Abstract
Background
Accumulated evidences indicate that long non‐coding RNAs (lncRNAs) participate in many biological mechanisms. Moreover, it acts as an essential regulator in various human diseases such as gastric cancer (GC). Nevertheless, the comprehensive regulatory roles and clinical significance of most lncRNAs in GC are not fully understood.
Methods
In this research, our aim was to investigate the underlying mechanism of lncRNA LINC01234 in GC. Firstly, the usage of qRT‐PCR helped to establish expression pattern of LINC01234 in GC tissues. Following this, appropriate statistical tests were applied to analyze the relation between expression level and clinicopathological factors. Ultimately, potential functions and regulatory network of LINC01234 were concluded via GSEA and a series of bioinformatics tools or databases, respectively.
Results
Consequently, at the end of research we found LINC01234 is up‐regulated in GC tissues in comparison with adjacent normal tissues. Furthermore, its expression level is correlated with differentiation of patients with GC. It is also important to highlight bioinformatics analysis revealed that LINC01234 is involved in cancer‐associated pathways such as cell cycle and mismatch repair. Also, regulatory network of LINC01234 presented a probability in the involvement of tumorigenesis through regulating cancer‐associated genes.
Conclusion
Overall, our results suggested that LINC01234 may play a crucial role in GC.
Keywords: gastric cancer, LINC01234, long non‐coding RNA, regulatory network
Abbreviations
- AUC
area under the ROC curve
- EMT
epithelial‐to‐mesenchymal transition
- ESCC
esophageal squamous cell carcinoma
- FDR
false discovery rate
- FPKM
Fragments per Kilobase of transcript per Million fragments mapped
- GC
gastric cancer
- GSEA
gene set enrichment analysis
- lncRNA
long non‐coding RNA
- qRT‐PCR
real‐time quantitative reverse transcription‐polymerase chain reaction
- ROC
receiver operating characteristic
- TCGA
the cancer genome atlas
1. INTRODUCTION
Gastric cancer (GC) is a complex disease caused by accumulation of both genetic and epigenetic factors and imposes a considerable global health burden.1 In fact, in 2016, there were 1.2 million cases of GC with 834 000 deaths worldwide and 18.3 million DALYs (disability‐adjusted life years).2 Even though scientists are making great efforts and there is a steady decline in GC incidence and mortality rates, it is still hard to diagnose GC patients at early stage. To illustrate, this means most GC patients are missing their opportunity for radical gastrectomy, which is currently the best way to cure GC when diagnosed.3, 4 Additionally, many patients have a significant risk of metastasis and low survival time even after curative resection. Thus, it is vital to identify new effective biomarkers and therapeutic target agents for the treatment and early diagnosis of GC.
Long non‐coding RNAs (lncRNAs) are a kind of non‐coding RNAs (lacking the ability of encoding protein) with a length larger than 200 nt. Even though the same as mRNAs, lncRNAs are also transcribed out of DNA by RNA polymerase II; they were initially thought to be a noise in transcriptome when first found.5 Despite this, accumulated studies established that lncRNAs function as regulators of gene expression, stability, and location at the epigenetic, transcriptional, and post‐transcriptional levels.6, 7 Thus, aberrance of lncRNA expression is involved in numerous biological processes such as cell cycle, cell differentiation, proliferation, apoptosis, metastasis, invasion, and migration in several kinds of cancer including GC.8, 9 Also, some lncRNAs can indeed be used as a biomarker for the diagnosis and prognosis in many kinds of cancers such as breast cancer10 and GC.11 For example, LINC1006 12 was found to be a novel biomarker for GC previously. Above all, lncRNAs are important in both the initiation and development of GC.
Over the last decades, numerous experimental researches have identified several lncRNAs that play crucial role in GC such as imprinted maternally expressed transcript (H19),13 small nucleolar RNA host gene 5 (SNHG5),14 homeobox transcript antisense RNA (HOTAIR),15 AGAP2 antisense RNA 1 (AGAP2‐AS1),16 and Pvt1 oncogene (PVT1).17 Yet they were only a tip of iceberg, there remain a large number of lncRNAs with unknown functions and regulation mechanism in GC. Due to the advances of sequencing technology, more and more high‐throughput data of transcriptome in GC were carried out. The Cancer Genome Atlas (TCGA) collects sequencing data of genome, transcriptome, and epigenome from many patients with various kinds of cancer including stomach adenocarcinoma (STAD). It provides an opportunity to dig out unknown genes especially for those lncRNAs in GC.
In this research, we first analyzed gene expression profiles of STAD patients in TCGA and found a number of lncRNAs differently expressed in cancerous tissues compared with adjacent non‐cancerous tissues. Then, we verified one of the up‐regulated lncRNA, LINC01234, in GC tissues compared with adjacent non‐cancerous tissues by real‐time quantitative reverse transcription‐polymerase chain reaction (qRT‐PCR). Also, the association between expression level of LINC01234 and clinicopathological factors was analyzed. Subsequently, we annotated the functions of LINC01234 using Gene Set Enrichment Analysis (GSEA) method and constructed the LINC01234 regulatory network to well interpret the regulation mechanism of LINC01234 in GC.
