. GATT), ab-interno canaloplasty is trabecular meshwork (TM) sparing, requiring only a small goniotomy to access Schlemm’s canal. The atraumatic nature of canaloplasty ab interno maximizes the range of available future surgical options should additional intervention be required.
While most surgical procedures targeting the conventional outflow pathway address proximal outflow resistance associated with the TM and inner wall of Schlemm’s canal, canaloplasty addresses distal resistance (i.e. collapsed Schlemm’s canal, blocked collector channel ostia) in addition to presumed effects on the inner wall and TM due to dilation and stretching.4
The effectiveness of canaloplasty has been demonstrated in patients with primary open-angle glaucoma (POAG),3,5-9 secondary open-angle glaucomas,3,9 POAG in black patients,10 and in steroid-induced ocular hypertension.11 These include studies with large sample sizes (N > 100 to > 500) (3, 7) and follow-up periods to 5 years.9 Both ab-interno and ab-externo 360° canaloplasty provide similar IOP-lowering efficacy. This was confirmed in a paired-eye study where one eye was treated ab externo and the other ab interno. Results were essentially identical.12
The VISCO360® system (Sight Sciences Inc. Menlo Park, CA, USA) is a purpose designed, single-handed, non-implantable surgical device that automatically delivers a predetermined amount of viscoelastic fluid to dilate up to 360° of Schlemm’s canal. The present study is a retrospective chart review of effectiveness outcomes for 180 eyes from 130 patients with POAG (96% of eyes) operated on by a single surgeon (NR) and followed for 12 months post-surgery. This study cohort is the largest dataset to date of eyes treated by circumferential viscodilation with the VISCO360 device.
Methods
The study is considered non-interventional in that it is a retrospective review of patient data accumulated in the normal conduct of standard medical practice; no prospective treatment assignments were made, and all assessments were routine standard of care. The study was reviewed and approved by the Sterling IRB. Waiver of consent was granted due to the retrospective, non-interventional nature of the trial and all patient data were treated with confidentiality, in accordance with the Declaration of Helsinki. All surgeries were performed by the same surgeon at a single outpatient surgery center located in the Bronx, NY.
Study Patients
Study patients were drawn from consecutive surgeries combining phacoemulsification cataract surgery with viscodilation using the VISCO360 device performed by a single surgeon (NR) between June 2015 and December 2017. Patients were adults of either gender and any race, the majority with a diagnosis of mild to moderate POAG as defined by International Statistical Classification of Diseases and Related Health Problems, revision 10 (ICD-10); in brief, optic nerve abnormalities consistent with glaucoma but no visual field abnormalities (Mild), or with glaucomatous visual field abnormalities in one hemifield but not within 5 degrees of fixation (Moderate). Key exclusion criteria included advanced glaucoma, and prior penetrating glaucoma surgery. Both eyes from a patient were included if both met eligibility criteria.
Per practice protocol, prior to surgery, a full ophthalmic exam was carried out including best-corrected visual acuity (BCVA), Goldmann intraocular pressure (IOP), slit-lamp exam including dilated fundus, and gonioscopic assessment (all four quadrants). For this retrospective review, the above data were collected as well as the number of ocular hypotensive medications with fixed combinations recorded as the number of component drugs. IOP-lowering medications were continued until the day of surgery and tapered postoperatively as warranted by IOP.
Surgical Procedure
For all cases, viscodilation was performed following cataract extraction and implantation of the posterior capsule intraocular lens (IOL). After IOL implantation, the anterior chamber (AC) was deepened with viscoelastic (Provisc®, Alcon, Fort Worth, TX), the head was tilted away from the surgeon and the microscope was tilted towards the surgeon for gonioscopic visualization. The VISCO360 device was introduced through the temporal clear corneal cataract incision into the AC. The device was advanced across the AC, positioned at the desired location nasally and a small <1 mm goniotomy was created with the cannula tip. The cannula was placed into the goniotomy, and the microcatheter was advanced into Schlemm’s canal for 180°. As the microcatheter was retracted a controlled amount of viscoelastic was delivered providing viscodilation of the canal and distal outflow pathway. This was repeated for the second 180°. The AC was irrigated by bimanual aspiration to remove viscoelastic, the chamber was pressurized, and 0.2 mL of TriMoxi (triamcinolone 15 mg/mL; moxifloxacin 1 mg/mL) was injected intravitreally. No postoperative anti-inflammatory or antibiotic drops were routinely used.
