Abstract
Background
Heartland virus (HRTV) was first described as a human pathogen in 2012. From 2013 to 2017, the Centers for Disease Control and Prevention (CDC) implemented a national protocol to evaluate patients for HRTV disease, better define its geographic distribution, epidemiology, and clinical characteristics, and develop diagnostic assays for this novel virus.
Methods
Individuals aged ≥12 years whose clinicians contacted state health departments or the CDC about testing for HRTV infections were screened for recent onset of fever with leukopenia and thrombocytopenia. A questionnaire was administered to collect data on demographics, risk factors, and signs and symptoms; blood samples were tested for the presence of HRTV RNA and neutralizing antibodies.
Results
Of 85 individuals enrolled and tested, 16 (19%) had evidence of acute HRTV infection, 1 (1%) had past infection, and 68 (80%) had no infection. Patients with acute HRTV disease were residents of 7 states, 12 (75%) were male, and the median age (range) was 71 (43–80) years. Illness onset occurred from April to September. The majority reported fatigue, anorexia, nausea, headache, confusion, arthralgia, or myalgia. Fourteen (88%) cases were hospitalized; 2 (13%) died. Fourteen (88%) participants reported finding a tick on themselves in the 2 weeks before illness onset. HRTV-infected individuals were significantly older (P < .001) and more likely to report an attached tick (P = .03) than uninfected individuals.
Conclusions
Health care providers should consider HRTV disease testing in patients with an acute febrile illness with either leukopenia or thrombocytopenia not explained by another condition or who were suspected to have a tickborne disease but did not improve following appropriate treatment.
Keywords: phlebovirus, tickborne diseases, Heartland virus
Heartland virus (HRTV) is a novel phlebovirus that was first isolated in 2009 from 2 residents of Northwestern Missouri hospitalized for acute onset of febrile illness with fatigue, anorexia, diarrhea, leukopenia, and thrombocytopenia [1]. They received doxycycline; however, no significant improvement was noted. Following 10 to 12 days in the hospital, the patients were released home. Both reported multiple tick exposures in the days before their illness.
Human pathogens of the genus Phlebovirus are predominantly transmitted by arthropod vectors (eg, ticks, mosquitoes, sandflies) [2]. Field and laboratory work performed following the documentation of the original 2 cases identified Amblyomma americanum ticks as a vector for HRTV [3–5]. A. americanum ticks are widely distributed across the Eastern and Central United States, and HRTV antibodies have been detected in wild animals in 13 Eastern and Central states [6].
In 2013, the Centers for Disease Control and Prevention (CDC) implemented a national protocol to evaluate patients throughout the United States for evidence of HRTV disease, better define its geographic distribution, describe its epidemiology and clinical characteristics, and develop diagnostic assays for this novel virus. This report summarizes the findings of HRTV disease cases identified through this protocol.
METHODS
From June 2013 to December 2017, individuals were identified for possible enrollment when a clinician or health department anywhere in the United States contacted the CDC to request testing for a possible HRTV infection. CDC personnel reviewed the patient’s clinical history to ensure they met the eligibility criteria: (1) aged ≥12 years; (2) recent onset of fever (≥38°C); (3) leukopenia (white blood cells <4500 cells/µL); (4) thrombocytopenia (platelets <150 000 cells/mL); and 5) having a sample collected within 4 weeks of their illness onset available for testing. Patients were excluded if they had a known noninfectious etiology or condition (eg, cancer or chemotherapy) that could explain their clinical findings or had been seen at 1 of the 6 institutions in Missouri taking part in a separate protocol [7]. Children aged <12 years were excluded due to potential limitations of obtaining assent.
If an individual met the enrollment criteria, they, their parent/guardian, or their legal health care proxy was contacted, provided with information about HRTV and protocol procedures, and asked to provide oral consent/assent. After obtaining consent, a standardized questionnaire was administered to collect information on demographics, possible risk factors for HRTV exposure, and clinical signs and symptoms occurring from illness onset to enrollment. In 2016, no individuals were consented and enrolled due to the Zika virus outbreak, which limited CDC staff time and laboratory resources. The study was reviewed and approved annually by the CDC’s Institutional Review Board.
One EDTA tube of whole blood was collected at the time of enrollment. A convalescent serum sample was requested 3‒6 weeks after the initial blood specimen. All specimens were stored at 4°C and shipped cold to the CDC Arboviral Diseases Branch. At the CDC, an aliquot of the whole-blood specimen was spun to separate serum. Both the acute whole blood and serum separated from the same sample were tested for HRTV RNA using a real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay, per the RNA virus detection methods and primer sequences previously described [5]. Acute whole blood also was placed into cell culture for potential virus isolation. If the patient was noted to be immunocompromised, convalescent serum samples were also tested for HRTV RNA using rRT-PCR assay.
