Abstract
Background
This study aimed to investigate the correlation of A‐kinase interacting protein 1 (AKIP1) expression with disease risk, clinical characteristics, and prognosis of acute myeloid leukemia (AML).
Methods
291 de novo AML patients and 97 controls were consecutively recruited, and bone marrow samples were collected to detect AKIP1 expression using quantitative polymerase chain reaction prior to initial treatment. Treatment response, event‐free survival (EFS), and overall survival (OS) in AML patients were evaluated.
Results
A‐kinase interacting protein 1 expression was higher in AML patients than that in controls; meanwhile, receiver operating characteristic curve displayed that AKIP1 was able to distinguish AML patients from controls (area under the curve:0.772, 95%CI: 0.720‐0.823). Among AML patients, AKIP1 high expression was correlated with −7 or 7q−, monosomal karyotype, and worse risk stratification. Moreover, AKIP1 expression was negatively correlated with complete remission achievement, while no correlation of AKIP1 expression with hematopoietic stem cell transplantation achievement was observed. AKIP1 high expression was associated with shorter EFS and OS in total patients, and further subgroup analysis exhibited that AKIP1 high expression correlated with worse EFS and OS in intermediate‐risk and poor‐risk patients but not in better‐risk patients. Besides, subsequent analysis revealed that AKIP1 high expression was an independent factor predicting unfavorable EFS and OS.
Conclusion
A‐kinase interacting protein 1 has the potential to be a novel marker for assisting AML management.
Keywords: acute myeloid leukemia, A‐kinase interacting protein 1, risk stratification, survival
1. INTRODUCTION
Acute myeloid leukemia (AML), an aggressive hematological malignancy, is characterized by abnormal cell proliferation and differentiation of the immature myeloid cells.1, 2 It accounts for 20% of all hematological malignancy related deaths, with a 5‐year survival rate of 40% for younger patients (18‐60 years) and 5‐year survival rate of 10% for the elder patients (age above 60 years).3, 4, 5 Despite there are potentially curative treatments for AML patients, including intensive chemotherapy and allogeneic stem cell transplantation, only a minority of eligible AML patients would benefit from these therapies, and half of patients received transplantation finally relapse.6, 7 Hence, it is needed to explore novel biomarkers for diagnosis, disease progression, and prognosis of AML, so as to conduct effective surveillance and therapies of AML patients at early stage.
A‐kinase interacting protein 1 (AKIP1), a 23 kDa protein located in the cytoplasm, nucleus and mitochondria, is initially discovered in breast cancer cells to facilitate the nuclear translocation of catalytic subunit of protein kinase A.8 Currently, AKIP1 has been shown to interact with a broad range of proteins involved in various cellular processes (such as cell apoptosis and oxidative stress).9 Besides, a few studies have shown that AKIP1 is overexpressed in tumor tissues (such as non‐small cell lung cancer (NSCLC), esophageal squamous cell carcinoma, and colorectal cancer), meanwhile, AKIP is reported to contribute to progression of cancers, and its overexpression correlates with aggravated disease progression (such as tumor size, TNM stage, and lymph node metastasis) and worse survival profiles in these cancers.10, 11, 12 For hematological malignancies, the role of AKIP1 is largely unknown, while AKIP1 has been found enriched in human bone marrow (https://www.ncbi.nlm.nih.gov/gene/56672); moreover, AKIP1 promotes solid tumor growth via regulating various proteins or pathways (such as chemokine (C‐X‐C motif) ligand 2 (CXCL2), NF‐κB pathway, and Akt pathway), which are also crucial steps in the initiation and progression of leukemia.8, 13, 14, 15 Taken together, we speculated that AKIP1 might also be related to disease initiation or progression of hematological malignancies, including AML, whereas the direct evidence about the role of AKIP1 in AML is rarely reported.
Thus, this study was conducted to investigate the correlation of AKIP1 expression with disease risk, clinical characteristics, and prognosis of AML based on a large‐sample‐size participants, and we envisaged that these data would provide information for prediction of AML prognosis and establishment of personalized therapies.
2. MATERIALS AND METHODS
2.1. Patients and controls
This study prospectively recruited 291 de novo AML patients and 97 controls from our hospital between January 2016 and May 2019. AML patients were enrolled in the study if they had confirmed diagnosis of primary AML based on the World Health Organization (WHO) Classification of Tumors of Hematopoietic and Lymphoid Tissues (2008), age more than 18 years old, no concurrent malignancies, and no history of radiotherapy or chemotherapy, while patients with acute promyelocytic leukemia or patients in pregnant or lactating were excluded from the study. Controls consisted of healthy bone marrow (BM) donors and subjects who underwent BM biopsy for the diagnosis of non‐hematopoietic malignancies. All controls were older than 18 years, not in pregnant or lactating, and had no history of malignant hematological diseases or tumors and serious infection. The current study was approved by the Ethics Committee of Bayannur Chinese Medicine Hospital and performed in line with the principles of Declaration of Helsinki. And the written informed consents were obtained from all AML patients and controls.
