Abstract
Chronic HIV-infected children suffer from premature aging and aging-related diseases. Viral replication induces an ongoing inflammation process, with the release of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), the activation of the immune system, and the production of proinflammatory cytokines. Although combined highly active antiretroviral therapy (ART) has significantly modified the natural course of HIV infection, normalization of T and B cell phenotype is not completely achievable; thus, many HIV-infected children display several phenotypical alterations, including higher percentages of activated cells, that favor an accelerated telomere attrition, and higher percentages of exhausted and senescent cells. All these features ultimately lead to the clinical manifestations related to premature aging and comorbidities typically observed in older general population, including non-AIDS-related malignancies. Therefore, even under effective treatment, the premature aging process of HIV-infected children negatively impacts their quality and length of life. This review examines the available data on the impact of HIV and ART on immune and biological senescence of HIV-infected children.
1. Introduction
The natural history of HIV infection has greatly changed over the course of the last 20 years due the great improvement of the combined highly active antiretroviral therapy (ART). Compared to the past, life expectancy of HIV-infected individuals on ART has drastically increased; however, ART does not eradicate the infection; therefore, HIV will persist in infected individuals, becoming a chronic disease [1]. Although some studies suggested that, under optimal treatment, life expectancy could be similar to that of the uninfected population [2–4], other studies evidenced that this goal has not been achieved yet, and life expectancy in Western countries can be shortened of up to 10 years [5–8]. AIDS-related complications, among which opportunistic infections and AIDS-defining malignancies, are reduced compared to the past; however, HIV-infected individuals on ART still have a higher risk of non-AIDS-related morbidity and mortality, due to an increased incidence of a wide range of illnesses associated with aging [9, 10].
Aging is a natural process that involves the loss of physiological integrity with a generalized organ decline that ultimately leads to death [11]; an aging system faces a decreasing ability to deal with stress and increasing frailty [12–15], inflammation [12], and age-related comorbidities, including cardiovascular disease, neuropathy, anemia, osteoporosis, and liver and kidney disease [11, 16]. The persistence of HIV, causing chronic immune activation, is likely a key determinant of the premature senescent pathway. Indeed, viral persistence induces activation of immune system cells, which undergo continuous expansion as a response to the antigen, eventually reaching the senescent stage, when they lose their functions [17]. A direct consequence of cellular replication is the shortening of their telomeres, until they reach a critical length under which the replicative capacity of the cell is lost [18–20], fueling the cells' premature senescence and the development of those age-related diseases that are involved the loss of the regenerative capacity of different tissues [21]. It is nowadays well established that there is a link between telomere shortening, cellular senescence, and aging [22]. In addition, HIV itself can impair the activity of telomerase (a ribonucleoprotein enzyme complex that synthesizes the telomeric repeats TTAGGG [23, 24]) specifically in CD4 cells [25]. The importance of this adverse effect resides on the fact that although telomerase is usually not expressed in somatic cells, it can be transiently upregulated in lymphocytes upon cell activation [26, 27]; the impairment of this upregulation can therefore increase the apoptotic propensity of hematologic cells and lead to immune system dysfunction.
Currently about 38 million people are living with HIV; 2 million of them are children under 15 years of age. Although new HIV infections among children are steadily decreasing, still, 160000 new infections occurred in 2018, the vast majority of them being mother-to-child transmission (MTCT) in African countries [28]. The clinical complications of HIV infection in children are more serious than those in adults [29–32]. Indeed, they experience a poorer control of the disease, which progresses to AIDS faster [29, 33], and the acute stage of the infection is characterized by higher levels of viremia, which is controlled slower and less effectively than in adults [33–35]. Several studies conducted in children [36–39] suggest that possible causes of the differences mentioned above include the very early exposure to HIV and pathogens when the infants' immune system is still under development, an interaction that could also influence the evolution of their incomplete immune system.
Thanks to the continuously increasing coverage of ART-based prophylaxis and treatment, in 2018, MTCT incidence was under 2%, and about 50% of HIV-infected children were receiving ART [28]. Therefore, an increasing number of children start ART at a very young age and will be receiving antiretroviral drugs for all their lifetime. ART greatly improved their survival and the quality of life [40, 41], but on the other hand, they now face the consequences of a lifelong chronic condition, suffering from pathogenic mechanisms typical of premature aging [42–46]; i.e., they show an increased risk of age-associated comorbidities, identified as non-AIDS-related diseases [47–49], compared to healthy individuals [50–52]. It has been suggested that antiretroviral drugs themselves can impact on accelerated aging, mainly due to the inhibitory effect of nucleoside reverse transcriptase inhibitors (NRTIs) on telomerase [53, 54]; however, more recent studies argued that the effect of prophylaxis and therapy is negligible compared to that of the HIV infection itself [55, 56].
In this review, we examine the available data on how HIV and/or ART impact on immune and biological senescence of HIV-infected children. The impact of HIV with and without ART is schematized in Figure 1.
Figure 1.
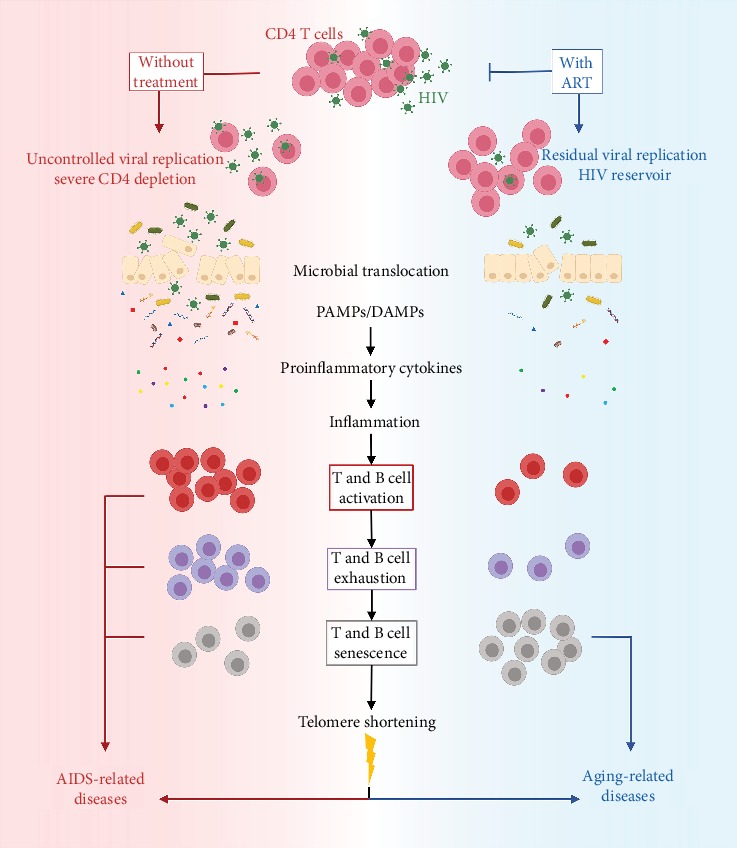
Schematic representation of the impact of HIV without (left side, red) and with (right side, blue) ART. HIV infects primarily CD4 cells, and without ART, there is a severe CD4 cell depletion. Microbial translocation from the damaged mucosa, releasing PAMPs (bacterial LPS, 16S rDNA, and CpG DNA) and DAMPs (mtDNA, HMGB1 protein, and defensins), stimulates the production of proinflammatory cytokines (IL-1, IL-6, IL-10, INF-α, and TNF-α) that promote the activation/inflammatory status, a critical hallmark of HIV infection. Immunodeficiency leads to AIDS-related diseases, including AIDS-defining malignancies. With ART, a small fraction of the virus escapes control and establishes the residual reservoir, which promotes a state of chronic low-grade inflammation/activation, where T and B cell phenotype is altered, with increased expression of senescence markers. Accelerated telomere shortening promotes premature aging and may induce genetic instability. This scenario leads to the development of aging-related illnesses, including non-AIDS-defining malignancies.
2. Clinical Conditions Related to Premature Aging of HIV-Infected Children
Despite the significant improvements due to ART introduction, the life span of HIV-infected children is not yet comparable to that of uninfected ones: their premature aging exposes them to a higher risk of acquiring and developing age-related chronic diseases. The continued release of virions by residual replicating virus (that persists at low levels even in the presence of effective ART) promotes a chronic inflammatory status, in which the release of proinflammatory cytokines favors premature cellular aging and the pathophysiological scenario typically observed in elderly persons. This includes renal and cardiovascular diseases, metabolic and endocrine alterations, cerebrovascular diseases, and malignancies [42–46, 57–59]. Here, we focus on malignancies diagnosed in HIV-infected children.
As happens for HIV-infected adults [60], HIV-infected children show a higher frequency of malignancies compared to the general population [45, 61–65]. In the pre-ART era, the risk of malignancy occurrence was mainly linked to the immunological dysfunction per se and lack of adaptive immune response against oncogenic viruses. Kaposi sarcoma (KS) and non-Hodgkin lymphoma (NHL), the two AIDS-defining malignancies (ADM) most frequent in children [61], are indeed associated with Human gammaherpesvirus 8 (HHV8) and Epstein-Barr Virus (EBV), respectively [45, 66–68]. ART introduction led to a change in the nature of HIV-related malignancies, with a reduced incidence of ADM (e.g., KS -87% and NHL -60% [69]) and increased incidence of non-ADM (including Hodgkin's disease, anal cancer, oral squamous carcinoma, hepatocarcinoma, leiomyosarcoma, and Merkel cell carcinoma [45]), especially among immunocompromised HIV-infected children who have received ART for a reduced period of time [61, 70]. The decrease in the incidence of ADM may be attributable to recovery of CD4 cells, partial restoration of immune functions, and lower immune activation, induced by effective ART. As an example, Petrara et al. [71] suggested that limiting HIV replication and related microbial translocation and immune activation may prevent superinfection with EBV or lower EBV viremia, thus reducing the risk of EBV-associated NHL. ART, however, can only partially revert the increased expression of all factors leading to chronic immune activation [72], due to the persistence of residual viremia. Chronic immune activation, with increased cell turnover and premature immune senescence, is indeed associated with the increased risk of non-ADM malignancies in HIV-infected children [17, 67, 73]. The genetic instability conferred by accelerated telomere erosion and the hampered immune surveillance may promote cancer development [74]. Moreover, senescent cells furtherly fuel tumor growth by secretion of inflammatory cytokines, growth factors, and proteases [75, 76], establishing a tumorigenic microenvironment.