Standard follow-up visits were generally scheduled for 1 day, 1 week, and 1, 6, and 12 months postoperatively. At each of these visits IOP, ocular hypotensive medication usage, BCVA, and any adverse events were assessed and recorded. IOP ≥10 mmHg than pre-op baseline IOP and >30 days post-surgery was captured as an adverse event.
Outcome Measures
Treatment goals for patients with mild to moderate POAG differ depending on the baseline IOP, the desired target IOP, and the medication burden. For the purposes of this study outcomes were stratified into two cohorts; patients with baseline IOP ≥ 18 mmHg where the main goal of treatment was pressure reduction (Group 1), and patients with baseline IOP < 18 mmHg where the main goal was maintenance of IOP control while reducing medication burden (Group 2). IOP (and mean IOP reduction from baseline) at 12 months was the primary outcome measure for both groups. Secondary outcomes were mean reduction in medication use at 12 months and the proportion of patients medication free at 12 months.
Statistical Analysis
This was a descriptive chart review study from a single surgical practice. It was not a hypothesis testing study and there was no comparator therefore sample size was based only on the number of available cases and not on statistical power calculations. Nevertheless, with 180 eyes, the dataset should provide sufficient information to be clinically helpful. The eye was the unit of analysis in this descriptive study, i.e., more than one eye from a single patient was included if both eyes met eligibility criteria. While this would violate assumptions of independence of variates required for inferential parametric statistics, the descriptive nature of this study and the resultant large sample size justify this approach. To assess the effect of including both eyes, a sensitivity analysis in which only one eye per patient was included (the first of the two eyes to be treated, or in the few instances where both eyes were treated the same day, the right eye) was also carried out.
Descriptive statistics were used to summarize the data including mean, standard deviation, max and min, and 95% confidence intervals. Means and standard deviations were calculated based on the total available eyes for each time point. Paired t-tests were used to evaluate differences between baseline and 12-month IOP and medication usage. Differences with P< 0.05 were considered statistically significant.
Results
Patient Demographics
A total of 180 eyes from 130 patients that met eligibility criteria were treated with ab-interno canal viscodilation. Slightly over half of eyes (52%) were from female patients. Mean age was 70 years (range 48–87). Most eyes were diagnosed with mild-to-moderate POAG. Mean visual field mean deviation was −9.6 dB for Group 1 and −9.4 dB for Group 2. Overall, 78% of eyes had a MD of ≥ −12 dB and 86% ≥ −16 dB. All viscodilation procedures were combined with phacoemulsification cataract surgery. Group 1 had 111 eyes with baseline IOP ≥18 mmHg and Group 2 had 69 eyes with baseline IOP <18 mmHg. Patients’ demographics and baseline characteristics are presented below (Table 1). Across both groups, 95 eyes were available for analysis at 12 months and 122 at six months. As this was a retrospective chart without a protocol mandated or patient agreed to schedule of visits, the observed attrition rate due to loss to follow-up was not unexpected.
Table 1.
