Abstract
Oral cancer (OC) is a devastating disease that takes the lives of lots of people globally every year. The current spectrum of treatment modalities does not meet the needs of the patients. The disease heterogeneity demands personalized medicine or targeted therapies. Therefore, there is an urgent need to identify potential targets for the treatment of OC. Abundant evidence has suggested that the components of the protein kinase B (AKT)/ mammalian target of rapamycin (mTOR) pathway are intrinsic factors for carcinogenesis. The AKT protein is central to the proliferation and survival of normal and cancer cells, and its downstream protein, mTOR, also plays an indispensable role in the cellular processes. The wide involvement of the AKT/mTOR pathway has been noted in oral squamous cell carcinoma (OSCC). This axis significantly regulates the various hallmarks of cancer, like proliferation, survival, angiogenesis, invasion, metastasis, autophagy, and epithelial-to-mesenchymal transition (EMT). Activated AKT/mTOR signaling is also associated with circadian signaling, chemoresistance and radio-resistance in OC cells. Several miRNAs, circRNAs and lncRNAs also modulate this pathway. The association of this axis with the process of tumorigenesis has culminated in the identification of its specific inhibitors for the prevention and treatment of OC. In this review, we discussed the significance of AKT/mTOR signaling in OC and its potential as a therapeutic target for the management of OC. This article also provided an update on several AKT/mTOR inhibitors that emerged as promising candidates for therapeutic interventions against OC/head and neck cancer (HNC) in clinical studies.
Keywords: Akt, mTOR, oral cancer, inhibitors, treatment, pathway
1. Introduction
Oral cancer (OC), one of the most common forms of head and neck cancer (HNC), comprises of cancers of the lip, tongue, gum, palate, mouth, the floor of the mouth, gingiva and other parts of the oral cavity. The most common variant of OC is oral squamous cell carcinoma (OSCC), which comprises ~91% of all OC cases [1]. OC is a global health concern as it is one of the highest mortality causing cancers in the world [2]. As per reports from Globocan in 2018, lip and oral cavity cancer account for 354,864 new cases and 177,384 death cases worldwide annually [3]. The incidence of this cancer is significantly high in Southern Asia and the Pacific islands, among which countries like India and Sri Lanka hold the highest position in regards to the male death toll due to OC [3].
The current strategies for the treatment of OC include surgical resection combined with or without radiotherapy or adjuvant chemotherapy. Immunotherapy is another promising approach, but it has not remarkably yielded yet, in the case of OC. Advancements in surgical techniques and the associated therapies for the treatment of OC have undoubtedly increased the five-year survival rate in patients; however, the scenario is still terrifying in particular regions of Asia where the traditional habits of tobacco chewing, smoking, alcohol drinking and betel quid chewing are highly predominant and tertiary healthcare is limited or unavailable for a majority of the population [4,5,6,7,8,9]. These countries demand organized prevention and more access to early detection and treatment of OC.
The high incidence of OC in low resource countries is due to several customary habits such as alcohol consumption, smoking, tobacco chewing and areca nut chewing [10]. OC may also occur due to human papillomavirus (HPV) infection, poor dental care, poor hygiene and consumption of unhealthy food. Additionally, gene polymorphisms and other genetic aberrations also contribute to the pathogenesis of OC. The cancer genome atlas (TCGA) study has revealed that a greater number of the head and neck squamous cell carcinoma (HNSCC) cases have shown an alteration in the protein kinase B (AKT)/ mammalian target of rapamycin (mTOR) pathway [11]. Moreover, compounds like nicotine present in the major risk factor tobacco and the synthetic carcinogen, 4-NQO, have also been reported to induce the activation of the AKT/mTOR pathway [12]. Thus, this pathway is remarkably associated with the development and progression of OC.