3. Premature Immune Senescence
The main target of HIV virus is immune cells, in particular CD4 lymphocytes, monocytes, and macrophages [77]. HIV infection leads indeed to a severe depletion of CD4 cells and a progressive loss of function of the innate and adaptive immune system [78]. Despite ART effectiveness, residual HIV infection still has consequences on the immune phenotype of the host: HIV-driven immune senescence is indeed one of the leading contributors to the premature aging displayed by HIV patients [79, 80]. A senescent immune system, characterized by the accumulation of functionally impaired differentiated immune cells, compromises the immune response [81], hampering the ability to react to novel antigen challenges and contributing to frailty [82].
One of the main peculiar features of the children immune system, compared to that of the adults, is their much higher thymic output [23] that constitutes an advantage over adults in the context of HIV infection. On the other hand, the immune system of children exposed to HIV has not yet fully developed when they meet the virus, therefore, leaving them unable to mount an efficient immune response; therefore, the consequences of HIV in this case are more complex and the disease has a faster progression [29]. Moreover, children are exposed to the virus from an early age, in most cases from birth: despite the benefits of an effective therapy, the lifelong exposure to the virus and to the drugs promotes a chronic activation of the immune system, contributing to its premature aging. The main alterations on the immune phenotype of HIV-infected children are described in detail below.
3.1. T Cell Compartment
Many alterations in the CD4 and CD8 cell subsets have been reported for all HIV-infected individuals and in particular for children and infants [83–86]. The main alteration in the immune phenotype is the inversion of the CD4/CD8 ratio, which is considered a hallmark of disease progression: whereas a normal CD4/CD8 ratio is above 1, untreated individuals with HIV undergo CD4 depletion, which results in a CD4/CD8 ratio below 1. Several children and adults on ART, despite reaching virological suppression, may not recover to a normal CD4/CD8 ratio, an effect attributable both to incomplete recovery of CD4 and to the increase of CD8 cells due to persistent immune activation. About 66% of HIV-infected children, however, succeed to recover to a normal CD4/CD8 ratio [87], a percentage higher than the one observed in adults [88]; the 33% of children who do not recover are usually older and/or have started ART later. This difference in the ability to restore a normal CD4/CD8 ratio has been partially explained with the increased expansion of T regulatory (Treg) cells of children compared to adults and by the better proliferative capacity of their HIV-specific T cells [89]. Conversely, a study [90] showed that defective recovery of the CD4/CD8 ratio is instead associated to increased levels of activated, senescent and effector memory T cells, with decreased naive T cells. Overall, the whole CD4 and CD8 T cell populations are affected by HIV, even in individuals on effective ART [59].
Naïve T cells undergo a drastic reduction due to thymus involution [91] and to frequent stimulation and expansion of preexisting populations of antigen-specific T cells in the struggle to regenerate the T cell pool [81]. Several studies [84, 92–94] pointed out, indeed, a loss of naïve CD4 and CD8 (CD45RA+CCR7+) cells both in adults and in children; at the same time, a decrease of central memory in favor to the effector memory (CD45RA-CCR7-), with the expansion of CD27-, marker of effector type T cells [95], was detected. Another study [36] compared HIV-infected, HIV-exposed uninfected (HEU), and HIV-unexposed uninfected (HUU) infants, showing that, over the first year of life, CD8 naïve, memory, effector, terminally differentiated, and senescent T cells were significantly altered in HIV-infected infants compared to the other two groups; in particular, CD8 naïve cells were significantly lower, while CD8 effector memory, terminally differentiated (CD45RA+CCR7-), and senescent (CD28-CD57+) cells were significantly higher. A study on 57 perinatally HIV-infected adolescents [39] showed increased levels of senescence and proliferation (Ki67+) markers in the memory CD4 cell subset, compared to healthy subjects; their effector memory cells were also positive for activation marker HLA-DR. A recent study [56] compared 71 HIV-infected children below 5 years of age to HEU and HUU cohorts, observing an accelerated senescence of both their CD4 and CD8 cell compartments, with significantly higher percentages of activated (CD38+HLA-DR+) and exhausted (programmed cell death, PD-1+) CD4 cells and of activated, senescent, and exhausted CD8 cells; interestingly, the exposure to ART prophylaxis of HEU children did not negatively affect their immune phenotype.
Other studies confirmed that HIV-infected children display higher percentages of exhausted T cells, which often fail to recover despite treatment [96, 97]; PD-1 expression, the principal marker of HIV-related cell exhaustion, has indeed been proposed as a disease progression marker [97]. Recently, additional immune checkpoint inhibitors (ICIs), including CTLA-4, TIM3, LAG3, TIGIT, 2B4, and CD160, were identified; they were found to coexpress especially in viremic progressors, furtherly inhibiting T cell function [98]. In spite of the promising potential of ICIs as progression markers, exploring their expression in HIV-infected children is still an open field of research.
Treg (CD4+CD25+CD127-FoxP3+) cells limit excessive or inappropriate immune activation against antigens, and in particular, they prevent responsiveness to self-antigens [99–101]. In the HIV-infection context, different studies highlight two opposite effects of Tregs; they are suggested not only to decrease excessive immune activation [102–104] but, on the other hand, also to suppress HIV-specific immune response [105–108]. A study on 6-14-year-old HIV-infected children [109] showed that viremia significantly correlates with the percentage of Tregs; higher percentage of Tregs is also associated with higher immune activation and higher HIV-DNA levels, suggesting that the regulatory function of this subset does not suffice to limit immune activation.
T follicular helper (Tfh) cells are specialized CD4 cell subset whose signaling function allows the generation of long-lived B cells during the immune response. Indeed, they are considered a biomarker of vaccine response. The equilibrium between their different subsets (i.e., Th1, Th2, and Th17) and their function is perturbed during HIV infection, even under virological control, resulting in an impaired response to vaccination. In particular, in HIV-infected children, a negative response to vaccination has been associated with Tfh cells coexpressing multiple activation markers [110, 111].
Increased immune senescent phenotype in HIV-infected children has been pointed out by many studies [38, 112, 113]. Moreover, the persistent immune activation and exhaustion, together with alterations of memory T cells, may also have an impact on the efficacy of childhood vaccination and have been linked to poor response to vaccines and higher risk to acquire vaccine-preventable diseases [36, 111–117].
3.2. B Cell Compartment
The B cell compartment is impacted by similar alterations as those affecting T cells: indeed, several studies [114, 118, 119] demonstrated that HIV-infected children, even with undetectable viral load, show B cell alterations typical of older healthy controls, such as an increased number of mature-activated (CD10- CD21-) and senescent double-negative (IgD- CD27-) B cells. Other B cell alterations linked with chronic HIV persistence include increased percentages of immature transitional (CD10+/++ CD21low/high CD27-), activated memory (CD10- CD21low CD27+), and exhausted memory (CD10- CD21low CD27-) B cells and decreased percentages of resting memory (CD10- CD21high CD27+) B cell subset [120, 121]. A study [122] on ART-naïve children below 2 years of age revealed many alterations of their B cell phenotype compared to the uninfected control group: they had significant depletion of naïve (IgD+ CD27-), nonswitched memory (NSM, IgD+ CD27+), naïve mature (CD21high CD27-), and activated (CD25+) B cells and significant expansion of double negative, activated memory (CD21low CD27+), tissue-like memory (TLM, CD21low CD27-), and apoptosis-prone (CD95+) B cells. ART-naïve children suffered a progressive deterioration over 1-year follow-up, with further depletion of naïve and NSM cells and expansion of double-negative B cell subset. On the other hand, one year of ART could only partially restore these alterations: there was an increase in the naïve, NSM, and naïve mature cell subsets and a decrease in the double-negative, activated memory, and TLM subsets; however, no improvement was found in the resting memory, activated, and apoptosis-prone B cell subsets that remained significantly altered. Thus, as for T cell compartment, ART does not fully restore B cell functionality. Notably, despite ART, homing of B cells to germinal center is defected with the consequent impaired vaccine responses in HIV-infected children [114, 123, 124]. In addition, the expression of B cell genes, including those involved in the inflammation and aging, that could predict the response to vaccination [125, 126], remains perturbed even after a stable and long virological control.
4. Chronic Immune Activation and Persistent Inflammation
Persistent inflammation and chronic immune activation are leading causes of the senescent pathway that favors the risk of non-AIDS morbidity and mortality in HIV-infected children. T cell activation, marked by CD38 and HLA-DR coexpression on CD8 T cells, is a prognostic indicator for disease progression at different stages of HIV infection [127]. Moreover, HIV infection drives the microbial translocation [128]: the massive depletion of CD4 cells associated with HIV infection induces an impairment of mucosal surface integrity in the gut and leads to the release of pathogen-associated molecular patterns (PAMPs, such as bacterial lipopolysaccharide, 16S ribosomal DNA, and CpG DNA [55]) and damage-associated molecular patterns (DAMPs, such as mitochondrial DNA, high-mobility group box 1 protein, and defensins [129, 130]) into the circulation. PAMPs and DAMPs activate the immune system by binding to the extra- or intracellular domain of Toll-like receptors (TLRs), which are involved in the host inflammatory response, initiating a complex-signal transduction cascade which, via the NF-κB pathway [131], ultimately leads to increased transcription of proinflammatory cytokines (such as IL-6, IL-10, and interferon-α) that may play a role in establishing a protumorigenic inflammatory environment [66].
Several studies [132–134] have suggested that, despite viral suppression, children with perinatally acquired HIV have higher levels of inflammation, immune activation, and alterations in intestinal permeability, compared to HEU and HUU children. Notably, immune activation is higher in viremic than aviremic children, but microbial translocation may occur regardless of viremia and T cell activation. While ART in HIV-infected subjects generally allows for immune reconstitution in peripheral blood, reconstitution of the gastrointestinal tract occurs at a much slower pace, and both immunological and structural abnormalities persist in the gastrointestinal tract, thus explaining the residual inflammation and heightened morbidities in HIV-infected ART recipients [135]. In a cohort of HIV-infected children [136], ART initiation rapidly and persistently reversed T cell activation but failed to normalize CD4/CD8 ratios and plasma sCD14 levels. However, another study on a cohort of perinatally HIV-infected children [137] showed that ART initiation normalized sCD163 (marker of monocyte activation) levels and improved long-term pediatric outcomes. A recent study [138] agreed that immune activation decreases over time in children after starting ART, which does not have adverse effects itself on microbial translocation.
To support the concept that persistent immune activation and cellular exhaustion are closely linked to accelerated biological aging and immune senescence, Gianesin et al. [56] found that HIV-infected children accumulate activated and exhausted CD8 T cells together with a higher percentage of senescent CD8 T cells, which are all inversely correlated with telomere length. The immune exhaustion is also increased in HIV-infected individuals despite viral suppression [139]. Indeed, PD-1 is the eligible marker of immune exhaustion of T cells, and its increased expression levels predict the rate of HIV disease progression in adults [140, 141]. HIV-infected children have increased PD-1 expression on CD8 T cells that correlates with immune activation [142, 143]. It was recently demonstrated that CD4 cells expressing PD-1 constitute an important source of persistent viral replication in ART-treated individuals, and the contribution of PD-1+ CD4 cells to the persistent reservoir progressively decreased with increased length of ART [144].