2.2. Sample and data collection
Prior to the initial treatment, AML patients’ BM samples were collected, and the density gradient centrifugation was immediately performed to isolate BM mononuclear cells (BMMCs), which was then stored at −80℃ for further detection. Controls’ BM samples were obtained when they underwent BM biopsy, and the BMMCs were separated and stored in the same condition as well. In addition, clinical characteristics of AML patients were also collected after the diagnostic work‐up was completed, mainly including age, gender, French‐American‐British (FAB) classification, cytogenetic abnormalities, molecular genetic abnormalities, and risk stratification. The risk stratification was based on cytogenetics and molecular genetics, according to the NCCN Guidelines (Version 1.2014).
2.3. Detection of AKIP1 expression
To detect the expression of AKIP1, total RNA was extracted from BMMCs using RNeasy Protect Mini Kit (Qiagen). Then, the reverse transcription was performed with ReverTra Ace® qPCR RT Kit (Toyobo, Osaka), followed by the qPCR process using KOD SYBR® qPCR Mix (Toyobo, Osaka), which was performed under the following condition: 95℃ for 5 minutes, 94℃ for 5 seconds, and then 61℃ for 30 seconds. The final results were calculated with 2−△△CT formula, and GAPDH was used as the internal reference. Information of primers applied in the qPCR was as follows: AKIP1, forward: AGAACATCTCTAAGGACCTCTACAT, reverse: CCAGAATCAACTGCTACCACAT; GAPDH, forward: GGAGCGAGATCCCTCCAAAAT; reverse: GGCTGTTGTCATACTTCTCATGG.
2.4. Treatment, response evaluation, and follow‐up
Three days of an anthracycline (eg, daunorubicin, at least 60 mg/m2, idarubicin, 10‐12 mg/m2, or the anthracenedione mitoxantrone, 10‐12 mg/m2) and 7 days of cytarabine (100‐200 mg/m2 cont. iv) or therapies of comparable intensity were administered to the AML patients for induction therapy. Response of AML to induction treatment was monitored clinically, with serial peripheral blood counts and repeat BM examinations. The complete remission (CR) was defined as blast clearance in the bone marrow to <5% of all nucleated cells, morphologically normal hematopoiesis, and return of peripheral blood cell counts to normal levels. The subsequent treatment, such as consolidation therapy, hematopoietic stem cell transplantation (HSCT), best supportive care, or palliative systemic treatment, was performed on the basis of response status after induction therapy. Besides, AML patients were followed up 3‐6 months or as clinically indicated, with a median follow‐up duration of 18.0 months (until deadline of 2019/5/1). Totally 42 patients lost follow‐up in our study. According to the follow‐up records, event‐free survival (EFS) and overall survival (OS) were evaluated.
2.5. Statistical analysis
SPSS 24.0 statistical software (IBM) and GraphPad Prism 7.02 statistical software (GraphPad Software Inc) were used for data analysis and figures processing. Normality determination for continuous variable was performed using the Kolmogorov‐Smirnov test, and the continuous variable was described as mean and standard deviation (SD) if normally distributed, or as median and interquartile (IQR) if not normally distributed. Categorized variable was displayed as count (percentage). Comparison was performed using chi‐square test or Wilcoxon rank‐sum test as appropriate. Receiver operating characteristic (ROC) curves and the area under the ROC curve (AUC) were used to assess the feasibility of using AKIP1 expression to distinguish different subjects. And the sensitivity and specificity at median value of AKIP1 expression were calculated as well. EFS was calculated from the date of initiation of therapy to the date of induction treatment failure, or relapse from CR, or death, and patients not known to have any of these events were censored on the date they were last examined. OS was calculated from the date of initiation of therapy to the date of death, and patients who lost follow‐up were censored on the date they were last known to be alive. Both EFS and OS were displayed by Kaplan‐Meier curves and were determined by the log‐rank test between subgroups. Univariate and forward stepwise multivariate Cox's proportional hazard regression model analyses were carried out to assess the factors related to EFS or OS. All reported tests were two‐tailed, and P value < .05 was considered statistically significant.
3. RESULTS
3.1. AKIP1 expression in AML patients and controls
A‐kinase interacting protein 1 expression was higher in AML patients than that in controls (P < .001; Figure 1A). Moreover, ROC curve displayed that AKIP1 was able to distinguish AML patients from controls (AUC:0.772, 95%CI: 0.720‐0.823), and the sensitivity and specificity at median value (1.759) of AKIP1 expression of all participants were 58.4% and 75.3%, respectively (Figure 1B).
Figure 1.