2. MATERIALS AND METHODS
2.1. Differently expression analysis of lncRNAs in STAD from TCGA
Fragments per Kilobase of transcript per Million fragments mapped (FPKM) expression profiles and clinical information of STAD patients were downloaded from TCGA website (https://portal.gdc.cancer.gov/). There are 375 cancerous samples and 32 adjacent normal samples (Table S1). Long intergenic non‐coding RNA (lincRNA) and antisense RNAs were selected as lncRNAs and were analyzed by t test. False discovery rate (FDR) method was used to correct P values. Those with FDR < 0.05 and fold change larger than 1.5 were considered to be as differently expressed lncRNAs.
2.2. Collection of GC samples and patients' clinical information
Paired cancerous and adjacent normal tissues of 83 GC patients were collected during surgery in the span of 2010 to 2015 at Zhejiang Cancer Hospital. The adjacent normal tissues were defined as those tissues located 5 cm away from the edge of the tumor. All the samples with a size of around 0.1 cm3 were immediately preserved in RNA fixer (BioTeke) and stored at −80°C until use. For each GC patient, the clinical information consisted of age, gender, invasion depth, differentiation, lymphatic metastasis, distal metastasis, and TNM stage. It is important to state no patient had undergone preoperative radiotherapy or chemotherapy. Also, each patient had handed over a written consent with a signed name indicating they are willing to participate in this research and the ethics committee of Ningbo University approved for this investigation.
2.3. Total RNA extraction and qRT‐PCR
The methods for total RNA preparation and qRT‐PCR were analogous to our previous study.18 For instance, we extracted the total RNA using TRIzol reagent (Thermo Fisher Scientific) from each cancer tissue and adjacent normal tissue. From here, we were able to detect total RNA by using a protein‐nucleic acid spectrophotometer according to A260/280 ratio. Hereafter, 2 μg RNA was reverse‐transcribed into cDNA with GoTaq qPCR Master Mix (Promega) and the process of qRT‐PCR was performed on LightCycler 480 (Roche). The sequences of PCR primers for β‐actin were 5′‐CATGTACGTTGCTATCCAGGC‐3′ (forward) and 5′‐CTCCTTAATGTCACGCACGAT‐3′ (reverse). On the other hand, the sequences of PCR primers for LINC01234 were 5‐TCTACTAGAGCCTCCAGAAGG‐3′ (forward) and 5‐CTACTCTTCACGCAGAGGA‐3′ (reverse). Importantly, the conditions of thermal cycling were as follows: predegeneration at 95°C in 10 minutes, after which 45 cycles at 95°C for 15 seconds, 55°C for 30 seconds, and 72°C for 30 seconds. The expression level of LINC01234 was calculated using the ΔCt method with β‐actin expression value as control, which was calculated by subtracting the Ct values of β‐actin from the Ct values of LINC01234. Then, ΔΔCt of LINC01234 was calculated by subtracting ΔCt of adjacent non‐cancerous tissue from that of the paired cancer tissue. At last, the fold change of LINC01234 was calculated by the equation 2−ΔΔCt. All results were described as the expression of mean ± standard deviation of three independent experiments.
2.4. Gene set enrichment analysis of LINC01234
By using the median expression level of LINC01234 as cutoff, STAD patients were divided into two groups: with low expression and high expression of LINC01234, respectively. Subsequently, FPKM expression profiles for STAD patients and group labels of samples were put into GSEA software.19 Gene Ontology (GO) Biological Process (BP) term and KEGG pathway datasets were selected to calculate the enriched functions and pathways associated with LINC01234. Adjusted P‐value < .05 was considered to be statistically significant.
2.5. Construction of LINC01234 regulatory network
2.5.1. TF‐LINC01234 regulation
We downloaded the genomic location of peaks of transcription factor (TF) from Cistrome databases,20 which re‐calculated ChIP‐seq datasets for TF and histone modification from GEO database.21 Next, we compared the chromosome position of these binding regions with that of LINC01234 , only those with the binding sites locating promoters of LINC01234 were considered as TF‐LINC01234 regulation relationships. Then, TFs were filtered by differently expressed protein‐coding genes in GC identified from STAD expression profiles from TCGA by using t test. False discovery rate (FDR) method was used to correct P‐values for multiple comparisons, and .05 was set as a cutoff.
2.5.2. miRNA‐LINC01234 interactions
The conclusion of miRNA‐LINC01234 interactions was established upon reliable miRNA target prediction tool known as miRanda set on default parameters.22 Due to the up‐regulation of LINC01234 in GC, only those miRNAs down‐regulated in GC were obtained according to miRCancer database.23
2.5.3. RBP‐LINC01234 interactions
Likewise, prediction of RBP‐LINC01234 interactions was set by utilizing a model called lncPro24 using sequence information downloaded from UniProt.25 Afterward, RBP was also filtered by removing non‐differently expressed protein‐coding genes in STAD from TCGA.