Immune activation also results in chronic stimulation and expansion of B cells. ART allows, at least partially, the normalization of activated B cell subsets and age-dependent accumulation of resting memory B cells [145]. However, as for T cell, ART does not eliminate B cell activation. In HIV-infected adults, immune activation persists over time and is susceptible to therapy; indeed, compared to classical combined ART, a monotherapy with protease inhibitors has a lower control on DAMP levels and B cell hyperactivation, so it may have lower control on EBV reactivation and/or polyclonal expansion of EBV-infected B cells and, thus, on the onset of EBV-related malignancies [146]. Few data are available about B cell activation in HIV-infected children. A study [71] demonstrated that children on ART have significant lower levels of microbial translocation and EBV levels than ART-naïve children. Recently, a study [147] showed that pre-ART progressors had higher percentages of mature activated and TLM cells and higher plasma levels of IL-4, IL-6, IL-10, and IgA compared to seronegative controls. After ART initiation, levels of proinflammatory cytokines IL-4, IL10, and IgG significantly lowered.
Overall, all these reports agree that, despite ART, microbial translocation persists and leads to a chronic low-grade inflammation. In HIV-infected children, the monitoring of persistent inflammation/immune activation and immune exhaustion will be of clinical importance to estimate the rate of premature aging and its associated production of inflammatory cytokines, as pivotal factors acting in the pathogenesis of premature aging and malignancies.
5. Premature Biological Aging
Telomeres are involved in cellular aging and immune senescence mechanisms. Telomeres are long tandem repeated DNA sequences (TTAGGG) at the end of chromosomes that are essential for protection of chromosome integrity, preventing end-to-end fusion and DNA degradation [148]. The ribonucleoprotein complex telomerase has the function of maintaining telomeres by synthesizing new telomeric repeats; its activity is usually not detected in somatic cells due to the downregulation of its catalytic protein TERT, which is instead expressed during embryogenesis, in rapidly dividing tissues and in the vast majority of tumors [148]. During each cell division, DNA polymerase is unable to copy the end of chromosomes (the end-replication problem); thus, some of the telomere repeats are lost. After several cell divisions, telomere length reaches a critical threshold, below which cells stop dividing and physiologically undergo senescence or trigger genomic instability, that may promote age-associated diseases and tumor development [149].
Telomeres get naturally shorter with age [150]; telomere length is therefore a valid biomarker of aging in the general population, and accelerated telomere shortening leads to premature aging, which is correlated with several pathologies [151, 152]. The causes leading to accelerated telomere shortening can be, however, diverse. Indeed, telomere length and their shortening rate are not only associated with genetic factors, gender, and ethnicity, but they are also influenced by different behavioral and environmental factors, such as stress, physical activity, dietary habits, smoke, and alcohol consumption.
5.1. Telomere Implications in Diseases
Telomere length is associated with age-related diseases and decreased life span. Several studies linked shorter telomeres and telomere attrition with increased risk and increased severity of cardiovascular diseases, stroke, heart attack, and mortality [153–159]. As an example, a study on elderly patients [153] showed that, among the 143 studied patients, the 71 with shortest telomeres had a 3- and 8-fold higher mortality rate due to heart and infectious diseases, respectively. Premature aging disorders, among which progeria, Nijmegen breakage, Cockayne and Down syndromes, and dyskeratosis congenita are associated with shorter telomeres; instead, others like Werner and Boom syndromes and Ataxia telangiectasia are associated with an accelerated telomere shortening [160]. In contrast to the clear association of the aforementioned aging conditions with telomere length or erosion, similar studies on other conditions raised conflicting results: it is not yet fully cleared whether type II diabetes [161, 162] and Alzheimer's [163–167] and Parkinson's [168, 169] diseases are associated with telomere shortening or if having shorter telomeres is a risk factor for such conditions. Moreover, telomere dynamics is intrinsically related with the tumorigenesis mechanism. Indeed, shortening of telomeres below a critical level triggers the pathways that lead to cell senescence, when genomic instability is increased [170]. Should the apoptotic mechanism fail, cells may acquire immortality (mainly through the upregulation of telomerase) and, thus, tumorigenesis mechanisms may begin. Telomeres have therefore the potential to be both beneficial and detrimental factors, whether they are recognized in the signaling pathway resulting in cell apoptosis or not. In agreement with this dual role, some types of cancers have been associated with shortened telomeres while others with elongated telomeres [171].
Telomeres are not only involved in diseases affecting the elderly population, but they also have a role in conditions affecting children and adolescents. A recent study [172] on 62 children and adolescents diagnosed with AATD (α1-antitrypsin deficiency) and with intermediate to high risk for developing lung or liver damage showed that they had significantly shorter telomeres and increased oxidative stress than controls; high-risk patients showed not only shorter telomeres but also lower TERT expression and decreased telomerase activity than the other groups. Another study [173] on 44 patients, among which 26 children, with inherited telomere biology disorders (such as dyskeratosis congenita, Hoyeraal-Hreidarsson, and Revesz syndrome) showed that 57% of them had at least one structural brain abnormality or variant; they also had psychiatric diagnoses and other diseases more frequently than the general population. Another confirmation comes from a study [174] conducted on 47 young adults (17-24 years old) diagnosed with the premature aging syndrome of Prader-Willi (PWS). They displayed significantly shorter telomere length compared to age-matched healthy controls; they also showed a mild association with lower IQ. Childhood cancers sometimes need to be addressed differently from adult ones; one of the reasons resides in the differences in the mutational landscapes and the prevalence of telomere maintenance mechanisms [175]. Environmental and behavioral factors might also impact on telomere length and erosion in newborns, children, and adolescents. For example, a study on 762 mother-newborn pairs in China [176] demonstrated that prenatal exposure to some phthalate metabolites was associated with shorter cord blood telomere length; this study was also an evidence that intrauterine environment has the potential to impact newborns' telomere length. Different studies on European cohorts [177, 178] showed that higher child adiposity indicators are associated with short telomeres in children; overweight and obesity in childhood and adolescence are associated with shorter telomeres; therefore, an increased BMI early in life may be associated with accelerated biological aging and may have an adverse impact on future health during adulthood.
To summarize, telomere length has the potential to have a valid diagnostic significance in specific settings. A recent work by Alder et al. [179] measured telomeres of 100 individuals with known pathogenic mutations in telomerase and other telomere maintenance genes, compared with those of 636 healthy individuals of all ages. All of the 100 patients had age-adjusted telomere length below the 50th percentile: this indicated a 100% negative predictive value for identifying a clinically relevant mutation in telomerase/telomere maintenance genes. Moreover, a significant correlation was found between faster telomere attrition and earlier onset of idiopathic bone marrow failure; 25% of the idiopathic bone marrow failure patients had their treatment regimen choice modified based on their telomere length measurement, resulting in an improvement of their clinical outcomes.
5.2. Telomere Shortening in HIV-Infected Children and Impact of ART
There is nowadays evidence that HIV-infected individuals have overall shorter telomeres than uninfected controls [180–183], implicating that HIV directly influences telomere attrition, which occurs early after infection [184]. It has also been suggested that antiretroviral drugs themselves can impact on accelerated aging. Indeed, HIV reverse transcriptase shares homology with TERT [148, 185, 186]; therefore, nucleoside reverse transcriptase inhibitors (NRTIs), such as zidovudine (ZDV) or abacavir, might also inhibit TERT. In vitro studies showed that NRTIs inhibit telomerase causing an accelerated erosion of telomeres [53, 54, 187, 188]; recent studies argued that the effect of prophylaxis and therapy is negligible compared to that of the infection itself [56]. However, two studies published in 2018 still reported apparently conflicting results on the impact of different NRTI regimens on patients' telomere length and their change over time [189, 190]. The main findings of the papers presented in this paragraph are also summarized in Table 1.
Table 1.
Summary of recent findings on telomeres on HIV-infected children, adolescents, and young adults.
| Patients | Median age [IQR] (y.o.) | Telomere length median [IQR] | Method of telomere measure | Main findings | Authors and reference |
|---|---|---|---|---|---|
| 94 pHIV | 13.3 [9.9-15.8] | n.d. | Relative TL by rtPCR | Telomere attrition is similar for pHIV, HEU, and HUU. Older age and male gender are correlated with shorter TL. Detectable viremia and absence of ART are correlated with shorter TL. | Cote et al. [37] |
| 177 HEU | 1.7 [0.6-4.0] | n.d. | |||
| 104 HUU | 10.6 [5.3-14.2] | n.d. | |||
| 71 pHIV | 3.11 [1.40-4.48] | 2.21 [1.94-2.58] | Relative TL by rtPCR | pHIV have significantly shorter TL than HEU and HUU. ART-naïve pHIV have shorter TL than pHIV on ART. Percentages of senescent, activated, and exhausted CD8 cells are higher in pHIV than in HEU and HUU. | Gianesin et al. [56] |
| 65 HEU | 1.74 [0.99-3.31] | 2.63 [2.25-3.21] | |||
| 56 HUU | 1.85 [0.84-3.46] | 2.88 [2.49-3.10] | |||
| 324 HEU | ≥1 samples 0-3 y.o. | n.d. | Relative TL by rtPCR | TL is similar between HEU and HUU. HIV and cART exposure in utero does not appear to alter telomere dynamics during early life. | Ajaykumar et al. [191] |
| 306 HUU | 1 sample 0-3 y.o. | n.d. | |||
| 120 pHIV | 6.4 ± 1.4 | 4.14 ± 0.85 | Absolute TL by rtPCR | There was no evidence of accelerated biological aging by mDNA levels. Absolute telomere length was shorter in pHIV and HEU compared to HUU but did not differ between pHIV and HEU. | Shiau et al. [193] |
| 33 HEU | 6.1 ± 1.5 | 4.05 ± 0.74 | |||
| 25 HUU | 6.9 ± 1.1 | 4.53 ± 0.79 | |||
| 94 HEU ZDV+ | 1.0 [0.0-7.0] days | 0.85 ± 0.23 | Relative TL by rtPCR | TL of ZDV+HEU infants is longer compared to that of ZDV-HEU. | Wang et al. [192] |
| 85 HEU ZDV- | 1.0 [0.0-6.9] days | 0.65 ± 0.19 | |||
| 21 pHIV | 27 [24-29] | 1.0 [0.8-1.2]∗ | Relative TL by rtPCR | TL and telomere shortening rate of pHIV and npHIV is significantly lower than that of HUU. pHIV and npHIV maintain a normal thymic output, with a continuous shift of the naïve pool into memory subsets. This phenomenon may allow to control viral infection and maintain peripheral homeostasis. | Paghera et al. [194] |
| 19 npHIV | 27 [24-29] | 0.9 [0.7-1.2]∗ | |||
| 40 HUU | 28 [24-31] | 1.5 [1.3-1.9]∗ |
pHIV: perinatally HIV-infected children; npHIV: nonperinatally HIV-infected children; HEU: HIV-exposed uninfected children; HUU: HIV-unexposed uninfected children; HUU: HIV unexposed uninfected; ZDV: zidovudine; TL: telomere length; n.d.: values not reported in the original papers; ∗values estimated from Figure 2A of Paghera et al. [194].
