Abstract
Purpose
To investigate the effects of a combined herbal medicine Miodesin™ on the inflammatory response of key cells involved in the acute and chronic inflammatory processes as well as the possible epigenetic involvement.
Methods
After the establishment of the IC50 dose, the chondrocyte, keratinocyte, and macrophage cell lines were pretreated for 2 hours with Miodesin™ (200 μg/mL) and stimulated with LPS (1 μg/mL) for 24 hours. The supernatant was used to measure the levels of cytokines (IL-1β, IL-6, IL-8, and TNF-α) and chemokines (CCL2, CCL3, and CCL5), and the cells were used to extract the mRNA for the transcription factor (NF-κβ), inflammatory enzymes (COX-1, COX-2, PLA2, and iNOS), and chemokines (CCL2, CCL3, and CCL5).
Results
Miodesin™ inhibited the release of LPS-induced cytokines (IL-1β, IL-6, IL-8, and TNF-α; p < 0.01) and chemokines (CCL2, CCL3, and CCL5; p < 0.01) and the expression of the transcription factor (NF-κβ; p < 0.01), inflammatory enzymes (COX-1, COX-2, PLA2, iNOS; p < 0.01), and chemokines (CCL2, CCL3, and CCL5; p < 0.01). In addition, the evaluation of epigenetic mechanism revealed that Miodesin™ did not induce changes in DNA methylation, assuring the genetic safeness of the compound in terms of the inflammatory response.
Conclusions
Miodesin™ presents anti-inflammatory properties, inhibiting hyperactivation of chondrocytes, keratinocytes, and macrophages, involving epigenetics in such effects.
1. Introduction
Although the inflammatory process is a natural response to an offending agent aiming to promote healing and repair, an exacerbated and/or unresolved inflammatory process underlies several acute and chronic diseases [1]. The inflammatory process is complex and involves a group of glycoproteins called cytokines, which coordinate, amplify, and regulate the magnitude and duration of inflammatory events [1].
Acute dermatitis and atopic dermatitis are examples of acute and chronic skin diseases, respectively, in which keratinocytes present a key role [2]. In this context, it has already been demonstrated that keratinocytes express different surface alarming receptors against pathogens, being the trigger for cytokines and reactive oxygen species (ROS) and reactive nitrogen species (RNS) release [2, 3]. In addition, lipopolysaccharides (LPS) are among the main players for skin infection, which may be installed during skin acute and chronic inflammatory processes [3]. Beyond keratinocytes, skin macrophages also represent the first line of defense during skin infections, contributing to the inflammatory process, releasing, for instance, cytokines and ROS and RNS [4]. Thus, in the last years, a growing number of studies are being developed to identify effective agents capable of preventing and treating infectious processes in the skin [5].
Similarly, acute and chronic joint diseases, for instance, arthritis and arthrosis, respectively, are modulated by inflammatory processes [6]. These inflammatory processes are also modulated by cytokine synthesis and release, activating degradative enzymes, such as different matrix metalloproteinases (MMPs), which present a central role in the physiopathology of arthritis and arthrosis [7]. Furthermore, this inflammatory cascade is centrally regulated by increased amounts of nitric oxide (NO), concomitantly with epigenetic regulation [7]. From the cellular point of view, chondrocytes can release massive amounts of cytokines, presenting a key role in the physiopathology of arthritis [8]. In the same direction, macrophages are also hyperactivated in arthritis, also releasing cytokines, MMPs, and NO, contributing to disease perpetuation [9].
Natural products have played an important role in the prevention and treatment of human diseases for thousands of years, and in recent decades, great efforts have been made to make natural products more effective and less toxic. Among the plant species present in the Brazilian territory, especially in the Amazon rainforest, we can find Uncaria tomentosa, known as cat's claw [10], a potential natural agent with anti-inflammatory activity, capable of reducing, for example, the expression of TNF-alpha in monocytes [11]. In addition, the specie Endopleura uchi, known as yellow uxi [12], is also found, which presents a marked antioxidant and anti-inflammatory potential [13]. Thus, many other pharmacologically active plant species with anti-inflammatory potential have been also investigated [14].
Miodesin™, a patented phytocomplex prepared by Fagron Pharmaceutical™ (Brazil) from Uncaria tomentosa, Endopleura uchi, and astaxanthin, a natural antioxidant carotenoid, has been shown to exert anti-inflammatory effects [15]. In 2018 and 2019, Maia et al. published the first manuscripts showing the role of Miodesin™ in reducing pelvic pain in patients with endometriosis and leiomyoma, confirming the central role of inflammation in the pathogenesis of endometriosis. In the same way, these findings support the role of anti-inflammatory herbal medicines in the treatment of this disease [16, 17]. Therefore, the present study is aimed at evaluating the anti-inflammatory activity of Miodesin™, using different cell lines, besides verifying a possible interaction with epigenetic mechanisms.
2. Material and Methods
2.1. Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, and phosphate-buffered saline (PBS) were obtained from Gibco BRL (Grand Island, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA). The Miodesin™ was supplied by Fagron Pharmaceutical™ (Brazil). The purity and quality of the raw materials used, as well as the formulation of Miodesin™ (Uncaria tomentosa, Endopleura uchi, and astaxanthin), were monitored by the Fagron Pharmaceutical™ quality control department.
2.2. Culture and Cytotoxicity Evaluation of Miodesin by MTT Assay
The human skin keratinocyte cell line (HaCaT) and human chondrocyte cell line (CHON-001) were obtained from the American Type Culture Collection (ATCC, USA). The murine macrophage (RAW 264.7) cells were obtained from the Rio de Janeiro Cell Bank (UFRJ, RJ, Brazil). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM high glucose) supplemented with 10% v/v fetal bovine serum (FBS), 1% L-glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin and maintained at 37°C in a humidified atmosphere of 5% CO2. The cells were trypsinized every 72 h using 0.01% trypsin and 1 mmol ethylenediaminetetraacetic acid (EDTA). For all the experiments, the Miodesin™ was dissolved in the culture medium in appropriate concentrations. The cell viability of the control and Miodesin™ (1–1.000 μg/mL)-treated chondrocyte, human keratinocyte (HaCaT), and macrophage RAW 264.7 cells was measured using a standard MTT assay. Briefly, 5 × 104 viable cells were seeded into clear 96-well flat-bottom plates (Corning) in the RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) and incubated with different concentrations of the extract for 24 h. Then, 10 μL/well of MTT (5 mg/mL) was added and the cells were incubated for 4 h. Following incubation, 100 μL of 10% sodium dodecyl sulfate (SDS) solution in deionized water was added to each well and left overnight. The absorbance was measured at 595 nm in a benchtop multimode reader (Molecular Device).