In this era of precision medicine, progress has been made in the development of personalized, targeted therapies that might block an individual pathway or a combination of pathways and rescue cancer development or progression. The current commercially available targeted regimen for OC patients includes the epidermal growth factor receptor (EGFR) inhibitor and cetuximab (Cet), also known as Erbitux®. Despite promising results in preclinical studies, resistance to EGFR therapy is a noteworthy limitation for the use of this drug in clinical settings. The increasing incidence of OC thus requires more efficient targeted therapies to be formulated. The AKT/mTOR pathway is a critical signaling axis for cell growth, survival, motility and metabolism in OC. Inhibitors of this pathway have shown positive outcomes in other cancers in the clinic. Therefore, this pathway might serve as an important therapeutic target and targeting this pathway singly or in combination with chemotherapeutic drugs or other targeted therapies might help in the prevention and treatment of OC. This review was an attempt to highlight the significance of the AKT/mTOR pathway in the development and progression of OC and also provide a summary of the natural and synthetic inhibitors of this axis, identified through various preclinical and clinical studies, for the prevention and treatment of OC.
2. The AKT/mTOR Signaling Pathway
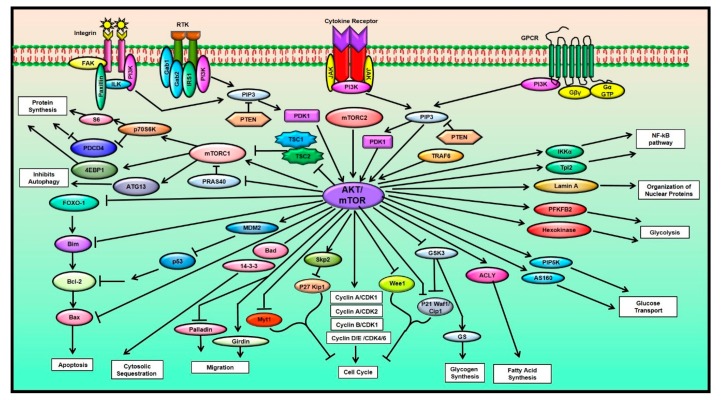
Many different cellular processes such as protein synthesis, autophagy, cell cycle regulation, glycogen metabolism, fatty acid synthesis, nutrient uptake, organization of nuclear proteins, regulation of different hallmarks of cancer like proliferation, survival, angiogenesis, invasion, migration and apoptosis of the cancer cells and the modulation of other signaling pathways such as the nuclear factor kappa-B (NF-κB), extracellular-signal-regulated kinase (ERK), Janus kinase (JAK)-signal transducer and activator of transcription (STAT), are all influenced by the AKT/mTOR signaling pathway [1,13,14,15,16,17] (Figure 1). The key proteins involved in the regulation of this pathway are phosphoinositide 3-kinase (PI3K), AKT and the mTOR proteins. When different growth factors and ligands such as integrins, receptor tyrosine kinases (RTKs), cytokine receptors and G protein-coupled receptors (GPCRs) bind to their respective receptors on the cell membrane, they generate a stimulus and cause the activation of the cell surface receptors, subsequently leading to the phosphorylation of PI3K [18,19,20,21,22,23].
Figure 1.