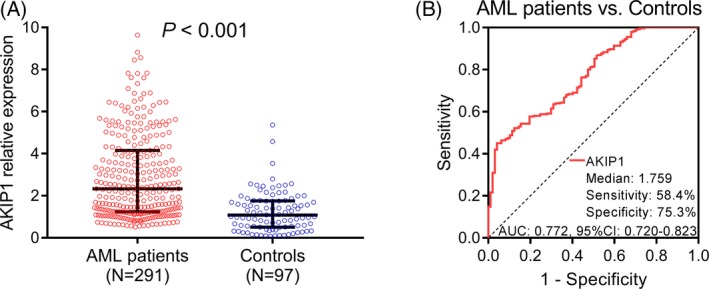
Comparison of AKIP1 expression between AML patients and controls. A, AKIP1 expression in AML patients and controls. B, ROC curve for AKIP1 predicting AML risk. Comparison between groups was determined by Wilcoxon rank‐sum test. P < .05 was considered significant. AKIP1, A‐kinase interacting protein 1; AML, acute myeloid leukemia; AUC, area under the curve; ROC curve, receiver operating characteristic curve
3.2. Correlation of AKIP1 expression with characteristics of AML patients
Acute myeloid leukemia patients were classified as AKIP1 high expression patients and AKIP1 low expression patients according to the median value of AKIP1 expression among them. And AKIP1 high expression was associated with −7 or 7q− (P = .003), monosomal karyotype (P = .021), and worse risk stratification (P = .002; Table 1). Meanwhile, AKIP1 high expression was numerically associated with presence of −5 or 5q− (P = .083) and absence of t (8;21; P = .050), while lacked statistical significance.
Table 1.
Correlation of AKIP1 expression with characteristics of AML patients
| Characteristics | AML patients | AKIP1 expressiona | P value | |
|---|---|---|---|---|
| High | Low | |||
| No. of patients | 291 | 146 | 145 | |
| Age (y), No. (%) | ||||
| <45 | 153 (52.6) | 71 (46.4) | 82 (53.6) | .176 |
| ≥45 | 138 (47.4) | 75 (54.3) | 63 (45.7) | |
| Gender, No. (%) | ||||
| Male | 156 (53.6) | 79 (50.6) | 77 (49.4) | .863 |
| Female | 135 (46.4) | 67 (49.6) | 68 (50.4) | |
| FAB classification, No. (%) | ||||
| M1 | 1 (0.3) | 0 (0.0) | 1 (100.0) | .410 |
| M2 | 108 (37.1) | 50 (46.3) | 58 (53.7) | |
| M4 | 73 (25.1) | 38 (52.1) | 35 (47.9) | |
| M5 | 93 (32.0) | 47 (50.5) | 46 (49.5) | |
| M6 | 16 (5.5) | 11 (68.8) | 5 (31.3) | |
| Normal karyotype, No. (%) | ||||
| No | 142 (48.8) | 71 (50.0) | 71 (50.0) | .954 |
| Yes | 149 (51.2) | 75 (50.3) | 74 (49.7) | |
| inv(16) or t(16;16), No. (%) | ||||
| No | 268 (92.1) | 135 (50.4) | 133 (49.6) | .815 |
| Yes | 23 (7.9) | 11 (47.8) | 12 (52.2) | |
| t(8;21), No. (%) | ||||
| No | 273 (93.8) | 141 (51.6) | 132 (48.4) | .050 |
| Yes | 18 (6.2) | 5 (27.8) | 13 (72.2) | |
| t(9;11), No. (%) | ||||
| No | 283 (97.3) | 142 (50.2) | 141 (49.8) | .992 |
| Yes | 8 (2.7) | 4 (50.0) | 4 (50.0) | |
| t(9;22), No. (%) | ||||
| No | 287 (98.6) | 145 (50.5) | 142 (49.5) | .311 |
| Yes | 4 (1.4) | 1 (25.0) | 3 (75.0) | |
| Complex karyotype, No. (%) | ||||
| No | 263 (90.4) | 131 (49.8) | 132 (50.2) | .705 |
| Yes | 28 (9.6) | 15 (53.6) | 13 (46.4) | |
| +8, No. (%) | ||||
| No | 278 (95.5) | 139 (50.0) | 139 (50.0) | .786 |
| Yes | 13 (4.5) | 7 (53.8) | 6 (46.2) | |
| −7 or 7q−, No. (%) | ||||
| No | 279 (95.9) | 135 (48.4) | 144 (51.6) | .003 |
| Yes | 12 (4.1) | 11 (91.7) | 1 (8.3) | |
| 11q23, No. (%) | ||||
| No | 284 (97.6) | 141 (49.6) | 143 (50.4) | .255 |
| Yes | 7 (2.4) | 5 (71.4) | 2 (28.6) | |
| −5 or 5q−, No. (%) | ||||
| No | 288 (99.0) | 143 (49.7) | 145 (50.3) | .083 |
| Yes | 3 (1.0) | 3 (100.0) | 0 (0.0) | |
| Others (not included in better or poor risk), No. (%) | ||||
| No | 262 (90.0) | 134 (51.1) | 128 (48.9) | .318 |
| Yes | 29 (10.0) | 12 (41.4) | 17 (58.6) | |
| Monosomal karyotype, No. (%) | ||||
| No | 271 (93.1) | 131 (48.3) | 140 (51.7) | .021 |
| Yes | 20 (6.9) | 15 (75.0) | 5 (25.0) | |
| FLT3‐ITD mutation, No. (%) | ||||
| No | 228 (78.4) | 112 (49.1) | 116 (50.9) | .496 |
| Yes | 63 (21.6) | 34 (54.0) | 29 (46.0) | |
| Biallelic CEBPA mutation, No. (%) | ||||
| No | 260 (89.3) | 132 (50.8) | 128 (49.2) | .555 |
| Yes | 31 (10.7) | 14 (45.2) | 17 (54.8) | |
| NPM1 mutation, No. (%) | ||||
| No | 191 (65.6) | 98 (51.3) | 93 (48.7) | .592 |
| Yes | 100 (34.4) | 48 (48.0) | 52 (52.0) | |
| Risk stratification, No. (%) | ||||
| Better‐risk | 80 (27.5) | 29 (36.2) | 51 (63.8) | .002 |
| Intermediate‐risk | 121 (41.6) | 60 (49.6) | 61 (50.4) | |
| Poor‐risk | 90 (30.9) | 57 (63.3) | 33 (36.7) | |
Comparison was determined by chi‐square test or Wilcoxon rank‐sum test.