2.3. Cell Viability after LPS Treatment
Briefly, cells were seeded in 96-well culture plates at a density of 5 × 104 viable cells/well and incubated for 24 h, then exposed to IC50 concentration of Miodesin (200 μg/mL), previously determined in Section 2.1 in the presence of LPS (1 μg/mL) for 60 minutes and incubated for another 24 h with Miodesin at 37°C. The MTT solution was added to the final concentration of 0.5 mg/mL and then incubated for 2 h at 37°C followed by the addition of 0.1 mL of dimethyl sulfoxide to dissolve the MTT-formazan. The amount of MTT-formazan was then determined by measuring the absorbance at 595 nm.
2.4. Cytokine and Chemokine Analysis in Cell Culture Supernatants
The concentrations of IL-1β, IL-6, IL-8, TNF-α, MCP-1 (CCL2), MIP-1α (CCL3), and RANTES (CCL5) in the cell culture supernatants were analyzed by using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions. Cells were pretreated with LPS (1 μg/mL) for 60 minutes with or without Miodesin (200 μg/mL) for 6 h. The cell culture supernatant (100 μL) was collected to determine the levels of cytokines and chemokines, according to the manufacturer's instructions.
2.5. NO Analysis
NO levels were determined by measuring the quantity of nitrite in the cell culture supernatant using a Griess reagent. Chondrocytes, keratinocytes and RAW 264.7 (macrophages) cells were pretreated with LPS (1 μg/mL) for 60 minutes with or without Miodesin (200 μg/mL) for 24 h. The cell culture supernatant (100 μL) was mixed with a 100 μL Griess reagent, and the absorbance was measured at 540 nm. The nitrite concentrations were calculated using a standard calibration curve prepared from different concentrations of sodium nitrite.
2.6. Reverse Transcription-Quantitative PCR (RT-qPCR)
Total RNA extracted from cell samples was converted to cDNA using a SuperScript® III RT kit (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. The concentration of RNA was detected using a NanoDrop 2000 (Thermo Fisher Scientific, Inc.). GAPDH and 18S rRNA were used as the internal control. The thermocycling conditions were as follows: 95°C for 10 min followed by 35 cycles of 95°C for 15 sec and 55°C for 40 sec. The 2-ΔΔCq method was used to quantify the relative gene expression levels of the target genes. Relative standard curves were generated by serial dilutions, and all samples were run in triplicate. Table 1 indicates the sense and antisense sequences of primers used in qRT-PCR analysis.
Table 1.
| Gene | Primer sequences |
|---|---|
| NF-κβ | Forward 5′-ATGGCTTCTATGAGGCTGAG-3′ |
| Reverse 5′-GTTGTTGTTGGTCTGGATGC-3′ | |
| COX-1 | Forward 5′-AGGAGATGGCTGCTGAGTTGG-3′ |
| Reverse 5′-AATCTGACTTTCTGAGTTGCC-3′ | |
| COX-2 | Forward 5′-ACACCTTCAACATTGAAGACC-3′ |
| Reverse 5′-ATCCCTTCACTAAATGCCCTC-3′ | |
| PLA2 | Forward 5′-AAAGAACACTATAGGGAGAG-3′ |
| Reverse 5′-AAAGAGGTAAAGGGCATTGT-3′ | |
| iNOS | Forward 5′-CTATCAGGAAGAAATGCAGGAGAT-3′ |
| Reverse 5′-GAGCACGCTGAGTACCTCATT-3′ | |
| CCL2 | Forward 5′-GATCCCAATGAGTAGGCTGG-3′ |
| Reverse 5′-CGGGTCAACTTCACATTCAAAG-3′ | |
| CCL3 | Forward 5′-ACACCAGAAGGATACAAGCAG-3′ |
| Reverse 5′-CGATGAATTGGCGTGGAATC-3′ | |
| CCL5 | Forward 5′-CCCACGTCAAGGAGTATTTCTAC-3′ |
| Reverse 5′-CTAGGACTAGAGCAAGCGATG-3′ | |
| Dnmt1 | Forward 5′-GGTTCTTCCTCCTGGAGAATGTC-3′ |
| Reverse 5′-GGGCCACGCCGTACTG-3′ | |
| Dnmt3A | Forward 5′-CAATGACCTCTCCATCGTCAAC-3′ |
| Reverse 5′-CATGCAGGAGGCGGTAGAA-3′ | |
| Dnmt3B | Forward 5′-CCATGAAGGTTGGCGACAA-3′ |
| Reverse 5′-TGGCATCAATCATCACTGGATT-3′ | |
| 18S | Forward 5′-AACTGCGGAATGGCTCATTAAATC-3′ |
| Reverse 5′-TTGATCTGATAAATGCACGCATC-3′ | |
| GAPDH | Forward 5′-CGGTGTGAACGGATTTGGC-3′ |
| Reverse 5′-GTGAGTGGAGTCATACTGGAAC-3′ |
2.7. Quantification of the 5-mC Content in Genomic DNA
The genomic DNA cytosine methylation levels in cell lines exposed to Miodesin (200 μg/mL) at 24 h were assessed by using an enzyme-linked immunosorbent assay-based commercial kit (MDQ1, Imprint® Methylated DNA Quantification Kit, Sigma-Aldrich). DNA at a concentration of 150 ng was diluted with 30 μL of binding buffers and incubated at 60°C. The samples were incubated with capture and detection antibodies, and the absorbance was read at 450 nm. Quantification of DNA methylation was obtained by calculating the amount of methylated cytosines in the sample relative to methylation in a positive control, which was provided by the manufacturer.
2.8. Statistical Analysis
The obtained results were expressed as mean ± standard error of mean (SEM) from at least three independent experiments, unless stated otherwise. Paired data was evaluated by Student's t-test. One-way analysis of variance (ANOVA) was used for multiple comparisons, followed by the Bonferroni test for comparison among the groups. A p value of <0.05 was considered significant.
3. Results
3.1. Effects of Miodesin™ on Cell Viability and on LPS-Induced Cell Cytotoxicity
Figure 1 shows the different concentrations of Miodesin™, which were tested for cell toxicity to determine the IC50 value. The dose of 200 μg/mL was defined as the study dose. Figures 1(a)– 1(c) show the results for keratinocytes, chondrocytes, and macrophages, respectively. In addition, from the IC50 dose (200 μg/mL), Miodesin™ was able to reverse the cytotoxicity caused by LPS in all these cell lines (Figure 1(d)).
Figure 1.
