Abstract
Introduction
Increasing bacteria resistance to antibiotics is a major problem of healthcare system. There is a need for solutions that broaden the spectrum of bactericidal agents improving the efficacy of commonly used antibiotics. One of the promising directions of search are silver nanoparticles (obtained by different methods and displaying diversified physical and chemical properties), and their combination with antibiotics.
Purpose
In this study, we tested the role of reactive oxygen species in the mechanism of synergistic antibacterial activity of gentamicin and Tween-stabilized silver nanoparticles against gentamicin-resistant clinical strains of Staphylococcus epidermidis.
Methods
Synergistic bactericidal activity of gentamicin and silver nanoparticles stabilized with non-ionic detergent (Tween 80) was tested by the checkerboard titration method on microtiter plates. Detection of reactive oxygen species was based on the chemiluminescence of luminol.
Results
Hydrophilic non-ionic surface functionalization of silver nanoparticles enabled the existence of non-aggregated active nanoparticles in a complex bacterial culture medium. Tween-stabilized silver nanoparticles in combination with gentamicin exhibited bactericidal activity against multidrug-resistant biofilm forming clinical strains of Staphylococcus epidermidis. A synergistic effect significantly decreased the minimal inhibitory concentration of gentamicin (the antibiotic with numerous undesirable effects). Gentamicin significantly enhanced the generation of reactive oxygen species by silver nanoparticles.
Conclusion
Generation of reactive oxygen species by Tween-coated metallic silver nanoparticles was significantly enhanced by gentamicin, confirming the hypothesis of oxidative-associated mechanism of the synergistic antibacterial effect of the gentamicin-silver nanoparticles complex.
Keywords: gentamicin, silver nanoparticles, reactive oxygen species, multidrug-resistant, Staphylococcus epidermidis
Introduction
Untargeted therapy as well as abuse of antibacterial drugs has led to the selection of bacterial strains resistant to available antibiotics. Microorganisms resistant to many different groups of antibiotics pose a particular danger.1 There is a need for alternative agents to combat bacterial infections.2 New antibacterial compounds should be effective in eradicating microorganisms, easily accessible, cheap, harmless to the environment and, above all, safe for the patient. Special interest is currently associated with nanomaterials, including silver nanoparticles (SNPs), as well as their combination with antibiotics.3,4
The antibacterial properties of silver were known from ancient times. In ancient Egypt, silver solutions were used to treat peptic ulcer disease. Food was stored in silver vessels for longer protection. Soldiers put silver coins on the wounds, what accelerated the healing process.5 Nowadays silver is widely used in medicine (silver coating on implants and catheter tubes), cosmetology, biotechnology, food and textile industries.6
The antibacterial properties of silver nanoparticles depend primarily on their size, shape, surface modification and concentration. The mechanism of bactericidal action of SNPs has not been clearly explained. Under the influence of their action, the process of wall and cell membrane formation is disturbed, protein synthesis is also inhibited. Nanoparticles affect nucleic acids and cellular metabolic processes. It is also assumed that the bactericidal effect may result from the induction of the reactive oxygen species (ROS) production by silver nanoparticles.7
Gentamicin and other antibiotics may form chemical complexes by binding of the active hydroxyl or amine group with metals. This may affect their biological activity.8 The combination of silver nanoparticles with antibiotics used in therapy may increase the bactericidal activity of both nanoparticles and antibiotics, even in relation to strains resistant to their action.9–13 The mechanism leading to the increased bactericidal activity of the drug-nanoparticle complex may be related to production of reactive oxygen species by silver nanoparticles under the influence of an antibiotic.14–16
Silver nanoparticles display diverse stability in complex solutions such as media used in microbiological tests. In order to provide adequate stability of SNPs, various types of stabilizing agents are used, both ionic and non-ionic. In this study Tween 80, non-ionic surfactant and emulgator, was used as a silver nanoparticles stabilizing agent.17
We demonstrated synergistic antibacterial activity of gentamicin and Tween-stabilized silver nanoparticles against gentamicin resistant clinical strains of Staphylococcus epidermidis. Antimicrobial activity of the Tween-stabilized silver nanoparticles was accompanied by production of reactive oxygen species as demonstrated by luminol chemiluminescence (CL).
Materials and Methods
Materials
Polysorbate 80 (Tween 80), silver nitrate, sodium borohydride, luminol and gentamicin sulfate were purchased from Sigma Aldrich Co. The Mueller-Hinton Broth II (Becton Dickinson) was used as a microbiological medium. UV-Vis spectra were recorded on Multiskan Sky Reader (Thermo Scientific). For the incubation and horizontal shaking of the microtiter plates containing bacterial cultures ES-20 Shaker (Biosan, Poland) was used.
All of the tested strains of Staphylococcus epidermidis were isolated from blood of newborns with low birth weight and catheter-related sepsis. Blood was collected during routine diagnostic tests, after obtaining written informed parental consent. No additional blood sample was taken, instead, the strains isolated in a hospital laboratory during routine diagnostic tests were used for the purpose of the study. This study was approved by the Bioethical Committee of the Jagiellonian University no. KBET/263/B/2013.
Synthesis of Silver Nanoparticles
Tween-stabilized silver nanoparticles (diameter 20–40 nm established by TEM - transmission electron microscopy) were prepared by thermal reduction of silver nitrate by Tween 80.18 Briefly, silver salt solution prepared by dissolving 50 mg of AgNO3 in 0.2 mL of deionized water was mixed with 2 mL of Tween 80 and incubated at 100°C for 72 hours. The color of reaction solution changed from yellow to orange and in the end to dark red-brown. The basic working solution of silver nanoparticles was prepared by 5x dilution of the reaction mixture with deionized water. The molar concentration of the working solution of SNPs was estimated on the basis of the LSPR (Localized Surface Plasmon Resonance) maximum and the molar extinction coefficient (4.18 x 109 M–1 cm–1),19 to be 60 nM. The concentration of metallic silver in the solution was 1400 µg/mL.
To address the problem of SNPs stability, citrate-stabilized silver nanoparticles were used. Nanoparticles were synthesized by reducing silver nitrate with sodium borohydride in the presence of sodium citrate.20 Briefly, 0.5 mL of AgNO3 (0.01 M) was mixed with 19 mL of sodium citrate (0.01 M). In the next step, 0.6 mL of sodium borohydride (0.01 M) was added gradually. Reaction was carried out at 0°C. The molar concentration of 10 nm SNPs solution was estimated, on the basis of the LSPR maximum and the molar extinction coefficient (5.56 x 108 M–1 cm–1)19 to be 10 nM.
The Assessment of Antimicrobial Activity of Combination of Gentamicin and Silver Nanoparticles
Nine multidrug-resistant (MDR) clinical strains of Staphylococcus epidermidis were isolated from blood cultures and stored at −80ºC until use. The drug resistance of the isolates were confirmed according to the protocols of European Committee on Antimicrobial Susceptibility Testing (EUCAST).21
The minimal inhibitory concentration (MIC) values of gentamicin for selected strains were assessed according to the recommendations of EUCAST.22 The analogous test was conducted to assess the antimicrobial activity of silver nanoparticles.