Demographic and Baseline Characteristics
| Variable | IOP < 18 mmHg (N= 69) | IOP ≥ 18 mmHg (N= 111) |
|---|---|---|
| Gender (n, %) | ||
| Male | 34 (49) | 51 (46) |
| Female | 35 (51) | 59 (53) |
| Not reported | – | 1 (1) |
| Age | ||
| Mean (SD) | 69.7 (9.4) | 70.1 (8.2) |
| Min, Max | 49.2, 86.8 | 48.5, 87.3 |
| Diagnosis (n, %) | ||
| POAG | 65 (94) | 108 (97) |
| PXF | 1 (1) | 1 (1) |
| PG | – | 1 (1) |
| Uveitic | 2 (3) | – |
| Neovascular | 1 (1) | 1 (1) |
| Mean Deviation, dB | ||
| Mean (SD) | −9.4 (6.3) | −9.6 (7.4) |
| Min, Max | −32.7, −2.3 | −32.0, −0.04 |
| Race (n, %) | ||
| Caucasian | 5 (7) | 1 (1) |
| Black | 17 (25) | 28 (25) |
| Asian | – | – |
| Other | 3 (4) | 6 (5) |
| Not reported | 44 (64)a | 76 (68)a |
| Ethnicity (n, %) | ||
| Hispanic | 44 (64) | 76 (68) |
| Not Hispanic | 25 (36) | 35 (32) |
| Not reported | – | – |
| Baseline IOP | ||
| Mean (SD) | 14.3 (2.3) | 22.0 (5.5) |
| Min, Max | 9, 17 | 18, 60 |
| Baseline Medications | ||
| Mean (SD) | 1.1 (0.9) | 0.9 (0.9) |
| Min, Max | 0, 3 | 0, 4 |
Notes: aRace and ethnicity were self-reported. Many patients reported race and ethnicity as “Hispanic”.
Abbreviations: POAG, primary open-angle glaucoma; PXG, pseudoexfoliative glaucoma; PG, pigmentary glaucoma; SD, standard deviation; IOP, intraocular pressure.
Effectiveness Outcomes
Intraocular Pressure
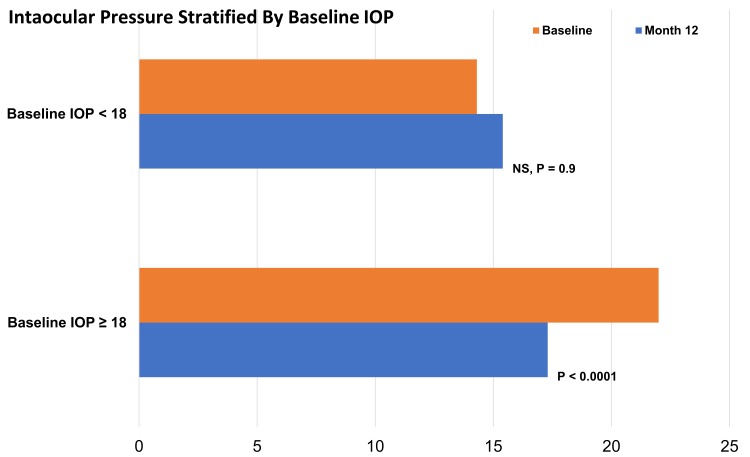
Mean medicated intraocular pressure at baseline and 12 months for both Groups 1 and 2 is presented in Figure 1. Eyes in Group 1 had a statistically significant reduction in mean IOP compared to baseline for both Month 6 and at Month 12 (P < 0.0001). The mean baseline IOP of 22.0 ± 5.5 mmHg (n = 111) was reduced to 15.7 ± 4.3 mmHg at six months and 17.2 ± 5.1 mmHg at 12 months, 29% and 22% reductions from baseline, respectively. The increase in mean IOP between Months 6 and 12 (1.5 mmHg) did not reach statistical significance (P = 0.058).
Figure 1.
Intraocular pressure at baseline and 12 months postoperative stratified by baseline IOP.
Group 2 eyes were, on average, well controlled at baseline (mean IOP 14.3 ± 2.3 mmHg). Pressure control was sustained over the follow-up period with mean IOP being 15.0 ± 3.3 and 15.4 ± 4.1 mmHg at six and 12 months, respectively.