2.6. Statistical analysis
IBM SPSS 21.0 software (SPSS) and R 3.3.3 were the two software used to perform statistical analysis. Comparison of “expression values” among three or more groups was analyzed by one‐way analyses of variance (ANOVAs), while that between two groups was performed by Student's t test. Statistical differences were set at *P < .05, **P < .01, and ***P < .001. P < .05 was set to analyze the statistical significances.
3. RESULTS
3.1. Experimental verification of LINC01234 up‐regulation in GC tissues
Firstly, we downloaded the expression profiles of STAD from TCGA and investigated the differently expressed lncRNAs. Consequently, 1016 up‐regulated and 140 down‐regulated lncRNAs in GC compared with non‐cancer tissues were found (Figure 1A, Table S2). Among them, one of up‐regulated lncRNAs, LINC01234, was selected to study deeply because of poor knowledge of it in GC. We then verified the disorder expression pattern of LINC01234 using qRT‐PCR in 83 GC tissues and adjacent normal tissues (Figure 1B). Hence, by comparing the adjacent non‐cancerous tissues, it is concluded that LINC01234 is strictly up‐regulated in 61 of 83 GC tissues (73.5%, Figure 1C, P < .001).
Figure 1.
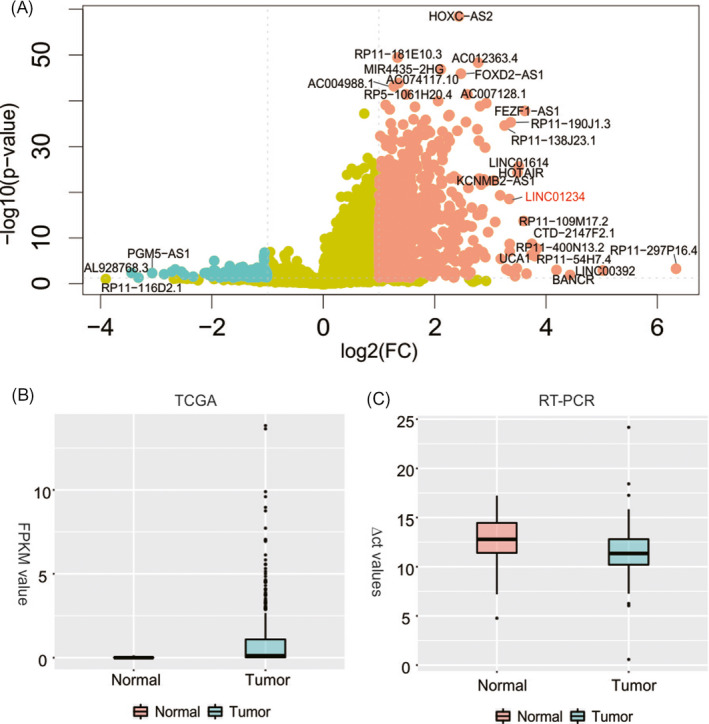
A, Differently expressed lncRNAs in STAD. B, Expression level (FPKM) of LINC01234 in STAD tissues compared with adjacent normal tissues. C, Expression level (△Ct value) of LINC01234 in GC tissues compared with adjacent normal tissues
3.2. Association analysis between expression level of LINC01234 and clinicopathological factors in GC patients
In the previous study, LINC01234 was considered to be a potential diagnostic marker in GC based on the data of TCGA.26 Consequently, we evaluated the likely diagnostic value of LINC01234 based on our own dataset. Initially, we performed a statistical analysis to examine the relationship between the clinicopathological factors and the expression level of LINC01234. As a result, we found differentiation of GC was associated with LINC01234 expression, that means the lower the LINC01234 expression is, the more the possibility for poor differentiation of GC tissues is (P < .05, Table 1). Besides, P value of the test for association between distal metastasis and LINC01234 expression is <0.05. However, the sample size of GC patients with M1 stage is not enough (n = 5) that the result may be unbelievable. Other clinicopathological factors including age, gender, greatest tumor dimension, invasion depth, lymphatic metastasis, and TNM stage are not related to LINC01234 level. In addition, we further explored the ability of differentiation of GC tissues from the normal adjacent tissues by a receiver operating characteristic (ROC) curve. The area under the ROC curve (AUC) was 0.888 for TCGA dataset (95% CI, 0.848‐0.929; P < .05, Figure 2A) while 0.664 for our qRT‐PCR results (95% CI, 0.581‐0.748; P < .05, Figure 2B), indicating that LINC01234 plays a prominent role in GC tumorigenesis.
Table 1.
