Abstract
Background
Ethnicity and environmental factors can influence the percentages of lymphocyte subpopulations. This study aimed to assess the percentages of lymphocyte subpopulations according to age in Thai children.
Methods
This was a cross‐sectional study. The percentages of lymphocyte subpopulations were measured in umbilical cord blood and peripheral blood of healthy Thai children aged 1 month‐15 years. The participants were stratified into five age groups: (a) cord blood; (b) age < 2 years; (c) age 2‐5 years; (d) age 5‐10 years; and (e) age 10‐15 years.
Results
Of 182 total samples, 32, 39, 41, 28, and 42 were from cord blood, children aged <2 years, children aged 2‐5 years, children aged 5‐10 years, and children aged 10‐15 years, respectively. The percentages of most lymphocyte subpopulations including CD8 + T cells, CD19 + cells, γδ T cells, double‐negative T cells, NK cells, and NK T cells increased significantly with age. Only the CD4+ T‐cell percentage decreased in older children. Moderate correlations were observed between age and the percentages of CD4+ T cells, γδ T cells, NK cells, NK T cells, and double‐negative T cells. Weak correlations were observed between age and the percentages of CD8+ T cells and CD19+ cells.
Conclusion
Our study demonstrated age‐related changes in the percentages of lymphocyte subpopulations in Thai children, which differed from those described in other countries. Therefore, the establishment of age‐specific reference values for lymphocyte subsets in each country is recommended.
Keywords: flow cytometry, immunology, lymphocyte subsets, reference values, T cells
1. INTRODUCTION
The reference ranges for major lymphocyte subpopulations have varied across previous studies, potentially resulting from differences in age, gender, ethnicity, and environmental factors.1, 2, 3, 4 These factors have varying influences on the percentages of lymphocyte subpopulations. Gender is not an important factor affecting the percentages of lymphocyte subsets, as shown by many studies.3, 5, 6, 7, 8 Ethnicity and geography both influence the percentages of lymphocyte subsets because people in different regions are differentially exposed to infections, as well as environmental and nutritional factors.3, 5, 9, 10 Age has the greatest impact on the percentages of lymphocyte subsets compared with other factors. In addition, age‐related changes in peripheral lymphocyte subsets have been demonstrated,11, 12, 13, 14, 15 especially during early life. Therefore, it is essential to establish reference values for lymphocyte subpopulations in various age groups to enable appropriate immunological assessments.
Apart from the major lymphocyte subsets, minor lymphocyte subpopulations, such as gamma delta T cells (γδ T cells) and regulatory T cells (Tregs), play many important roles in immunological and infectious diseases. Reference values for these subpopulations in individual age groups, particularly in Asian populations, are still lacking. γδ T cells are mainly present in the epithelium where they participate in early responses to pathogens and contribute to mucosal immune defense.16, 17 Multiple lines of evidence have demonstrated the vital roles of γδ T cells in the pathogenesis of inflammatory bowel disease and the use of these cells for anti‐tumor immunotherapy.18, 19, 20, 21, 22, 23 Tregs also have a central role in various immune responses and are involved in the pathogenesis of autoimmune diseases, cancer, infectious diseases, and allergic diseases.24, 25, 26, 27, 28 Furthermore, immunotherapeutic strategies using Tregs for treatment and prevention of inflammatory diseases are very promising.29, 30, 31 Since normal reference ranges for all lymphocyte subsets are essential for diagnosis of immunological diseases and the basis for further research, this study aimed to define age‐specific references for lymphocyte subsets (CD4+ T cells, CD8+ T cells, double‐negative T cells, γδ T cells, CD19+ B cells, NK cells, NK T cells, and Tregs) in Thai children.
2. MATERIALS AND METHODS
This cross‐sectional study was undertaken at Ramathibodi Hospital, Bangkok, Thailand, a tertiary care center, between August 2017 and December 2018. Written informed consent was obtained from the legal guardians of the study participants prior to enrollment. This study was approved by the Ethics Committee of Ramathibodi Hospital and conformed to the principles laid out in the World Medical Association's Declaration of Helsinki.