Abbreviations: AKIP1, A‐kinase interacting protein 1; AML, acute myeloid leukemia; CEBPA, CCAAT/enhancer‐binding protein α; FAB classification, French‐American‐British classification; FLT3‐ITD, internal tandem duplications in the FMS‐like tyrosine kinase 3; NPM1, nucleophosmin; SD, standard deviation.
High or low expression of AKIP1 was classified by the median value of AML patients.
3.3. Correlation of AKIP1 expression with CR and HSCT achievements
Among total AML patients, 231 (79.4%) patients achieved CR while 60 (20.6%) patients failed to achieve CR. AKIP1 expression was decreased in CR patients than that in non‐CR patients (P < .001; Figure 2A). Besides, ROC curve showed that AKIP1 expression was able to distinguish CR patients from non‐CR patient with the relatively low AUC value (0.646, 95% CI: 0.574‐0.718), and the sensitivity and specificity at median value (2.330) of AKIP1 expression in AML patients were 54.1% and 65.0%, respectively (Figure 2B). Furthermore, among CR patients, there were 31 patients received HSCT as well as 200 patients did not receive HSCT, and no difference of AKIP1 expression was observed between HSCT patients and non‐HSCT patients (P = .318; Figure 2C). Meanwhile, ROC curve showed that AKIP1 expression was unable to distinguish HSCT patients from non‐HSCT patients (AUC: 0.556, 95% CI: 0.439‐0.672; Figure 2D). These results indicated that AKIP1 high expression correlated with reduced CR achievement, while no correlation of AKIP1 expression with HSCT achievement was found.
Figure 2.
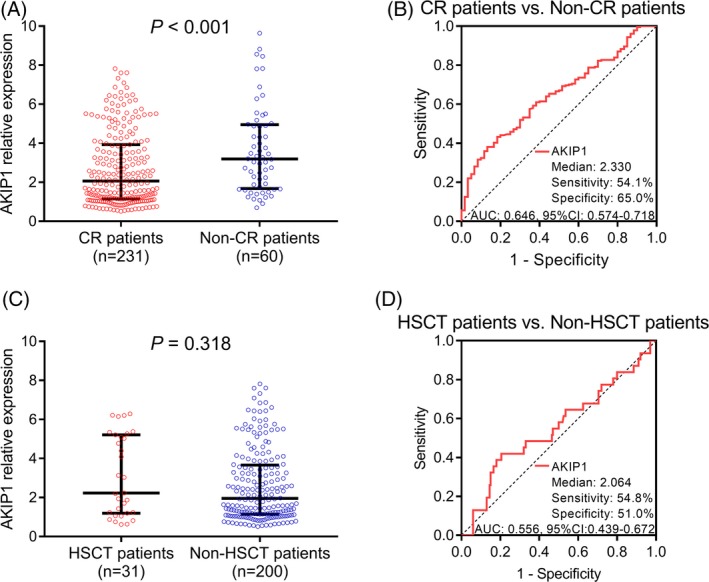
Comparison of AKIP1 expression between CR and non‐CR patients, HSCT and non‐HSCT patients. A, AKIP1 expression in CR patients and non‐CR patients. B, ROC curve for AKIP1 predicting CR achievement. C, AKIP1 expression in HSCT patients and non‐HSCT patients. D, ROC curve for AKIP1 predicting HSCT achievement. Comparison between groups was determined by Wilcoxon rank‐sum test. P < .05 was considered significant. AKIP1, A‐kinase interacting protein 1; AUC, area under the curve; CR, complete remission; HSCT, hematopoietic stem cell transplantation; ROC curve, receiver operating characteristic curve
3.4. Correlation of AKIP1 expression with EFS and OS in AML patients
Kaplan‐Meier curves disclosed that AML patients with AKIP1 high expression had shorter EFS (P < .001; Figure 3A) and decreased OS (P < .001; Figure 3B) than those with AKIP1 low expression. To further assess the correlation of AKIP1 expression with survival profiles, patients were divided into three subgroups according to different risk stratification. In intermediate‐risk patients (P < .001; Figure 4B) and poor‐risk patients (P = .005; (Figure 4C), AKIP1 expression was negatively correlated with EFS, while no correlation of AKIP1 expression with EFS was found in better‐risk patients (P = .955; Figure 4A). Besides, AKIP1 expression was also negatively correlated with OS in intermediate‐risk patients (P = .002; Figure 5B) and poor‐risk patients (P = .010; Figure 5C), while no correlation of AKIP1 expression with OS was observed among better‐risk patients (P = .084; Figure 5A).