Relationship between LINC01234 expression level (ΔCt value) and clinicopathological factors of GC patients
| Characteristics | Groups | Number of Patients (%) | Expression level (Mean ± SE) | P‐value |
|---|---|---|---|---|
| Age (y) | .079 | |||
| ≧60 | 33 (39.76) | 17.55 ± 2.14 | ||
| <60 | 50 (60.24) | 18.34 ± 1.88 | ||
| Gender | .619 | |||
| Male | 63 (75.9) | 17.96 ± 1.92 | ||
| Female | 20 (24.1) | 18.22 ± 2.31 | ||
| Greatest tumor dimension (cm) | .474 | |||
| ≧5 | 40 (48.19) | 18.19 ± 1.91 | ||
| <5 | 43 (51.81) | 17.87 ± 2.12 | ||
| Invasion depth | .804 | |||
| T1/T2 | 14 (16.87) | 18.15 ± 1.76 | ||
| T3/T4 | 69 (88.13) | 18 ± 2.07 | ||
| Differentiation | <.001* | |||
| Well/Moderate | 37 (44.58) | 17.17 ± 1.65 | ||
| Poor | 46 (55.42) | 18.71 ± 2.03 | ||
| Lymphatic metastasis | .377 | |||
| N0/N1 | 28 (33.73) | 18.3 ± 1.93 | ||
| N2/N3 | 55 (66.27) | 17.88 ± 2.06 | ||
| Distal metastasis | .013a | |||
| M0 | 78 (93.98) | 18.16 ± 1.99 | ||
| M1 | 5 (6.02) | 15.88 ± 0.818 | ||
| TNM stage | .87 | |||
| I/II | 22 (26.51) | 18.09 ± 1.95 | ||
| Ⅲ/Ⅳ | 61 (73.49) | 18 ± 2.05 |
Abbreviation: SE, standard error.
The sample size is so small that the result may be unbelievable even though P < .05.
P < .05.
Figure 2.
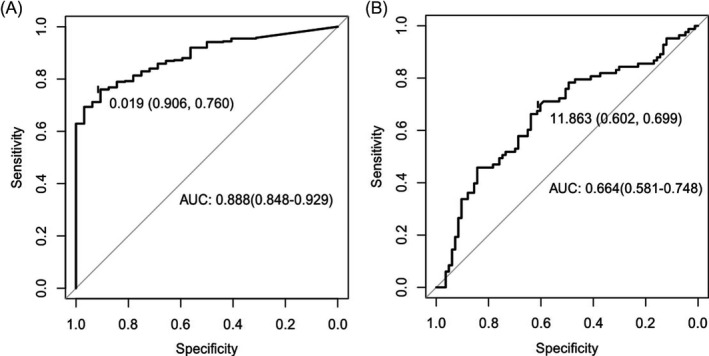
Diagnostic value of LINC01234 in GC. A, ROC curve of LINC01234 predicting STAD samples using FPKM value from TCGA. B, ROC curve of LINC01234 predicting GC samples using △Ct value detected by qRT‐PCR
3.3. Potential functions of LINC01234
To explore the potential functions of LINC01234 in GC, we firstly divided the STAD patients from TCGA into two groups, low expression and high expression of LINC01234 in cancer tissues, respectively. Secondly, GSEA was performed to investigate biological processes or pathways that were associated with LINC01234. Thus, the results showed LINC01234 may be involved in cancer and immune‐related pathways such as cell cycle (Figure 3A), mismatch repair (Figure 3B), intestinal immune network for IgA production (Figure 3C), and B‐cell receptor signaling pathway (Figure 3D). In the case of GO BP, cancer‐associated functions were found such as negative regulation of tumor factor–mediated signaling pathway (Figure 3E) and positive regulation of cell migration are involved in sprouting angiogenesis (Figure 3F). These findings present a strong evidence that LINC01234 has a major role in GC formation.
Figure 3.