To evaluate the MIC value of gentamicin in combination with SNPs, the antimicrobial activity of antibiotic and silver nanoparticles was investigated by checkerboard titration method on 96-well microtiter plates.23 The checkerboard method was based on microdilution assay. Briefly, 100 μL of Mueller-Hinton Broth II (MHB II) was added to each well on the plate, the dilutions of gentamicin (in range of 0.97 to 250 μg/mL) were made from left to the right side of the plate, dilutions of SNPs (in range of 21.5 to 1400 μg/mL) were made from upper to lower rows of the plate. To microtiter wells, containing different combinations of gentamicin and SNPs, 10 μL of 1.0 McFarland bacterial saline suspension was added. The final concentration of bacteria in each well was 1.5 × 107 CFU/mL (colony-forming unit). To determine the microbial growth inhibition the microtiter plates were incubated at 37°C for 24 hours with horizontal shaking (120 rpm/min). To reduce the SNPs related optical background of SNPs, after first incubation, 10 μL of suspension from each well on the plate was transferred to 190 μL of MHB II on another plate and incubated at 37°C for 24 hours with horizontal shaking (120 rpm/min). To assess the growth of the strains, the optical density of the solutions was measured with microplate reader Sunrise (Tecan, Switzerland) at wavelength 600 nm. The checkerboard tests was performed for all of tested strains in duplicates. The growth control and negative control were maintained during the test. The growth control was maintained to confirm the normal growth of the bacteria in test condition. Briefly, 10 µL of 1.0 McFarland bacterial saline suspension was added to 190 µL of MHB II. The negative control (200 µL of MHB II) was maintained to confirm the purity of microbiological medium and to prove lack of contamination of the solutions during the test. Additional control was carried out to confirm that Tween 80, detergent used during the synthesis of SNPs, does not show the bactericidal effect. Briefly, 100 µL of MHB II was mixed with 100 µL of 20% v/v Tween 80 solution and 10 µL of bacterial saline suspension.
The interpretation of the checkerboard test was based on the value of a Fractional Inhibitory Concentration (FIC) Index:24
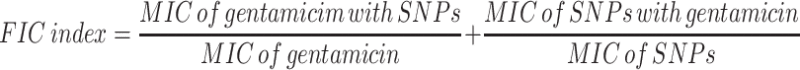 |
The FIC values have been interpreted as follows:
FIC ≤ 0.5 - synergistic effect
> 0.5 and ≤ 1 - additive effect
> 1 and <4 - no action
≥ 4 - antagonistic effect
The capability of tested strains to biofilm formation was evaluated according to procedure of Christensen et al, based on the assessment of bacterial cells’ adherence to 96-well tissue culture plates. Bacterial saline suspension (1.0 McFarland) was incubated with the plate for 18 hours, 37ºC. After incubation, the suspension was removed and wells were washed with phosphate-buffered saline. Adherent cells were fixed and stained with 1% crystal violet water solution. Interpretation of the test was based on the value of optical density measured with microplate reader at 600 nm wavelength.25 The positive (Staphylococcus aureus SA RN450) and negative control (Staphylococcus epidermidis SE ATCC12228) were maintained during the test. Eight of nine of the investigated strains of Staphylococcus epidermidis displayed the ability to biofilm formation.
Detection of Reactive Oxygen Species
Detection of ROS was based on the chemiluminescence of luminol.26 The generation of ROS was investigated by mixing 100 µL of luminol solution (5 mM in phosphate-buffered saline) with 100 µL Tween-stabilized silver nanoparticles (working solution was diluted 25x with deionized water) and 2 µL of gentamicin water solution (40 mg/mL) to obtain final antibiotic concentration of 0.4 mg/mL. Chemiluminescence was measured on a LUMAT LB 9507 (Berthold Technologies) luminometer at 1-s intervals.
Results
Stability of SNP Solutions in the Presence of Bacterial Growth Medium and Gentamicin
Silver nanoparticles require adequate stabilization to eliminate precipitation in growth media due to the destabilization of the surface charge of the molecules. Citrate-stabilized silver nanoparticles precipitate in the broth, yielding dark precipitate of metallic silver. The study compared the stability of silver nanoparticles, both Tween and citrate-stabilized in Mueller-Hinton Broth II.
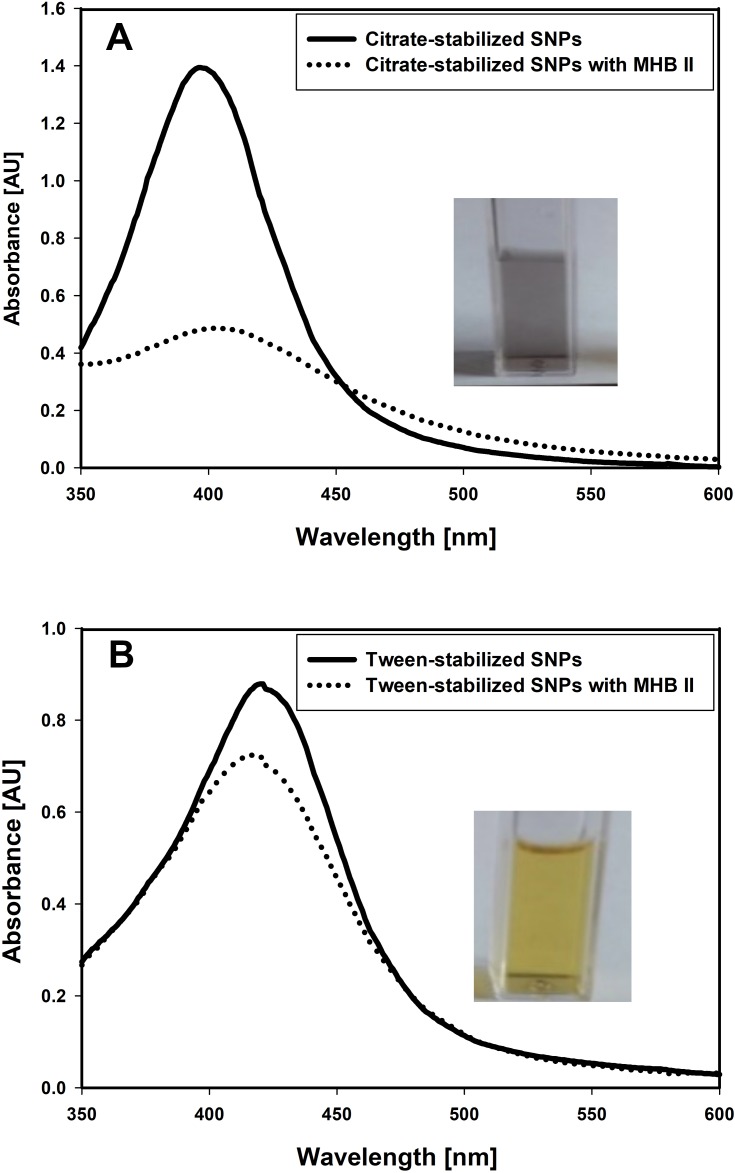
Figure 1 presents plasmon resonance related UV-Vis spectrum of the solutions of Tween and citrate-stabilized silver nanoparticles made with and without addition of broth (1:1 volume ratio). Changes in the spectrum of citrate-stabilized silver nanoparticles accompanied by metallic silver precipitation in the presence of broth confirms instability of citrate-stabilized SNPs (Figure 1A). Contrarily, Tween-stabilized silver nanoparticles are stable in the presence of broth solution as confirmed by UV-Vis spectrum of Tween-stabilized silver nanoparticles in the presence of MHB II (Figure 1B).
Figure 1.
Differences in stability of citrate and Tween-stabilized silver nanoparticles in bacterial growth medium.