A study [37] on 94 0-19-year-old HIV-infected children found no significant differences in their relative telomere length compared to that of exposed (HEU) and unexposed (HUU) uninfected controls. However, in the HIV-positive group, higher viral load was associated with shortening of telomeres. To investigate the impact of NRTIs on children's telomeres, a study was conducted on 114 HEU infants exposed to ZDV prophylaxis [191]; their telomeres at birth were similar to those of HUU controls, and no association was found between telomere length and maternal ART regimen. Among the 114 HEU children, those exposed to maternal ZDV+lamivudine+nelfinavir/nevirapine regimen had longer telomeres at birth. Moreover, telomere attrition in HEU children was more rapid in their first year of life compared to that in HUU children, but then it normalized. In agreement with these findings, a recent study [192] on 94 HEU ZDV-exposed and 85 HEU ZDV-unexposed newborns revealed that telomere length of the ZDV-exposed infants was longer compared to that of ZDV-unexposed ones. This study also found a correlation between high maternal plasma viremia levels and shorter infants' telomeres. In partial conflict with these evidences, a recent study [193] on 120 HIV-infected children below 6 years of age who started ART before 2 years of age found that telomeres of HUU children were significantly longer than those of HIV-infected and HEU children; instead, HIV-infected and HEU children had similar telomere lengths. In addition, this study did not find any relationship between telomere length and markers of inflammation (IL-6, TNF-α, high-sensitivity CRP, and sCD14). Only one study [56] analyzed both the immune senescence profile and telomere length in 0-5-year-old HIV-infected, HEU, and HUU children. In this study, telomeres were significantly shorter in HIV-infected children compared to HEU and HUU ones; moreover, among the HIV-infected children, telomeres were shorter in ART-naïve than in ART-treated children. HIV-infected children also displayed significantly higher percentages of senescent (CD28- CD57+), activated (CD38+ HLADR+), and exhausted (PD1+) CD8 T cells, while these percentages were comparable between HEU and HUU children. The inverse correlation found between activated, exhausted, and senescent CD8 cells and telomere length corroborated the idea that persistent immune activation is closely linked to accelerated biological aging and immune senescence. Given the need of maintaining children with HIV on ART for their entire life span, it is of interest to investigate the long-term consequences of perinatally acquired HIV. A 2019 study [194] investigated telomere length and erosion, together with thymic and bone marrow output, of young adults (median age 27 years old) who acquired HIV perinatally compared to age-matched individuals infected later in life. Both groups showed a normal thymic output and normal CD4 count; however, both groups had shorter telomeres and a faster telomere erosion compared to uninfected age-matched controls. This apparent discrepancy has been explained proposing that the attempt to control the infection continuously recruits naïve cells, which shift to the memory phenotype. Moreover, the positive correlation that was found between CD4 count and telomere length in both HIV-infected groups furtherly supports the concept that CD4 cells are newly recruited cells which underwent fewer cell divisions.
The collection of these data supports the idea that HIV infection itself has the major detrimental impact on cellular aging, and ART benefits strongly outweigh the negative effects on telomeres.
6. Conclusions
ART has changed the natural history of HIV, which is now considerably a chronic disease.
Despite effectiveness of treatments, HIV-infected children still do not have the same life expectancy and quality of life compared to the general population. Perinatally infected children acquire the virus early, when their immune system has not yet reached full development and they are unable to mount efficient immune response, so the disease has a faster progression. A persistent state of inflammation and activation of the immune system contributes to establishing a premature aging profile. This cannot be fully reverted by ART, representing one of the main causes of comorbidities/malignancies in treated HIV-infected children. Accumulating evidence demonstrated that the beneficial effects of ART greatly outweigh the potential side effects of NRTI use; indeed, it is mainly HIV that induces telomere attrition and premature aging. In this setting, the monitoring of markers of inflammation/immune activation and premature aging is of great clinical relevance. Early-treated children with reduced inflammatory and senescent status could reveal optimal candidates for future treatments and vaccine trials.
Acknowledgments
This work was supported by Paediatric European Network for treatment of AIDS (PENTA) Foundation. AD was supported by PENTA, within the EPIICAL project. MRP was supported by University of Padova, BIRD (181981/18), and Associazione Italiana Ricerca sul Cancro (AIRC) (IG-19112).
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Deeks S. G., Lewin S. R., Havlir D. V. The end of AIDS: HIV infection as a chronic disease. The Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagawa F., May M., Phillips A. Life expectancy living with HIV. Current Opinion in Infectious Diseases. 2013;26(1):17–25. doi: 10.1097/QCO.0b013e32835ba6b1. [DOI] [PubMed] [Google Scholar]
- 3.Johnson L. F., Mossong J., Dorrington R. E., et al. Life Expectancies of South African Adults Starting Antiretroviral Treatment: Collaborative Analysis of Cohort Studies. PLoS Medicine. 2013;10(4, article e1001418) doi: 10.1371/journal.pmed.1001418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.May M. T., Gompels M., Delpech V., et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28(8):1193–1202. doi: 10.1097/QAD.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wing E. J. HIV and aging. International Journal of Infectious Diseases. 2016;53:61–68. doi: 10.1016/j.ijid.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 6.IeDEA Pediatric Working Group. Taking a critical look at the UNAIDS global estimates on paediatric and adolescent HIV survival and death. Journal of the International AIDS Society. 2017;20(1, article 21952) doi: 10.7448/IAS.20.1.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cockerham L., Scherzer R., Zolopa A., et al. Association of HIV Infection, Demographic and Cardiovascular Risk Factors With All-Cause Mortality in the Recent HAART Era. Journal of Acquired Immune Deficiency Syndromes. 2010;53(1):102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The Antiretroviral Therapy Cohort Collaboration. Mortality of HIV-infected patients starting potent antiretroviral therapy: comparison with the general population in nine industrialized countries. International Journal of Epidemiology. 2009;38(6):1624–1633. doi: 10.1093/ije/dyp306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rickabaugh T. M., Kilpatrick R. D., Hultin L. E., et al. The Dual Impact of HIV-1 Infection and Aging on Naïve CD4+ T-Cells: Additive and Distinct Patterns of Impairment. PLoS One. 2011;6(1, article e16459) doi: 10.1371/journal.pone.0016459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Effros R. B., Fletcher C. V., Gebo K., et al. Aging and Infectious Diseases: Workshop on HIV Infection and Aging: What Is Known and Future Research Directions. Clinical Infectious Diseases. 2008;47(4):542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy B. K., Berger S. L., Brunet A., et al. Geroscience: Linking Aging to Chronic Disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erlandson K. M., Allshouse A. A., Jankowski C. M., et al. Association of Functional Impairment with Inflammation and Immune Activation in HIV Type 1–Infected Adults Receiving Effective Antiretroviral Therapy. The Journal of Infectious Diseases. 2013;208(2):249–259. doi: 10.1093/infdis/jit147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piggott D. A., Muzaale A. D., Mehta S. H., et al. Frailty, HIV Infection, and Mortality in an Aging Cohort of Injection Drug Users. PLoS One. 2013;8(1, article e54910) doi: 10.1371/journal.pone.0054910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah K., Hilton T. N., Myers L., Pinto J. F., Luque A. E., Hall W. J. A New Frailty Syndrome: Central Obesity and Frailty in Older Adults with the Human Immunodeficiency Virus. Journal of the American Geriatrics Society. 2012;60(3):545–549. doi: 10.1111/j.1532-5415.2011.03819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desquilbet L., Jacobson L. P., Fried L. P., et al. HIV-1 Infection Is Associated With an Earlier Occurrence of a Phenotype Related to Frailty. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2007;62(11):1279–1286. doi: 10.1093/gerona/62.11.1279. [DOI] [PubMed] [Google Scholar]
- 16.Lagathu C., Cossarizza A., Béréziat V., Nasi M., Capeau J., Pinti M. Basic science and pathogenesis of ageing with HIV. AIDS. 2017;31(Supplement 2):S105–S119. doi: 10.1097/QAD.0000000000001441. [DOI] [PubMed] [Google Scholar]
- 17.Desai S., Landay A. Early Immune Senescence in HIV Disease. Current HIV/AIDS Reports. 2010;7(1):4–10. doi: 10.1007/s11904-009-0038-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewitt G., Jurk D., Marques F. D. M., et al. Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nature Communications. 2012;3(1):p. 708. doi: 10.1038/ncomms1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fumagalli M., Rossiello F., Clerici M., et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nature Cell Biology. 2012;14(4):355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olovnikov A. M. Telomeres, telomerase, and aging: Origin of the theory. Experimental Gerontology. 1996;31(4):443–448. doi: 10.1016/0531-5565(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 21.Armanios M., Blackburn E. H. The telomere syndromes. Nature Reviews Genetics. 2012;13(10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armanios M., Alder J. K., Parry E. M., Karim B., Strong M. A., Greider C. W. Short Telomeres are Sufficient to Cause the Degenerative Defects Associated with Aging. American Journal of Human Genetics. 2009;85(6):823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ballon G., Ometto L., Righetti E., et al. Human Immunodeficiency Virus Type 1 Modulates Telomerase Activity in Peripheral Blood Lymphocytes. The Journal of Infectious Diseases. 2001;183(3):417–424. doi: 10.1086/318072. [DOI] [PubMed] [Google Scholar]
- 24.Bostik P., Dodd G. L., Patel S. S., Kadivar H., Ansari A. A. Effect of Productive In Vitro Human Immunodeficiency Virus or Simian Immunodeficiency Virus Infection on Telomerase Activity in Lymphoid and Nonlymphoid Cells. The Journal of Infectious Diseases. 2002;185(7):999–1001. doi: 10.1086/339412. [DOI] [PubMed] [Google Scholar]
- 25.Franzese O., Adamo R., Pollicita M., et al. Telomerase activity, hTERT expression, and phosphorylation are downregulated in CD4+ T lymphocytes infected with human immunodeficiency virus type 1 (HIV-1) Journal of Medical Virology. 2007;79(5):639–646. doi: 10.1002/jmv.20855. [DOI] [PubMed] [Google Scholar]
- 26.Weng N. P., Levine B. L., June C. H., Hodes R. J. Regulated expression of telomerase activity in human T lymphocyte development and activation. The Journal of Experimental Medicine. 1996;183(6):2471–2479. doi: 10.1084/jem.183.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng N. P., Hathcock K. S., Hodes R. J. Regulation of Telomere Length and Telomerase in T and B Cells. Immunity. 1998;9(2):151–157. doi: 10.1016/s1074-7613(00)80597-x. [DOI] [PubMed] [Google Scholar]
- 28.UNAIDS 2019 estimates, Global AIDS Monitoring. 2019. http://aidsinfo.unaids.org.