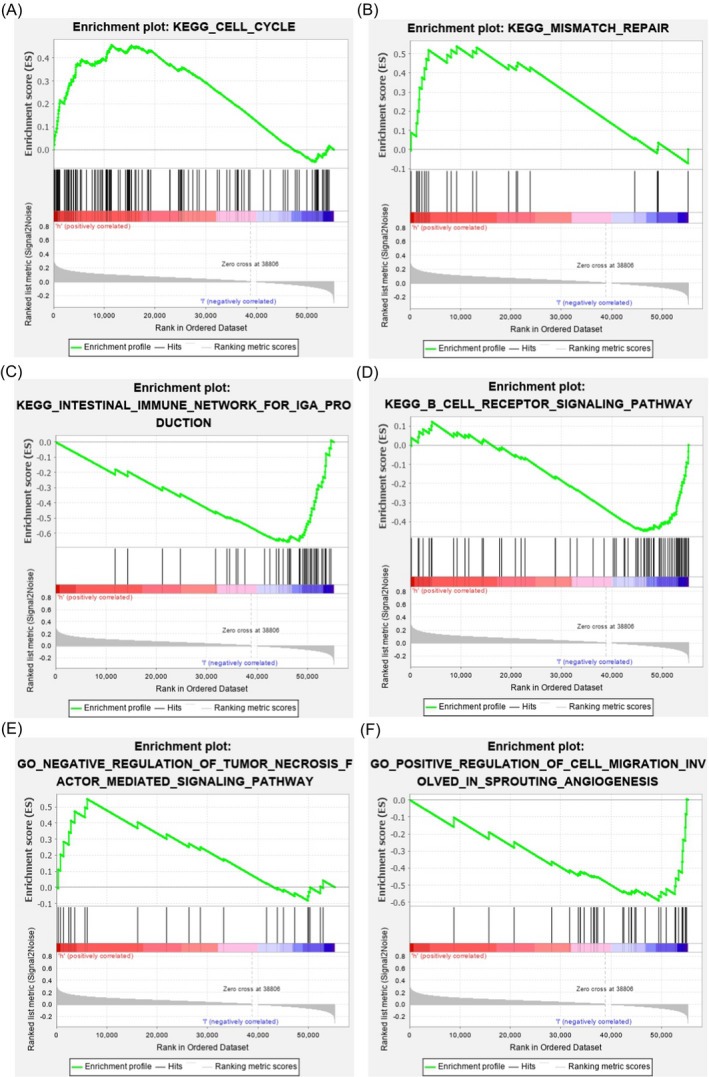
Potential functions of LINC01234 in GC. GSEA showed that aberrant expression of LINC01234 would affect the genes involved in cancer‐related pathways such as cell cycle (A), mismatch repair (B), intestinal immune network for IgA production (C), B‐cell receptor signaling pathway (D), negative regulation of tumor factor–mediated signaling pathway (E), and positive regulation of cell migration involved in sprouting angiogenesis (F)
3.4. Regulatory network of LINC01234
LncRNAs have been discovered to interact with various types of molecules including DNA, miRNA, mRNA, and protein. To analyze the regulation mechanism of LINC01234 in GC, we constructed a regulatory network of LINC01234 by utilizing a series of bioinformatics tools and databases. This network included TF‐lncRNA regulation, miRNA‐lncRNA relationship, as well as lncRNA‐RBP interactions. In total, 31 TFs, 49 miRNAs, and 138 RBPs associated with LINC01234 were achieved (Table S3, Figure 4).
Figure 4.
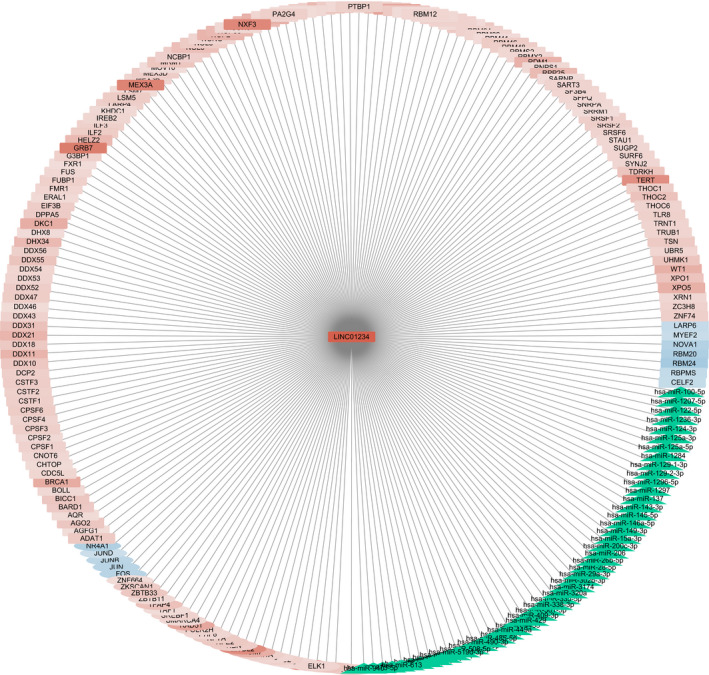
Regulatory network of LINC01234 in GC. The central node is LINC01234. Rectangle nodes represent TF, circle nodes represent RBP, and green triangle nodes represent miRNA. Red color represents high expression in GC, while blue color represents low expression in GC. Color strength represents log2 value of fold change in GC tissues to adjacent normal tissues
3.4.1. TF‐LINC01234 regulation
Some of the 31 TFs have thoroughly participated in GC development. For example, FOXK2 inhibited the proliferation, invasion, and migration of GC cells, and its down‐regulation is related to poor prognosis in GC patients.27 Besides, HDAC2 was significantly up‐regulated in various histopathologic grades of human GC, and the inactivation of HDAC2 has been confirmed to reduce cell motility, cell invasion, clonal expansion, and tumor growth.28 Specifically, 2 TFs were found to co‐express with LINC01234 according to the co‐expression network we previously constructed.29 They are ELK1 and ZNF664 (Table S4).