IOP-Lowering Medications
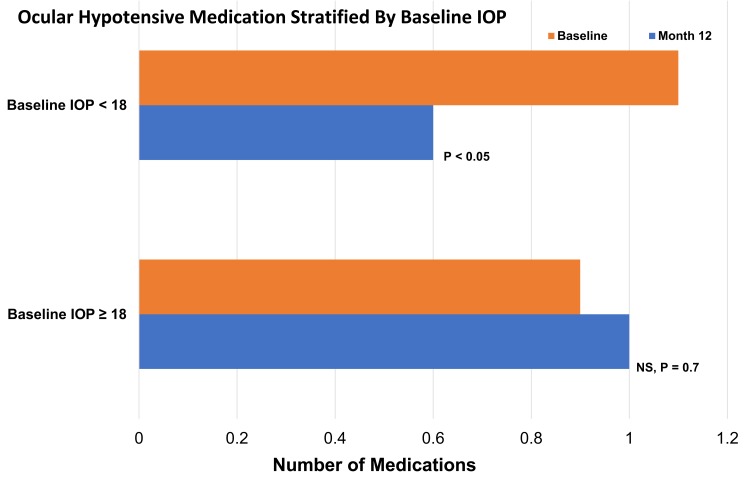
Patients were on a mean of 0.9 ± 0.9 (Group 1) and 1.1 ± 0.9 (Group 2) IOP-lowering medications at baseline. Postoperatively, at 12 months, there was a statistically significant reduction in the mean number of IOP-lowering medications compared to the baseline (P <0.05) for Group 2 but not for Group 1 (P = 0.7). At 12 months, patients were on a mean of 1.0 ± 0.9 and 0.6 ± 0.6 IOP-lowering medications for Groups 1 and 2, respectively (Figure 2).
Figure 2.
Ocular hypotensive medication usage at baseline and 12 months postoperative stratified by baseline IOP.
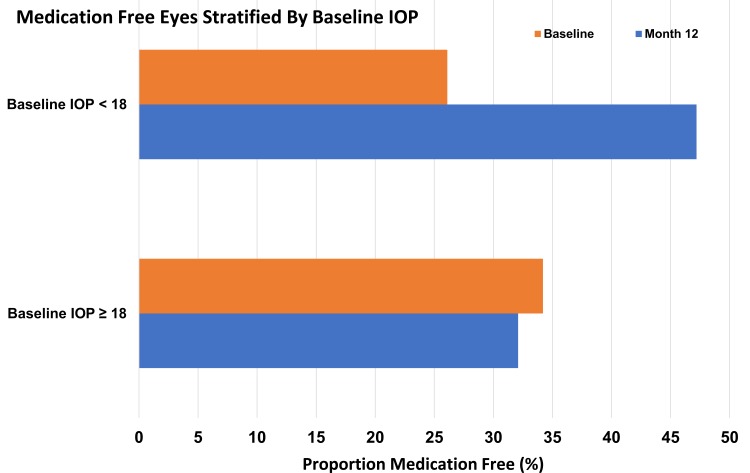
The proportion of eyes that were medication free increased nearly two-fold for Group 2 from 26% at baseline to 47% at month 12. For Group 1, the proportion of eyes that were medication free did not change (34%, 32%) (Figure 3).
Figure 3.
Proportion of eyes ocular hypotensive medication free at baseline and 12 months postoperative stratified by baseline IOP.
Sensitivity Analysis
When only a single eye from each patient was included in the analyses, the results were unchanged. Mean IOP at baseline, month 6 and month 12 was either identical to the analysis including fellow eyes or differed by <1%. Number of medications was identical at all time points and for both groups with one exception. At 12 months, the average number of medications for Group 2 was 0.5 when fellow eyes were excluded versus 0.6 for the full data set.
Safety Outcomes
There were few adverse events noted in either the early postoperative period (first 30 days) or thereafter. Transient IOP spikes were observed in 7% of eyes for Group 1, but none in Group 2 during the early postoperative period. After 30 days post-op, clinically significant hyphemas were observed in three eyes (1.7%), IOP spikes ≥10 mmHg above baseline IOP in two eyes (1.1%), and mild anterior chamber inflammation in a single eye (<1%).
Secondary surgical intervention to control IOP was not required for any eyes aside from a single case in Group 1 where the IOP was 40 mmHg the day after surgery (pre-op IOP was 18 mmHg). A paracentesis was performed and IOP was 6 mmHg on zero medications a week later. The same eye had IOP of 14 and 12 mmHg at 6 and 12 months, both on zero medications.