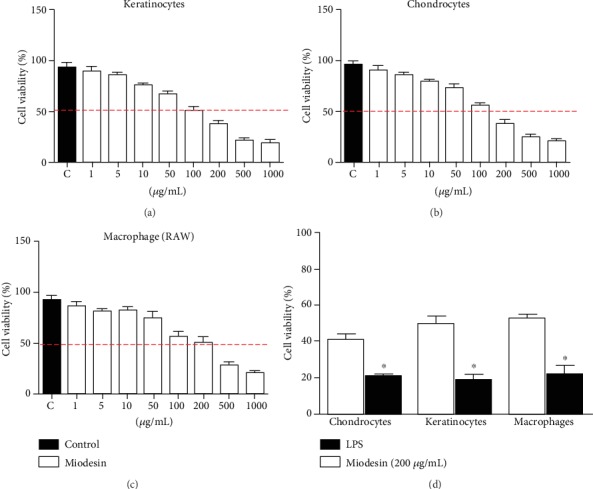
Effects of Miodesin™ on cell line viability and cell toxicity. Cells were treated with different concentrations of Miodesin™ for 24 h, and the IC50 was defined as the study test concentration (200 μg/mL) for all cell lines. (a) Chondrocytes, (b) keratinocytes, and (c) macrophages. Effects of Miodesin™ on LPS-induced cytotoxicity. Date shown are representative of three independent experiments. The values are expressed as mean ± SEM, and ∗p < 0.01 indicates statistical difference (unpaired t-test).
3.2. LPS-Induced Inflammatory Cytokine and Chemokine Release in Cell Lines Is Regulated by Miodesin™
Figure 2 shows the interleukin levels in the supernatant of chondrocytes (Figures 2(A.1) IL-1β, 2(A.2) IL-6, 2(A.3) IL-8, and 2(A.4) TNF-α), keratinocytes (Figures 2(B.1) IL-1β, 2(B.2) IL-6, 2(B.3) IL-8, and 2(B.4) TNF-α), and macrophages (Figures 2(C.1) IL-1β, 2(C.2) IL-6, 2(C.3) IL-8, and 2(C.4) TNF-α). The results demonstrated that while LPS significantly increased the levels of IL-1β, IL-6, IL-8, and TNF-α for all cell types (#p < 0.01), Miodesin™ significantly reduced the levels of IL-1β, IL-6, IL-8, and TNF-α for all cell types (∗p < 0.01).
Figure 2.
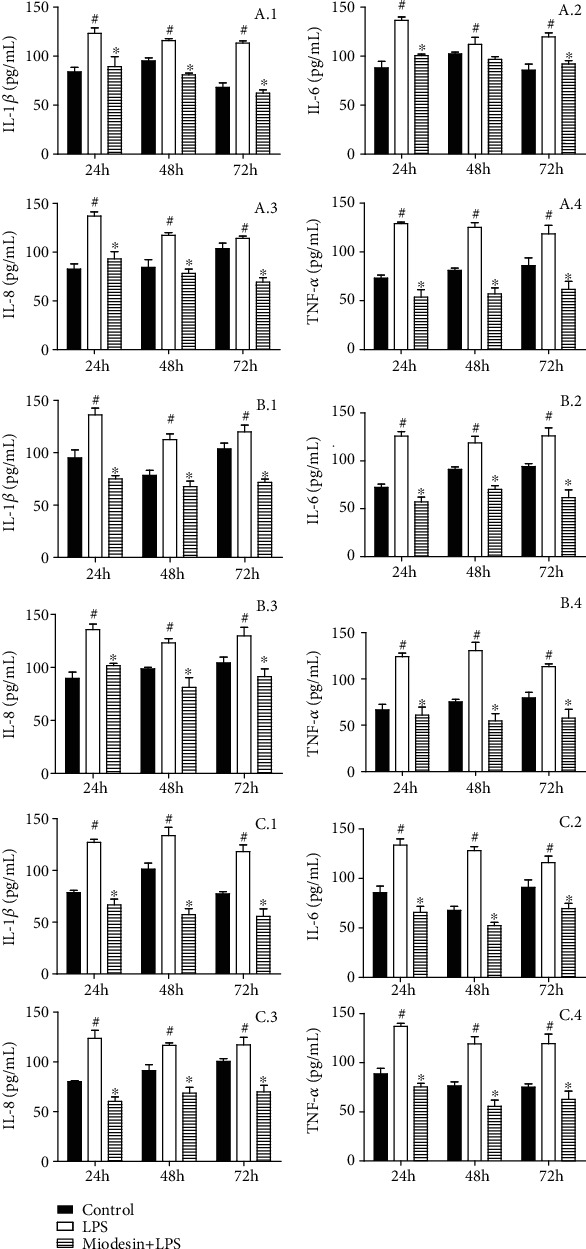
Cells were pretreated with Miodesin™ (200 μg/mL) for 2 h and treated with LPS (1 μg/mL) for 24 h. Values were expressed as mean ± SEM. #p < 0.01 vs. control (nontreated cells), and ∗p < 0.01 vs. LPS-treated cells.
In addition, Figure 3 shows that all cell types responded similarly to LPS stimulation, since LPS increased the levels of CCL2 (Figure 3(a); #p < 0.01), CCL3 (Figure 3(b); #p < 0.01), and CCL5 (Figure 3(c); #p < 0.01), while Miodesin™ significantly reduced the levels of CCL2 (Figure 3(a); ∗p < 0.01), CCL3 (Figure 3(b); ∗p < 0.01), and CCL5 (Figure 3(c); ∗p < 0.01).
Figure 3.
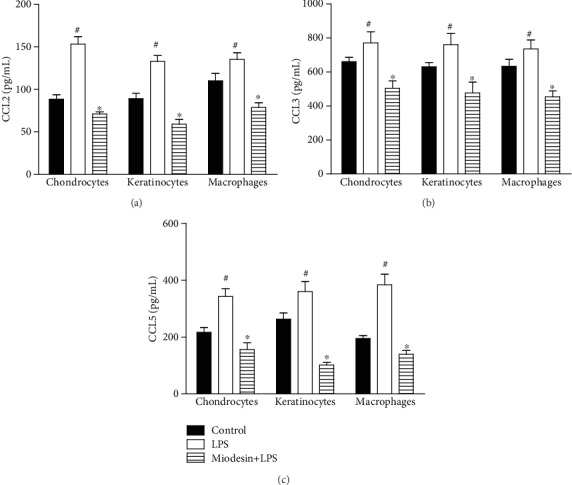
Cells were pretreated with Miodesin™ (200 μg/mL) for 2 h and treated with LPS (1 μg/mL) for 24 h. Values were expressed as mean ± SEM. #p < 0.01 vs. control (nontreated cells), and ∗p < 0.01 vs. LPS-treated cells.