- 29.Prendergast A. J., Klenerman P., Goulder P. J. The impact of differential antiviral immunity in children and adults. Nature Reviews Immunology. 2012;12(9):636–648. doi: 10.1038/nri3277. [DOI] [PubMed] [Google Scholar]
- 30.Ricci E., Malacrida S., Zanchetta M., Montagna M., Giaquinto C., de Rossi A. Role of β-Defensin-1 Polymorphisms in Mother-to-Child Transmission of HIV-1. Journal of Acquired Immune Deficiency Syndromes. 2009;51(1):13–19. doi: 10.1097/QAI.0b013e31819df249. [DOI] [PubMed] [Google Scholar]
- 31.Freguja R., Gianesin K., Zanchetta M., de Rossi A. Cross-talk between virus and host innate immunity in pediatric HIV-1 infection and disease progression. The New Microbiologica. 2012;35(3):249–257. [PubMed] [Google Scholar]
- 32.Gianesin K., Freguja R., Carmona F., et al. The Role of Genetic Variants of Stromal Cell-Derived Factor 1 in Pediatric HIV-1 Infection and Disease Progression. PLoS One. 2012;7(9, article e44460) doi: 10.1371/journal.pone.0044460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Rossi A., Masiero S., Giaquinto C., et al. Dynamics of viral replication in infants with vertically acquired human immunodeficiency virus type 1 infection. The Journal of Clinical Investigation. 1996;97(2):323–330. doi: 10.1172/JCI118419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntosh K., Shevitz A., Zaknun D., et al. Age- and Time-related Changes in Extracellular Viral Load in Children Vertically Infected by Human Immunodeficiency Virus. The Pediatric Infectious Disease Journal. 1996;15(12):1087–1091. doi: 10.1097/00006454-199612000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Henrard D. R., Phillips J. F., Muenz L. R., et al. Natural history of HIV-1 cell-free viremia. JAMA. 1995;274(7):554–558. [PubMed] [Google Scholar]
- 36.Mansoor N., Abel B., Scriba T. J., et al. Significantly skewed memory CD8+ T cell subsets in HIV-1 infected infants during the first year of life. Clinical Immunology. 2009;130(3):280–289. doi: 10.1016/j.clim.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Côté H. C. F., Soudeyns H., Thorne A., et al. Leukocyte Telomere Length in HIV-Infected and HIV-Exposed Uninfected Children: Shorter Telomeres with Uncontrolled HIV Viremia. PLoS One. 2012;7(7, article e39266) doi: 10.1371/journal.pone.0039266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Díaz L., Méndez-Lagares G., Correa-Rocha R., et al. Detectable Viral Load Aggravates Immunosenescence Features of CD8 T-Cell Subsets in Vertically HIV-Infected Children. Journal of Acquired Immune Deficiency Syndromes. 2012;60(5):447–454. doi: 10.1097/QAI.0b013e318259254f. [DOI] [PubMed] [Google Scholar]
- 39.Méndez-Lagares G., Díaz L., Correa-Rocha R., et al. Specific patterns of CD4-associated immunosenescence in vertically HIV- infected subjects. Clinical Microbiology and Infection. 2013;19(6):558–565. doi: 10.1111/j.1469-0691.2012.03934.x. [DOI] [PubMed] [Google Scholar]
- 40.Gibb D. M., Duong T., Tookey P. A., et al. Decline in mortality, AIDS, and hospital admissions in perinatally HIV-1 infected children in the United Kingdom and Ireland. BMJ. 2003;327(7422):p. 1019. doi: 10.1136/bmj.327.7422.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Judd A., Doerholt K., Tookey P. A., et al. Morbidity, Mortality, and Response to Treatment by Children in the United Kingdom and Ireland with Perinatally Acquired HIV Infection during 1996-2006: Planning for Teenage and Adult Care. Clinical Infectious Diseases. 2007;45(7):918–924. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 42.Deeks S. G., Phillips A. N. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ. 2009;338, article a3172 doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 43.Hazra R., Siberry G. K., Mofenson L. M. Growing Up with HIV: Children, Adolescents, and Young Adults with Perinatally Acquired HIV Infection. Annual Review of Medicine. 2010;61:169–185. doi: 10.1146/annurev.med.050108.151127. [DOI] [PubMed] [Google Scholar]
- 44.Brady M. T., Oleske J. M., Williams P. L., et al. Declines in Mortality Rates and Changes in Causes of Death in HIV-1-Infected Children During the HAART Era. Journal of Acquired Immune Deficiency Syndromes. 2010;53(1):86–94. doi: 10.1097/QAI.0b013e3181b9869f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiappini E., Berti E., Gianesin K., et al. Pediatric Human Immunodeficiency Virus infection and cancer in the Highly Active Antiretroviral Treatment (HAART) era. Cancer Letters. 2014;347(1):38–45. doi: 10.1016/j.canlet.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 46.Guaraldi G., Palella F. J., Jr. Clinical implications of aging with HIV infection. AIDS. 2017;31(Supplement 2):S129–S135. doi: 10.1097/QAD.0000000000001478. [DOI] [PubMed] [Google Scholar]
- 47.Klein R. S. Trends Related to Aging and Co-Occurring Disorders in HIV-Infected Drug Users. Substance Use & Misuse. 2011;46(2-3):233–244. doi: 10.3109/10826084.2011.522843. [DOI] [PubMed] [Google Scholar]
- 48.Brouilette S. W., Moore J. S., McMahon A. D., et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. The Lancet. 2007;369(9556):107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 49.Takata Y., Kikukawa M., Hanyu H., et al. Association Between ApoE Phenotypes and Telomere Erosion in Alzheimer’s Disease. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2012;67A(4):330–335. doi: 10.1093/gerona/glr185. [DOI] [PubMed] [Google Scholar]
- 50.Deeks S. G., Verdin E., McCune J. Immunosenescence and HIV. Current Opinion in Immunology. 2012;24(4):501–506. doi: 10.1016/j.coi.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Guaraldi G., Orlando G., Zona S., et al. Premature Age-Related Comorbidities Among HIV-Infected Persons Compared With the General Population. Clinical Infectious Diseases. 2011;53(11):1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 52.Smith R. L., Boer R., Brul S., Budovskaya Y., Spek H. Premature and accelerated aging: HIV or HAART? Frontiers in Genetics. 2013;3:p. 328. doi: 10.3389/fgene.2012.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu X., Takahashi H., Harada Y., et al. 3'-Azido-2',3'-dideoxynucleoside 5'-triphosphates inhibit telomerase activity in vitro, and the corresponding nucleosides cause telomere shortening in human HL60 cells. Nucleic Acids Research. 2007;35(21):7140–7149. doi: 10.1093/nar/gkm859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hukezalie K. R., Thumati N. R., Côté H. C. F., Wong J. M. Y. In Vitro and Ex Vivo Inhibition of Human Telomerase by Anti-HIV Nucleoside Reverse Transcriptase Inhibitors (NRTIs) but Not by Non-NRTIs. PLoS One. 2012;7(11, article e47505) doi: 10.1371/journal.pone.0047505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marks M. A., Rabkin C. S., Engels E. A., et al. Markers of microbial translocation and risk of AIDS-related lymphoma. AIDS. 2013;27(3):469–474. doi: 10.1097/QAD.0b013e32835c1333. [DOI] [PubMed] [Google Scholar]
- 56.Gianesin K., Noguera-Julian A., Zanchetta M., et al. Premature aging and immune senescence in HIV-infected children. AIDS. 2016;30(9):1363–1373. doi: 10.1097/QAD.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antiretroviral Therapy Cohort Collaboration, Gill J., May M., et al. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996-2006: collaborative analysis of 13 HIV cohort studies. Clinical Infectious Diseases. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith C. J., Ryom L., Weber R., et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. The Lancet. 2014;384(9939):241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 59.Chiappini E., Bianconi M., Dalzini A., et al. Accelerated aging in perinatally HIV-infected children: clinical manifestations and pathogenetic mechanisms. Aging. 2019;10 doi: 10.18632/aging.101622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biggar R. J., Chaturvedi A. K., Goedert J. J., Engels E. A., For the HIV/AIDS Cancer Match Study AIDS-Related Cancer and Severity of Immunosuppression in Persons With AIDS. Journal of the National Cancer Institute. 2007;99(12):962–972. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 61.Chiappini E., Galli L., Tovo P. A., et al. Cancer Rates After Year 2000 Significantly Decrease in Children With Perinatal HIV Infection: A Study by the Italian Register for HIV Infection in Children. Journal of Clinical Oncology. 2007;25(1):97–101. doi: 10.1200/JCO.2006.06.6506. [DOI] [PubMed] [Google Scholar]
- 62.Pollock B. H., Jenson H. B., Leach C. T., et al. Risk Factors for Pediatric Human Immunodeficiency Virus–Related Malignancy. JAMA. 2003;289(18):2393–2399. doi: 10.1001/jama.289.18.2393. [DOI] [PubMed] [Google Scholar]
- 63.Mueller B. U. Cancers in Children Infected With the Human Immunodeficiency Virus. The Oncologist. 1999;4(4):309–317. doi: 10.1634/theoncologist.4-4-309. [DOI] [PubMed] [Google Scholar]
- 64.Granovsky M. O., Mueller B. U., Nicholson H. S., Rosenberg P. S., Rabkin C. S. Cancer in human immunodeficiency virus-infected children: a case series from the Children's Cancer Group and the National Cancer Institute. Journal of Clinical Oncology. 1998;16(5):1729–1735. doi: 10.1200/JCO.1998.16.5.1729. [DOI] [PubMed] [Google Scholar]
- 65.Biggar R. J., Frisch M., Goedert J. J. Risk of Cancer in Children With AIDS. JAMA. 2000;284(2):205–209. doi: 10.1001/jama.284.2.205. [DOI] [PubMed] [Google Scholar]
- 66.Petrara M. R., Freguja R., Gianesin K., Zanchetta M., Rossi A. D. Epstein-Barr virus-driven lymphomagenesis in the context of human immunodeficiency virus type 1 infection. Frontiers in Microbiology. 2013;4:p. 311. doi: 10.3389/fmicb.2013.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Breen E. C., Hussain S. K., Magpantay L., et al. B-Cell Stimulatory Cytokines and Markers of Immune Activation Are Elevated Several Years Prior to the Diagnosis of Systemic AIDS-Associated Non-Hodgkin B-Cell Lymphoma. Cancer Epidemiology, Biomarkers & Prevention. 2011;20(7):1303–1314. doi: 10.1158/1055-9965.EPI-11-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ambinder R. F., Bhatia K., Martinez-Maza O., Mitsuyasu R. Cancer biomarkers in HIV patients. Current Opinion in HIV and AIDS. 2010;5(6):531–537. doi: 10.1097/COH.0b013e32833f327e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Simard E. P., Shiels M. S., Bhatia K., Engels E. A. Long-term Cancer Risk among People Diagnosed with AIDS during Childhood. Cancer Epidemiology Biomarkers & Prevention. 2012;21(1):148–154. doi: 10.1158/1055-9965.EPI-11-0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kest H., Brogly S., McSherry G., Dashefsky B., Oleske J., Seage G. R., III Malignancy in Perinatally Human Immunodeficiency Virus-Infected Children in the United States. The Pediatric Infectious Disease Journal. 2005;24(3):237–242. doi: 10.1097/01.inf.0000154324.59426.8d. [DOI] [PubMed] [Google Scholar]