2.1. Subjects and samples
One hundred and eighty‐two participants were enrolled in this study. Thirty‐two samples were obtained from the cord blood of healthy newborns, and 150 samples were obtained from the leftover ethylenediaminetetraacetic acid (EDTA)‐treated blood from healthy children aged between 1 month and 15 years; the samples were secured at outpatient clinics during routine healthcare checkups of healthy volunteer school children. Children who had chronic illnesses, such as infection, malnutrition, autoimmune diseases, or malignancies, or who were taking immunosuppressive drugs, were excluded.
Samples were stratified according to the participants' age into five groups: (a) cord blood; (b) age < 2 years (from 30 days old to the day before the second birthday); (c) age 2‐5 years (from the second birthday to the day before the fifth birthday); (d) age 5‐10 years (from the fifth birthday to the day before the 10th birthday); and (e) age 10‐15 years (from the 10th birthday to the day before the 15th birthday). Cell subtype frequencies were calculated as percentages of total lymphocyte counts.
2.2. Immunophenotyping
Cellular phenotypes were analyzed using fluorochrome‐conjugated antibodies. All antibodies were obtained from eBioscience, Inc. The fluorochrome‐conjugated antibodies against cell‐surface antigens were diluted 1:100 and 10‐20 µL of EDTA‐treated peripheral blood were stained for 15 minutes at room temperature protected from light. Erythrocytes were lysed using a lysis buffer (BD Biosciences) at room temperature for 10 minutes. The samples were centrifuged to remove unbound antibodies and then resuspended in phosphate‐buffered saline. The cells were analyzed using a flow cytometer (BD FACSVerse™, BD Bioscience).
For Treg staining, 10‐20 µL of EDTA‐treated blood were first stained with Phycoerythrin‐Cy™7‐conjugated anti‐CD25 and allophycocyanin‐conjugated anti‐CD4 antibodies (1:100 dilution) for 15 minutes at room temperature. The samples were then incubated with a fixation/permeabilization buffer (eBioscience) for 15 minutes as per the manufacturer's protocol. The cells were pelleted, resuspended in a permeabilization buffer (eBioscience), stained with fluorescein isothiocyanate‐conjugated anti‐FoxP3 antibody (1:100 dilution), and then incubated at room temperature for 45 minutes. The samples were centrifuged to remove unbound antibodies and then analyzed by flow cytometry.
The gating strategy for each cell type is described in Table 1. The gating strategy for each lymphocyte subset was as follows: CD4+ T cells (CD3+ CD4+); CD8+ T cells (CD3+ CD8+); double‐negative T cells (CD3+ CD4− CD8−); γδ T cells (CD3+ CD4− CD8− γδ TCR+); B cells (CD3− CD19+); NK cells (CD56+); NK T cells (CD3+ CD56+); and Tregs (CD4+ CD25+ FoxP3+). All cells (except for the Tregs) were first gated from the CD45+ population and then gated from the lymphocyte population, as determined from the forward scatter/side scatter plot. Tregs were first gated from the CD4+ population and then gated from CD25 + and FoxP3 + cells, as shown in Figure 1. The percentage of each T cell subset among total lymphocytes was calculated using FlowJo v10.
Table 1.
Fluorochrome‐conjugated antibodies used in this study
| Markers | Fluorochromes |
|---|---|
| CD3 | Fluorescein isothiocyanate (FITC) |
| CD4 | Allophycocyanin (APC) |
| CD8 | APC‐eFluor®780 |
| γδ TCR | Phycoerythrin (PE) |
| CD19 | APC |
| CD56 | PE |
| CD45 | PE‐Cy™7 |
| CD25 | PE‐Cy™7 |
| FoxP3 | FITC |
Figure 1.