Discussion
Ab-interno microcatheterization and 360° viscodilation of Schlemm’s canal combined with cataract surgery, resulted in lower mean IOP 12 months postoperative for eyes with an elevated, medicated baseline IOP ≥18 mmHg (Group1). In eyes with a medicated baseline IOP <18 mmHg (Group2), ab-interno microcatheterization and 360° viscodilation of Schlemm’s canal in conjunction with cataract surgery, was effective in maintaining the baseline IOP while lowering the need for medications for up to 12 months.
It is known that phacoemulsification cataract surgery provides a modest IOP-lowering effect and should be considered as a possible contributor to the overall pressure reduction observed. However, under conditions similar to the present study (real-world, retrospective chart review) this pressure reduction was <2 mmHg.13
Our results confirm and extend those of Ondrejka and Körber from a smaller European study. Like the present study, results were stratified by baseline IOP; Group 1 included 61 eyes with medicated baseline IOP ≥ 18 mmHg and Group 2 included 33 eyes with IOP < 18 mmHg. In accord with the results presented herein, mean IOP at 12 months for Group 2 eyes was not significantly different from the well-controlled baseline however ocular hypotensive medication usage was significantly decreased. Where the main treatment goal was IOP reduction (Group 1 eyes), substantial reductions (41% from baseline) were achieved along with significant medication reduction.8
Successful outcomes have also been achieved using other technologies to viscodilate Schlemm’s canal. Vastardis et al presented retrospective 12-month results for over 500 POAG eyes treated ab externo with the iTrack system (Ellex Medical). In the “early” to “moderate” glaucoma subgroups (N = 90) treated with canaloplasty along with cataract surgery (similar to the present study population), 12-month IOP was generally reduced about 5 mmHg from baseline IOP of approximately 19.5 mmHg.7 This is similar to the 5 mmHg reduction observed in the present study (Group 1) although likely due to differences in baseline IOP, about 19 mmHg in the Vastardis study and 22 mmHg in the present study, along with different racial characteristics (the present study included primarily black and Hispanic patients known to have more intractable IOP elevations), the ultimate IOP at 12 months was about 2 mmHg higher here than for the Vastardis cohort.
The adverse events that were observed in this study are common to any minimally invasive glaucoma surgery (MIGS)14 nevertheless, the frequency (beyond the initial post-op period) for each was <2%. The rate of elevated IOP after one month (≥10 mmHg than pre-op baseline) was 1.0%, identical to that reported by Ondrejka and Körber, and lower than that reported for many other MIGS studies.15 No patient experienced an AE for BCVA loss (loss of 2 lines or more compared to baseline). It is interesting that there was a significantly higher rate (P=0.026, Chi-square test) of postoperative IOP spikes in the immediate postoperative period in Group 1 (7%) compared to Group 2 (0%). It could be that, despite being given the same intraoperative steroid treatment, Group 1, with higher baseline and presumably less tractable IOP, tended to have a more robust steroid response than Group 2. This phenomenon has been reported in the literature.16
Abnormally high resistance to outflow through the physiologic canalicular pathway results in elevated IOP, the most important and only treatable risk factor for glaucoma.17 The juxtacanalicular trabecular meshwork and inner wall of Schlemm’s canal may account for up to 75% of resistance18,19 however significant resistance has also been observed distally. The cross-sectional area of Schlemm’s canal averages 54% less and mean outflow facility 55% less in POAG eyes compared with normal, implicating canal atrophy as a source of resistance.20 Additionally, the ostia of the collector channels may themselves be occluded by herniations of the inner wall of Schlemm’s canal.21 A recent review article provides additional details of published studies of Schlemm’s canal viscodilation.22