Notes: (A) UV-Vis spectrum of citrate-stabilized silver nanoparticles before and after addition of Mueller-Hinton Broth II medium. Metallic silver precipitation is accompanied by suppression of characteristic nanoparticle-associated plasmon resonance spectrum; (B) UV-Vis spectrum of Tween-stabilized silver nanoparticles before and after addition of Mueller-Hinton Broth II medium. Strong plasmon resonance absorption band in the range of 400 nm confirms the presence of native metal nanoparticles.
Abbreviations: MHB II, Mueller-Hinton Broth II; SNPs, silver nanoparticles
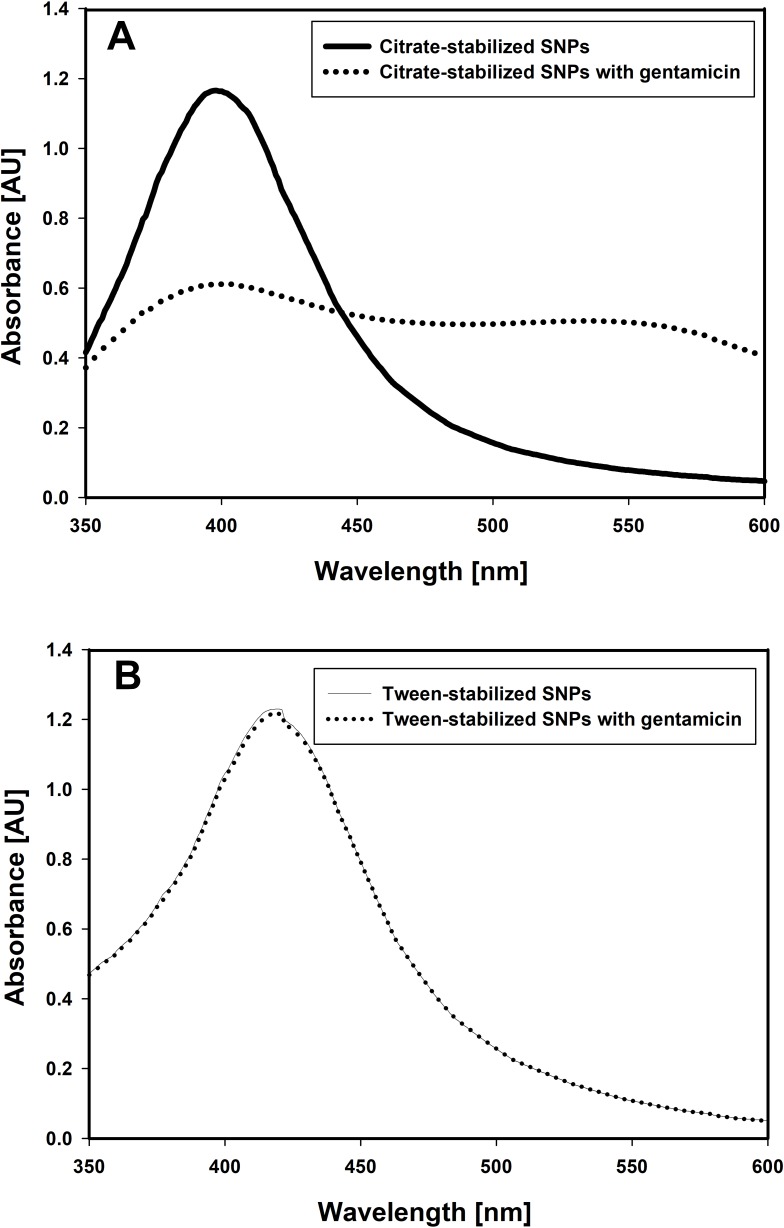
Stability of SNPs may be affected by many physical factors/reagents. Containing four amino groups, basic molecules of gentamicin have enhanced affinity to citrate-stabilized SNPs causing their massive aggregation and precipitation.27 To the opposite, Tween-stabilized SNPs are stable in the presence of gentamicin.
Changes in the spectrum of citrate-stabilized silver nanoparticles accompanied by metallic silver precipitation in the presence of gentamicin are presented on the Figure 2A. UV-Vis spectrum of Tween-stabilized silver nanoparticles in the presence of gentamicin (Figure 2B) confirms that Tween-stabilized silver nanoparticles are stable in that conditions. The method of synthesis/stabilization plays a crucial role in the biological activity of SNPs. Regarding the high stability of Tween-stabilized SNPs and the lack of the pronounced biological activity of the Tween itself, Tween-stabilized silver nanoparticles were used in further experiments.
Figure 2.
Differences in stability of citrate and Tween-stabilized silver nanoparticles in the presence of gentamicin (0.4 mg/mL).
Notes: (A) UV-Vis spectrum of citrate-stabilized silver nanoparticles before and after addition of gentamicin (0.4 mg/mL). Metallic silver precipitation is accompanied by suppression of characteristic nanoparticle-associated plasmon resonance spectrum; (B) UV-Vis spectrum of Tween-stabilized silver nanoparticles before and after addition of gentamicin (0.4 mg/mL). Strong plasmon resonance absorption band in the range of 400 nm confirms the presence of native metal nanoparticles.
Abbreviation: SNPs, silver nanoparticles
Antimicrobial Activity of Gentamicin in the Presence of SNPs
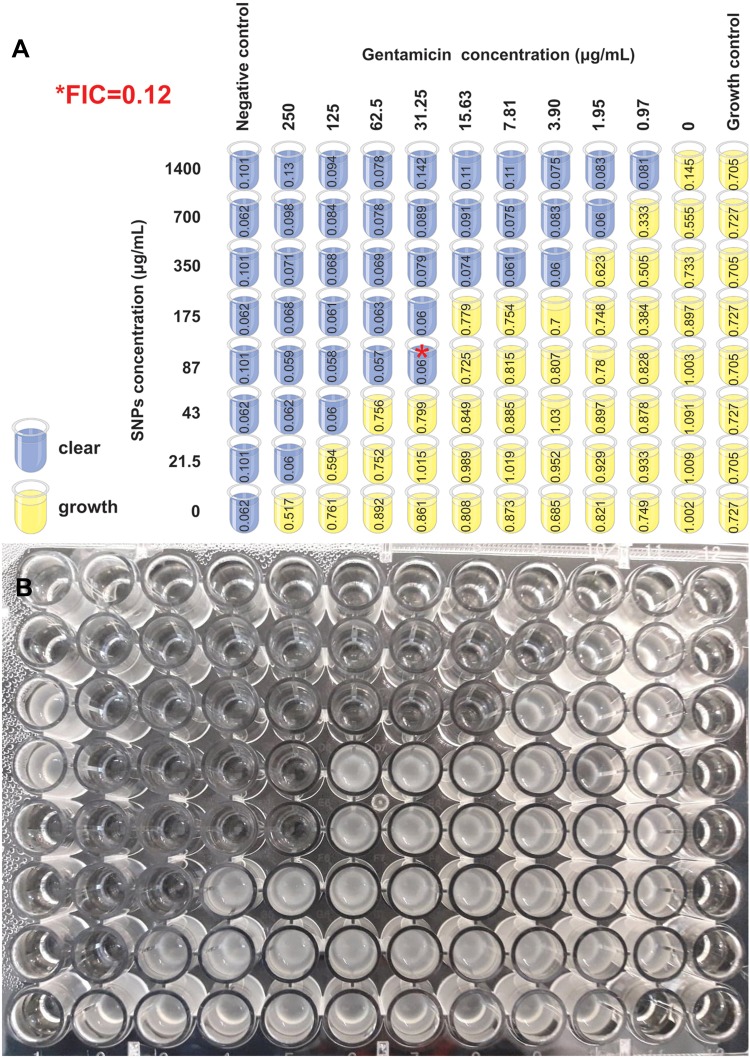
Addition of SNPs strongly affected the minimal inhibitory concentration of gentamicin for all gentamicin resistant clinical strains of Staphylococcus epidermidis. Combination of SNPs and gentamicin leads to synergistic decrease of gentamicin and SNPs concentrations. Figure 3 presents typical bacterial growth pattern in the presence of gentamicin and SNPs. Increase in gentamicin concentration resulted in a decrease of SNPs concentration necessary for bacterial growth inhibition and vice versa. Minimal FIC index for each bacterial strain identified the point where synergistic effect was the most prominent, establishing conditions of optimal concentrations of gentamicin and SNPs. Minimal inhibitory concentration for gentamicin and silver nanoparticles at minimal FIC index for all clinical strains are presented in Table 1. At minimal value of FIC index, MIC of gentamicin was significantly decreased (median 16 times). Simultaneously in the presence of gentamicin, bactericidal activity of SNPs was observed. Silver nanoparticles alone did not exhibit antimicrobial activity at a concentration of 1400 µg/mL of silver (or 60 nM of SNPs concentration). In the presence of gentamicin, at minimal FIC index value, median value of inhibitory concentrations of SNPs was 175 µg/mL.