- 71.Petrara M. R., Penazzato M., Massavon W., et al. Epstein-Barr Virus Load in Children Infected With Human Immunodeficiency Virus Type 1 in Uganda. The Journal of Infectious Diseases. 2014;210(3):392–399. doi: 10.1093/infdis/jiu099. [DOI] [PubMed] [Google Scholar]
- 72.Regidor D. L., Detels R., Breen E. C., et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011;25(3):303–314. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Douek D. C., Roederer M., Koup R. A. Emerging Concepts in the Immunopathogenesis of AIDS. Annual Review of Medicine. 2009;60(1):471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Effros R. B. Replicative senescence of CD8 T cells: potential effects on cancer immune surveillance and immunotherapy. Cancer Immunology, Immunotherapy. 2004;53(10):925–933. doi: 10.1007/s00262-004-0508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fumagalli M., d'Adda di Fagagna F. SASPense and DDRama in cancer and ageing. Nature Cell Biology. 2009;11(8):921–923. doi: 10.1038/ncb0809-921. [DOI] [PubMed] [Google Scholar]
- 76.Campisi J., Andersen J. K., Kapahi P., Melov S. Cellular senescence: a link between cancer and age-related degenerative disease? Seminars in Cancer Biology. 2011;21(6):354–359. doi: 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fauci A. S., Desrosiers R. C. Retroviruses. USA: Cold Spring Harbor Laboratory Press; 1997. [PubMed] [Google Scholar]
- 78.Pantaleo G., Fauci A. S. Immunopathogenesis of HIV infection. Annual Review of Microbiology. 1996;50(1):825–854. doi: 10.1146/annurev.micro.50.1.825. [DOI] [PubMed] [Google Scholar]
- 79.Franzese O., Barbaccia M. L., Bonmassar E., Graziani G. Beneficial and Detrimental Effects of Antiretroviral Therapy on HIV-Associated Immunosenescence. Chemotherapy. 2018;63(2):64–75. doi: 10.1159/000487534. [DOI] [PubMed] [Google Scholar]
- 80.Dock J. N., Effros R. B. Role of CD8 T Cell Replicative Senescence in Human Aging and in HIV-mediated Immunosenescence. Aging and Disease. 2011;2(5):382–397. [PMC free article] [PubMed] [Google Scholar]
- 81.Akbar A. N., Fletcher J. M. Memory T cell homeostasis and senescence during aging. Current Opinion in Immunology. 2005;17(5):480–485. doi: 10.1016/j.coi.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 82.Goronzy J. J., Fulbright J. W., Crowson C. S., Poland G. A., O'Fallon W. M., Weyand C. M. Value of Immunological Markers in Predicting Responsiveness to Influenza Vaccination in Elderly Individuals. Journal of Virology. 2001;75(24):12182–12187. doi: 10.1128/JVI.75.24.12182-12187.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Clerici M., Saresella M., Colombo F., et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96(12):3866–3871. doi: 10.1182/blood.V96.12.3866. [DOI] [PubMed] [Google Scholar]
- 84.McCloskey T. W., Kohn N., Lesser M., Bakshi S., Pahwa S. Immunophenotypic analysis of HIV-infected children: Alterations within the first year of life, changes with disease progression, and longitudinal analyses of lymphocyte subsets. Cytometry. 2001;46(3):157–165. doi: 10.1002/cyto.1100. [DOI] [PubMed] [Google Scholar]
- 85.Shearer W. T., Easley K. A., Goldfarb J., et al. Prospective 5-year study of peripheral blood CD4+, CD8+, and CD19+/CD20+ lymphocytes and serum Igs in children born to HIV-1+ women. The Journal of Allergy and Clinical Immunology. 2000;106(3):559–566. doi: 10.1067/mai.2000.109433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kharbanda M., McCloskey T. W., Pahwa R., Sun M., Pahwa S. Alterations in T-Cell Receptor Vβ Repertoire of CD4 and CD8 T Lymphocytes in Human Immunodeficiency Virus-Infected Children. Clinical and Diagnostic Laboratory Immunology. 2003;10(1):53–58. doi: 10.1128/CDLI.10.1.53-58.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seers T., Vassallo P., Pollock K., Thornhill J. P., Fidler S., Foster C. CD4:CD8 ratio in children with perinatally acquired HIV-1 infection. HIV Medicine. 2018;19(9):668–672. doi: 10.1111/hiv.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mussini C., Lorenzini P., Cozzi-Lepri A., et al. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. The Lancet HIV. 2015;2(3):e98–e106. doi: 10.1016/S2352-3018(15)00006-5. [DOI] [PubMed] [Google Scholar]
- 89.Muenchff M., Prendergast A. J., Goulder P. J. Immunity to HIV in early life. Frontiers in Immunology. 2014;12:p. 391. doi: 10.3389/fimmu.2014.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sainz T., Serrano-Villar S., Díaz L., et al. The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. AIDS. 2013;27(9):1513–1516. doi: 10.1097/QAD.0b013e32835faa72. [DOI] [PubMed] [Google Scholar]
- 91.Linton P. J., Dorshkind K. Age-related changes in lymphocyte development and function. Nature Immunology. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 92.Rabin R. L., Roederer M., Maldonado Y., Petru A., Herzenberg L. A., Herzenberg L. A. Altered representation of naive and memory CD8 T cell subsets in HIV-infected children. The Journal of Clinical Investigation. 1995;95(5):2054–2060. doi: 10.1172/JCI117891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Resino S., Correa R., Bellón J. M., Muñoz-Fernández M. A. Preserved immune system in long-term asymptomatic vertically HIV-1 infected children. Clinical and Experimental Immunology. 2003;132(1):105–112. doi: 10.1046/j.1365-2249.2003.02116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jordan K. A., Furlan S. N., Gonzalez V. D., et al. CD8 T cell effector maturation in HIV-1-infected children. Virology. 2006;347(1):117–126. doi: 10.1016/j.virol.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 95.Ahlers J. D., Belyakov I. M. Memories that last forever: strategies for optimizing vaccine T-cell memory. Blood. 2010;115(9):1678–1689. doi: 10.1182/blood-2009-06-227546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Foldi J., Kozhaya L., McCarty B., et al. HIV-Infected Children Have Elevated Levels of PD-1+ Memory CD4 T Cells With Low Proliferative Capacity and High Inflammatory Cytokine Effector Functions. The Journal of Infectious Diseases. 2017;216(6):641–650. doi: 10.1093/infdis/jix341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sperk M., Domselaar R., Neogi U. Immune Checkpoints as the Immune System Regulators and Potential Biomarkers in HIV-1 Infection. International Journal of Molecular Sciences. 2018;19(7):p. 2000. doi: 10.3390/ijms19072000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fenwick C., Joo V., Jacquier P., et al. T‐cell exhaustion in HIV infection. Immunological Reviews. 2019;292(1):149–163. doi: 10.1111/imr.12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nature Reviews Immunology. 2007;7(11):875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 100.Holmes D., Jiang Q., Zhang L., Su L. Foxp3 and Treg cells in HIV-1 infection and immuno-pathogenesis. Immunologic Research. 2008;41(3):248–266. doi: 10.1007/s12026-008-8037-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vignali D. A. A., Collison L. W., Workman C. J. How regulatory T cells work. Nature Reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aandahl E. M., Michaëlsson J., Moretto W. J., Hecht F. M., Nixon D. F. Human CD4+ CD25+ Regulatory T Cells Control T-Cell Responses to Human Immunodeficiency Virus and Cytomegalovirus Antigens. Journal of Virology. 2004;78(5):2454–2459. doi: 10.1128/jvi.78.5.2454-2459.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weiss L., Donkova-Petrini V., Caccavelli L., Balbo M̀., Carbonneil Ć., Levy Y. Human immunodeficiency virus–driven expansion of CD4+CD25+ regulatory T cells, which suppress HIV-specific CD4 T-cell responses in HIV-infected patients. Blood. 2004;104(10):3249–3256. doi: 10.1182/blood-2004-01-0365. [DOI] [PubMed] [Google Scholar]
- 104.Weiss L., Piketty C., Assoumou L., et al. Relationship between Regulatory T Cells and Immune Activation in Human Immunodeficiency Virus-Infected Patients Interrupting Antiretroviral Therapy. PLoS One. 2010;5(7, article e11659) doi: 10.1371/journal.pone.0011659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Eggena M. P., Barugahare B., Jones N., et al. Depletion of Regulatory T Cells in HIV Infection Is Associated with Immune Activation. Journal of Immunology. 2005;174(7):4407–4414. doi: 10.4049/jimmunol.174.7.4407. [DOI] [PubMed] [Google Scholar]
- 106.Kinter A., McNally J., Riggin L., Jackson R., Roby G., Fauci A. S. Suppression of HIV-specific T cell activity by lymph node CD25+ regulatory T cells from HIV-infected individuals. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3390–3395. doi: 10.1073/pnas.0611423104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kolte L., Gaardbo J. C., Skogstrand K., Ryder L. P., Ersbøll A. K., Nielsen S. D. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active anti-retroviral therapy may be due to increased thymic production of naive Tregs. Clinical and Experimental Immunology. 2009;155(1):44–52. doi: 10.1111/j.1365-2249.2008.03803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gaardbo J. C., Nielsen S. D., Vedel S. J., et al. Regulatory T cells in human immunodeficiency virus‐infected patients are elevated and independent of immunological and virological status, as well as initiation of highly active anti‐retroviral therapy. Clinical and Experimental Immunology. 2008;154(1):80–86. doi: 10.1111/j.1365-2249.2008.03725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Freguja R., Gianesin K., Mosconi I., et al. Regulatory T cells and chronic immune activation in human immunodeficiency virus 1 (HIV‐1)‐infected children. Clinical and Experimental Immunology. 2011;164(3):373–380. doi: 10.1111/j.1365-2249.2011.04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pallikkuth S., de Armas L., Rinaldi S., Pahwa S. T Follicular Helper Cells and B Cell Dysfunction in Aging and HIV-1 Infection. Frontiers in Immunology. 2017;8, article 1380 doi: 10.3389/fimmu.2017.01380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Armas L. R., Cotugno N., Pallikkuth S., et al. Induction of IL21in Peripheral T Follicular Helper Cells Is an Indicator of Influenza Vaccine Response in a Previously Vaccinated HIV-Infected Pediatric Cohort. Journal of Immunology. 2017;198(5):1995–2005. doi: 10.4049/jimmunol.1601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sallusto F., Geginat J., Lanzavecchia A. Central Memory and Effector Memory T Cell Subsets: Function, Generation, and Maintenance. Annual Review of Immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 113.Brenchley J. M., Karandikar N. J., Betts M. R., et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101(7):2711–2720. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 114.Cagigi A., Rinaldi S., di Martino A., et al. Premature immune senescence during HIV-1 vertical infection relates with response to influenza vaccination. The Journal of Allergy and Clinical Immunology. 2014;133(2):592–594.e1. doi: 10.1016/j.jaci.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 115.Hart M., Steel A., Clark S. A., et al. Loss of Discrete Memory B Cell Subsets Is Associated with Impaired Immunization Responses in HIV-1 Infection and May Be a Risk Factor for Invasive Pneumococcal Disease. Journal of Immunology. 2007;178(12):8212–8220. doi: 10.4049/jimmunol.178.12.8212. [DOI] [PubMed] [Google Scholar]