There are limitations to this study that should be acknowledged. Importantly, this was a single-center, single-surgeon series which has a greater risk of investigator bias than a multicenter trial. However, to mitigate this potential bias, the surgeon (NR) was not involved in chart review and data extraction or in the data analysis. Moreover, all cases were included in a sequential fashion within the sampling period. The retrospective nature of the study could also be considered a weakness. Prospective randomized trials are high-level scientific evidence and they excel at describing the performance of a treatment under “perfect” conditions with narrowly defined study populations and strict protocol requirements. A retrospective evaluation of a treatment that was delivered as part of a surgeon’s standard medical practice has the virtue of providing real-world outcomes that may be more generalizable to the kinds of patients other surgeons treat. Both eyes from a patient were included in the analysis in instances where both eyes met eligibility criteria therefore not all data points are independent statistically speaking. This should be considered when interpreting the results; however, a sensitivity analysis including only a single eye per patient showed essentially identical results for both IOP and medication usage outcomes. As this was a retrospective study, patients did not agree or consent prospectively to a defined follow-up schedule, attrition is to be expected. Patients may not return for follow-up for a variety of reasons including relocation, economic considerations, difficulty with transportation, and death. Moreover, a study that interviewed 300 patients with OAG showed a substantial number (14%) of patients to have no concern that they could lose vision from glaucoma if they were not compliant with treatment and doctor’s visits.23 The relatively large attrition of patients over the 12-month follow-up period could also be considered a limitation. Specifically, it could suggest a bias toward a 12-month cohort “enriched” in those responding well to the treatment. However, arguing against such bias, the proportion of patients in Group 1, those with higher baseline IOP, did not change between baseline and Month 12 (62% and 63%), and this group, with poorly controlled IOP, would seem more likely to include non-responders than the patients with well-controlled IOP at baseline (Group 2). Despite these limitations, we believe that this study provides important new data for surgeons to consider as they weigh minimally invasive surgical options to treat glaucoma.
Conclusion
Microcatheterization and viscodilation of Schelmm’s canal with the VISCO360® Viscosurgical System in conjunction with phacoemulsification cataract surgery provides clinically meaningful outcomes at up to 12 months post-surgery. For patients in need of additional IOP control, significant improvements in IOP and reductions in the need for IOP-lowering medications were observed through 12 months. For subjects where reduction in medication burden was desired, this was achieved. Adverse events were few and were like other established MIGS procedures. No additional surgical intervention for IOP control was required for any of the eyes.
Disclosure
Jaime E Dickerson Jr is an employee of Sight Sciences Inc. Nathan M Radcliffe reports personal fees from Sight Sciences, during the conduct of the study; personal fees from Glaukos, Ivantis, New World Medical, Beaver Visitec, Alcon, Allergan, Iridex, and Lumenis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Krasnov MM. Sinusotomy. Trans Am Acad Ophthalmol Otolaryngol. 1972;76:368–374. [PubMed] [Google Scholar]
- 2.Stegmann R, Pienaar A, Miller D. Viscocanalostomy for open-angle glaucoma in black African patients. J Cataract Refract Surg. 1999;25:316–322. doi: 10.1016/S0886-3350(99)80078-9 [DOI] [PubMed] [Google Scholar]
- 3.Lewis RA, von Wolff K, Tetz M, et al. Canaloplasty: circumferential viscodilation and tensioning of schlemm canal using a flexible microcatheter for the treatment of open-angle glaucoma in adults: two-year interim clinical study results. J Cataract Refract Surg. 2009;35:814–824. doi: 10.1016/j.jcrs.2009.01.010 [DOI] [PubMed] [Google Scholar]
- 4.Smit BA, Johnstone MA. Effects of viscoelastic injection into schlemm’s canal in primate and human eyes. Potential relevance to viscocanalostomy. Ophthalmology. 2002;109:786–792. doi: 10.1016/S0161-6420(01)01006-5 [DOI] [PubMed] [Google Scholar]