Figure 3.
Bacterial growth pattern in the presence of gentamicin and silver nanoparticles.
Notes: (A) Growth pattern of S.epidermidis K/8605/12 in the presence of gentamicin and SNPs. The dilutions of gentamicin (in range of 0.97 to 250 μg/mL) were made from left to the right side of the plate, dilutions of SNPs (in range of 21.5 to 1400 μg/mL) were made from upper to lower rows of the plate. The FIC index was established on the growth inhibition border. Minimal FIC index (0.12) identified the point where synergistic effect was the most prominent*. For each well, optical density (mean from duplicate measurements) were presented. (B)Photography of the growth of the presented strain of Staphylococcus epidermidis K/8605/12.
Abbreviations: FIC, fractional inhibitory concentration; SNPs, silver nanoparticles
Table 1.
Results of Checkerboard Assay
| Clinical Strain | Mechanism of Resistance | MIC (µg/mL) | Change of MIC of Gentamicin | Minimal FIC Index | ||
|---|---|---|---|---|---|---|
| Gentamicin | Gentamicin+ SNPs | SNPs+ Gentamicin |
||||
| K/8605/12 | MRSE, MLSB | 500 | 31.25 | 87 | 16 | 0.12 |
| K/4042/16 | MRSE, MLSB | 500 | 31.25 | 175 | 16 | 0.19 |
| K/8399/12 | MRSE, MLSB | 500 | 31.25 | 175 | 16 | 0.19 |
| K/15/928 | MRSE, MLSB | 500 | 7.81 | 87 | 64 | 0.08 |
| K/15/323 | MRSE | 125 | 0.97 | 350 | 128 | 0.26 |
| K/15/534 | MRSE, MLSB | 250 | 31.25 | 87 | 16 | 0.18 |
| K/15/428 | MRSE, MLSB | 500 | 62.5 | 175 | 8 | 0.25 |
| K/15/420 | MRSE, MLSB | 500 | 31.25 | 87 | 16 | 0.13 |
| K/16/3882 | MRSE, MLSB | 500 | 31.25 | 175 | 16 | 0.19 |
Note: Minimal inhibitory concentrations for gentamicin and silver nanoparticles and minimal FIC index for all clinical strains of Staphylococcus epidermidis.
Abbreviations: FIC, fractional inhibitory concentration; MIC, minimal inhibitory concentration; MLSB, macrolide-lincosamide-streptogramin resistance of Staphylococcus epidermidis; MRSE, methicillin-resistant Staphylococcus epidermidis; SNPs, silver nanoparticles.
Generation of ROS in the Presence of Gentamicin
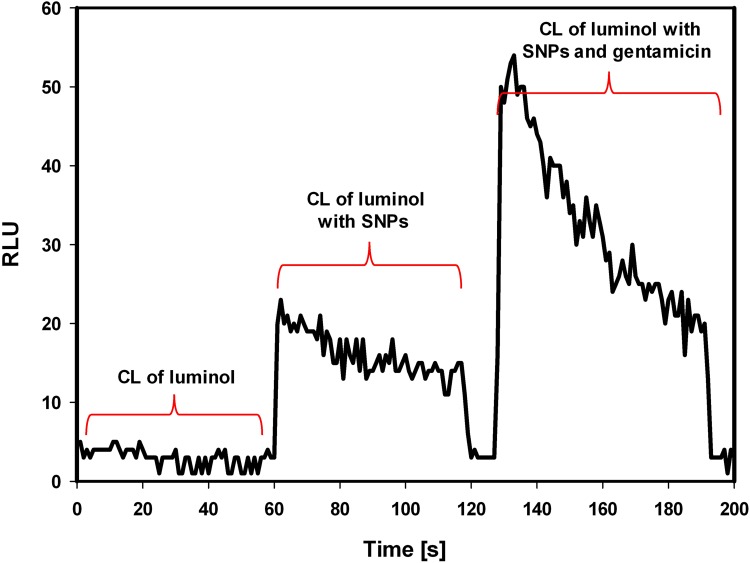
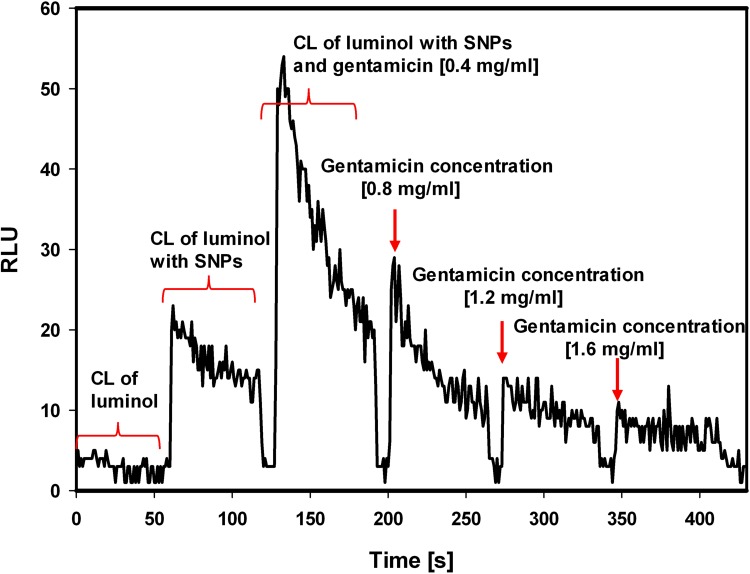
To assess the production of reactive oxygen species in the presence of SNPs and gentamicin, the chemiluminescence of luminol was tested. Addition of Tween-stabilized silver nanoparticles to luminol-based detection system stimulated production of reactive oxygen species. Consecutive addition of gentamicin significantly increased the generation of ROS (Figure 4). The generation of ROS induced by gentamicin decreased with time and further addition of gentamicin did not stimulate chemiluminescence, indicating that oxidizing potential of that system is SNPs dependent (Figure 5).