3.3. Miodesin™ Inhibits LPS-Induced Nitric Oxide (NO) Release
The NO levels released by chondrocytes, keratinocytes, and macrophages are presented in Figure 4. The results demonstrated that LPS significantly induced NO release by chondrocytes (Figure 4, #p < 0.01), keratinocytes (Figure 4, #p < 0.01), and macrophages (Figure 4, #p < 0.01), while Miodesin™ abolished such effects in all cell types tested: chondrocytes (Figure 4, ∗p < 0.01), keratinocytes (Figure 4, ∗p < 0.01), and macrophages (Figure 4, ∗p < 0.01).
Figure 4.
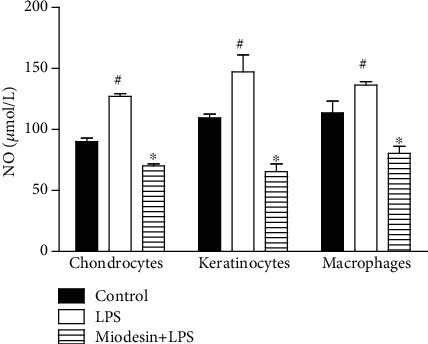
Cells were pretreated with Miodesin™ (200 μg/mL) for 2 h and treated with LPS (1 μg/mL) for 24 h. Values were expressed as mean ± SEM. #p < 0.01 vs. control (nontreated cells), and ∗p < 0.01 vs. LPS-treated cells.
3.4. Expression of Cytokine and Inflammatory Mediators at mRNA Levels in Cell Lines
Figure 5 shows the effects of Miodesin™ on the mRNA levels of the nuclear transcription factor (NF-κβ), inflammatory enzymes (COX-1, COX-2, PLA2, and iNOS), and chemokines (CCL2, CCL3, and CCL5) on chondrocytes (Figure 5(a)), keratinocytes (Figure 5(b)), and macrophages (Figure 5(c)). The results demonstrated that LPS significantly induced the expression of the nuclear transcription factor (NF-κβ; #p < 0.01), inflammatory enzymes (COX-1, COX-2, PLA2, and iNOS; #p < 0.01), and chemokines (CCL2, CCL3, and CCL5; #p < 0.01) on chondrocytes (Figure 5(a)), keratinocytes (Figure 5(b)), and macrophages (Figure 5(c)), while Miodesin™ inhibited such effects in all cell types tested: chondrocytes (Figure 5(a), ∗p < 0.01), keratinocytes (Figure 5(b), ∗p < 0.01), and macrophages (Figure 5(c), ∗p < 0.01).
Figure 5.
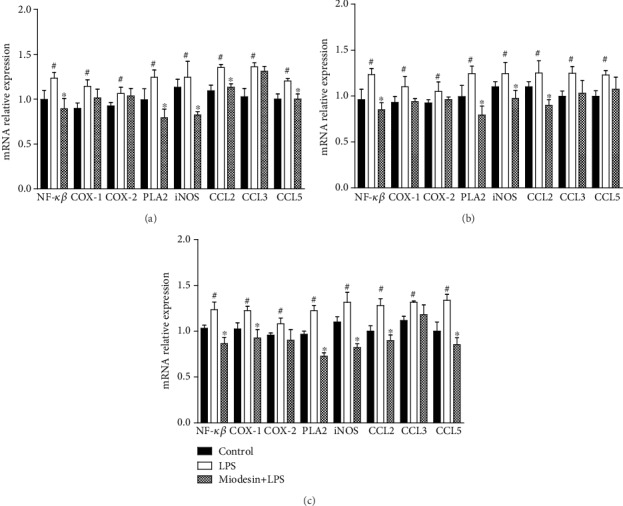
Effects of Miodesin™ on the expression of mRNA of the nuclear transcription factor (NF-κβ), inflammatory enzymes (COX-1, COX-2, PLA2, and iNOS), and chemokines (CCL2, CCL3, and CCL5) in chondrocytes (a), keratinocytes (b), and macrophages (c). Values are expressed as mean ± SEM. #p < 0.01 vs. control (nontreated cells), and ∗p < 0.01 vs. LPS-treated cells.
3.5. Miodesin™ Promotes Downregulation in Global DNA Methylation and Decreases Dnmt mRNA Levels
Cells were treated with 200 μg/mL of Miodesin™; the DNA methylation showed no significant reduction in any of the cell lines evaluated, in comparison to the control at 24 h as estimated using the Imprint® Methylated DNA Quantification Kit (Figure 6(a)). To verify whether the changes observed in DNA methylation was accompanied by changes in the expression of Dnmt genes, the mRNA levels of Dnmt1, Dnmt3A, and Dnmt3B genes in the studied cell lines were quantified after incubation with Miodesin™ 200 μg/mL for 24 h. Miodesin™ did not reduce significantly the endogenous Dnmt1, Dnmt3A, and Dnmt3B mRNA levels in cell lines (Figure 6(b)).
Figure 6.
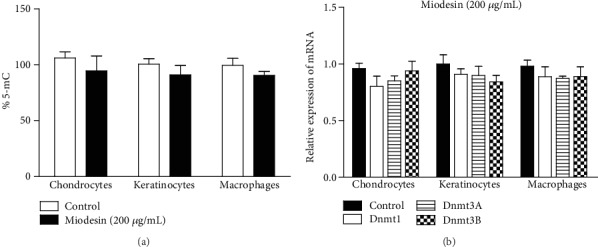
Global DNA methylation and mRNA levels of Dnmts. (a) Methylation status of genomic DNA cytosine methylation in Miodesin™-treated cells. Detection of 5-mC present in the genomic DNA of the control and Miodesin™-treated cells. Methylation was estimated by an enzyme-linked immunosorbent assay-based Imprint® Methylated DNA Quantification Kit (Sigma-Aldrich), which specifically detects 5-mC in the input DNA. Data are represented as percent cytosine methylation as compared with the control. (b) Representative results of fold change difference of Dnmt1, Dnmt3A, and Dnmt3B. Data are expressed as mean ± SEM in three independent experiments, representing the relative levels with normalization by 18S ribosomal RNA. The fold differences were calculated compared with the control groups and shown in the figure. No statistical differences were found.