Figure 3.
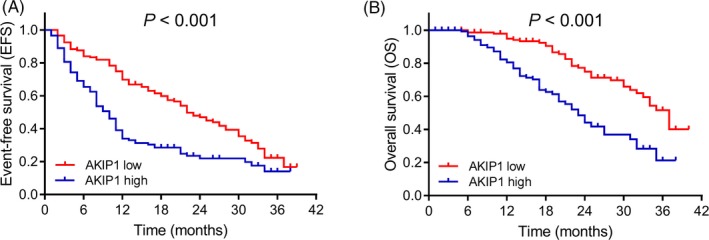
EFS and OS in AML patients. A, EFS in AKIP1 high expression and AKIP1 low expression patients. B, OS in AKIP1 high expression and AKIP1 low expression patients. K‐M curves were used to exhibit EFS and OS. Comparison between groups was determined by log‐rank test. P value < .05 was considered significant. AKIP1, A‐kinase interacting protein 1; EFS, event‐free survival; K‐M curves, Kaplan‐Meier curves; OS, overall survival
Figure 4.
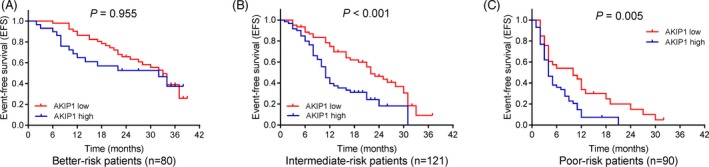
Subgroup analysis for correlation between AKIP1 and EFS. A, Correlation between AKIP1 expression with EFS in better‐risk AML patients. B, Correlation between AKIP1 expression with EFS in intermediate‐risk AML patients. C, Correlation between AKIP1 expression with EFS in poor‐risk AML patients. K‐M curves were used to exhibit EFS. Comparison between groups was determined by log‐rank test. P value < .05 was considered significant. AKIP1, A‐kinase interacting protein 1; AML, acute myeloid leukemia; EFS, event‐free survival; K‐M curves, Kaplan‐Meier curves
Figure 5.
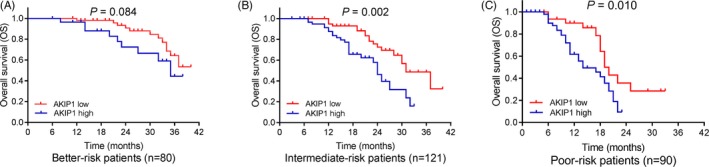
Subgroup analysis for correlation between AKIP1 expression and OS. A, Correlation between AKIP1 expression with OS in better‐risk AML patients. B, Correlation between AKIP1 expression with OS in intermediate‐risk AML patients. C, Correlation between AKIP1 expression with OS in poor‐risk AML patients. K‐M curves were used to exhibit OS. Comparison between groups was determined by log‐rank test. P value < .05 was considered significant. AKIP1, A‐kinase interacting protein 1; AML, acute myeloid leukemia; K‐M curves, Kaplan‐Meier curves; OS, overall survival
3.5. Analysis of factors affecting EFS and OS
Univariate Cox's regression displayed that AKIP1 high expression was associated with worse EFS in AML patients (P < .001), and age (≥45 years; P = .045), complex karyotype (P < .001), monosomal karyotype (P = .001), FLT3‐ITD mutation, and poor risk stratification were associated with worse EFS as well (Table 2). According to further multivariate Cox's regression analysis, AKIP1 high expression (P < .001) was verified as an independent predictive factor for poor EFS, and age (≥45 years; P = .038) as well as poor risk stratification (P < .001) also independently predicted unsatisfied EFS (Table 2). As for factors affecting OS, AKIP1 high expression was associated with decreased OS (P < .001), and age (≥45 years; P = .043), complex karyotype (P = .021), monosomal karyotype (P = .015), FLT3‐ITD mutation (P = .003), and poor risk stratification (P < .001) were also correlated with shorter OS; furthermore, multivariate Cox's regression analysis disclosed that AKIP1 high expression (P < .001) and poor risk stratification (P < .001) were independent factors predicting worse OS in AML patients (Table 3).
Table 2.