- 116.Siberry G. K., Patel K., Bellini W. J., et al. Immunity to Measles, Mumps, and Rubella in US Children With Perinatal HIV Infection or Perinatal HIV Exposure Without Infection. Clinical Infectious Diseases. 2015;61(6):988–995. doi: 10.1093/cid/civ440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moir S., Ho J., Malaspina A., et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. The Journal of Experimental Medicine. 2008;205(8):1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rinaldi S., Pallikkuth S., George V. K., et al. Paradoxical aging in HIV: immune senescence of B Cells is most prominent in young age. Aging. 2017;9(4):1307–1325. doi: 10.18632/aging.101229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Palma P., Rinaldi S., Cotugno N., et al. Premature B-cell senescence as a consequence of chronic immune activation: Implications for vaccination of immune compromised individuals. Human Vaccines & Immunotherapeutics. 2014;10(7):2083–2088. doi: 10.4161/hv.28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moir S., Fauci A. S. B cells in HIV infection and disease. Nature Reviews Immunology. 2009;9(4):235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moir S., Fauci A. S. B-cell responses to HIV infection. Immunological Reviews. 2017;275(1):33–48. doi: 10.1111/imr.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Singh R., Mukherjee A., Singla M., et al. Impact of HIV infection and highly active antiretroviral therapy (HAART) on B cell subpopulations in children. Journal of Medical Virology. 2018;90(7):1222–1231. doi: 10.1002/jmv.25074. [DOI] [PubMed] [Google Scholar]
- 123.Cagigi A., Rinaldi S., Santilli V., et al. Premature aging of the immune system relates with increased anti-lymphocyte antibodies (ALA) after an immunization in HIV-1 infected and kidney transplanted patients. Clinical and Experimental Immunology. 2013;174(2):274–280. doi: 10.1111/cei.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bekele Y., Lemma M., Bobosha K., et al. Homing defects of B cells in HIV-1 infected children impair vaccination responses. Vaccine. 2019;37(17):2348–2355. doi: 10.1016/j.vaccine.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 125.Cotugno N., de Armas L., Pallikkuth S., et al. Perturbation of B Cell Gene Expression Persists in HIV-Infected Children Despite Effective Antiretroviral Therapy and Predicts H1N1 Response. Frontiers in Immunology. 2017;8, article 1083 doi: 10.3389/fimmu.2017.01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.de Armas L. R., Pallikkuth S., Pan L., et al. Single Cell Profiling Reveals PTEN Overexpression in Influenza-Specific B cells in Aging HIV-infected individuals on Anti-retroviral Therapy. Scientific Reports. 2019;9(1, article 2482) doi: 10.1038/s41598-019-38906-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Giorgi J. V., Lyles R. H., Matud J. L., et al. Predictive Value of Immunologic and Virologic Markers After Long or Short Duration of HIV-1 Infection. Journal of Acquired Immune Deficiency Syndromes. 2002;29(4):346–355. doi: 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 128.Brenchley J. M., Price D. A., Schacker T. W., et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nature Medicine. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 129.Zhang Q., Raoof M., Chen Y., et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464(7285):104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Van Crombruggen K., Jacob F., Zhang N., Bachert C. Damage-associated molecular patterns and their receptors in upper airway pathologies. Cellular and Molecular Life Sciences. 2013;70(22):4307–4321. doi: 10.1007/s00018-013-1356-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Janeway C. A., Jr., Medzhitov R. Innate immune recognition. Annual Review of Immunology. 2002;20(1):197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 132.Sainz T., Álvarez-Fuente M., Navarro M. L., et al. Subclinical Atherosclerosis and Markers of Immune Activation in HIV-Infected Children and Adolescents. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;65(1):42–49. doi: 10.1097/QAI.0b013e3182a9466a. [DOI] [PubMed] [Google Scholar]
- 133.Persaud D., Patel K., Karalius B., et al. Influence of Age at Virologic Control on Peripheral Blood Human Immunodeficiency Virus Reservoir Size and Serostatus in Perinatally Infected Adolescents. JAMA Pediatrics. 2014;168(12):1138–1146. doi: 10.1001/jamapediatrics.2014.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dirajlal-Fargo S., El-Kamari V., Weiner L., et al. Altered intestinal permeability and fungal translocation in Ugandan children with human immunodeficiency virus. Clinical Infectious Diseases. 2019;(article ciz561) doi: 10.1093/cid/ciz561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mudd J. C., Brenchley J. M. Gut Mucosal Barrier Dysfunction, Microbial Dysbiosis, and Their Role in HIV-1 Disease Progression. The Journal of Infectious Diseases. 2016;214(Supplement 2):S58–S66. doi: 10.1093/infdis/jiw258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alvarez P., Mwamzuka M., Marshed F., et al. Immune activation despite preserved CD4 T cells in perinatally HIV-infected children and adolescents. PLoS One. 2017;12(12, article e0190332) doi: 10.1371/journal.pone.0190332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Generoso M., Álvarez P., Kravietz A., et al. High soluble CD163 levels correlate with disease progression and inflammation in Kenyan children with perinatal HIV-infection. AIDS. 2020;34(1):33–38. doi: 10.1097/QAD.0000000000002378. [DOI] [PubMed] [Google Scholar]
- 138.Fitzgerald F. C., Lhomme E., Harris K., et al. Microbial Translocation Does Not Drive Immune Activation in Ugandan Children Infected With HIV. The Journal of Infectious Diseases. 2019;219(1):89–100. doi: 10.1093/infdis/jiy495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Breton G., Chomont N., Takata H., et al. Programmed Death-1 Is a Marker for Abnormal Distribution of Naive/Memory T Cell Subsets in HIV-1 Infection. Journal of Immunology. 2013;191(5):2194–2204. doi: 10.4049/jimmunol.1200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Day C. L., Kaufmann D. E., Kiepiela P., et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 141.Trautmann L., Janbazian L., Chomont N., et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nature Medicine. 2006;12(10):1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 142.Prendergast A., O'Callaghan M., Menson E., et al. Factors Influencing T Cell Activation and Programmed Death 1 Expression in HIV-Infected Children. AIDS Research and Human Retroviruses. 2012;28(5):465–468. doi: 10.1089/aid.2011.0113. [DOI] [PubMed] [Google Scholar]
- 143.Ssewanyana I., Baker C. A. R., Ruel T., et al. The Distribution and Immune Profile of T Cell Subsets in HIV-Infected Children from Uganda. AIDS Research and Human Retroviruses. 2009;25(1):65–71. doi: 10.1089/aid.2008.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Banga R., Procopio F. A., Noto A., et al. PD-1+ and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nature Medicine. 2016;22(7):754–761. doi: 10.1038/nm.4113. [DOI] [PubMed] [Google Scholar]
- 145.Muema D. M., Macharia G. N., Hassan A. S., et al. Control of Viremia Enables Acquisition of Resting Memory B Cells with Age and Normalization of Activated B Cell Phenotypes in HIV-Infected Children. Journal of Immunology. 2015;195(3):1082–1091. doi: 10.4049/jimmunol.1500491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Petrara M. R., Cattelan A. M., Sasset L., et al. Impact of monotherapy on HIV-1 reservoir, immune activation, and co-infection with Epstein-Barr virus. PLoS One. 2017;12(9, article e0185128) doi: 10.1371/journal.pone.0185128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Aggarwal H., Khan L., Chaudhary O., et al. Alterations in B Cell Compartment Correlate with Poor Neutralization Response and Disease Progression in HIV-1 Infected Children. Frontiers in Immunology. 2017;8, article 1697 doi: 10.3389/fimmu.2017.01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Giunco S., Celeghin A., Gianesin K., Dolcetti R., Indraccolo S., de Rossi A. Cross talk between EBV and telomerase: the role of TERT and NOTCH2 in the switch of latent/lytic cycle of the virus. Cell Death & Disease. 2015;6(5, article e1774) doi: 10.1038/cddis.2015.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tchkonia T., Zhu Y., van Deursen J., Campisi J., Kirkland J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. Journal of Clinical Investigation. 2013;123(3):966–972. doi: 10.1172/JCI64098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shay J. W. Telomeres and aging. Current Opinion in Cell Biology. 2018;52:1–7. doi: 10.1016/j.ceb.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 151.Artandi S. E., DePinho R. Telomeres and telomerase in cancer. Carcinogenesis. 2010;31(1):9–18. doi: 10.1093/carcin/bgp268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Meyer T., Rietzschel E. R., De Buyzere M. L., Van Criekinge W., Bekaert S. Telomere length and cardiovascular aging: The means to the ends? Ageing Research Reviews. 2011;10(2):297–303. doi: 10.1016/j.arr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 153.Cawthon R. M., Smith K. R., O'Brien E., Sivatchenko A., Kerber R. A. Association between telomere length in blood and mortality in people aged 60 years or older. The Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 154.Willeit P., Willeit J., Brandstätter A., et al. Cellular Aging Reflected by Leukocyte Telomere Length Predicts Advanced Atherosclerosis and Cardiovascular Disease Risk. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(8):1649–1656. doi: 10.1161/ATVBAHA.110.205492. [DOI] [PubMed] [Google Scholar]