4. Discussion
The present study is the first to demonstrate the mechanism of action of Miodesin™ in reducing inflammatory markers such as interleukins, tumor necrosis factor-alpha (TNF-α), chemokines, and nitric oxide (NO) that are triggered by the chondrocytes, keratinocytes, and inflammatory cells [18]. In addition, a probable involvement in epigenetic mechanisms may be involved in the anti-inflammatory action of Miodesin™. The presence of the phytocomplex formed by Uncaria tomentosa (cat's claw), Endopleura uchi, known as uxi, and astaxanthin, the xanthophyll carotenoid, guarantees Miodesin™ anti-inflammatory and antioxidant activity.
The presence of Uncaria tomentosa in the formulation gives Miodesin™ a variety of bioactive secondary metabolites, including tetra- and pentacyclic oxindole alkaloids, glycosides, polyoxygenated triterpenes, and procyanidins [19]. Most investigators attribute the biological effects of this plant to the oxindole alkaloids, an assumption that has been corroborated by studies of several such alkaloids that indicated their antioxidant, immunomodulatory, and antineoplastic properties [20]. The anti-inflammatory effects of Uncaria tomentosa could be due to its inhibition of lipopolysaccharide- (LPS-) dependent expression of tumor necrosis factor-alpha (TNF-α) [11] by reduction of the expression of the transcription factor nuclear factor κ light-chain enhancer of activated B cells (NF-κβ) [21, 22], an effect that, in turn, regulates the expression of tumor necrosis factor-alpha (TNF-α). By reducing the expression of NF-κβ, Uncaria tomentosa also reduces the TNF-α levels and intensifies its anti-inflammatory action [21, 23].
Phytochemical studies with Endopleura uchi, which is an Amazon species traditionally used for the treatment of inflammations and female disorders, indicate that the compound bergenin (coumarin) is responsible for the anti-inflammatory action [12, 24], although it indicates the presence of tannins and saponins in the barks [25, 26]. Bergenin, the most abundant molecule, has anti-inflammatory action, apparently through mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-κβ) inhibition (N), in addition to inhibiting the action of cycloxygenase-2 [27].
Astaxanthin is a well-known carotenoid, since many studies in recent years have demonstrated its inhibitory role against oxidative stress and inflammation, dangerous processes at the basis of many chronic diseases [28]. Astaxanthin exerts its anti-inflammatory effect by inhibiting nuclear translocation of NF-κβ p65 and by preventing ROS accumulation in NRF2-dependent and NRF2-independent mechanisms [29].
The treatment of chondrocytes with Miodesin™ reduced the secretion of interleukins and chemokines, corroborating data found in the literature that show the action of Uncaria tomentosa in osteoarthritis [30–33]. It is worth mentioning the reduction in the secretion of two other interleukins, IL-17 and IL-23, that have been found in osteoarthritis joints which also cause destructive proteases and induction of the synthesis of NO [30], in addition to being considered those which play crucial roles in the induction of local inflammation and cartilage destruction diseases such as rheumatoid arthritis [34]. The effects of Miodesin™ on the expression of mRNA of inflammatory enzymes, NF-κβ, and chemokines in cell lines in the presence or absence of LPS were also evaluated. NF-κβ, PLA2, iNOS, and CCL5 showed significantly reduced mRNA levels, while COX-1, COX-2, and CCL3 showed a reduction, but not significant. It is noteworthy that CCL3 secretion was significantly reduced when chondrocytes were challenged with LPS. The reduction in mRNA expression of the iNOS enzyme, associated with a reduction in NO in the treated chondrocytes, suggests an important role of Miodesin™ in NO modulation [35]. Our results also showed that treatment with Miodesin™ reduced the levels of NF-κβ mRNA. Thus, we suggest that Miodesin™ can regulate the function of NF-κβ, which has a mechanism associated with the destruction of cartilaginous tissue [36]. Also, in this sense, NF-κβ regulates the production of matrix metalloproteinases by chondrocytes that are released promoting matrix degradation [37]. Chemokine CCL5 was increased in chondrocytes after in vitro treatment with LPS. However, when chondrocytes were previously treated with Miodesin™, they showed a significant reduction in the secretion of this chemokine, as well as mRNA levels, suggesting an important role of Miodesin™ in the modulation of this chemokine, which has a primary function of attracting lymphocytes and monocytes as well as other cell types [38, 39] and of activating rheumatoid arthritis synovial fibroblasts (RASFs) to promote MMP-1 and MMP-13 mediated ECM destruction [40].
Keratinocytes form the first line of defense against microorganisms, physical or chemical tissue damage, in addition to producing cytokines that regulate the migration of inflammatory cells, activation of immune responses, and proliferation and differentiation of keratinocytes and fibroblasts [3]. In addition, these cells mediate the skin's immune response by secreting various proinflammatory cytokines [41] and recruit immune cells to the site of insult [42]. Regarding keratinocytes, Miodesin™ reduced the secretion of IL-8, IL-1B, and IFN-γ. IL-17 showed a reduction after 72 hours of treatment. Regarding the evaluated chemokines, although all showed a reduction, only CCL2 (MCP-1) showed a significant reduction compared to the control (cells without treatment). CCL2 mRNA levels were also significantly reduced after previous treatment with Miodesin™. Monocyte chemoattractant protein-1 (MCP-1/CCL2) is one of the key chemokines that regulate migration and infiltration of monocytes/macrophages [43]. Upregulated expression of chemokines such as RANTES/CCL5 and MCP-1/CCL2 has been detected in the epidermis of patients with both atopic dermatitis and psoriasis [44]; in addition, with TNF-α being a potent inducer of IL-8, whose high production is associated with psoriasis, but not in the skin of patients with atopic dermatitis or healthy skin, we can suggest that, due to the reduced production of TNF-α by Miodesin™, the phytocomplex could present itself as an interesting adjunctive therapy option in the treatment of psoriasis [45, 46]. Interestingly, a reduction in IFN-γ levels was observed, since IFN-γ-activated keratinocytes express a wide range of cytokines, chemokines, and membrane molecules that direct the recruitment, activation, and retention of specific leukocyte subpopulations in the skin [47].