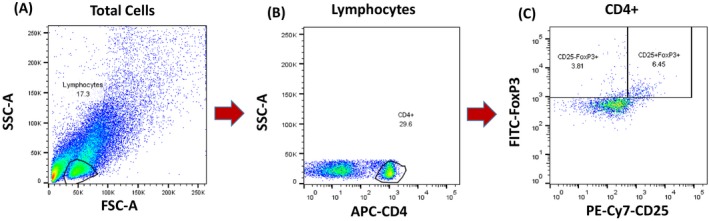
Gating strategy for regulatory T cells. A, The lymphocyte population was selected from total cells as determined by the forward scatter/side scatter plot. B, CD4+ T cells were then selected from the lymphocyte population. C, Regulatory T cells were gated as CD4+ CD25+ FoxP3+ cells
2.3. Statistical analysis
The sample size was calculated according to the results of previous studies.32, 33 The calculation was based on the standard deviation of the percentage of cells in each age group, a significance level of 0.05, and a power of 80%. Based on this calculation, the required sample size in this study was approximately 25 samples for each age group.
Baseline characteristics were presented as median percentages along with 25th–75th percentile ranges. The Kruskal‐Wallis test was used to assess differences between groups. Spearman correlations were used to analyze the relationships between age and lymphocyte subset percentages. Correlations were considered weak, moderate, or strong when Spearman's rank correlation coefficient was <0.4, 0.4‐0.7, or >0.7, respectively. All data were analyzed using SPSS version 23.0 (IBM Corp). Values of P < .05 were judged as statistically significant.
3. RESULTS
One hundred and eighty‐two samples were collected from 182 participants and stratified into five different age groups: (a) 32 cord blood samples; (b) 39 samples from children aged <2 years; (c) 41 samples from children aged 2‐5 years; (d) 28 samples from children aged 5‐10 years; and (e) 42 samples from children aged 10‐15 years. Of the 150 participants (excluding cord blood samples), 71 (47.3%) were female and 79 (52.7%) were male. Females made up 41.0% of participants aged <2 years, 41.5% of participants aged 2‐5 years, 42.9% of participants aged 5‐10 years, and 61.9% of participants aged 10‐15 years.
The overall percentages of lymphocyte subsets in the five age groups are shown as medians along with 25th–75th percentile ranges in Table 2. Statistical differences across groups are described in Figure 2.
Table 2.
Percentages of lymphocyte subpopulations in each age group
| Population |
Cord blood (N = 32) |
<2 y (N = 39) |
2‐5 y (N = 41) |
5‐10 y (N = 28) |
10‐15 y (N = 42) |
P‐value |
|---|---|---|---|---|---|---|
| % CD3+ T cells | 64.1 (57.7‐73.0) | 62.2 (55.6‐66.1) | 64.9 (60.1‐69.5) | 62.0 (51.8‐66.3) | 63.1 (59.3‐66.5) | .09 |
| % CD4+ T cells | 42.5 (37.0‐49.0) | 36.0 (29.5‐39.8) | 33.5 (30.3‐37.5) | 26.8 (24.8‐29.3) | 28.7 (26.9‐35.0) | <.01* |
| % CD8+ T cells | 17.1 (13.1‐24.2) | 18.9 (14.7‐23.9) | 21.4 (18.7‐24.8) | 21.5 (17.4‐26.0) | 23.9 (20.5‐25.7) | <.01* |
| % Regulatory T cells/CD4+ T cells | 2.7 (1.7‐4.2) | 4.9 (4.2‐6.3) | 5.9 (4.6‐6.9) | 4.6 (3.6‐7.3) | 4.0 (3.2‐5.1) | <.01* |
|
% Regulatory T cells (CD4+ CD25+Foxp3+) |
1.0 (0.7‐1.7) | 1.8 (1.3‐2.5) | 2.0 (1.5‐2.4) | 1.4 (1.1‐1.9) | 1.0 (0.9‐1.4) | <.01* |
|
% γδ T cells (CD45+ CD3+ CD4−CD8− γδ TCR+) |
0.6 (0.4‐1.0) | 3.3 (2.4‐4.7) | 6.1 (4.0‐8.2) | 7.4 (5.6‐9.9) | 5.9 (4.5‐9.5) | <.01* |
|
% Double‐negative T cells (CD45+ CD3+ CD4− CD8−) |
1.2 (0.8‐1.5) | 4.6 (3.7‐6.2) | 7.7 (6.0‐9.4) | 10.5 (7.6‐13.2) | 7.6 (5.7‐10.8) | <.01* |
|
% NK cells (CD56+) |
5.0 (2.6‐10.9) | 4.5 (3.5‐7.2) | 7.6 (5.6‐11.5) | 12.6 (8.7‐15.4) | 14.1 (10.0‐17.0) | <.01* |
|
% NK T cells (CD3+ CD56+) |
0.1 (0.0‐0.3) | 0.2 (0.1‐0.4) | 0.7 (0.4‐1.0) | 0.8 (0.5‐1.6) | 0.8 (0.6‐1.5) | <.01* |
| % CD19+ B cells | 12.2 (8.6‐16.0) | 17.8 (13.3‐22.3) | 19.9 (17.5‐22.8) | 21.6 (19.6‐24.2) | 17.1 (14.3‐20.4) | <.01* |
Values were presented as medians along with 25th–75th percentile ranges. Percentages are given with reference to the total number of lymphocytes.