3.4.2. miRNA‐LINC01234 regulation
A total of 49 miRNAs were predicted to regulate LINC01234 in GC. Several of them have already been accepted to be correlated with GC progression. Also, the overexpression of miR‐1284 was reported to be a suppressor for GC by controlling over cell proliferation and apoptosis.30 In fact, a prior study showed miR‐1284 might modulate multidrug resistance of GC cells by targeting specific genes.31 The miR‐1297 expression found to be remarkably lower in GC tissue and suppress GC cell growth by inhibiting the expression of CREB1.32
3.4.3. RBP‐LINC01234 regulation
A total of 138 RBPs were predicted to likely interact with LINC01234. Among them, a part of RBPs was already shown to be related to GC. For example, reports revealed DDX21 could affect the proliferation of GC cells by up‐regulating levels of cyclin D1 and CDK2.33 Likewise, the suppression of EIF3B inhibits the proliferation and metastasis of GC by effectively modulating the expression of cancer‐related genes.34 Likewise, we also found 15 co‐expression relationships in RBP interactions such as CPSF3, DDX18, DKC1, and FUS (Table S4).
4. DISCUSSION
LncRNA is a type of non‐coding RNAs without the ability of encoding proteins; despite this, it has a regulating gene expression at chromatin modification, transcriptional, or post‐transcriptional levels.35 In addition, the polymorphisms in lncRNAs could be a risk of disease or cancer.36 In fact, an increasing number of lncRNAs have been identified to be related to numerous kinds of diseases,37 including GC. For example, through the expression analysis of metabolic pathway‐related lncRNAs and protein‐coding genes, a dozen of lncRNAs were functionally annotated and discovered to be important in GC.38 DGCR9, another lncRNA up‐regulated in GC, was shown to promote the tumorigenesis of GC. 39 Some of lncRNAs were even indeed considered as biomarkers for diagnosis or prognosis of GC. For instance, a metabolism‐related lncRNA, RP11‐555H23.1, was found to be a potential diagnostic biomarker in GC.40 Also, the expression level of H19 in plasma could be served as a biomarker for patients with GC.41 Due to the regulatory role of lncRNAs, exploring new lncRNA biomarkers can help to explain the initiation and progression mechanism of GC. Nevertheless, still there are many unknown lncRNAs in GC nowadays.
The previous study found LINC01234 is highly expressed in esophageal squamous cell carcinoma (ESCC). Also, it is one of the three lncRNAs that can be a signature to predict the survival time of ESCC patients accurately.42, 43, 44 Besides, it was likewise discovered to be up‐regulated in the GC in prior study.26 However, the functions and regulatory role of LINC01234 need to be studied.
In this research, we identified that LINC01234 was also highly expressed in GC tissues compared with adjacent non‐cancerous tissues. Later, we explored the associations between the expression level of LINC01234 and clinical features through which we found LINC01234 was correlated with differentiation of GC. LncRNAs usually interact with other kinds of molecules to involve in multiple biological processes. For example, the binding of lncRNA OLC8 and IL‐11 will impair the degradation of IL‐11 mRNAs to accelerate GC development.45 Besides, combination of lncRNA and its target may increase the diagnostic value of lncRNA. Just as it was found by previous report that the combined use of RP11‐19P22.6‐001 and its target NOS2 may be useful to diagnose patients with GC.46 Thus, we further explored the potential functions and regulatory network of LINC01234. We identified 218 relationships of LINC01234 in total. Among them, 17 associations including two pairs of TF regulation and 17 pairs of RBP interaction were found to be co‐expressed. One of 2 TFs, ELK1, is an important regulator and known to activate many lncRNAs including TRPM2‐AS, MIR100HG, and HOXA10‐AS in cancer until now,47, 48, 49 indicating ELK1 may induce expression of LINC01234 to promote tumor progression in GC too.
In conclusion, the results of this present study indicated that LINC01234 expression is linked with the diagnostics of patients with GC and similarly may be involved in differentiation in GC through cell cycle or other cancer‐related pathways.
Supporting information
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China [Grant Numbers 31970630], the Natural Science Foundation of Ningbo [Grant Numbers 2017A610154, 2016A610121], Zhejiang Key Laboratory of Pathophysiology [Grant Numbers 201812], The Scientific Innovation Team Project of Ningbo [Grant Numbers 2016C51001, 2017C110019], and KC Wong Magna Fund in Ningbo University.
Zhu Y, Luo C, Korakkandan AA, et al. Function and regulation annotation of up‐regulated long non‐coding RNA LINC01234 in gastric cancer. J Clin Lab Anal. 2020;34:e23210 10.1002/jcla.23210
Yinyin Zhu and Cong Luo contributed equally to this study and share first authorship.