Univariate and multivariate Cox's proportional hazard regression model analyses of factors related to EFS
| Items | Cox's proportional hazard regression model | |||
|---|---|---|---|---|
| P value | HR | 95%CI | ||
| Lower | Higher | |||
| Univariate Cox's regression analysis | ||||
| AKIP1 high expression | <.001 | 2.021 | 1.515 | 2.696 |
| Age (≥45 y) | .010 | 1.451 | 1.092 | 1.926 |
| Gender (male) | .138 | 1.242 | 0.933 | 1.652 |
| Complex karyotype | <.001 | 2.406 | 1.504 | 3.849 |
| Normal karyotype | .992 | 1.001 | 0.755 | 1.328 |
| Monosomal karyotype | .001 | 2.383 | 1.418 | 4.005 |
| FLT3‐ITD mutation | <.001 | 2.104 | 1.517 | 2.919 |
| Biallelic CEBPA mutation | .428 | 1.206 | 0.759 | 1.917 |
| NPM1 mutation | .174 | 0.812 | 0.601 | 1.096 |
| Poor risk stratificationa | <.001 | 2.600 | 2.109 | 3.204 |
| Forward stepwise multivariate Cox's regression | ||||
| AKIP1 high expression | <.001 | 1.897 | 1.413 | 2.546 |
| Age (≥45 y) | .038 | 1.352 | 1.017 | 1.798 |
| Poor risk stratificationa | <.001 | 2.576 | 2.081 | 3.188 |
Abbreviations: AKIP1, A‐kinase interacting protein 1; CEBPA, CCAAT/enhancer‐binding protein α; CI, confidence interval; EFS, event‐free survival; FAB classification, French‐American‐British classification; FLT3‐ITD, internal tandem duplications in the FMS‐like tyrosine kinase 3; HR, hazard ratio; NPM1, nucleophosmin.
Risk stratification was included into the Cox's regression analysis in the form of ordered categorized variables, which was encoded as better‐risk = 1, intermediate‐risk = 2, poor‐risk = 3.
Table 3.
Univariate and multivariate Cox's proportional hazard regression model analyses of factors predicting OS
| Items | Cox's proportional hazard regression model | |||
|---|---|---|---|---|
| P value | HR | 95%CI | ||
| Lower | Higher | |||
| Univariate Cox's regression | ||||
| AKIP1 high expression | <.001 | 2.815 | 1.875 | 4.225 |
| Age (≥45 y) | .043 | 1.501 | 1.012 | 2.225 |
| Gender (male) | .926 | 1.019 | 0.689 | 1.507 |
| Complex karyotype | .021 | 2.262 | 1.132 | 4.520 |
| Normal karyotype | .919 | 1.020 | 0.692 | 1.505 |
| Monosomal karyotype | .015 | 2.654 | 1.212 | 5.810 |
| FLT3‐ITD mutation | .003 | 2.008 | 1.273 | 3.168 |
| Biallelic CEBPA mutation | .920 | 1.036 | 0.522 | 2.057 |
| NPM1 mutation | .056 | 0.661 | 0.432 | 1.011 |
| Poor risk stratificationa | <.001 | 2.816 | 2.110 | 3.759 |
| Forward stepwise multivariate Cox's regression | ||||
| AKIP1 high expression | <.001 | 2.666 | 1.759 | 4.040 |
| Poor‐risk stratificationa | <.001 | 2.788 | 2.071 | 3.754 |
Abbreviations: AKIP1, A‐kinase interacting protein 1; CEBPA, CCAAT/enhancer‐binding protein α; CI, confidence interval; FAB classification, French‐American‐British classification; FLT3‐ITD, internal tandem duplications in the FMS‐like tyrosine kinase 3; HR, hazard ratio; NPM1, nucleophosmin; OS, overall survival.
Risk stratification was included into the Cox's regression analysis in the form of ordered categorized variables, which was encoded as better‐risk = 1, intermediate‐risk = 2, poor‐risk = 3.
4. DISCUSSION
Our results indicated that (a) AKIP1 presented with increased expression in AML patients compared to controls, and it was able to distinguish AML patients from controls; (b) in AML patients, AKIP1 high expression correlated with −7 or 7q−, monosomal karyotype, and worse risk stratification; (c) AKIP1 high expression correlated with reduced CR achievement, and it was an independent predictive factor for worse EFS and OS in total AML patients; meanwhile, further subgroup analysis showed that AKIP1 presented with good predictive value for shorter EFS and OS in intermediate‐ and poor‐risk patients but had no predictive value for prognosis in better‐risk patients.