Statistically significant at the P < .05 levels.
Figure 2.
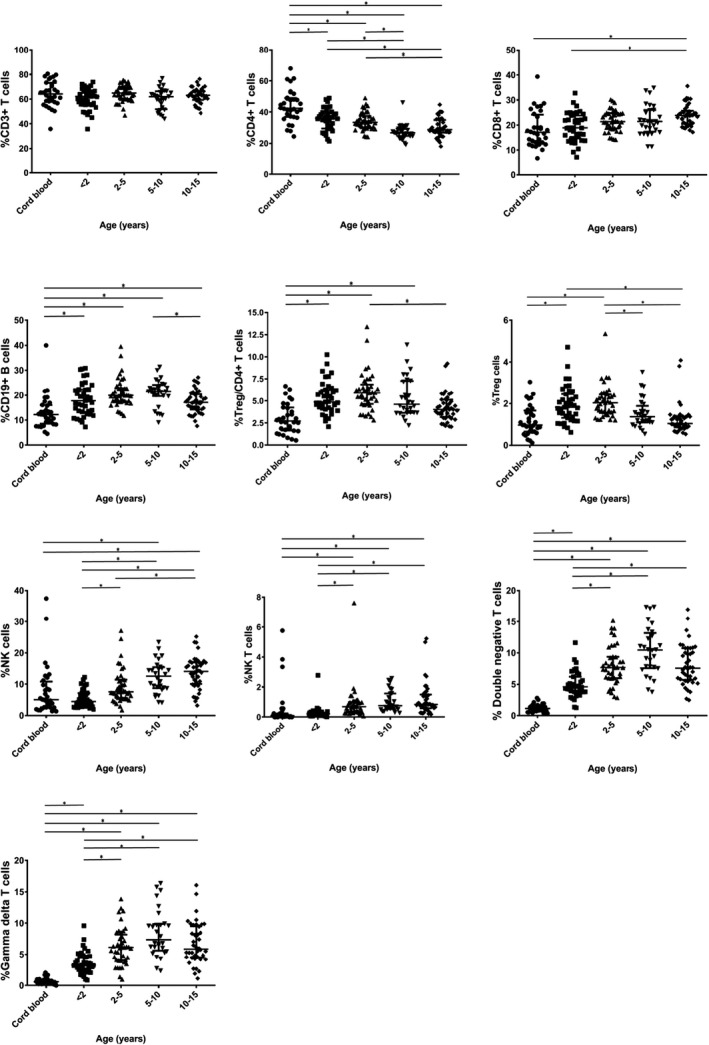
Comparison of percentages of lymphocyte subpopulations among the different age groups. Lines represent medians and interquartile ranges, *P < .05
3.1. T‐cell subsets
The percentage of CD3+ T cells remained stable from birth to adolescence, unlike the percentages of CD4+ and CD8+ T cells. The CD4+ T‐cell percentage was highest in cord blood and then declined with age until 10 years of age, with significant differences observed among age groups. The CD8+ T‐cell percentage was the lowest in cord blood and increased with age. The percentage of Tregs was also the lowest in the cord blood. These cells increased in frequency during the first 5 years of life and then declined. The percentage of γδ T cells was similar to the percentage of double‐negative T cells; both were lowest in cord blood, but then increased with age until 10 years of age.