Acute and convalescent serum samples were tested for HRTV-specific neutralizing antibodies using plaque reduction neutralization testing (PRNT) with a 90% plaque reduction criterion. Standard PRNT methods were used as previously described with use of Vero E6 cells, which based on experience in the laboratory lead to the best plaques for Heartland virus [8, 9]. Starting in 2015, all prospectively collected samples also were tested for anti-HRTV immunoglobulin (Ig) M antibodies using a newly developed microsphere-based immunoassay (MIA) technology, which involves the identification of a microsphere set (bead set) and measurement of the fluorescence associated with the reaction when antibody is coupled to the beads, as previously described [10].
For the purpose of this evaluation, acute HRTV infection was defined as (1) HRTV RNA detected in a whole-blood or serum specimen or (2) a ≥4-fold increase in HRTV-neutralizing antibodies measured by PRNT between acute and convalescent serum specimens. Past HRTV infection was defined as detection of HRTV-neutralizing antibodies but a <4-fold increase in titers between acute and convalescent specimens. Participants were considered to have no evidence of HRTV infection if their specimens were negative for HRTV RNA and neutralizing antibodies. HRTV IgM antibody results were not used to classify cases, as the assay was being validated at the time samples were tested and not all individuals had IgM antibody testing performed on their samples.
Electronic case report forms and data management were performed using the REDCap Study Data Management System [11]. Categorical variables were summarized using counts and proportions, and continuous variables were summarized using median and range or interquartile range (IQR). Mapping was performed using ArcGIS, version 10.3 (ESRI, Redlands, CA, USA). The Fisher exact, chi-square, and Kruskal-Wallis tests were used to evaluate for differences in the demographics, exposure history, and clinical signs and symptoms between individuals with and without evidence of acute HRTV infection.
RESULTS
From June 2013 to December 2017, 85 patients were enrolled and provided ≥1 specimen for testing. Patients were seen at 63 medical institutions by 72 providers. Of the 85 patients, 56 (66%) were male, and their median age (range) was 53 (18–89) years. Enrolled subjects were residents of 23 states, with 56 (66%) residing in 5 states (Arkansas, Kansas, Kentucky, Missouri, and Oklahoma).
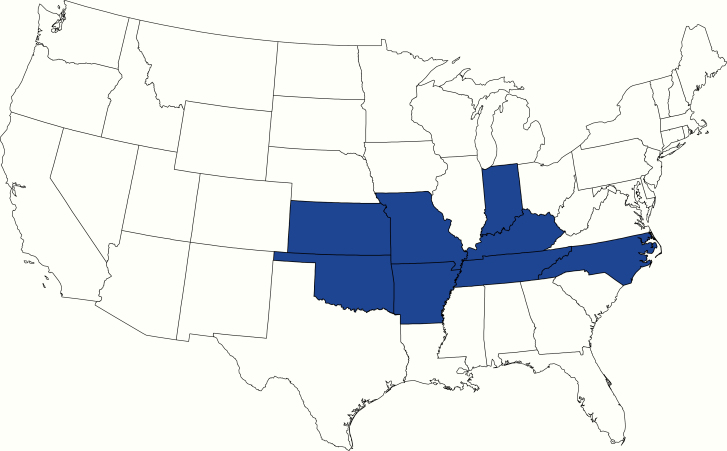
Of the 85 enrolled patients, 16 (19%) had laboratory evidence of acute HRTV infection, 1 (1%) had evidence of past infection, and 68 (80%) had no evidence of infection. Of the 16 participants with acute HRTV infection, 12 (75%) were male and their median age (range) was 71 (43–80) years (Table 1). The acutely infected individuals were residents of 7 states (Figure 1); however, 2 patients reported traveling out of state in the 2 weeks before their illness onset, 1 to a state where another acute HRTV infection was confirmed and the second to a state without identified cases. The 16 participants with acute HRTV infection were at 14 medical institutions attended by 15 providers. When the demographic features were compared, patients with acute HRTV infection were significantly older than patients without evidence of acute infection (P < .001) (Table 1).
Table 1.