- 155.Haycock P. C., Heydon E. E., Kaptoge S., Butterworth A. S., Thompson A., Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349 doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fitzpatrick A. L., Kronmal R. A., Gardner J. P., et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 157.Salpea K. D., Humphries S. E. Telomere length in atherosclerosis and diabetes. Atherosclerosis. 2010;209(1):35–38. doi: 10.1016/j.atherosclerosis.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fitzpatrick A. L., Kronmal R. A., Kimura M., et al. Leukocyte Telomere Length and Mortality in the Cardiovascular Health Study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;66A(4):421–429. doi: 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Epel E. S., Merkin S. S., Cawthon R., et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men: a novel demonstration. Aging. 2008;1(1):81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Turner K. J., Vasu V., Griffin D. Telomere Biology and Human Phenotype. Cell. 2019;8(1):p. 73. doi: 10.3390/cells8010073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Willeit P., Raschenberger J., Heydon E. E., et al. Leucocyte Telomere Length and Risk of Type 2 Diabetes Mellitus: New Prospective Cohort Study and Literature-Based Meta-Analysis. PLoS One. 2014;9(11, article e112483) doi: 10.1371/journal.pone.0112483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.You N. C. Y., Chen B. H., Song Y., et al. A Prospective Study of Leukocyte Telomere Length and Risk of Type 2 Diabetes in Postmenopausal Women. Diabetes. 2012;61(11):2998–3004. doi: 10.2337/db12-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thomas P., O’ Callaghan N. J., Fenech M. Telomere length in white blood cells, buccal cells and brain tissue and its variation with ageing and Alzheimer's disease. Mechanisms of Ageing and Development. 2008;129(4):183–190. doi: 10.1016/j.mad.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 164.Panossian L., Porter V. R., Valenzuela H. F., et al. Telomere shortening in T cells correlates with Alzheimer's disease status. Neurobiology of Aging. 2003;24(1):77–84. doi: 10.1016/S0197-4580(02)00043-X. [DOI] [PubMed] [Google Scholar]
- 165.Honig L. S., Schupf N., Lee J. H., Tang M. X., Mayeux R. Shorter telomeres are associated with mortality in those with APOE ϵ4 and dementia. Annals of Neurology. 2006;60(2):181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- 166.Grodstein F., van Oijen M., Irizarry M. C., et al. Shorter Telomeres May Mark Early Risk of Dementia: Preliminary Analysis of 62 Participants from the Nurses' Health Study. PLoS One. 2008;3(2, article e1590) doi: 10.1371/journal.pone.0001590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hochstrasser T., Marksteiner J., Humpel C. Telomere length is age-dependent and reduced in monocytes of alzheimer patients. Experimental Gerontology. 2012;47:160–163. doi: 10.1016/j.exger.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Watfa G., Dragonas C., Brosche T., et al. Study of telomere length and different markers of oxidative stress in patients with Parkinson’s disease. The Journal of Nutrition, Health & Aging. 2011;15(4):277–281. doi: 10.1007/s12603-010-0275-7. [DOI] [PubMed] [Google Scholar]
- 169.Guan J. Z., Maeda T., Sugano M., et al. A Percentage Analysis of the Telomere Length in Parkinson's Disease Patients. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63(5):467–473. doi: 10.1093/gerona/63.5.467. [DOI] [PubMed] [Google Scholar]
- 170.Blasco M. A. Telomeres and human disease: ageing, cancer and beyond. Nature Reviews Genetics. 2005;6(8):611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 171.Giunco S., Rampazzo E., Celeghin A., Petrara M. R., de Rossi A. Telomere and Telomerase in Carcinogenesis: Their Role as Prognostic Biomarkers. Current Pathobiology Reports. 2015;3(4):315–328. doi: 10.1007/s40139-015-0087-x. [DOI] [Google Scholar]
- 172.Escribano A., Pastor S., Reula A., et al. Accelerated telomere attrition in children and teenagers with α1-antitrypsin deficiency. The European Respiratory Journal. 2016;48(2):350–358. doi: 10.1183/13993003.00176-2016. [DOI] [PubMed] [Google Scholar]
- 173.Bhala S., Best A. F., Giri N., et al. CNS manifestations in patients with telomere biology disorders. Neurology Genetics. 2019;5(6):p. 370. doi: 10.1212/NXG.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Donze S. H., Codd V., Damen L., et al. Evidence for Accelerated Biological Aging in Young Adults with Prader–Willi Syndrome. The Journal of Clinical Endocrinology & Metabolism. 2020;105(6) doi: 10.1210/clinem/dgz180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ackermann S., Fischer M. Telomere Maintenance in Pediatric Cancer. International Journal of Molecular Sciences. 2019;20(23):p. 5836. doi: 10.3390/ijms20235836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Song L., Liu B., Wu M., et al. Prenatal Exposure to Phthalates and Newborn Telomere Length: A Birth Cohort Study in Wuhan, China. Environmental Health Perspectives. 2019;127(8, article 087007) doi: 10.1289/EHP4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Clemente D. B. P., Maitre L., Bustamante M., et al. Obesity is associated with shorter telomeres in 8 year-old children. Scientific Reports. 2019;9(1) doi: 10.1038/s41598-019-55283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lamprokostopoulou A., Moschonis G., Manios Y., et al. Childhood obesity and leucocyte telomere length. European Journal of Clinical Investigation. 2019;49(12, article e13178) doi: 10.1111/eci.13178. [DOI] [PubMed] [Google Scholar]
- 179.Alder J. K., Hanumanthu V. S., Strong M. A., et al. Diagnostic utility of telomere length testing in a hospital-based setting. Proceedings of the National Academy of Sciences of the United States of America. 2018;155(10):2358–2365. doi: 10.1073/pnas.1720427115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu J. C. Y., Leung J. M., Ngan D. A., et al. Absolute Leukocyte Telomere Length in HIV-Infected and Uninfected Individuals: Evidence of Accelerated Cell Senescence in HIV-Associated Chronic Obstructive Pulmonary Disease. PLoS One. 2015;10(4, article e0124426) doi: 10.1371/journal.pone.0124426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Srinivasa S., Fitch K. V., Petrow E., et al. Soluble CD163 Is Associated With Shortened Telomere Length in HIV-Infected Patients. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;67(4):414–418. doi: 10.1097/QAI.0000000000000329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zanet D. A. L., Thorne A., Singer J., et al. Association Between Short Leukocyte Telomere Length and HIV Infection in a Cohort Study: No Evidence of a Relationship With Antiretroviral Therapy. Clinical Infectious Diseases. 2014;58(9):1322–1332. doi: 10.1093/cid/ciu051. [DOI] [PubMed] [Google Scholar]
- 183.Pathai S., Lawn S. D., Gilbert C. E., et al. Accelerated biological ageing in HIV-infected individuals in South Africa. AIDS. 2013;27(15):2375–2384. doi: 10.1097/QAD.0b013e328363bf7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gonzalez-Serna A., Ajaykumar A., Gadawski I., et al. Rapid Decrease in Peripheral Blood Mononucleated Cell Telomere Length After HIV Seroconversion, but Not HCV Seroconversion. Journal of Acquired Immune Deficiency Syndromes. 2017;76(1):e29–e32. doi: 10.1097/QAI.0000000000001446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Peng Y., Mian I. S., Lue N. F. Analysis of Telomerase Processivity. Molecular Cell. 2001;7(6):1201–1211. doi: 10.1016/S1097-2765(01)00268-4. [DOI] [PubMed] [Google Scholar]
- 186.Gillis A. J., Schuller A. P., Skordalakes E. Structure of the Tribolium castaneum telomerase catalytic subunit TERT. Nature. 2008;455(7213):633–637. doi: 10.1038/nature07283. [DOI] [PubMed] [Google Scholar]
- 187.Leeansyah E., Cameron P. U., Solomon A., et al. Inhibition of Telomerase Activity by Human Immunodeficiency Virus (HIV) Nucleos(t)ide Reverse Transcriptase Inhibitors: A Potential Factor Contributing to HIV-Associated Accelerated Aging. The Journal of Infectious Diseases. 2013;207(7):1157–1165. doi: 10.1093/infdis/jit006. [DOI] [PubMed] [Google Scholar]
- 188.Gomez D. E., Armando R. G., Alonso D. F. AZT as a telomerase inhibitor. Frontiers in Oncology. 2012;2:p. 113. doi: 10.3389/fonc.2012.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Stella-Ascariz N., Montejano R., Rodriguez-Centeno J., et al. Blood Telomere Length Changes After Ritonavir-Boosted Darunavir Combined With Raltegravir or Tenofovir-Emtricitabine in Antiretroviral-Naive Adults Infected With HIV-1. The Journal of Infectious Diseases. 2018;218(10):1523–1530. doi: 10.1093/infdis/jiy399. [DOI] [PubMed] [Google Scholar]
- 190.Montejano R., Stella-Ascariz N., Monge S., et al. Impact of Nucleos(t)ide Reverse Transcriptase Inhibitors on Blood Telomere Length Changes in a Prospective Cohort of Aviremic HIV–Infected Adults. The Journal of Infectious Diseases. 2018;218(10):1531–1540. doi: 10.1093/infdis/jiy364. [DOI] [PubMed] [Google Scholar]
- 191.Ajaykumar A., Soudeyns H., Kakkar F., et al. Leukocyte Telomere Length at Birth and During the Early Life of Children Exposed to but Uninfected With HIV After In Utero Exposure to Antiretrovirals. The Journal of Infectious Diseases. 2018;217(5):710–720. doi: 10.1093/infdis/jix618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Y., Brummel S. S., Beilstein-Wedel E., et al. Association between zidovudine-containing antiretroviral therapy exposure in utero and leukocyte telomere length at birth. AIDS. 2019;33(13):2091–2096. doi: 10.1097/QAD.0000000000002317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Shiau S., Strehlau R., Shen J., et al. Biomarkers of Aging in HIV-Infected Children on Suppressive Antiretroviral Therapy. Journal of Acquired Immune Deficiency Syndromes. 2018;78(5):549–556. doi: 10.1097/QAI.0000000000001714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Paghera S., Quiros-Roldan E., Sottini A., Properzi M., Castelli F., Imberti L. Lymphocyte homeostasis is maintained in perinatally HIV-infected patients after three decades of life. Immunity & Ageing. 2019;16(1):p. 26. doi: 10.1186/s12979-019-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