3.2. B cells
The percentage of CD19+ cells was lowest in the cord blood, but increased during the first 2 years of life and then remained stable until 10 years of age. After 10 years of age, the B‐cell percentage declined significantly until age 15 years.
3.3. NK cells and NK T cells
The percentages of NK cells and NK T cells increased significantly with age following the first two years of life. The percentages of NK cells and NK T cells were the highest in the 10‐15 year age group.
3.4. Correlations between age and percentages of lymphocyte subsets
The percentage of CD4+ T cells was moderately and inversely correlated with age, whereas the percentages of γδ T cells, double‐negative T cells, NK cells, and NK T cells were moderately and positively correlated with age. The percentages of CD8+ T cells and CD19+ cells were weakly positively correlated with age, while percentages of other cell types (CD3+ T cells and Tregs) showed no correlation with age (Table 3).
Table 3.
Correlations between age and percentages of lymphocyte subpopulations
| Population | Spearman's correlation | Population | Spearman's correlation |
|---|---|---|---|
| % CD3 + T cells | −.00 | % γδ T cells (CD45 + CD3+Double Negative TCR γδ) | 0.67* |
| % CD4 + T cells | −.55* | % Double‐negative T cells (CD45 + CD3+Double Negative) | 0.66* |
| % CD8 + T cells | .37* | %NK cells (CD56+) | 0.56* |
| % Regulatory T cells/ CD4 + T cells | .08 | % NK T cells (CD3 + CD56+) | 0.61* |
| % Regulatory T cells (CD4 + CD25+Foxp3+) | −.13 | % CD19 + B cells | 0.22* |
Correlation was significant at the P < .01 level.
4. DISCUSSION
The results of our study showed that dynamic changes in lymphocyte subset percentages occurred in Thai children from birth to adolescence. Age‐related changes in lymphocyte subset percentages represent important information for the diagnosis of immunological disorders. When we compared our results with those of previous studies, we found that age‐specific percentages of lymphocyte subsets in different populations varied. Thus, establishment of age‐specific references for lymphocyte subset percentages in each country, or each population, may be necessary for the accurate interpretation of lymphocyte subset percentages.
The percentages of CD3+ and CD4+ T cells in our study were lower than those reported in previous studies from Europe and the United States.3, 6, 7, 8, 15, 34, 35, 36, 37 However, our results were similar to other studies conducted in Asian countries.5, 32, 33 The percentage of CD8+ T cells calculated here was in line with the results of previous studies, except that a higher percentage of CD8+ T cells was detected in a study from India.7, 8, 15, 32, 33 The percentage of CD19+ cells observed in our study was similar to that of American children, but slightly higher than that of Dutch, Indian, and Polish children older than 5 years of age.3, 6, 15, 32, 36 The percentage of NK cells observed in our study was similar to that of Indian children32 but lower than that of Chinese children.5, 37 However, the percentage of NK cells in our study was higher than that of European and American children older than 5 years of age.6, 7, 8, 15 Infections such as dengue virus were proposed to be potential causes of the increased frequencies of NK cells with advancing age, since Thailand is a dengue‐endemic area and most Thai children become infected before 5 years of age. These findings suggest that ethnicity is not the only important factor affecting percentages of lymphocyte subsets. Environmental factors, geographical variation, exposure to infectious diseases, and vaccinations may be other factors associated with these differences.1, 2, 3, 4 Although CD56 was the only surface marker used to identify NK cells in our study, both CD56+ bright and CD56+ dim cells were classified as NK cells, which included the majority of NK cells. These results have been supported by data for Thai children as reported by Mandala et al.9
In addition to the major lymphocyte subsets, γδ T cells, Tregs, and double‐negative T cells also play essential roles in immune responses. Age‐specific reference values for these cells are still scarce. In our study, the percentages of γδ T cells and double‐negative T cells increased with age and reached their highest levels around 5‐10 years of age, in line with the results from a Chinese study.33 In another previous study, the percentages of these cells were found to be highest at 2‐6 years of age in Italian children.7 Foxp3 is the major transcription factor responsible for the development and function of Tregs and has been used as a marker for these cells.38, 39 In our study, therefore, we established age‐specific reference values for Tregs defined as CD4+ CD25+ FoxP3+ cells. Due to limited data on these cells in different age groups and the use of different markers in a previous study (CD3+ CD4+ CD25+ CD127+), we did not attempt the frequency of these cells with the results of other studies.