Demographics, Clinical Characteristics, and Exposures for Participants With and Without Evidence of Acute Heartland Virus Infection
| HRTV-Infected | Noninfected | ||||
|---|---|---|---|---|---|
| n = 16 | n = 69 | ||||
| No. | (%) | No. | % | ||
| Demographics | |||||
| Male sex | 12 | (75) | 44 | (64) | |
| Age groups, y | |||||
| <40 | 0 | (0) | 24 | (35) | |
| 40–49 | 1 | (6) | 12 | (17) | |
| 50–59 | 2 | (13) | 11 | (16) | |
| 60–69 | 4 | (25) | 14 | (20) | |
| 70–79 | 8 | (50) | 7 | (10) | |
| ≥80 | 1 | (6) | 1 | (1) | |
| State of residence | |||||
| Missouri | 6 | (38) | 18 | (26) | |
| Arkansas | 2 | (13) | 4 | (6) | |
| Indiana | 2 | (13) | 1 | (1) | |
| Kentucky | 2 | (13) | 3 | (4) | |
| Oklahoma | 2 | (13) | 3 | (4) | |
| Kansas | 1 | (6) | 15 | (22) | |
| North Carolina | 1 | (6) | 2 | (3) | |
| Illinois | 0 | (0) | 4 | (6) | |
| Iowa | 0 | (0) | 3 | (4) | |
| Tennessee | 0 | (0) | 3 | (4) | |
| Othera | 0 | - | 12 | - | |
| Exposures | |||||
| Average number of hours outside per day | |||||
| <1 | 0 | (0) | 10 | (14) | |
| 1–4 | 6 | (38) | 28 | (41) | |
| 5–8 | 4 | (25) | 17 | (10) | |
| >8 | 6 | (38) | 13 | (19) | |
| Unknown | 0 | (0) | 1 | (1) | |
| Outdoor activities | |||||
| Yard work/gardening | 12 | (75) | 45 | (65) | |
| Walking | 5 | (42) | 37 | (54) | |
| Hunting | 3 | (25) | 14 | (20) | |
| Hiking/camping | 1 | (8) | 14 | (20) | |
| Vector exposure | |||||
| Attached tick | 13 | (81) | 34 | (49) | |
| Crawling tick | 10 | (63) | 33 | (48) | |
| Mosquito bite | 5 | (31) | 23 | (33) | |
| No known exposure | 2 | (13) | 13 | (20) | |
| Sandfly/midge bite | 1 | (6) | 3 | (4) | |
| Clinical characteristics | |||||
| Solicited | |||||
| Feverb | 16 | (100) | 69 | (100) | |
| Leukopeniab | 16 | (100) | 69 | (100) | |
| Thrombocytopeniab | 16 | (100) | 69 | (100) | |
| Fatigue | 15 | (94) | 69 | (100) | |
| Anorexia | 13 | (81) | 57 | (83) | |
| Nausea | 12 | (75) | 45 | (65) | |
| Headache | 11 | (69) | 58 | (84) | |
| Confusion | 11 | (69) | 26 | (38) | |
| Arthralgia | 10 | (63) | 39 | (57) | |
| Myalgia | 9 | (56) | 48 | (70) | |
| Diarrhea | 8 | (50) | 26 | (38) | |
| Cough | 5 | (31) | 23 | (33) | |
| Rash | 2 | (13) | 24 | (35) | |
| Easy bruising | 1 | (6) | 14 | (20) | |
| Unsolicitedc | |||||
| Chills | 3 | (19) | 27 | (39) | |
| Gait disturbance | 3 | (19) | 2 | (3) | |
| Dizziness | 2 | (13) | 0 | (0) | |
| Vomiting | 2 | (13) | 4 | (6) | |
| Altered/lost taste | 2 | (13) | 0 | (0) | |
aOther includes 1 individual each for Colorado, Florida, Georgia, Louisiana, Massachusetts, Nebraska, North Dakota, Ohio, Texas, Virginia, and West Virginia; percentage not calculated but would be 1% for each of the 12 states.
bRequired signs and symptoms for testing.
cOnly symptoms reported by >1 individual with acute Heartland virus infection are listed.
Figure 1.
State of residence for participants with acute Heartland virus disease (n = 16).