Figure 4.
Silver nanoparticles-associated generation of ROS in the presence of gentamicin.
Note: The chemiluminescence of luminol (stage 1) in the presence of Tween-stabilized silver nanoparticles (stage 2) and stabilized silver nanoparticles combined with gentamicin (2 µL of gentamicin, the final antibiotic concentration 0.4 mg/mL) (stage 3).
Abbreviations: CL, chemiluminescence; RLU, relative luminescence units; ROS, reactive oxygen species; SNPs, silver nanoparticles.
Figure 5.
Silver nanoparticles-associated generation of ROS stimulated by subsequent doses of gentamicin.
Note: Luminol chemiluminescence in the presence of Tween-stabilized silver nanoparticles after addition of subsequent amounts of gentamicin (2µL of gentamicin, delta concentration 0.4 mg/mL).
Abbreviations: CL, chemiluminescence; RLU, relative luminescence units; ROS, reactive oxygen species; SNPs, silver nanoparticles.
Discussion
Silver nanoparticles are increasingly used in various fields of medicine and biotechnology.28 SNPs with controlled size and shapes determined by synthesis conditions, are used in biochemical diagnostics systems, bioimaging strategies, phototherapy of cancer cells and drugs delivery. Antibacterial properties of silver were observed from ancient times. Extraordinary antibacterial activity of functionalized SNPs acquire increasing interest in 21st century.29,30
The new resistance mechanisms developed by bacteria limited the amount of available medicines used in therapy. Bacterial strains isolated from patients are increasingly characterized by resistance to more than one group of antibiotics. These multidrug-resistant bacterial strains are a serious challenge for health care.31 Searching for new antibacterial agents became one of main goals of modern medicine.32 Promising results were obtained with metal nanoparticles, including silver nanoparticles. There are many studies evaluating antimicrobial activity of silver nanoparticles. Silver nanoparticles are applied on the surface of surgical instruments, implants, catheters and dental prostheses to inhibit their microbiological colonization.33,34 The main advantages of silver nanoparticles used as a antimicrobial agents are: gradual release of silver ions (which provides long-term activity), low toxicity to eukaryotic cells (lower than silver ions) and lack of mechanism of resistance developed by bacteria.35,36
The bactericidal activity of silver nanoparticles and the mechanisms of bactericidal action raise a lot of controversy. The results of published studies conducted to elucidate the antibacterial activity of silver nanoparticles differ significantly.37 It is very difficult to draw specific conclusions, as the published studies utilized various methods of nanoparticle synthesis, including so called green methods, based on undefined extract of natural products, and tested the variety of bacterial strains and various methods for assessing biocidal activity.38,39
The exact mechanism of bactericidal activity of silver nanoparticles has not been fully determined.40,41 The possible modes of action of metal nanoparticles include: (a) production of reactive oxygen species inside microbial cells including inhibition of microbial proteins/enzymes by increased production of H2O2. This mechanism is supported by the lack of many antioxidant mechanisms in bacterial cells as compared to eukaryotic cells; (b) disruption of vital enzymes in bacterial respiratory chain via forming silver complexes with electron donors containing sulfur, oxygen, nitrogen or thiols, (c) damage of biomolecules such as DNA or proteins, (d) targeting the bacterial membrane by SNPs. The accumulation of metal ions in microbial membranes alters the bacterial membrane potential and increases the cell membrane permeability; (e) electrostatic attraction between metal nanoparticles and microbial cells which disrupt metabolic activities.42–44
In order to be used in therapy, compounds with bactericidal activity must exhibit not only high biocidal activity but also should be safe for human cells. Many of antibiotics are highly toxic to the patient. Gentamicin, an aminoglycoside antibiotic that exhibits bactericidal activity against aerobic bacteria commonly used to treat respiratory and urinary tract infections, skin and eyes diseases, causes a variety of undesirable side effects.45 Nephrotoxicity, neurotoxicity, ototoxicity, hematological disorders and impairment of liver function are among of the most deteriorating effects. One of the main problems of pharmacology is to use therapeutic compounds in the lowest possible doses, as well as to search for alternative therapeutic agents with similar efficacy but less toxicity.46 Gentamicin is a part of the basic panel of susceptibility testing for Staphylococcus epidermidis strains isolated from blood. Staphylococcus epidermidis is the most common coagulase-negative staphylococci responsible for nosocomial infections observed among newborns with very low birth weight and associated with colonization of medical devices. Research conducted at intensive care units in Poland showed that 95% of Staphylococcus epidermidis strains isolated from blood of neonates with very low birth weight were resistant to gentamicin.47 In treatment of Staphylococcus epidermidis infections, gentamicin is very often used in combination with other antibiotics.
The effect of silver nanoparticles on human cells is not entirely known. The small diameter and surface of the nanoparticles enables to their transport with blood to all organs and tissues.48,49 Research has been done to assess the toxic activity of silver nanoparticles.50,51 It is not entirely clear whether the nanoparticles themselves are responsible for their toxic activity, or whether the silver ions that are gradually released contribute to the toxic effect.52,53 The effect of silver nanoparticles on human cells is similar to their effect on bacterial cells. They change the structure of the cell wall, affect the process of protein synthesis and reduce the potential of the mitochondrial membrane.54–56, Questionable safety of SNPs enforces administrating of silver nanoparticles in the lowest possible concentrations. To achieve this effect, various methods of stabilizing and functionalizing the surface of silver nanoparticles are sought, as well as their combinations with other agents with bactericidal activity. All this technological solutions contribute to the increase in the bactericidal activity of nanoparticles, while reducing their concentration.57,58 Our study utilized relatively high concentrations of SNPs. However, such concentrations might be used eg in preparations designed for external use, such as ointments. This is clinically relevant, as gentamicin is one of the antibiotics used externally to treat skin infections. It is noteworthy that the synthesis of SNPs is relatively inexpensive, broadening their universal use.