A‐kinase interacting protein 1 is initially reported as human breast cancer‐associated gene 3 (BCA3) whose biological role is largely unknown.12 Recently, some studies have identified AKIP1 as a gene overexpressed in multiple human cancer cells, and it might participate in the tumorigenesis and invasiveness.10, 14, 16, 17 For example, an experiment shows that AKIP1 induces the autocrine effect of CXCL1, CXCL2, and CXCL8 to enhance cervical cancer cell proliferation in vitro and promote tumor growth in vivo.16 Besides, a study displays that AKIP1 promotes cell proliferation, cell migration, and cell invasion via activating Slug‐induced epithelial‐mesenchymal transition (EMT) in gastric cancer cells.17 Also, a study shows that AKIP1 knockdown represses NSCLC cell migration and cell invasion via transactivating zinc finger E‐box binding homeobox 1 (ZEB1).10 Another study discloses that AKIP1 downregulation inhibits cell motility and cell invasion through suppressing Akt/GSK‐3β/Snail pathway in breast cancer cells.14 These data indicate that AKIP1 might regulate some factors such as CXC chemokines and ZEB1 or mediate some processes such as Slug‐induced EMT and Akt/GSK‐3β/Snail pathway to promote cell proliferation and cell invasion, thereby facilitates tumorigenesis and invasiveness in some solid cancers.14, 16, 17
Accumulating evidences have displayed that AKIP1 is overexpressed in tumor tissues of several human malignancies, such NSCLC, gastric cancer, and colorectal cancer, and its high expression also correlates with severer tumor features in these cancers.10, 11, 14 For instance, a study displays that AKIP1 is positively associated with TNM stage and lymph node metastasis in NSCLC patients.10 Besides, a study shows that AKIP1 expression is positively associated with tumor size, TNM stage, and lymph node metastasis in colorectal cancer.11 These data disclose the positive correlation of AKIP1 expression with disease progression in some solid tumors. As for the role of AKIP1 in hematological malignancies, related evidence is limited. Whereas AKIP1 presents with abundant expression in human bone marrow samples, moreover, AKIP1 promotes tumorigenesis and invasiveness in solid tumors through regulating some chemokines or pathways (such as CXCL1 and Wnt/β‐catenin pathway), leading to the increased risk and accelerate progression, and the regulation on these chemokines or pathways is also critical to promote initiation and progression of hematological malignancies, especially AML.16, 18 Based on these indications, we speculated that AKIP1 might have potential correlation with disease risk and progression in AML, whereas the direct evidence about the role of AKIP1 in AML is rarely reported. In our study, we found that AKIP1 expression was increased in AML patients compared to controls, and it showed predictive value for AML risk, which might result from that AKIP1 activated Akt/GSK‐3β/Snail signal pathway or CXC chemokines to promote cell proliferation, and further facilitated disease initiation of AML, thereby it predicted increased AML risk.14, 16 Furthermore, we observed that AKIP1 high expression was associated with presence of −7 or 7q−, monosomal karyotype, and worse risk stratification in AML patients. The following reasons might explain these results: AKIP1 might have influence on genes or signaling pathways that contribute to cytogenetic or molecular mutations; thus, its high expression led to increased possibilities of −7 or 7q−, monosomal karyotype, and worse risk stratification in AML patients, while its detailed effect underlying cytogenetic or molecular mutations needed to be further explored.
The development of useful prognostic markers is important to predict outcomes of cancer patients, and AKIP1 has been identified as a biomarker for poor prognosis in some cancers.11, 12 For example, a study displays that AKIP1 is negatively correlated with OS in colorectal cancer patients.11 Also, another study shows the negative correlation of AKIP1 expression with OS in esophageal squamous cell carcinoma patients.12 Considering AKIP1 had shown the predictive value for poor prognosis in these solid tumors and it had interaction with CXCL1, NF‐κB pathway, and Akt pathway, which were also critical in the mechanism underlying AML progression, we hypothesized that AKIP1 might also play a role in the prediction of AML prognosis, while direct evidence is rarely seen. In our study, we observed that AKIP1 high expression correlated with decreased CR achievement, EFS, and OS in AML patients, which might due to the following reasons: (a) Patients with AKIP1 high expression presented severer disease condition than those with AKIP1 low expression; thus, AKIP1 high expression patients might have less possibility to achieve CR.10, 17 (b) AKIP1 might promote cell proliferation (via regulating CXCL1, CXCL2, and CXCL8) or enhance cell invasion to accelerate disease progression and retard treatment efficacy, which further led to worse EFS and OS in AML patients10, 14, 16, 17; (c) considering AKIP1 expression negatively correlated with CR achievement and independently predicted survival profiles, we speculated that AKIP1 might cause drug resistance through activating NF‐κB pathway or Akt pathway, which impaired the treatment efficacy, aggravated disease progression, and eventually reduced survival profiles in AML patients.19, 20 Furthermore, we performed subgroup analysis and observed that AKIP1 high expression correlated with shorter EFS and OS in intermediate‐ and poor‐risk patients but not in better‐risk patients, indicating that AKIP1 could be a biomarker for assisting prognosis prediction in intermediate‐risk and as poor‐risk AML patients.
Our data provided clinical evidences about the role of AKIP1 in AML, while some limitations still existed in our study: (a) The follow‐up duration (median follow‐up duration of 18.0 months) was relatively short; thus, long‐term assessment about the role of AKIP1 in AML prognosis needed further exploration; (b) the underlying mechanism of AKIP1 in AML is largely unknown; thus, further study investigating the molecular mechanism of AKIP1 in AML is needed; (c) AML patients in this study were de novo AML patients, and the role of AKIP1 in refractory and relapsed AML patients needed to be further investigated; (d) sample size, especially sample size in controls, was relatively small, and further study with equal sample size in controls and AML patients would be better; and (e) further study with AKIP1 detection using more methods would be better.
In conclusion, AKIP1 is overexpressed, and its high expression correlates with −7 or 7q−, monosomal karyotype, worse risk stratification, reduced CR achievement, and worse survival profiles in AML patients. Therefore, AKIP1 has the potential to be a novel marker for assisting AML management.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
None.