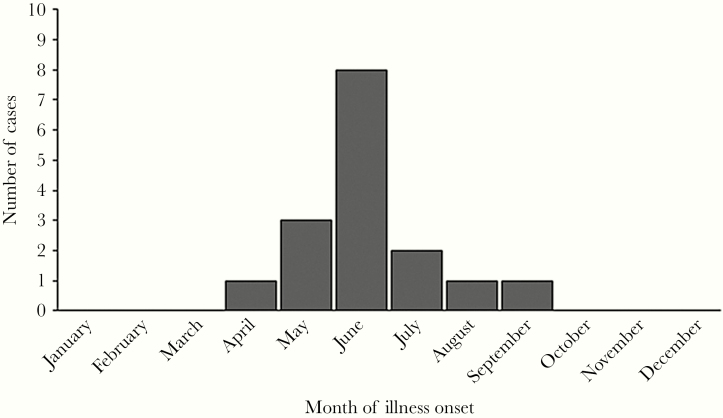
Participants with evidence of acute HRTV infection had onset of illness from April through September, with 8 (50%) occurring in June (Figure 2). All individuals reported spending ≥1 hour outside per day, and 6 (38%) spent an average of ≥8 hours outside per day (Table 1). Seven (44%) of the participants with acute infection were not employed (eg, retired or disabled). Of those reporting an occupation, 3 (19%) worked predominantly outdoors (ie, farmer, lawn care, construction). The most commonly reported outdoor activities in the 2 weeks before illness onset included gardening or yard work (12, 75%), walking (5, 32%), or hunting (3, 19%). Fourteen (88%) participants reported either finding a tick attached to them (n = 13) or crawling on them (n = 10) in the 2 weeks before their illness onset. The 2 individuals without known tick exposure also did not have reported mosquito or midge bites; for both, their spouses were interviewed as they were too sick to provide a history. When exposures histories were compared, patients with acute HRTV infection were significantly more likely to report an attached tick than patients without evidence of acute infection (P = .03) (Table 1).
Figure 2.
Month of illness onset for participants with acute Heartland virus disease (n = 16).
Per enrollment criteria, all cases had fever (median [IQR], 38.9°C [38.5°C–39.3°C]), thrombocytopenia (median [IQR], 48 000 [37 750–77 250] cells/mL), and leukopenia (median [IQR], 1750 [1400–2400] cells/µL). Of solicited clinical signs and symptoms, the majority of patients reported fatigue, anorexia, nausea, headache, confusion, arthralgia, myalgia, or diarrhea (Table 1). Two (13%) individuals reported a localized rash. When compared with patients without acute HRTV infection, patients with evidence of infection had significantly lower white blood cell counts (median, 1750 cells/µL vs 2700 cells/µL; P = 0.04) and lower platelet counts (median, 48 000 cells/mL vs 83 000 cells/mL; P = 0.05) at enrollment. The patient’s temperature at enrollment was not different between those with acute HRTV infection and those without evidence of infection. Of the various solicited and unsolicited clinical characteristics, patients with acute HRTV infection were significantly more likely to have confusion (P = .03), gait disturbance (P = .04), dizziness (P = .03), and altered taste (P = .03) than patients without evidence of acute HRTV infection.
Of the 16 patients with acute HRTV infection, 14 were tested for evidence of ehrlichiosis, usually with a combination of PCR and antibody testing. All 14 were either negative (n = 10) or had evidence of prior infection (n = 4). For the 2 remaining patients, it was unknown if they were tested for ehrlichiosis based on the information provided; however, 1 was RT-PCR-positive for HRTV during acute infection, and the second had a 4-fold increase in titers and no response to 14 days of doxycycline. Participants were not routinely asked about underlying medical conditions other than if they were immunocompromised or were taking an immunosuppressing medicine. Among the acute HRTV infection patients, 3 (19%) individuals reported being immunosuppressed. Fourteen (88%) patients were hospitalized, and 12 (75%) required a proxy to be interviewed as they were considered too sick to be interviewed. Two (13%) died after enrollment, including 1 of the individuals who was immunocompromised. The rates of hospitalization and death were not significantly different between those with and without evidence of acute HRTV infection.
Of the 16 participants with evidence of acute HRTV infection, the diagnosis was made by detection of viral RNA in 10 (63%) individuals and by serology in 6 (38%) (Supplementary Table 1). Of the 10 participants who had evidence of HRTV RNA, 6 had a convalescent sample submitted >14 days after illness and all 6 seroconverted between their acute and convalescent samples.
Of the 18 specimens tested by rRT-PCR from the 15 individuals, 13 (72%) were positive and 1 (6%) was equivocal (Table 2). Specimens positive by rRT-PCR testing were collected 5 to 23 days after illness onset, with 10 (77%) positive samples collected from 7 to 11 days post–illness onset. One rRT-PCR-positive specimen was collected 23 days after onset of illness from a patient who died and was immunocompromised due to receipt of a monoclonal antibody therapy. Virus was isolated from 5 individuals whose samples were collected from 5 to 9 days post–illness onset.
Table 2.