REFERENCES
- 1. Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis Primers. 2017;3:17036. [DOI] [PubMed] [Google Scholar]
- 2. Fitzmaurice C, Akinyemiju TF, Al Lami FH, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability‐adjusted life‐years for 29 cancer groups, 1990 to 2016. JAMA Oncol. 2018;4(11):1553‐1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87‐108. [DOI] [PubMed] [Google Scholar]
- 4. Miao RL, Wu AW. Towards personalized perioperative treatment for advanced gastric cancer. World J Gastroenterol. 2014;20:11586‐11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kugel JF, Goodrich JA. Non‐coding RNAs: key regulators of mammalian transcription. Trends Biochem Sci. 2012;37:144‐151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li J, Chen Y, Chen Z, et al. SPRY4‐IT1: a novel oncogenic long non‐coding RNA in human cancers. Tumour Biol. 2017;39:1010428317711406. [DOI] [PubMed] [Google Scholar]
- 7. Vance KW, Sansom SN, Lee S, et al. The long non‐coding RNA Paupar regulates the expression of both local and distal genes. EMBO J. 2014;33:296‐311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaikkonen MU, Lam MT, Glass CK. Non‐coding RNAs as regulators of gene expression and epigenetics. Cardiovasc Res. 2011;90:430‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang H, Chen Z, Wang X, Huang Z, He Z, Chen Y. Long non‐coding RNA: a new player in cancer. J Hematol Oncol. 2013;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nie ZL, Wang YS, Mei YP, et al. Prognostic significance of long noncoding RNA Z38 as a candidate biomarker in breast cancer. J Clin Lab Anal. 2018;32(1):e22193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao J, Liu Y, Huang G, Cui P, Zhang W, Zhang Y. Long non‐coding RNAs in gastric cancer: versatile mechanisms and potential for clinical translation. Am J Cancer Res. 2015;5:907‐927. [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu X, Chen F, Shao Y, Xu D, Guo J. Long intergenic non‐protein coding RNA 1006 used as a potential novel biomarker of gastric cancer. Cancer Biomark. 2017;21:73‐80. [DOI] [PubMed] [Google Scholar]
- 13. Li H, Yu B, Li J, et al. Overexpression of lncRNA H19 enhances carcinogenesis and metastasis of gastric cancer. Oncotarget. 2014;5:2318‐2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao L, Han T, Li Y, et al. The lncRNA SNHG5/miR‐32 axis regulates gastric cancer cell proliferation and migration by targeting KLF4. FASEB J. 2017;31:893‐903. [DOI] [PubMed] [Google Scholar]
- 15. Ma T, Wang RP, Zou X. Dioscin inhibits gastric tumor growth through regulating the expression level of lncRNA HOTAIR. BMC Complement Altern Med. 2016;16:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qi F, Liu X, Wu H, et al. Long noncoding AGAP2‐AS1 is activated by SP1 and promotes cell proliferation and invasion in gastric cancer. J Hematol Oncol. 2017;10:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu MD, Wang Y, Weng W, et al. A positive feedback loop of lncRNA‐PVT1 and FOXM1 facilitates gastric cancer growth and invasion. Clin Cancer Res. 2017;23:2071‐2080. [DOI] [PubMed] [Google Scholar]
- 18. Luo C, Tao Y, Zhang Y, et al. Regulatory network analysis of high expressed long non‐coding RNA LINC00941 in gastric cancer. Gene. 2018;662:103‐109. [DOI] [PubMed] [Google Scholar]
- 19. Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge‐based approach for interpreting genome‐wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545‐15550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mei S, Qin Q, Wu Q, et al. Cistrome data browser: a data portal for ChIP‐Seq and chromatin accessibility data in human and mouse. Nucleic Acids Res. 2017;45:D658‐D662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barrett T, Edgar R. Gene expression omnibus: microarray data storage, submission, retrieval, and analysis. Methods Enzymol. 2006;411:352‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Enright AJ, John B, Gaul U, Tuschl T, Sander C, Marks DS. MicroRNA targets in drosophila. Genome Biol. 2003;5:R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xie B, Ding Q, Han H, Wu D. miRCancer: a microRNA‐cancer association database constructed by text mining on literature. Bioinformatics. 2013;29:638‐644. [DOI] [PubMed] [Google Scholar]
- 24. Lu Q, Ren S, Lu M, et al. Computational prediction of associations between long non‐coding RNAs and proteins. BMC Genom. 2013;14:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The UniProt Consortium . UniProt: the universal protein knowledgebase. Nucleic Acids Res. 2018;46:2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gu J, Li Y, Fan L, et al. Identification of aberrantly expressed long non‐coding RNAs in stomach adenocarcinoma. Oncotarget. 2017;8:49201‐49216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu X, Wei X, Niu W, Wang D, Wang B, Zhuang H. Downregulation of FOXK2 is associated with poor prognosis in patients with gastric cancer. Mol Med Rep. 2018;18:4356‐4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JK, Noh JH, Eun JW, et al. Targeted inactivation of HDAC2 restores p16INK4a activity and exerts antitumor effects on human gastric cancer. Mol Cancer Res. 2013;11:62‐73. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Tao Y, Ji H, et al. Genome‐wide identification of the essential protein‐coding genes and long non‐coding RNAs for human pan‐cancer. Bioinformatics. 2019;35:4344‐4349. [DOI] [PubMed] [Google Scholar]