Innate immunity cells, such as macrophages, trigger a rapid immune response, being able to secrete various types of cytokines [48]. In this way, we use RAW 264.7 (macrophages) cells associated with LPS to stimulate these macrophages to produce inflammatory mediators. The results found showed that Miodesin™ inhibited NO production and suppressed iNOS mRNA levels in LPS-stimulated cells. In addition, Miodesin™ also significantly reduced the secretion of cytokines, such as TNF-α, IL-6, IL-8, and IL-1β. The results of the evaluated chemokines showed that CCL2/MCP-1 CCL3/MIP-1α were significantly reduced when compared to RAW 264.7 (macrophage) cells stimulated with LPS. The levels of NF-κβ, COX-1, PLA2, CCL2, and CCL5 mRNA were also reduced in RAW 264.7 (macrophage) cells when compared to cells stimulated with LPS. The results obtained suggest that the anti-inflammatory action of Miodesin™ is due, at least in part, to the action of the components of its formulation, either by the action of astaxanthin, reducing proinflammatory cytokine secretion, e.g., IL-1β, IL-6, and TNF-α, and reducing NF-κβ nuclear expression [49, 50]; by the action of Uncaria tomentosa, promoting the inhibition of interleukins such as IL-1β, IL-17, and TNF-α, in addition to the inhibition of NF-κβ [10, 51, 52]; or by the action of Endopleura uchi [27, 53], inhibiting the action of cyclooxygenases (COX-1 and COX-2) and phospholipase A2 (PLA2), reducing the production of proinflammatory cytokines (IFN-γ and TNF-α). Our results, therefore, show the anti-inflammatory activity of Miodesin™, by regulating the expression and/or secretion of several important inflammatory biomarkers.
Finally, we evaluated if Miodesin™ promotes changes in the global DNA methylation and in the levels of mRNA of the enzymes called methylases (Dnmt1, Dnmt3A, and Dnmt3B). The results obtained indicated no significant alterations in global DNA methylation in all cell lines evaluated. The levels of mRNA of the enzymes called methylases were not statistically reduced, corroborating the findings of global DNA methylation. These findings seem to be in accordance with the current evidence which suggests a potential role of epigenetics on the level of inflammatory markers reporting on the association of inflammation with global DNA methylation showing a hypomethylation trend [54–56]. The evidence reinforces the role of epigenetic changes in the modification and modulation of transcription factors, leading to the deregulation of multiple cascades of intracellular signaling [57]. In this sense, the literature shows that some bioactive molecules present in components of the formulation of Miodesin™ could have an epigenetic effect, as in the case of hirsutine from the genus Uncaria, such as Uncaria rhynchophylla [58] and Uncaria tomentosa [59], which exerts its epigenetic action inhibiting the activation of NF-κβ [58]. Thus, NF-κβ could become a possible target in anti-inflammatory therapy, since it is able to regulate epigenetic changes associated with inflammation [60]. The correct understanding of these aspects may generate strategies for the development of new therapeutic approaches, opening space for future studies with the aim of investigating the participation of Miodesin™ in epigenetic mechanisms related to the control of different inflammatory processes.
Acknowledgments
This study was supported by a research grant from Fagron do Brasil Pharmaceutical™ LTDA.
Data Availability
The authors declare that all raw data presented in this manuscript will be available upon request.
Conflicts of Interest
All authors disclose any influence of companies or manufacturers in the present study. In addition, all authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
References
- 1.Varela M. L., Mogildea M., Moreno I., Lopes A. Acute inflammation and metabolism. Inflammation. 2018;41(4):1115–1127. doi: 10.1007/s10753-018-0739-1. [DOI] [PubMed] [Google Scholar]
- 2.Asahina R., Maeda S. A review of the roles of keratinocyte-derived cytokines and chemokines in the pathogenesis of atopic dermatitis in humans and dogs. Veterinary Dermatology. 2017;28(1):p. 16-e5. doi: 10.1111/vde.12351. [DOI] [PubMed] [Google Scholar]
- 3.Lee J. H., Moon J. H., Lee Y. J., Park S. Y. SIRT1, a class III histone deacetylase, regulates LPS-induced inflammation in human keratinocytes and mediates the anti-inflammatory effects of hinokitiol. Journal of Investigative Dermatology. 2017;137(6):1257–1266. doi: 10.1016/j.jid.2016.11.044. [DOI] [PubMed] [Google Scholar]
- 4.Chambers E. S., Vukmanovic-Stejic M. Skin barrier immunity and ageing. Immunology. 2019 doi: 10.1111/imm.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falcone M., Concia E., Giusti M., et al. Acute bacterial skin and skin structure infections in internal medicine wards: old and new drugs. Internal and Emergency Medicine. 2016;11(5):637–648. doi: 10.1007/s11739-016-1450-6. [DOI] [PubMed] [Google Scholar]
- 6.Huang X., You Y., Xi Y., et al. p-Coumaric acid attenuates IL-1β-induced inflammatory responses and cellular senescence in rat chondrocytes. Inflammation. 2020;43(2):619–628. doi: 10.1007/s10753-019-01142-7. [DOI] [PubMed] [Google Scholar]
- 7.Shen J., Abu-Amer Y., O'Keefe R. J., McAlinden A. Inflammation and epigenetic regulation in osteoarthritis. Connective Tissue Research. 2017;58(1):49–63. doi: 10.1080/03008207.2016.1208655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao K., Zhu W., Li H., et al. Association between cytokines and exosomes in synovial fluid of individuals with knee osteoarthritis. Modern Rheumatology. 2019;19:1–7. doi: 10.1080/14397595.2019.1651445. [DOI] [PubMed] [Google Scholar]
- 9.Udalova I. A., Mantovani A., Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nature Reviews Rheumatology. 2016;12(8):472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- 10.Azevedo B. C., Morel L. J. F., Carmona F., et al. Aqueous extracts from Uncaria tomentosa (Willd. ex Schult.) DC. reduce bronchial hyperresponsiveness and inflammation in a murine model of asthma. Journal of Ethnopharmacology. 2018;218:76–89. doi: 10.1016/j.jep.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Allen L., Buckner A., Buckner C. A., Cano P., Lafrenie R. M. Uncaria tomentosa (Willd. ex Schult.) DC (Rubiaceae) sensitizes THP-1 cells to radiation-induced cell death. Pharmacognosy Research. 2017;9(3):221–229. doi: 10.4103/pr.pr_83_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peixoto H., Roxo M., Silva E., et al. Bark extract of the Amazonian tree Endopleura uchi (Humiriaceae) extends lifespan and enhances stress resistance in Caenorhabditis elegans. Molecules. 2019;24(5):p. 915. doi: 10.3390/molecules24050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Politi F. A. S., Mello J. C. P., Migliato K. F., Nepomuceno A. L. A., Moreira R. R. D., Pietro R. C. L. R. Antimicrobial, cytotoxic and antioxidant activities and determination of the total tannin content of bark extracts Endopleura uchi. International Journal of Molecular Sciences. 2011;12(4):2757–2768. doi: 10.3390/ijms12042757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recio M. C., Andujar I., Rios J. L. Anti-inflammatory agents from plants: progress and potential. Current Medicinal Chemistry. 2012;19(14):2088–2103. doi: 10.2174/092986712800229069. [DOI] [PubMed] [Google Scholar]