Number and Proportion of Samples Positive for Heartland Virus, Viral RNA, and Neutralizing Antibodies for Heartland Virus Disease Case Patients by Number of Days the Specimen Was Collected After Onset of Illnessa
| Virus by Culture | RNA by RT-PCR | Neutralizing Antibodies by PRNT | ||||
|---|---|---|---|---|---|---|
| Days Post–Illness Onset | No. Positive/No. Tested (%) | No. Positive/No. Tested (%) | No. Positive/No. Tested (%) | |||
| 1–7 | 2/4 | (50) | 4/5b | (80) | 0/5 | (0) |
| 8–14 | 3/7 | (45) | 9/12 | (75) | 7/14 | (50) |
| 15–21 | 0/0 | (0) | 0/1 | (0) | 3/3 | (100) |
| >21 | 0/0 | (0) | 1/1c | (100) | 8/8 | (100) |
Abbreviations: PRNT, plaque reduction neutralization test; RNA, ribonucleic acid; RT-PCR, reverse transcriptase polymerase chain reaction.
aImmunoglobulin M antibody test results are not presented, as the assay was developed during enrollment of participants.
bThree were RT-PCR-positive, 1 equivocal, and 1 negative; note that 1 patient had positive RT-PCR results for 2 specimens collected on days 5 and 7 post–illness onset. Data from only 1 sample are included in the table, as the results were the same.
cPostmortem whole-blood sample collected at 23 days from an individual who was immunocompromised due to receipt of monoclonal antibody therapy; PRNT could not be performed on the whole-blood sample.
Of 30 samples tested by PRNT, 18 (60%) were positive (Table 2). HRTV-specific neutralizing antibodies were detected in samples collected 8 to 173 days after illness onset. All samples tested beyond day 12 had detectable neutralizing antibody titers. Of the 26 samples tested for anti-HRTV IgM antibodies, 20 (77%) were positive from 13 individuals. Samples with detectable IgM antibodies were collected 2 to 94 days post–illness onset. All samples tested >10 days after onset had detectable IgM antibodies.
DISCUSSION
We report the demographics, geographic distribution, clinical features, initial outcomes, and laboratory results for 16 HRTV disease cases. Overall, the signs and symptoms for these patients were similar to those described in previous HRTV disease case reports and also to other tickborne diseases (eg, ehrlichiosis, anaplasmosis, and Colorado tick fever) [1, 7, 12–17]. However, we did identify cases in a broader geographic range than previously described and identified a higher proportion of cases in females. We were also able to confirm that older adults are most likely to be cases and that ticks appear to be the main if not the only vector for the virus. Laboratory findings from patients with evidence of acute HRTV disease demonstrated viremia for 1–2 weeks after illness onset with IgM, and neutralizing antibodies were detected consistently by 10 and 12 days after illness onset, respectively.
The current distribution of HRTV disease cases closely mirrors the distribution of A. americanum, and most patients we identified reported either finding a tick attached to or crawling on their body in the 2 weeks before illness onset [18, 19]. Several states with A. americanum have not yet identified HRTV disease cases. Although this might be due to differences in where the pathogen is located, it also could be due to differences in enrollment and testing of residents of their states for the disease. Currently, no other tick species have been found to be infected, and no other mode of transmission has been identified for HRTV [5, 20]. However, as several of these patients had culturable virus detected until 9 days post–illness onset, unprotected exposure to infected blood could represent a potential transmission risk, as has been reported with severe fever with thrombocytopenia syndrome (SFTS) virus, a closely related tickborne virus found in Asia [21–23].
All patients had illness onset from April to September, with the majority of patients developing symptoms in June. This seasonal distribution is consistent with other tickborne diseases and suggests that either nymphal or adult ticks are likely the source of human infection [17, 24–28]. This is supported by field investigations in Missouri and Kansas, where HRTV was identified in nymphal and adult ticks but no infection was identified in larval ticks, which tend to occur in mid- to late summer [4, 5, 20].
The majority of patients with HRTV disease were older adult males with no cases identified in persons aged <40 years. The finding of older adult males accounting for the majority of case patients has been reported for several other arboviral diseases (eg, West Nile and Powassan viruses). Higher rates of disease in older adults are also seen with SFTS virus, where 96% (5126/5360) of the cases identified in China from 2011 to 2016 were aged ≥40 years [29]. Although this might reflect increased exposure to the vectors that transmit the virus, there also is likely an increased risk of symptomatic infection in this group due to underlying medical comorbidities or differences in the immunologic response to the virus by sex and age [30]. The proportion of asymptomatic HRTV infections is currently unknown.