The percentage of CD4+ T cells, an important marker for monitoring pediatric HIV infections, significantly decreased with age. Therefore, establishment of reference values for these cells in each age group is crucial for management of pediatric HIV. Our findings are supported by the results of Valiathan et al6 showing that the percentage of CD4+ T cells decreased with age until adolescence, increased during adulthood, and then remained stable in old age. Therefore, the dynamics of these cells in adult and elderly populations differ from those in children. In addition, no significant changes were observed with age in the percentage of CD3+ T cells in our study. These findings were in line with a previous study conducted in Japan.40 The percentages of CD8+ T cells and NK cells increased significantly with age, in agreement with previous studies.35, 40 However, dynamic changes in NK cell percentages varied between regions. In American children, the percentage of NK cells8 remained stable from birth to adolescence, whereas in our study, the percentage of NK cells increased with age. This conflict is also found in the literature9 in studies comparing lymphocyte subsets between American, Malawian, and Thai children. The percentage of γδ T cells also increased with age, as shown in a previous study, reaching high levels in the peripheral blood by age 10 years and then further increasing until age 25 years.40 We found moderate correlations between age and the percentage of γδ T cells.
The results of our study illustrate the dynamic changes in the frequencies of T‐cell subpopulations that are associated with age in Thai children. Our study provides preliminary data for the percentage ranges of these cells in healthy Thai children. However, there were also some limitations to our study. We did not define absolute numbers of each lymphocyte subpopulation. Nevertheless, the percentages of lymphocytes can still document age‐related changes in lymphocyte subpopulations. In addition, due to the small sample size of the study, a future study with a larger sample size is needed to support our results.
5. CONCLUSION
In conclusion, differences in the percentages of all lymphocyte subsets were associated with age and ethnicity. Exposure to infectious diseases and environmental factors may play a pivotal role in these differences. Therefore, the establishment of reference ranges for the percentages of all lymphocyte subsets is key for the development of clinical and diagnostic criteria in immunological and infectious diseases, as well as for guiding future research in each country.
AUTHOR CONTRIBUTIONS
BL participated in the study design, data collection, data analysis, and drafting of the article. SV participated in the study design, data analysis, and editing of the article. NA participated in the data analysis and editing of the article. CK participated in the data collection and analysis. SH participated in editing of the article. All authors read and approved the final article.
ACKNOWLEDGMENTS
This work was supported by the Children Cancer Fund under the Patronage of HRH Princess Soamsawali and Ramathibodi Foundation (CF_61004). The authors thank the Hematology unit, Department of Pediatrics, Faculty of Medicine Ramathibodi hospital for handling of samples. We would like to thank Umaporn Udomsubpayakul, Section for Clinical Epidemiology and Biostatistics, Faculty of Medicine Ramathibodi Hospital, Mahidol University for statistical analysis. We also thank Dr Mahippathorn Chinnapha, Jeremy C. Pennington, Senior PRS Proofreading Practitioner, London, England, and Kevin Henry, Edanz Group (http://www.edanzediting.com/ac) for copy editing and proofreading a draft of the article.