- 30. Huang M, Wu L, Luo S, et al. MicroRNA‐1284 inhibits proliferation and induces apoptosis in SGC‐7901 human gastric cancer cells. Biotech Lett. 2017;39:33‐38. [DOI] [PubMed] [Google Scholar]
- 31. Cao W, Wei W, Zhan Z, Xie Y, Xiao Q. MiR‐1284 modulates multidrug resistance of gastric cancer cells by targeting EIF4A1. Oncol Rep. 2016;35:2583‐2591. [DOI] [PubMed] [Google Scholar]
- 32. Gao W, Cao Y, Guo P, et al. Downregulation of MiR‐1297 predicts poor prognosis and enhances gastric cancer cell growth by targeting CREB1. Biomed Pharmacother. 2018;105:413‐419. [DOI] [PubMed] [Google Scholar]
- 33. Cao J, Wu N, Han Y, et al. DDX21 promotes gastric cancer proliferation by regulating cell cycle. Biochem Biophys Res Comm. 2018;505:1189‐1194. [DOI] [PubMed] [Google Scholar]
- 34. Ma F, Li X, Ren J, et al. Downregulation of eukaryotic translation initiation factor 3b inhibited proliferation and metastasis of gastric cancer. Cell Death Dis. 2019;10:623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ricciuti B, Mencaroni C, Paglialunga L, et al. Long noncoding RNAs: new insights into non‐small cell lung cancer biology, diagnosis and therapy. Med Oncol. 2016;33:18. [DOI] [PubMed] [Google Scholar]
- 36. Abdollahzadeh S, Ghorbian S. Association of the study between LncRNA‐H19 gene polymorphisms with the risk of breast cancer. J Clin Lab Anal. 2019;33:e22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen G, Wang Z, Wang D, et al. LncRNADisease: a database for long‐non‐coding RNA‐associated diseases. Nucleic Acids Res. 2013;41:D983‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mo X, Li T, Xie Y, et al. Identification and functional annotation of metabolism‐associated lncRNAs and their related protein‐coding genes in gastric cancer. Mol Genet Genomic Med. 2018;6:728‐738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ni C, Yang P, Guo J, Ye M. Role of DiGeorge syndrome critical region gene 9, a long noncoding RNA, in gastric cancer. Onco Targets Ther. 2018;11:2259‐2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mo X, Wu Y, Chen L, et al. Global expression profiling of metabolic pathway‐related lncRNAs in human gastric cancer and the identification of RP11‐555H23.1 as a new diagnostic biomarker. J Clin Lab Anal. 2019;33:e22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hashad D, Elbanna A, Ibrahim A, Khedr G. Evaluation of the role of circulating long non‐coding RNA H19 as a promising novel biomarker in plasma of patients with gastric cancer. J Clin Lab Anal. 2016;30:1100‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tong YS, Wang XW, Zhou XL, et al. Identification of the long non‐coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer. 2015;14:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li J, Chen Z, Tian L, et al. LncRNA profile study reveals a three‐lncRNA signature associated with the survival of patients with oesophageal squamous cell carcinoma. Gut. 2014;63:1700‐1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guo W, Wang Q, Zhan Y, et al. Transcriptome sequencing uncovers a three‐long noncoding RNA signature in predicting breast cancer survival. Sci Rep. 2016;6:27931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhou R, Wu Z, Deng X, Chen H. The long non‐coding RNA OLC8 enhances gastric cancer by interaction with IL‐11. J Clin Lab Anal. 2019;33:e22962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sun W, Mo X, Li T, Xie Y, Guo J. Clinical significance of the long noncoding RNA RP11‐19P22.6‐001 in gastric cancer. Cancer Biomark. 2017;18:397‐403. [DOI] [PubMed] [Google Scholar]
- 47. Huang B, Chang C, Wang BL, Li H. ELK1‐induced upregulation of lncRNA TRPM2‐AS promotes tumor progression in gastric cancer by regulating miR‐195/ HMGA1 axis. J Cell Biochem. 2019;120:16921‐16933. [DOI] [PubMed] [Google Scholar]
- 48. Su X, Teng J, Jin G, et al. ELK1‐induced upregulation of long non‐coding RNA MIR100HG predicts poor prognosis and promotes the progression of osteosarcoma by epigenetically silencing LATS1 and LATS2. Biomed Pharmacother. 2019;109:788‐797. [DOI] [PubMed] [Google Scholar]
- 49. Sheng K, Lu J, Zhao H. ELK1‐induced upregulation of lncRNA HOXA10‐AS promotes lung adenocarcinoma progression by increasing Wnt/beta‐catenin signaling. Biochem Biophys Res Commun. 2018;501:612‐618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