Clinical signs and symptoms previously have been reported for 10 cases of HRTV disease, including 2 of the 16 patients in our cohort [1, 7, 12–14, 31]. All published cases presented with or had a history of fever. This is similar to our cases, but fever was an inclusion criterion for testing in our evaluation. Additional symptoms reported by our case patients were similar to other published cases and included fatigue, nausea, headache, myalgia, and arthralgia. Rash is typically not reported, though some case patients describe a localized rash associated with their original tick bite. Several published cases and our cases reported confusion or mental status changes. However, the 2 cases who underwent lumbar punctures had no evidence of inflammation in their cerebrospinal fluid [13, 14]. Although there were higher rates of confusion, gait disturbance, and dizziness in patients with acute HRTV infection, it is likely that these are all related and in part due to the older ages of the patients with acute HRTV infection compared with those who were not acutely infected.
All individuals with acute HRTV disease identified through this protocol had thrombocytopenia and leukopenia per enrollment criteria. Of the 10 other published cases, all had thrombocytopenia at presentation and 9 had leukopenia. The case without leukopenia was noted to have leukocytosis when measured later in their illness [13]. At least 1 additional case with initial leukopenia also developed leukocytosis later in the second week of their illness [12]. Anemia typically has not been reported with HRTV infections. For cases where liver function tests were measured, all had elevated aspartate aminotransferase (AST) and alanine aminotransferase (ALT), usually peaking later in the second week of illness with AST levels at least 2 times higher than ALT [1, 12–14]. Other noted laboratory abnormalities, particularly in more severe cases, include hyponatremia, elevated bilirubin, elevated creatinine kinase, elevated lactate dehydrogenase, and markedly elevated ferritin. Of the cases with elevated ferritin, 1 met the diagnostic criteria for hemophagocytic lymphohistiocytosis (HLH), and another was noted to have hemophagocytosis in their bone marrow with immunostaining of viral antigens in mononuclear cells [13, 14]. One additional fatal case did not have ferritin measured but had hemophagocytosis seen in a lymph node on autopsy [12]. HLH and hemophagocytosis also have occurred with SFTS virus infections as well as other tick-borne pathogens such as Ehrlichia chaffeensis [15, 32, 33].
The majority of HRTV disease cases reported here were hospitalized, and 2 (13%) died. Of the additional 10 published cases, 8 were hospitalized and 2 died. All deaths identified to date have been in adult males aged >60 years, with death occurring 2–3 weeks after their illness onset from multisystem organ failure [12–14]. All 4 fatal cases had a history of multiple underlying medical comorbidities. We suspect that the case fatality ratio for HRTV disease is lower than what is currently seen for documented cases (16%), as there is likely a case ascertainment bias with physicians requesting testing for patients with more severe illness where the etiology is unknown; 4 (6%) of the patients enrolled in our study without evidence of acute HRTV infection also died.
There are no known antiviral medications available to treat HRTV infection, and management consists of supportive care. Antipyretics and analgesics can be used to reduce fever and pain. More severe cases might need intravenous fluids, ventilator support, vasopressors, blood products, or dialysis. For SFTS virus, a number of therapeutics (eg, ribavirin, steroids) have been administered in attempts to lower disease morbidity and mortality, with limited success, particularly in a case–control trial [34–36].
The majority of cases were diagnosed by detection of viral RNA, which was present in many individuals’ samples into the second of week of their illness. Neutralizing antibodies were not consistently detectable until week 2 of illness; IgM antibodies were detected roughly 2 days before neutralizing antibodies. This pattern of prolonged viral RNA detection and delayed development of neutralizing antibodies is similar to that seen with Colorado tick fever virus, which is an intracellular red blood cell pathogen. The initial discovery of HRTV and subsequent animal and laboratory work suggest that the virus likely infects white blood cells that initially present immunodominant nucleocapsid protein, which is not projected on the surface, and the immune responses against this protein are non-neutralizing [2, 37, 38].
Although these cases represent the largest cohort of HRTV disease cases reported to date, they likely are not representative of all potential HRTV disease. First, all cases were identified by a clinician who considered HRTV infection and contacted public health officials about testing their patient. Most of the cases were hospitalized, and many were too ill at the time of enrollment to provide a history of their signs and symptoms. As noted above, all case patients described here had fever, leukopenia, and thrombocytopenia, as this was necessary for protocol inclusion. This provided some increased sensitivity to detect HRTV infections, particularly as the assays to diagnose the disease were being developed and validated. However, these inclusion criteria likely excluded individuals with milder disease and those who did not have a complete blood count performed. Finally, comprehensive testing was not performed for other pathogens, particularly those also transmitted by A. americanum, such as Spotted Fever Group Rickettsiae. Given this, some of the signs and symptoms noted for the individuals with evidence of acute HRTV infection could be caused by other pathogens if co-infection occurred.