To achieve bactericidal effect, nanoparticles must be stable and active in the complex microbiological media.59 Despite the fact that citrate-stabilized silver nanoparticles are widely used, their lack of stability in a complex microbiological environment excludes the possibility of their use in microbiological tests. The stability of nanoparticles can be achieved by using various types of ionic and non-ionic factors (polymers and surfactants).17,60 SNPs must also be stable in the presence of other compounds as antibiotics. Tween 80 ensures the stability of silver nanoparticles in a microbiological medium and in the presence of positively charged antibiotics, including gentamicin. Similar effect in stabilization of silver nanoparticles with Tween 80 was reported by Chen et al. For several tested surfactants, the most preferable effect was achieved with using Tween 80-stabilized silver nanoparticles, which have been recognized by the authors as a very promising antibacterial agent.61
In this study, the antibacterial activity of silver nanoparticles combined with gentamicin was investigated. Other studies on the mechanism of action of the nanoparticles-antibiotic combinations have suggested that the improvement in the antimicrobial activity could be caused by their chemical interaction. The underlying molecular mechanism of the effect, either synergistic or additive, still requires clarification.62,63 In former studies, it was postulated that combinatorial effect of antimicrobials drives synergy by membrane alterations generated by SNPs and no chemical interactions between SNPs and antibiotics were detected.64 We demonstrated that synergistic mechanism is based on direct specific chemical interactions between SNPs and gentamicin stimulating production of ROS. In our study, antimicrobial activity was assessed against well characterized clinical strains of Staphylococcus epidermidis with developed mechanisms of resistance to beta-lactam antibiotics (MRSE) and to macrolides, lincosamides and streptogramins groups of antibiotics (MLSB). Staphylococcus epidermidis is a species of Gram positive bacteria present on the skin and responsible for the most of nosocomial infections resistant to antibiotics used in the therapy.65 All clinical strains of multidrug-resistant Staphylococcus epidermidis were resistant to gentamicin. Eight of nine investigated strains displayed ability to bacterial biofilm formation, an additional virulence factor of external surface matrix consisted of proteins and oligosaccharides.66
We observed no antimicrobial effects of SNPs alone against tested strains, even at the highest concentration of SNPs (1400 µg/mL, 60 nM of SNPs concentration). This is in line with former evidence: the lack of significant results in the assessment of the antibacterial properties of weak, (citrate) stabilized silver nanoparticles against the reference strain of Staphylococcus aureus was described by Kin et al. There were no antimicrobial effect of silver nanoparticles even at the highest concentration of SNPs - 33 nM.67
On the contrary, in the presence of gentamicin, silver nanoparticles show antibacterial activity against tested strains of S. epidermidis. The interpretation of the checkerboard method, used to assess the antimicrobial activity of combination SNPs and gentamicin was based on the value of Fractional Inhibitory Concentration index. The FIC value provides information on whether the achieved bactericidal effect is associated with the synergistic effect of the compounds, or whether it can be an additive effect.24 Several studies indicated that SNPs may enhance the antibacterial effects of antibiotics. However, these synergistic effects of SNPs–antibiotic conjunction were observed against antibiotic susceptible bacteria. Only few studies confirmed a successful synergistic effect of SNPs–antibiotics combinations in MDR bacteria.68 The minimal value of FIC index is associated with the most prominent conditions for bactericidal effect. The combination of SNPs and gentamicin leads to decrease in the minimal inhibitory concentration of gentamicin for all of tested strains of S. epidermidis. The synergistic effect of silver nanoparticles and gentamicin against Escherichia coli and Staphylococcus aureus strains was reported by Wang et al.7 Authors demonstrated that the presence of gentamicin even at low concentration (1 µg/mL) stimulates antimicrobial activity of silver nanoparticles against reference strains. The authors explained the differences between E. coli and S. aureus susceptibility to silver nanoparticles and gentamicin by the different membrane structure of Gram-positive and Gram-negative bacteria. Gram-negative Escherichia coli strains present higher sensitivity to SNPs and gentamicin than Gram-positive Staphylococcus aureus strains.
It was suggested that one of the possible mechanisms of bactericidal activity of silver nanoparticles relies on stimulation of production of reactive oxygen species. Oxidative stress caused by silver nanoparticles through the increase in ROS production leads to damage of the proteins and nucleic acids, and consequently to inhibition of cell proliferative processes.44,69
There are many works confirming that SNPs in various conditions can produce toxic ROS including hydrogen peroxide (H2O2), superoxide anions (O2−), and most reactive hydroxyl radicals (OH*).7,66,69 Liu et al showed that silver nanoparticles stimulate the chemiluminescence of luminol and the intensity of the chemiluminescence is dependent on the size of SNPs, the most intense chemiluminescence was generated by the silver nanoparticles with the smallest diameter.70 On the contrary, investigation concerning the generation of ROS in SNPs-gentamicin system is controversial. Some of the studies conclude that ROS do not play a major role in the gentamicin potentiating activity of silver.71 Some recent works have shown the generation of ROS in the presence of silver ions (derived from silver nitrate) and gentamicin.72
In order to determine the mechanism of the combined antibacterial activity of silver nanoparticles and gentamicin, our study utilized a test based on the chemiluminescence of luminol to assess the production of reactive oxygen species both by the nanoparticles themselves and by their combination with gentamicin. In luminol model, Tween-stabilized silver nanoparticles generated reactive oxygen species. The presence of gentamicin significantly increased the generation of ROS (Figure 4). Decrease in ROS production over time, which is not stimulated by subsequent doses of gentamicin (Figure 5) confirms that the bactericidal oxidation potential of the system depends on silver nanoparticles.
Antibacterial effects of SNPs, either in metallic form or in gradually released ionic form yields multifactorial system operating on different cellular levels. That system, in combination with antibiotics influences on many bacterial structures and metabolic processes concurrently. In connection with different forms of ROS, it is an antimicrobial defending system characterized by low risk of bacterial resistance development.68
Conclusion
To ensure pronounced antibacterial activity, SNPs must be stable in complex bacteria environments. Tween-stabilized silver nanoparticles and gentamicin revealed synergistic antibacterial activity against multidrug-resistant strains of Staphylococcus epidermidis. The combination of silver nanoparticles and gentamicin allowed to decrease the minimal inhibitory concentration of gentamicin (median 16 times). Gentamicin increased the generation of SNPs derived ROS, one of the probable co-mechanisms of complex SNPs-antibiotic system.
Acknowledgments
The study was approved by the Bioethical Committee of the Jagiellonian University no. KBET/263/B/2013. Scientific works were financed from the research project no. 2018/31/N/NZ6/03339 (K/PBM/000649) financed by the National Science Centre, Poland.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Medina E, Pieper DH. Tackling threats and future problems of multidrug-resistant bacteria. Curr Top Microbiol Immunol. 2016;398:3–33. doi: 10.1007/82_2016_492 [DOI] [PubMed] [Google Scholar]