Lerkvaleekul B, Apiwattanakul N, Klinmalai C, Hongeng S, Vilaiyuk S. Age‐related changes in lymphocyte subpopulations in healthy Thai children. J Clin Lab Anal. 2020;34:e23156 10.1002/jcla.23156
REFERENCES
- 1. Chng WJ, Tan GB, Kuperan P. Establishment of adult peripheral blood lymphocyte subset reference range for an Asian population by single‐platform flow cytometry: influence of age, sex, and race and comparison with other published studies. Clin Diagn Lab Immunol. 2004;11(1):168‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsegaye A, Wolday D, Otto S, et al. Immunophenotyping of blood lymphocytes at birth, during childhood, and during adulthood in HIV‐1‐uninfected Ethiopians. Clin Immunol. 2003;109(3):338‐346. [DOI] [PubMed] [Google Scholar]
- 3. Valiathan R, Deeb K, Diamante M, Ashman M, Sachdeva N, Asthana D. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology. 2014;219(7):487‐496. [DOI] [PubMed] [Google Scholar]
- 4. Rudy BJ, Wilson CM, Durako S, Moscicki A‐B, Muenz L, Douglas SD. Peripheral blood lymphocyte subsets in adolescents: a longitudinal analysis from the REACH project. Clin Diagn Lab Immunol. 2002;9(5):959‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee BW, Yap HK, Chew FT, et al. Age‐and sex‐related changes in lymphocyte subpopulations of healthy Asian subjects: from birth to adulthood. Cytometry A. 1996;26(1):8‐15. [DOI] [PubMed] [Google Scholar]
- 6. Valiathan R, Ashman M, Asthana D. Effects of ageing on the immune system: infants to elderly. Scand J Immunol. 2016;83(4):255‐266. [DOI] [PubMed] [Google Scholar]
- 7. Tosato F, Bucciol G, Pantano G, et al. Lymphocytes subsets reference values in childhood. Cytometry A. 2015;87(1):81‐85. [DOI] [PubMed] [Google Scholar]
- 8. Shearer WT, Rosenblatt HM, Gelman RS, et al. Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS Clinical Trials Group P1009 study. J Allergy Clin Immunol. 2003;112(5):973‐980. [DOI] [PubMed] [Google Scholar]
- 9. Mandala WL, Ananworanich J, Apornpong T, et al. Control lymphocyte subsets: Can one country's values serve for another's? J Allergy Clin Immunol. 2014;134(3):759‐761.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dhaliwal J, Balasubramaniam T, Quek C, Gill H, Nasuruddin B. Reference ranges for lymphocyte subsets in a defined Malaysian population. Singapore Med J. 1995;36(3):288‐291. [PubMed] [Google Scholar]
- 11. Shahabuddin S, Al‐Ayed I, El‐Rab MG, Qureshi M. Age‐related changes in blood lymphocyte subsets of Saudi Arabian healthy children. Clin Diagn Lab Immunol. 1998;5(5):632‐635. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12. Comans‐Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhood: reference values for lymphocyte subpopulations. J Pediatr. 1997;130(3):388‐393. [DOI] [PubMed] [Google Scholar]
- 13. Heldrup J, Kalm O, Prellner K. Blood T and B lymphocyte subpopulations in healthy infants and children. Acta Paediatr. 1992;81(2):125‐132. [DOI] [PubMed] [Google Scholar]
- 14. Robinson M, O'donohoe J, Dadian G, Wankowicz A, Barltrop D, Hobbs JR. An analysis of the normal ranges of lymphocyte subpopulations in children aged 5–13 years. Eur J Pediatr. 1996;155(7):535‐539. [DOI] [PubMed] [Google Scholar]
- 15. Piatosa B, Wolska‐Kusnierz B, Siewiera K, Grzduk H, Galkowska E, Bernatowska E. Distribution of leukocyte and lymphocyte subsets in peripheral blood. Age related normal values for preliminary evaluation of the immune status in Polish children. Cent Eur J Immunol. 2010;35(3):168‐175. [Google Scholar]
- 16. Zheng J, Liu Y, Lau Y‐L, Tu W. γδ‐T cells: an unpolished sword in human anti‐infection immunity. Cell Mol Immunol. 2013;10(1):50‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chien Y‐H, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol. 2014;32:121‐155. [DOI] [PubMed] [Google Scholar]
- 18. Kadivar M, Petersson J, Svensson L, Marsal J. CD8αβ+ γδ T cells: a novel T cell subset with a potential role in inflammatory bowel disease. J Immunol. 2016;197:4584‐4592. [DOI] [PubMed] [Google Scholar]