- 5.Cameron B, Field M, Ball S, Kearney J. Circumferential viscodilation of schlemm’s canal with a flexible microcannula during non-penetrating glaucoma surgery. Digit J Ophthalmol. 2006;12(1). Available from: http://www.djo.harvard.edu/site.php?urlZ/physicians/oa/929. Accessed, December 18, 2019. [Google Scholar]
- 6.Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd. 2018;32:223–227. doi: 10.1007/s00717-018-0416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vastardis I, Fili S, Gatzioufas Z, Kohlhaas M. Ab externo canaloplasty results and efficacy: a retrospective cohort study with a 12-month follow-up. Eye Vis. 2019;6:9. doi: 10.1186/s40662-019-0134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ondrejka S, Körber N. 360°ab-interno schlemm’s canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol. 2019;13:1235–1246. doi: 10.2147/OPTH.S203917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voykov B, Blumenstock G, Leitritz MA, et al. Treatment efficacy and safety of canaloplasty for open-angle glaucoma after 5 years. Clin Exp Ophthalmol. 2015;43(8):768–771. doi: 10.1111/ceo.12549 [DOI] [PubMed] [Google Scholar]
- 10.Grieshaber MC, Pienaar A, Olivier J, Stegmann R. Comparing two tensioning suture sizes for 360° viscocanalostomy (canaloplasty): a randomized controlled trial. Eye. 2010;24:1220–1226. doi: 10.1038/eye.2009.317 [DOI] [PubMed] [Google Scholar]
- 11.Brusini P, Tosoni C, Zeppieri M. Canaloplasty in corticosteroid-induced glaucoma. Preliminary results. J Clin Med. 2018;7:31. doi: 10.3390/jcm7020031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallardo MJ, Supnet RA, Ahmed IIK. Circumferential viscodilation of Schlemm’s canal for open-angle glaucoma: ab-interno vs ab-externo canaloplasty with a tensioning suture. Clin Ophthalmol. 2018;12:2493–2498. doi: 10.2147/OPTH.S178962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shingleton BJ, Pasternack JJ, Hung JW, O’Donoghue MW. Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients. J Glaucoma. 2006;15:494–498. doi: 10.1097/01.ijg.0000212294.31411.92 [DOI] [PubMed] [Google Scholar]
- 14.Yook E, Vinod K, Panarelli JF. Complications of micro-invasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29:147–154. doi: 10.1097/ICU.0000000000000457 [DOI] [PubMed] [Google Scholar]
- 15.Lavia C, Dallorto L, Maule M, Ceccarelli M, Fea AM, Virgili G. Minimally-invasive glaucoma surgeries (MIGS) for open angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. doi: 10.1371/journal.pone.0183142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smithen LM, Ober MD, Maranan L, Spaide RF. Intravitreal triamcinolone acetonide and intraocular pressure. Am J Ophthalmol. 2004;138:740–743. doi: 10.1016/j.ajo.2004.06.067 [DOI] [PubMed] [Google Scholar]
- 17.Van Buskirk EM, Cioffi GA. Glaucomatous optic neuropathy. Am J Ophthalmol. 1992;113:447–452. doi: 10.1016/S0002-9394(14)76171-9 [DOI] [PubMed] [Google Scholar]
- 18.Grant WM. Experimental aqueous perfusion in enucleated human eyes. Arch Ophthalmol. 1963;69:783–801. doi: 10.1001/archopht.1963.00960040789022 [DOI] [PubMed] [Google Scholar]
- 19.Mäepea O, Bill A. Pressures in the juxtacanalicular tissue and schlemm’s canal in monkeys. Exp Eye Res. 1992;54:879–883. doi: 10.1016/0014-4835(92)90151-H [DOI] [PubMed] [Google Scholar]
- 20.Allingham RR, De Kater AW, Ethier CR. Schlemm’s canal and primary open angle glaucoma: correlation between schlemm’s canal dimensions and outflow facility. Exp Eye Res. 1996;62:101–109. doi: 10.1006/exer.1996.0012 [DOI] [PubMed] [Google Scholar]
- 21.Battista SA, Lu Z, Hofmann S, et al. Reduction of the available area for aqueous humor outflow and increase in meshwork herniations into collector channels following acute IOP elevation in bovine eyes. Invest Ophthalmol Vis Sci. 2008;49(12):5346–5352. doi: 10.1167/iovs.08-1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickerson JE Jr, Brown RH. Circumferential canal surgery: a brief history. Curr Opin Ophthalmol. 2020;31:139–146. doi: 10.1097/ICU.0000000000000639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedman DS, Hahn SR, Gelb L, et al. Doctor-patient communication, health-related beliefs, and adherence in glaucoma. Ophthalmology. 2008;115:1320–1327. doi: 10.1016/j.ophtha.2007.11.023 [DOI] [PubMed] [Google Scholar]