- 2.Eleraky NE, Allam A, Hassan SB, Omar MM. Nanomedicine fight against antibacterial resistance: an overview of the recent pharmaceutical innovations. Pharmaceutics. 2020;12(2):1–49. doi: 10.3390/pharmaceutics12020142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh P, Kim YJ, Singh H, et al. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanome. 2015;10:2567–2577. doi: 10.2147/IJN.S72313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hajipour MJ, Fromm KM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trend Biotechnol. 2012;30(10):499–511. doi: 10.1016/j.tibtech.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 5.Domínguez AV, Algaba RA, Canturri AM, Villodres AR, Smani Y. Antibacterial activity of colloidal silver against gram-negative and gram-positive bacteria. Antibiotics. 2020;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahamed M, AlSalhi MS, Siddiqui MKJ. Silver nanoparticles applications and human health. Clin Chim Acta. 2010;411:1841–1848. doi: 10.1016/j.cca.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Tang H, Wu D, et al. Enhanced bactericidal toxicity of silver nanoparticles by the antibiotic gentamicin. Environ Sci Nano. 2016;3:788–798. [Google Scholar]
- 8.Katva S, Das S, Moti HS, Jyoti A, Kaushik S. Antibacterial synergy of silver nanoparticles with gentamicin and chloramphenicol against Enterococcus faecalis. Pharmacogn Mag. 2017;13(4):828–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hwang I-S, Hwang JH, Choi H, Kim K-J, Lee DG. Synergistic effects between silver nanoparticles and antibiotics and the mechanisms involved. J Med Microbiol. 2012;61:1719–1726. doi: 10.1099/jmm.0.047100-0 [DOI] [PubMed] [Google Scholar]
- 10.Naqvi SZH, Kiran U, Ali MI, et al. Combined efficacy of biologically synthesized silver nanoparticles and different antibiotics against multidrug-resistant bacteria. Int J Nanome. 2013;8:3187–3195. doi: 10.2147/IJN.S49284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figueiredo EP, Ribeiro JM, Nishio EK, et al. New approach for simvastatin as an antibacterial: synergistic effect with bio-synthesized silver nanoparticles against multidrug-resistant bacteria. Int J Nanome. 2019;14:7975–7985. doi: 10.2147/IJN.S211756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Carrizales M, Velasco KI, Castillo C, et al. In vitro synergism of silver nanoparticles with antibiotics as an alternative treatment in multiresistant uropathogens. Antibiotics. 2018;7(50):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abo-Shama UH, El-Gendy H, Mousa WS, et al. Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect Drug Resist. 2020;13:351–362. doi: 10.2147/IDR.S234425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brochmann RP, Helmfrid A, Jana B, Magnowska Z, Guardabassi L. Antimicrobial synergy between carprofen and doxycycline against methicillin-resistant Staphylococcus pseudintermedius ST71. BMC Vet Res. 2016;12:126–134. doi: 10.1186/s12917-016-0751-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan G, Ruan L, Yin Y, Yang T, Ge M, Cheng X. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. Int J Nanome. 2016;11:3789–3800. doi: 10.2147/IJN.S104166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogowska A, Rafińska K, Pomastowski P, et al. Silver nanoparticles functionalized with ampicillin. Electrophoresis. 2017;38:2757–2764. doi: 10.1002/elps.201700093 [DOI] [PubMed] [Google Scholar]
- 17.Fernando I, Qian T, Zhou Y. Long term impact of surfactants & polymers on the colloidal stability, aggregation and dissolution of silver nanoparticles. Environ Res. 2019;179:1–9. doi: 10.1016/j.envres.2019.108781 [DOI] [PubMed] [Google Scholar]
- 18.Li H-J, Zhang A-Q, Hu Y, Sui L, Qian D-J, Chen M. Large-scale synthesis and self-organization of silver nanoparticles with Tween 80 as a reductant and stabilizer. Nanoscale Res Lett. 2012;7(1):612–625. doi: 10.1186/1556-276X-7-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paramelle D, Sadovoy A, Gorelik S, Free P, Hobley J, Fernig DG. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst. 2014;139:4855–4861. doi: 10.1039/C4AN00978A [DOI] [PubMed] [Google Scholar]
- 20.Zhang W, Qiao X, Chen Q, Cai Y, Chen H, The influence of synthesis condition and aging process of silver nanocrystals on the formation of silver nanorods. Appl Surf Sci. 2012;258(15):5909–5913. doi: 10.1016/j.apsusc.2012.02.138 [DOI] [Google Scholar]
- 21.The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 10.0, 2020. http://www.eucast.org. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint_Tables.pdf. Accessed April 24, 2020.
- 22.EUCAST reading guide for broth microdilution. Available from: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2020_manuals/Reading_guide_BMD_v_2.0_2020.pdf. Accessed April 24, 2020.
- 23.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother. 2003;52:1. doi: 10.1093/jac/dkg301 [DOI] [PubMed] [Google Scholar]
- 24.Chin NX, Weitzman I, Della-Latta P. In vitro activity of fluvastatin, a cholesterol-lowering agent, and synergy with fluconazole and itraconazole against Candida species and Cryptococcus neoformans. Antimicrob Agents Chemother. 1997;41(4):850–852. doi: 10.1128/AAC.41.4.850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christensen GD, Simpson WA, Younger JJ, et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22(6):996–1006. doi: 10.1128/JCM.22.6.996-1006.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Drozdz R, Parmentier C, Hachad H, Leroy P, Siest G, Wellman M. γ-glutamyltransferase dependent generation of reactive oxygen species from a glutathione/transferrin system. Free Radic Biol Med.1998;25(7):786–792. doi: 10.1016/S0891-5849(98)00127-0. [DOI] [PubMed] [Google Scholar]
- 27.Krause KM, Serio AW, Kane TR, Connolly LE. Aminoglycosides: an overview. Cold Spring Harb Perspect Med. 2016;6(6):1–19. doi: 10.1101/cshperspect.a027029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh M, Singh S, Prasad S, Gambhir IS. Nanotechnology in medicine and antimicrobial effect of silver nanoparticles. Dig J Nanomater Bios. 2008;3(3):115–122. [Google Scholar]
- 29.Wong KKY, Liu X. Silver nanoparticles – the real „silver bullet” in clinical medicine? MedChemComm. 2010;1:125–131. doi: 10.1039/c0md00069h [DOI] [Google Scholar]
- 30.Marassi V, Di Cristo L, Smith SGJ, et al. Silver nanoparticles as a medical device in healthcare settings: a five-step approach for candidate screening of coating agents R. Soc Open Sci. 2018;5:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Magiorakos AP, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- 32.Tacconelli E, Carrara E, Savoldi A, et al.; the WHO Pathogens Priority List Working Group. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 33.Prabhu S, Poulose EK. Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Letter. 2012;2(32):1–10. doi: 10.1186/2228-5326-2-32 [DOI] [Google Scholar]
- 34.Barkat MA, Harshita, Beg S, et al. Current progress in synthesis, characterization and applications of silver nanoparticles: precepts and prospects. Recent Pat Antiinfect Drug Discov. 2018;13(1):53–69. doi: 10.2174/1574891X12666171006102833 [DOI] [PubMed] [Google Scholar]
- 35.Corrêa JM, Mori M, Sanches HL, et al. Silver nanoparticles in dental biomaterials. Int J Biomater. 2015;2015:1–9. doi: 10.1155/2015/485275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Savithramma N, Linga Rao M, Rukmini K. Suvarnalatha devi P. Antimicrobial activity of silver nanoparticles synthesized by using medicinal plants. Int J Chemtech Res. 2011;3(3):1394–1402. [Google Scholar]
- 37.Martínez-Castañón GA, Niño-Martínez N, Martínez-Gutierre F, Martínez-Mendoza JR, Ruiz F. Synthesis and antibacterial activity of silver nanoparticles with different sizes. J Nanoparticle Res. 2010;10(8):1343–1348. doi: 10.1007/s11051-008-9428-6 [DOI] [Google Scholar]