- 15.Wan F. C., Zhang C., Jin Q., et al. Protective effects of astaxanthin on lipopolysaccharide-induced inflammation in bovine endometrial epithelial cells. Biology of Reproduction. 2020;102(2):339–347. doi: 10.1093/biolre/ioz187. [DOI] [PubMed] [Google Scholar]
- 16.Maia H., Saback W., Haddad C., Sitya P. R. Treatment of endometriosis and leiomyoma with the association of Miodesin and Gestrinone in Pentravan through the vaginal route. Journal of Clinical Review & Case Reports. 2018;3(7) doi: 10.33140/jcrc/03/07/00011. [DOI] [Google Scholar]
- 17.Maia H., Saback W., Haddad C., Sitya P. R. Effect of vaginal Miodesin™ in Pentravan™ on the response to progestin therapy in patients with deep endometriosis and adenomyosis. Journal of Clinical Review & Case Reports. 2019;3(7):1–5. [Google Scholar]
- 18.Kumar V., Abbas A., Fausto N. Robbins and Cotran Pathologic Basis of Disease. 7th. St. Louis, MO, USA: Elsevier Inc; 2005. [Google Scholar]
- 19.de Paula L. C. L., Fonseca F., Perazzo F., et al. Uncaria tomentosa (cat’s claw) improves quality of life in patients with advanced solid tumors. The Journal of Alternative and Complementary Medicine. 2015;21(1):22–30. doi: 10.1089/acm.2014.0127. [DOI] [PubMed] [Google Scholar]
- 20.Dreifuss A. A., Bastos-Pereira A. L., Fabossi I. A., et al. Uncaria tomentosa exerts extensive anti-neoplastic effects against the Walker-256 tumour by modulating oxidative stress and not by alkaloid activity. PLoS One. 2013;8(2, article e54618) doi: 10.1371/journal.pone.0054618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sandoval-Chacon. Antiinflammatory actions of cat’s claw: the role of NF-κB. Alimentary Pharmacology & Therapeutics. 1998;12(12):1279–1289. doi: 10.1046/j.1365-2036.1998.00424.x. [DOI] [PubMed] [Google Scholar]
- 22.Akesson C., Lindgren H., Pero R. W., Leanderson T., Ivars F. An extract of Uncaria tomentosa inhibiting cell division and NF-κB activity without inducing cell death. International Immunopharmacology. 2003;3(13-14):1889–1900. doi: 10.1016/j.intimp.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Allen-Hall L., Arnason J. T., Cano P., Lafrenie R. M. Uncaria tomentosa acts as a potent TNF-α inhibitor through NFκB. Journal of Ethnopharmacology. 2010;127(3):685–693. doi: 10.1016/j.jep.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Silva L. R., Teixeira R. Phenolic profile and biological potential of Endopleura uchi extracts. Asian Pacific Journal of Tropical Medicine. 2015;8(11):889–897. doi: 10.1016/j.apjtm.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 25.de Sá Hyacienth Beatriz M., Brenda S. O., Arlindo C. M. P., et al. Endopleura uchi (Huber) Cuatrec, a medicinal plant with potential anti-inflammatory activity: a review of its phytochemistry and biological activities. African Journal of Pharmacy and Pharmacology. 2019;13(7):76–83. doi: 10.5897/AJPP2019.5000. [DOI] [Google Scholar]
- 26.Politi F. A. Z. Estudos farmacognósticos e avaliação de atividades biológicas de extratos obtidos das cascas pulverizadas de Endopleura uchi (Huber) Cuatrec. Araraquara: (Humiriaceae) Universidade Estadual Paulista; 2009. [Google Scholar]
- 27.Nunomura R. C. S., Oliveira V. G., da Silva S. L., Nunomura S. M. Characterization of bergenin in Endopleura uchi bark and its anti-inflammatory activity. Journal of the Brazilian Chemical Society. 2009;20(6):1060–1064. doi: 10.1590/S0103-50532009000600009. [DOI] [Google Scholar]
- 28.Galasso C., Orefice I., Pellone P., et al. On the neuroprotective role of astaxanthin: new perspectives? Marine Drugs. 2018;16(8):p. 247. doi: 10.3390/md16080247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farruggia C., Kim M.-B., Bae M., et al. Astaxanthin exerts anti-inflammatory and antioxidant effects in macrophages in NRF2-dependent and independent manners. The Journal of Nutritional Biochemistry. 2018;62:202–209. doi: 10.1016/j.jnutbio.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 30.Hardin S. R. Cat's claw: an Amazonian vine decreases inflammation in osteoarthritis. Complementary Therapies in Clinical Practice. 2007;13(1):25–28. doi: 10.1016/j.ctcp.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Akhtar N., Miller M. J. S., Haqqi T. M. Effect of a herbal-leucine mix on the IL-1β-induced cartilage degradation and inflammatory gene expression in human chondrocytes. BMC Complementary and Alternative Medicine. 2011;11(1):p. 66. doi: 10.1186/1472-6882-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castilhos L. G., Rezer J. F., Ruchel J. B., et al. Effect of Uncaria tomentosa extract on purinergic enzyme activities in lymphocytes of rats submitted to experimental adjuvant arthritis model. BMC Complementary and Alternative Medicine. 2015;15:p. 189. doi: 10.1186/s12906-015-0694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed S., Anuntiyo J., Malemud C. J., Haqqi T. M. Biological basis for the use of botanicals in osteoarthritis and rheumatoid arthritis: a review. Evidence-Based Complementary and Alternative Medicine: Ecam. 2005;2(3):301–308. doi: 10.1093/ecam/neh117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Askari A., Naghizadeh M. M., Homayounfar R., et al. Increased serum levels of IL-17A and IL-23 are associated with decreased vitamin D3 and increased pain in osteoarthritis. PLoS one. 2016;11(11, article e0164757) doi: 10.1371/journal.pone.0164757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cassini-Vieira P., Araújo F. A., da Costa Dias F. L., et al. iNOS activity modulates inflammation, angiogenesis, and tissue fibrosis in polyether-polyurethane synthetic implants. Mediators of Inflammation. 2015;2015:9. doi: 10.1155/2015/138461.138461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beg A. A., Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274(5288):782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 37.Miller M. J. S., Mehta K., Kunte S., et al. Early relief of osteoarthritis symptoms with a natural mineral supplement and a herbomineral combination: a randomized controlled trial. Journal of Inflammation. 2005;2(1):p. 11. doi: 10.1186/1476-9255-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed S., Pakozdi A., Koch A. E. Regulation of interleukin-1beta-induced chemokine production and matrix metalloproteinase 2 activation by epigallocatechin-3-gallate in rheumatoid arthritis synovial fibroblasts. Arthritis and Rheumatism. 2006;54(8):2393–2401. doi: 10.1002/art.22023. [DOI] [PubMed] [Google Scholar]