CONCLUSIONS
Based on the data described here and elsewhere for HRTV disease, health care providers should consider HRTV testing in patients presenting with an acute febrile illness with either leukopenia or thrombocytopenia not explained by another condition or who were suspected to have a tickborne disease but did not improve following appropriate treatment (eg, doxycycline) [7]. Testing should be limited to patients who either resided in or traveled to an area with previous evidence of HRTV or had a known tick exposure [6, 19]. Because the virus is transmitted by infected ticks, prevention likely will depend on using insect repellents, wearing long sleeves and pants, avoiding bushy and wooded areas, and performing tick checks after spending time outdoors. Future research is needed to understand the clinical spectrum and further geographic distribution of HRTV disease, including determination of whether asymptomatic infections can occur.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We would like to thank the vector-borne disease coordinators in the state health departments who had residents enrolled in the protocol for their assistance with identifying and facilitating screening of several of the individuals included in the study; Laura Youngblood for her guidance on human subjects research; Karen Trott for her assistance with database management; Alison Jane Basile for diagnostic testing support; and Robert Lanciotti for feedback on laboratory test result interpretations.
Financial support. There was no external funding to support this work.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. McMullan LK, Folk SM, Kelly AJ, et al. A new phlebovirus associated with severe febrile illness in Missouri. N Engl J Med 2012; 367:834–41. [DOI] [PubMed] [Google Scholar]
- 2. Brault AC, Savage HM, Duggal NK, Eisen RJ, Staples JE. Heartland virus epidemiology, vector association, and disease potential. Viruses. 2018; 10:piiE48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Godsey MS, Savage HM, Burkhalter KL, et al. Transmission of Heartland virus (Bunyaviridae: Phlebovirus) by experimentally infected Amblyomma americanum (Acari: Ixodidae). J Med Entomol 2016; 53:1226–33. [DOI] [PubMed] [Google Scholar]
- 4. Savage HM, Godsey MS Jr, Panella NA, et al. Surveillance for Heartland virus (Bunyaviridae: Phlebovirus) in Missouri during 2013: first detection of virus in adults of Amblyomma americanum (Acari: Ixodidae). J Med Entomol 2016; 53:607–12. [DOI] [PubMed] [Google Scholar]
- 5. Savage HM, Godsey MS, Lambert A, et al. First detection of heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg 2013; 89:445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Riemersma KK, Komar N. Heartland virus neutralizing antibodies in vertebrate wildlife, United States, 2009–2014. Emerg Infect Dis 2015; 21:1830–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pastula DM, Turabelidze G, Yates KF, et al. Notes from the field: Heartland virus disease - United States, 2012–2013. MMWR Morb Mortal Wkly Rep 2014; 63:270–1. [PMC free article] [PubMed] [Google Scholar]
- 8. Beaty BJ, Calisher CH, Shope RE. Arboviruses. In: Lennette EH, Lennette DA, Lennette ET. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. 7th ed. Washington, DC: American Public Health Association; 1995:189–212. [Google Scholar]
- 9. Bosco-Lauth AM, Panella NA, Root JJ, et al. Serological investigation of heartland virus (Bunyaviridae: Phlebovirus) exposure in wild and domestic animals adjacent to human case sites in Missouri 2012–2013. Am J Trop Med Hyg 2015; 92:1163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson AJ, Noga AJ, Kosoy O, et al. Duplex microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin M antibodies. Clin Diagn Lab Immunol 2005; 12:566–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muehlenbachs A, Fata CR, Lambert AJ, et al. Heartland virus-associated death in Tennessee. Clin Infect Dis 2014; 59:845–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fill MA, Compton ML, McDonald EC, et al. Novel clinical and pathologic findings in a Heartland virus-associated death. Clin Infect Dis 2017; 64:510–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carlson AL, Pastula DM, Lambert AJ, et al. Heartland virus and hemophagocytic lymphohistiocytosis in immunocompromised patient, Missouri, USA. Emerg Infect Dis 2018; 24:893–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biggs HM, Behravesh CB, Bradley KK, et al. Diagnosis and management of tickborne rickettsial diseases: Rocky Mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States. MMWR Recomm Rep 2016; 65:1–44. [DOI] [PubMed] [Google Scholar]
- 16. Brackney MM, Marfin AA, Staples JE, et al. Epidemiology of Colorado tick fever in Montana, Utah, and Wyoming, 1995–2003. Vector Borne Zoonotic Dis 2010; 10:381–5. [DOI] [PubMed] [Google Scholar]
- 17. Goodpasture HC, Poland JD, Francy DB, Bowen GS, Horn KA. Colorado tick fever: clinical, epidemiologic, and laboratory aspects of 228 cases in Colorado in 1973–1974. Ann Intern Med 1978; 88:303–10. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention. Geographic distribution of ticks that bite humans Available at: https://www.cdc.gov/ticks/geographic_distribution.html. Accessed 23 October 2019.