- 38.Rimal Isaac RS, Sakthivel G, Murthy C. Green synthesis of gold and silver nanoparticles using Averrhoa bilimbi fruit extract. J Nanotechnol. 2013;2013:1–6. doi: 10.1155/2013/906592 [DOI] [Google Scholar]
- 39.Le Ouay B, Stallacci F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today. 2015;10:339–354. doi: 10.1016/j.nantod.2015.04.002 [DOI] [Google Scholar]
- 40.Yan X, He B, Liu L, et al. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: proteomics approach. Metallomics. 2018;10(4):515–652. doi: 10.1039/C7MT00328E [DOI] [PubMed] [Google Scholar]
- 41.Lara HH, Garza-Treviño EN, Ixtepan-Turrent L, Singh DK. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J Nanobiotechnol. 2011;9(30):1–8. doi: 10.1186/1477-3155-9-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nisar P, Ali N, Rahman L, Ali M, Shinwari ZK. Antimicrobial activities of biologically synthesized metal nanoparticles: an insight into the mechanism of action. J Biol Inorg Chem. 2019;7:929–941. [DOI] [PubMed] [Google Scholar]
- 43.Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, OU-Yang Y-S, Chen Y-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl Microbiol Biotechnol. 2010;85:1115–1122. doi: 10.1007/s00253-009-2159-5 [DOI] [PubMed] [Google Scholar]
- 44.Durán N, Durán M, de Jesus MB, Seabra AB, Fávaro WJ, Nakazato G. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomed. 2016;12(3):789–799. doi: 10.1016/j.nano.2015.11.016 [DOI] [PubMed] [Google Scholar]
- 45.Libert J, Ketelbant-Balasse PE, Van Hoof F, Aubert-Tulkens G, Tulkens P. Cellular toxicity of gentamicin. Am J Ophthalmol. 1979;87:405–411. doi: 10.1016/0002-9394(79)90085-0 [DOI] [PubMed] [Google Scholar]
- 46.Schentag JJ, Cumbo TJ, Jusko WJ. Gentamicin tissue accumulation and nephrotoxic reactions. JAMA. 1978;240(19):2067–2069. doi: 10.1001/jama.1978.03290190045027 [DOI] [PubMed] [Google Scholar]
- 47.Brzychczy-Wloch M, Borszewska-Kornacka M, Gulczynska E, et al. Prevalence of antibiotic resistance in multi-drug resistant coagulase-negative staphylococci isolated from invasive infection in very low birth weight neonates in two Polish NICUs. Ann Clin Microbiol Antimicrob. 2013;12:41–48. doi: 10.1186/1476-0711-12-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teodoro JS, Simões AM, Duarte FV, et al. Assessment of the toxicity of silver nanoparticles in vitro: a mitochondrial perspective. Toxicol in Vitro. 2011;25(3):664–670. doi: 10.1016/j.tiv.2011.01.004 [DOI] [PubMed] [Google Scholar]
- 49.Rodriguez-Garraus A, Azqueta A, Vettorazzi A. López de Cerain A. Genotoxicity of silver nanoparticles. Nanomater. 2020;10(2). doi: 10.3390/nano10020251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lojk J, Repas J, Veranič P, Bregar VB, Pavlin M. Toxicity mechanisms of selected engineered nanoparticles on human neural cells in vitro. Toxicol. 2020;432:152364. doi: 10.1016/j.tox.2020.152364 [DOI] [PubMed] [Google Scholar]
- 51.Murugesan K, Koroth J, Srinivasan PP, et al. Effects of green synthesized silver nanoparticles (ST06-AgNPs) using curcumin derivative (ST06) on human cervical cancer cells (HeLa) in vitro and EAC tumor bearing mice models. Int J Nanome. 2019;14:5257–5270. doi: 10.2147/IJN.S202404 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Beer C, Foldbjerg R, Hayashi Y, Sutherland DS, Autrup H. Toxicity of silver nanoparticles—nanoparticle or silver ion? Toxicol Lett. 2012;208(3):286–292. doi: 10.1016/j.toxlet.2011.11.002 [DOI] [PubMed] [Google Scholar]
- 53.Vrček IV, Žuntar I, Petlevski R, et al. Comparison of in vitro toxicity of silver ions and silver nanoparticles on human hepatoma cells. Environ Toxicol. 2016;31(6):679–692. doi: 10.1002/tox.22081 [DOI] [PubMed] [Google Scholar]
- 54.Hwang ET, Lee JH, Chae YJ, et al. Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small. 2008;4(6):746–750. doi: 10.1002/smll.200700954 [DOI] [PubMed] [Google Scholar]
- 55.Laloy J, Minet V, Alpan L, et al. Impact of silver nanoparticles on haemolysis, platelet function and coagulation. Nanomed. 2014;1(4):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gliga AR, Di Bucchianico S, Lindvall J, Fadeel B, Karlsson HL. RNA-sequencing reveals long-term effects of silver nanoparticles on human lung cells. Nature Sci Rep. 2018;8(1):6668–6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guével XL, Wang FY, Stranik O, et al. Synthesis, stabilization, and functionalization of silver nanoplates for biosensor applications. J Phys Chem C. 2009;113(37):16380–16386. doi: 10.1021/jp904761p [DOI] [Google Scholar]
- 58.Neouze MA, Schubert U. Surface modification and functionalization of metal and metal oxide nanoparticles by organic ligands. Monatsh Chem. 2008;139:183–195. doi: 10.1007/s00706-007-0775-2 [DOI] [Google Scholar]
- 59.Burdus AC, Gherasim O, Grumezescu AM, Mogoanta L, Ficai A, Andronescu E. Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomater. 2018;8:681–706. doi: 10.3390/nano8090681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kvítek L, Panáček A, Soukupová J, et al. Effect of surfactants and polymers on stability and antibacterial activity of silver nanoparticles (NPs). J Phys Chem C. 2008;112(15):5825–5834. doi: 10.1021/jp711616v [DOI] [Google Scholar]
- 61.Chen J, Li S, Luo J, Wang R, Ding W. Enhancement of the antibacterial activity of silver nanoparticles against phytopathogenic bacterium Ralstonia solanacearum by stabilization. J Nanomater. 2016;1:1–15. [Google Scholar]
- 62.Deng H, McShan D, Zhang Y, et al. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ Sci Technol. 2016;50:8840–8848. doi: 10.1021/acs.est.6b00998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li P, Li J, Wu C, Wu Q, Li J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnol. 2005;16:1912–1917. doi: 10.1088/0957-4484/16/9/082 [DOI] [Google Scholar]
- 64.Vazquez-Muñoz R, Meza-Villezcas A, Fournier PGJ, et al. Enhancement of antibiotics antimicrobial activity due to the silver nanoparticles impact on the cell membrane. PLoS One. 2019;14(11):e0224904. doi: 10.1371/journal.pone.0224904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziebuhr W, Hennig S, Eckart M, Kränzler H, Batzilla C, Kozitskaya S. Nosocomial infections by Staphylococcus epidermidis: how a commensal bacterium turns into a pathogen. Int J Antimicrob Agents. 2006;28:14–20. doi: 10.1016/j.ijantimicag.2006.05.012 [DOI] [PubMed] [Google Scholar]
- 66.Abbas WS, Atwan ZW, Abdulhussein ZR, Mahdi MA. Preparation of silver nanoparticles as antibacterial agents through DNA damage. Mater Technol. 2019;34(14):867–879. doi: 10.1080/10667857.2019.1639005 [DOI] [Google Scholar]
- 67.Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomed. 2007;3:95–101. doi: 10.1016/j.nano.2006.12.001 [DOI] [PubMed] [Google Scholar]
- 68.Panáček A, Smékalová M, Večeřová R, et al. Silver nanoparticles strongly enhance and restore bactericidal activity of inactive antibiotics against multiresistant Enterobacteriaceae. Colloids Surf B Biointerfaces. 2016;142:392–399. doi: 10.1016/j.colsurfb.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 69.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182. doi: 10.1016/j.jcis.2004.02.012 [DOI] [PubMed] [Google Scholar]
- 70.Liu C, Li B. Silver nanoparticle-initiated chemiluminescence reaction of luminol-AgNO3 and its analytical application. Anal Bioanal Chem. 2011;401:229–235. doi: 10.1007/s00216-011-5071-7 [DOI] [PubMed] [Google Scholar]
- 71.Herisse M, Duverger Y, Martin-Verstraete I, Barras F, Ezraty B. Silver potentiates aminoglycoside toxicity by enhancing their uptake. Mol Microbiol. 2017;105(1):115–126. doi: 10.1111/mmi.13687 [DOI] [PubMed] [Google Scholar]
- 72.Zou L, Wang J, Gao Y, et al. Synergistic antibacterial activity of silver with antibiotics correlating with the upregulation of the ROS production. Nature. 2018;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]