- 39.Koch A. E. Chemokines and their receptors in rheumatoid arthritis: future targets? Arthritis and Rheumatism. 2005;52(3):710–721. doi: 10.1002/art.20932. [DOI] [PubMed] [Google Scholar]
- 40.Agere S. A., Akhtar N., Watson J. M., Ahmed S. RANTES/CCL5 induces collagen degradation by activating MMP-1 and MMP-13 expression in human rheumatoid arthritis synovial fibroblasts. Frontiers in Immunology. 2017;8 doi: 10.3389/fimmu.2017.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutowska-Owsiak D., Selvakumar T. A., Salimi M., Taylor S., Ogg G. S. Histamine enhances keratinocyte-mediated resolution of inflammation by promoting wound healing and response to infection. Clinical and Experimental Dermatology. 2014;39(2):187–195. doi: 10.1111/ced.12256. [DOI] [PubMed] [Google Scholar]
- 42.Pastore S., Mascia F., Mariani V., Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. The Journal of Investigative Dermatology. 2008;128(6):1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 43.Deshmane S. L., Kremlev S., Amini S., Sawaya B. E. Monocyte chemoattractant protein-1 (MCP-1): an overview. Journal of Interferon & Cytokine Research. 2009;29(6):313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giustizieri M. L., Mascia F., Frezzolini A., et al. Keratinocytes from patients with atopic dermatitis and psoriasis show a distinct chemokine production profile in response to T cell–derived cytokines. Journal of Allergy and Clinical Immunology. 2001;107(5):871–877. doi: 10.1067/mai.2001.114707. [DOI] [PubMed] [Google Scholar]
- 45.Bos J. D., De Rie M. A. The pathogenesis of psoriasis: immunological facts and speculations. Immunology Today. 1999;20(1):40–46. doi: 10.1016/s0167-5699(98)01381-4. [DOI] [PubMed] [Google Scholar]
- 46.Nickoloff B. J., Karabin G. D., Barker J. N., et al. Cellular localization of interleukin-8 and its inducer, tumor necrosis factor-alpha in psoriasis. The American Journal of Pathology. 1991;138(1):129–140. [PMC free article] [PubMed] [Google Scholar]
- 47.Federici M., Giustizieri M. L., Scarponi C., Girolomoni G., Albanesi C. Impaired IFN-γ-dependent inflammatory responses in human keratinocytes overexpressing the suppressor of cytokine signaling 1. The Journal of Immunology. 2002;169(1):434–442. doi: 10.4049/jimmunol.169.1.434. [DOI] [PubMed] [Google Scholar]
- 48.Zhong T. Y., Arancibia S., Born R., et al. Hemocyanins stimulate innate immunity by inducing different Temporal patterns of proinflammatory cytokine expression in macrophages. Journal of Immunology. 2016;196(11):4650–4662. doi: 10.4049/jimmunol.1501156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Speranza L., Pesce M., Patruno A., et al. Astaxanthin treatment reduced oxidative induced pro-inflammatory cytokines secretion in U937: SHP-1 as a novel biological target. Marine Drugs. 2012;10(4):890–899. doi: 10.3390/md10040890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kimble L., Mathison B., Chew B. P. Astaxanthin mediates inflammatory biomarkers associated with arthritis in human chondrosarcoma cells induced with interleukin-1β. The FASEB Journal. 2013;27(Supplement 1):638.6–638. 6. [Google Scholar]
- 51.Rojas-Duran R., González-Aspajo G., Ruiz-Martel C., et al. Anti-inflammatory activity of mitraphylline isolated from Uncaria tomentosa bark. Journal of Ethnopharmacology. 2012;143(3):801–804. doi: 10.1016/j.jep.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 52.Sandoval M., Okuhama N. N., Zhang X.-J., et al. Anti-inflammatory and antioxidant activities of cat's claw (Uncaria tomentosa and Uncaria guianensis) are independent of their alkaloid content. Phytomedicine. 2002;9(4):325–337. doi: 10.1078/0944-7113-00117. [DOI] [PubMed] [Google Scholar]
- 53.Patel D. K., Patel K., Kumar R., Gadewar M., Tahilyani V. Pharmacological and analytical aspects of bergenin: a concise report. Asian Pacific Journal of Tropical Disease. 2012;2(2):163–167. doi: 10.1016/S2222-1808(12)60037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao M., Tang J., Gao F., et al. Hypomethylation of IL10 and IL13 promoters in CD4+ T cells of patients with systemic lupus erythematosus. Journal of Biomedicine & Biotechnology. 2010;2010 doi: 10.1155/2010/931018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang Y., Lo M., Cai X., Kuo H. Epigenetic hypomethylation and upregulation of NLRC4 and NLRP12 in Kawasaki disease. Oncotarget. 2018;9(27):18939–18948. doi: 10.18632/oncotarget.24851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez-Jaramillo V., Portilla-Fernandez E., Glisic M., et al. Epigenetics and inflammatory markers: a systematic review of the current evidence. International Journal of Inflammation. 2019;2019:14. doi: 10.1155/2019/6273680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ratovitski E. A. Anticancer natural compounds as epigenetic modulators of gene expression. Current Genomics. 2017;18(2):175–205. doi: 10.2174/1389202917666160803165229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lou C., Takahashi K., Irimura T., Saiki I., Hayakawa Y. Identification of hirsutine as an anti-metastatic phytochemical by targeting NF-κB activation. International Journal of Oncology. 2014;45(5):2085–2091. doi: 10.3892/ijo.2014.2624. [DOI] [PubMed] [Google Scholar]
- 59.Honório I. C. G., Bertoni B. W., Telles M. P. C., et al. Genetic and chemical diversity of Uncaria tomentosa (Willd. ex. Schult.) DC. in the Brazilian Amazon. PLoS One. 2017;12(5, article e0177103) doi: 10.1371/journal.pone.0177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakshatri H., Appaiah H., Anjanappa M., et al. NF-κB-dependent and -independent epigenetic modulation using the novel anti-cancer agent DMAPT. Cell Death & Disease. 2015;6, article e1608 doi: 10.1038/cddis.2014.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all raw data presented in this manuscript will be available upon request.