- 19. McCarthy NE, Hedin CR, Sanders TJ, et al. Azathioprine therapy selectively ablates human Vδ2+ T cells in Crohn's disease. J Clin Invest. 2015;125(8):3215‐3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mann E, McCarthy N, Peake S, et al. Skin‐and gut‐homing molecules on human circulating γδ T cells and their dysregulation in inflammatory bowel disease. Clin Exp Immunol. 2012;170(2):122‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andreu‐Ballester JC, Amigó‐García V, Catalán‐Serra I, et al. Deficit of gammadelta T lymphocytes in the peripheral blood of patients with Crohn’s disease. Dig Dis Sci. 2011;56(9):2613‐2622. [DOI] [PubMed] [Google Scholar]
- 22. Silva‐Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol. 2015;15(11):683‐691. [DOI] [PubMed] [Google Scholar]
- 23. Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: a systematic review of clinical trials. Oncoimmunology. 2014;3(1):e27572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self‐tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531‐562. [DOI] [PubMed] [Google Scholar]
- 25. Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self‐tolerance and autoimmune disease. Immunol Rev. 2006;212(1):8‐27. [DOI] [PubMed] [Google Scholar]
- 26. Levings MK, Allan S, d'Hennezel E, Piccirillo CA. Functional dynamics of naturally occurring regulatory T cells in health and autoimmunity. Adv Immunol. 2006;92:119‐155. [DOI] [PubMed] [Google Scholar]
- 27. Zou W. Regulatory T cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295‐307. [DOI] [PubMed] [Google Scholar]
- 28. Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116(5):961‐968. [DOI] [PubMed] [Google Scholar]
- 29. Hori S, Takahashi T, Sakaguchi S. Control of autoimmunity by naturally arising regulatory CD4+ T cells. Adv Immunol. 2003;81:331‐371. [DOI] [PubMed] [Google Scholar]
- 30. Ahmadzadeh M, Rosenberg SA. IL‐2 administration increases CD4+ CD25hi Foxp3+ regulatory T cells in cancer patients. Blood. 2006;107(6):2409‐2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223(1):371‐390. [DOI] [PubMed] [Google Scholar]
- 32. Swaminathan S, Hanna LE, Raja A, Sankaran K, Arunkumar N. Age‐related changes in blood lymphocyte subsets of south Indian children. Nati Med J India. 2003;16(5):249‐252. [PubMed] [Google Scholar]
- 33. Lin SC, Chou CC, Tsai MJ, et al. Age‐related changes in blood lymphocyte subsets of Chinese children. Pediatr Allergy Immunol. 1998;9(4):215‐220. [DOI] [PubMed] [Google Scholar]
- 34. İkincioğulları A, Kendirli T, Doğu F, et al. Peripheral blood lymphocyte subsets in healthy Turkish children. Turk J Pediatr. 2004;46(2):125‐130. [PubMed] [Google Scholar]
- 35. Hannet I, Erkeller‐Yuksel F, Lydyard P, Deneys V, DeBruyère M. Developmental and maturational changes in human blood lymphocyte subpopulations. Immunol Today. 1992;13(6):215‐218. [DOI] [PubMed] [Google Scholar]
- 36. Schatorjé EJ, Gemen EF, Driessen GJ, Leuvenink J, van Hout RW, de Vries E. Paediatric reference values for the peripheral T cell compartment. Scand J Immunol. 2012;75(4):436‐444. [DOI] [PubMed] [Google Scholar]
- 37. Hulstaert F, Hannet I, Deneys V, et al. Age‐related changes in human blood lymphocyte subpopulations: II. Varying kinetics of percentage and absolute count measurements. Clin Immunol Immunopathol. 1994;70(2):152‐158. [DOI] [PubMed] [Google Scholar]
- 38. Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3‐dependent programme of regulatory T‐cell differentiation. Nature. 2007;445(7129):771‐775. [DOI] [PubMed] [Google Scholar]
- 39. Wan YY, Flavell RA. Regulatory T‐cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766‐770. [DOI] [PubMed] [Google Scholar]
- 40. Osugi Y, Hara J, Kurahashi H, et al. Age‐related changes in surface antigens on peripheral lymphocytes of healthy children. Clin Exp Immunol. 1995;100(3):543‐548. [DOI] [PMC free article] [PubMed] [Google Scholar]