Yan Y, Li X, Gao J. Identification of A‐kinase‐interacting protein 1 as a potential biomarker for risk and prognosis of acute myeloid leukemia. J Clin Lab Anal. 2020;34:e23055 10.1002/jcla.23055
Yan Yan and Xiaoyan Li contributed equally to this work.
REFERENCES
- 1. Liu Y, Cheng Z, Pang Y, et al. Role of microRNAs, circRNAs and long noncoding RNAs in acute myeloid leukemia. J Hematol Oncol. 2019;12(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldman SL, Hassan C, Khunte M, et al. Epigenetic modifications in acute myeloid leukemia: prognosis, treatment, and heterogeneity. Front Genet. 2019;10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bohl SR, Bullinger L, Rucker FG. New targeted agents in acute myeloid leukemia: new hope on the rise. Int J Mol Sci. 2019;20(8):1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136‐1152. [DOI] [PubMed] [Google Scholar]
- 5. Loew A, Kohnke T, Rehbeil E, Pietzner A, Weylandt KH. A role for lipid mediators in acute myeloid leukemia. Int J Mol Sci. 2019;20(10):2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shallis RM, Wang R, Davidoff A, Ma X, Zeidan AM. Epidemiology of acute myeloid leukemia: Recent progress and enduring challenges. Blood Rev. 2019;36:70‐87. [DOI] [PubMed] [Google Scholar]
- 7. Rautenberg C, Germing U, Haas R, Kobbe G, Schroeder T. Relapse of acute myeloid leukemia after allogeneic stem cell transplantation: prevention, detection, and treatment. Int J Mol Sci. 2019;20(1):228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gao N, Hibi Y, Cueno M, Asamitsu K, Okamoto T. A‐kinase‐interacting protein 1 (AKIP1) acts as a molecular determinant of PKA in NF‐kappaB signaling. J Biol Chem. 2010;285(36):28097‐28104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keprova A, Korinkova L, Krizova I, et al. Various AKIP1 expression levels affect its subcellular localization but have no effect on NF‐kappaB activation. Physiol Res. 2019;68(3):431‐443. [DOI] [PubMed] [Google Scholar]
- 10. Guo X, Zhao L, Cheng D, Mu Q, Kuang H, Feng K. AKIP1 promoted epithelial‐mesenchymal transition of non‐small‐cell lung cancer via transactivating ZEB1. Am J Cancer Res. 2017;7(11):2234‐2244. [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang W, Yang W, Yuan L, Liu F. Upregulation of AKIP1 contributes to metastasis and progression and predicts poor prognosis of patients with colorectal cancer. Onco Targets Ther. 2018;11:6795‐6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin C, Song L, Liu A, et al. Overexpression of AKIP1 promotes angiogenesis and lymphangiogenesis in human esophageal squamous cell carcinoma. Oncogene. 2015;34(3):384‐393. [DOI] [PubMed] [Google Scholar]
- 13. Burgess M, Cheung C, Chambers L, et al. CCL2 and CXCL2 enhance survival of primary chronic lymphocytic leukemia cells in vitro. Leuk Lymphoma. 2012;53(10):1988‐1998. [DOI] [PubMed] [Google Scholar]
- 14. Mo D, Li X, Li C, et al. Overexpression of AKIP1 predicts poor prognosis of patients with breast carcinoma and promotes cancer metastasis through Akt/GSK‐3beta/Snail pathway. Am J Transl Res. 2016;8(11):4951‐4959. [PMC free article] [PubMed] [Google Scholar]
- 15. Bosman MC, Schuringa JJ, Vellenga E. Constitutive NF‐kappaB activation in AML: causes and treatment strategies. Crit Rev Oncol Hematol. 2016;98:35‐44. [DOI] [PubMed] [Google Scholar]
- 16. Zhang W, Wu Q, Wang C, Yang L, Liu P, Ma C. AKIP1 promotes angiogenesis and tumor growth by upregulating CXC‐chemokines in cervical cancer cells. Mol Cell Biochem. 2018;448(1–2):311‐320. [DOI] [PubMed] [Google Scholar]
- 17. Chen D, Cao G, Liu Q. A‐kinase‐interacting protein 1 facilitates growth and metastasis of gastric cancer cells via Slug‐induced epithelial‐mesenchymal transition. J Cell Mol Med. 2019;23(6):4434‐4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cui Y, Wu X, Lin C, et al. AKIP1 promotes early recurrence of hepatocellular carcinoma through activating the Wnt/beta‐catenin/CBP signaling pathway. Oncogene. 2019;38(27):5516‐5529. [DOI] [PubMed] [Google Scholar]
- 19. Huy H, Kim TD, Kim WS, et al. TLR4/NF‐kappaB axis induces fludarabine resistance by suppressing TXNIP expression in acute myeloid leukemia cells. Biochem Biophys Res Commun. 2018;506(1):33‐40. [DOI] [PubMed] [Google Scholar]
- 20. Chen P, Jin Q, Fu Q, et al. Induction of multidrug resistance of acute myeloid leukemia cells by cocultured stromal cells via upregulation of the PI3K/Akt signaling pathway. Oncol Res. 2016;24(4):215‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]