- 19. Centers for Disease Control and Prevention. Heartland virus: statistics & maps Available at: https://www.cdc.gov/heartland-virus/statistics/index.html. Accessed 23 October 2019.
- 20. Savage HM, Godsey MS Jr, Tatman J, et al. Surveillance for Heartland and bourbon viruses in Eastern Kansas, June 2016. J Med Entomol 2018; 55:1613–6. [DOI] [PubMed] [Google Scholar]
- 21. Chen Y, Jia B, Huang R, et al. Occupational severe fever with thrombocytopenia syndrome following needle-stick injury. Infect Control Hosp Epidemiol 2017; 38:760–2. [DOI] [PubMed] [Google Scholar]
- 22. Gai Z, Liang M, Zhang Y, et al. Person-to-person transmission of severe fever with thrombocytopenia syndrome bunyavirus through blood contact. Clin Infect Dis 2012; 54:249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tang X, Wu W, Wang H, et al. Human-to-human transmission of severe fever with thrombocytopenia syndrome bunyavirus through contact with infectious blood. J Infect Dis 2013; 207:736–9. [DOI] [PubMed] [Google Scholar]
- 24. Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. Surveillance for Lyme disease - United States, 2008–2015. MMWR Surveill Summ 2017; 66:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nichols Heitman K, Dahlgren FS, Drexler NA, Massung RF, Behravesh CB. Increasing incidence of ehrlichiosis in the United States: a summary of national surveillance of Ehrlichia chaffeensis and Ehrlichia ewingii infections in the United States, 2008–2012. Am J Trop Med Hyg 2016; 94:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Krow-Lucal ER, Lindsey NP, Fischer M, Hills SL. Powassan virus disease in the United States, 2006–2016. Vector Borne Zoonotic Dis 2018; 18:286–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Semtner PJ, Hair JA. The ecology and behavior of the lone star tick (Acarina: Ixodidae). V. Abundance and seasonal distribution in different habitat types. J Med Entomol 1973; 10:618–28. [DOI] [PubMed] [Google Scholar]
- 28. Kollars TM Jr, Oliver JH Jr, Durden LA, Kollars PG. Host association and seasonal activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J Parasitol 2000; 86:1156–9. [DOI] [PubMed] [Google Scholar]
- 29. Sun J, Lu L, Wu H, Yang J, Ren J, Liu Q. The changing epidemiological characteristics of severe fever with thrombocytopenia syndrome in China, 2011–2016. Sci Rep 2017; 7:9236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016; 16:626–38. [DOI] [PubMed] [Google Scholar]
- 31. Hevey MA, O’Halloran JA, Jagger BW, et al. Heartland virus infection in a heart transplant recipient from the heartland. Transpl Infect Dis 2019; 21:e13098. [DOI] [PubMed] [Google Scholar]
- 32. Oh HS, Kim M, Lee JO, et al. Hemophagocytic lymphohistiocytosis associated with SFTS virus infection: a case report with literature review. Medicine (Baltimore) 2016; 95:e4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim N, Kim KH, Lee SJ, et al. Bone marrow findings in severe fever with thrombocytopenia syndrome: prominent haemophagocytosis and its implication in haemophagocytic lymphohistiocytosis. J Clin Pathol 2016; 69:537–41. [DOI] [PubMed] [Google Scholar]
- 34. Li H, Lu QB, Xing B, et al. Epidemiological and clinical features of laboratory-diagnosed severe fever with thrombocytopenia syndrome in China, 2011-17: a prospective observational study. Lancet Infect Dis 2018; 18:1127–37. [DOI] [PubMed] [Google Scholar]
- 35. Liu Q, He B, Huang SY, et al. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis 2014; 14:763–72. [DOI] [PubMed] [Google Scholar]
- 36. Liu W, Lu QB, Cui N, et al. Case-fatality ratio and effectiveness of ribavirin therapy among hospitalized patients in China who had severe fever with thrombocytopenia syndrome. Clin Infect Dis 2013; 57:1292–9. [DOI] [PubMed] [Google Scholar]
- 37. Bosco-Lauth AM, Calvert AE, Root JJ, et al. Vertebrate host susceptibility to Heartland virus. Emerg Infect Dis 2016; 22:2070–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Calvert AE, Brault AC. Development and characterization of monoclonal antibodies directed against the nucleoprotein of Heartland virus. Am J Trop Med Hyg 2015; 93:1338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.