The AKT/mTOR signaling and its role in various cellular processes (Abbreviations: 4E-BP1: Eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1; ACLY: ATP citrate lyase; AS160: Akt substrate of 160 kDa; ATG13: Autophagy-related protein 13; Bad: Bcl2-associated agonist of cell death; Bax: Bcl 2-associated X protein; Bcl-2: B-cell lymphoma 2; CDK: Cyclin-dependent kinase; Cip1: CDK-interacting protein 1; FAK: Focal adhesion kinase; FOXO-1: Forkhead box protein O1; Gab1: GRB2-associated-binding protein 1; Gab2: GRB2-associated-binding protein 2; Girdin: Girders of actin filament; GPCR: G protein-coupled receptor; GS: Glycogen synthase; GSK3: Glycogen synthase kinase-3; Gα GTP: GTP-bound Gα subunit; Gβγ: G beta-gamma complex; IKKα: IκB Kinase α; ILK: Integrin-linked kinase; IRS1: Insulin receptor substrate 1; JAK: Janus kinase; MDM2: Mouse double minute 2 homolog; mTOR: Mammalian target of rapamycin; mTORC1: Mammalian target of rapamycin complex 1; mTORC2: Mammalian target of rapamycin complex 2; Myt1: Myelin transcription factor 1; P70S6K: 70 kDa ribosomal protein S6 kinase; PDCD4: Programmed cell death protein 4; PDK1: Phosphoinositide-dependent kinase 1; PFKFB2: 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 2; PI3K: Phosphoinositide 3-kinase; PIP3: Phosphatidylinositol-(3,4,5)-trisphosphate; PIP5K: Phosphatidylinositol-4-phosphate 5-kinases; PRA S40: Proline-rich Akt substrate of 40 kDa; PTEN: Phosphatase and tensin homolog; RTK: Receptor tyrosine kinases; S6: Ribosomal protein S6; Skp2: S-Phase kinase-associated protein 2; Tpl2: Tumor progression locus 2; TRAF6: Tumor necrosis factor receptor-associated factor 6; TSC1: Tuberous sclerosis protein 1; TSC2: Tuberous sclerosis protein 2; Wee1: Wee1-like protein kinase).
Phosphoinositide 3-kinase (PI3K), a heterodimer, consists of mainly four classes, IA-PI3K, IB-PI3K, II-PI3K, and III-PI3K, among which class IA-PI3K plays the most crucial role in cancer progression and development [19,24,25]. PI3K helps in the phosphorylation of the D3 hydroxyl group present in the inositol ring of phosphatidyl inositols and catalyzes the transformation of membrane-bound phosphatidylinositol-(4,5)-bisphosphate (PIP2) to phosphatidylinositol-(3,4,5)-trisphosphate (PIP3) [26]. PIP3 acts as a secondary messenger that can be inactivated through the dephosphorylation by phosphatase and tensin homolog (PTEN). This protein mainly recruits two kinases that contain a pleckstrin homology domain (PH domain) to the membrane, i.e., phosphoinositide-dependent kinase 1 (PDK1) and serine/threonine kinase AKT [18,25].
AKT, a member of the AGC (cAMP-dependent protein kinase 1 (PKA)/ cGMP-dependent protein kinase (PKG)/ protein kinase C (PKC)) protein kinase family, consists of a PH domain at the N-terminal and a connecting hinge region at the C-terminal along with a kinase domain. The AKT protein resides in the cytoplasm of the cell in its inactive form. The PH domain of AKT has a high affinity towards PIP3. As already mentioned, PIP3 recruits AKT, and upon binding to AKT it causes the conformational change and exposure of the phosphorylation sites of AKT, which in turn activates the AKT protein. Studies have reported three isoforms of AKT, i.e., AKT1, AKT2 and AKT3. The AKT1 isoform mainly helps in cell survival and the inhibition of apoptosis [27,28,29,30,31,32,33]. The AKT2 isoform modulates the intrinsic mitochondrial pathway of apoptosis, metabolism, cell invasion and migration, while the third isoform, AKT3, functions in the regulation of the migration in tumor cells [13,34]. In a recent study conducted in our laboratory, we demonstrated the isoform-specific role of AKT in OC. The results suggested that the AKT1 and AKT2 isoforms were overexpressed in OC and silencing these isoforms reduced cell survival and caused cell cycle arrest at the G2/M phase besides inhibiting the expression of proteins involved in cell proliferation such as cyclooxygenase-2 (COX-2), cyclin D1 and cell survival such as survivin and the anti-apoptotic protein, B-cell lymphoma 2 (Bcl-2) [1].
In the case of cancers, the enhancement of AKT activity due to somatic mutations results in the impairment of the downstream elements of AKT. The activation of AKT causes the inhibition of proapoptotic proteins, Bcl-2-associated death promoter (Bad) and Bcl-2-associated X protein (Bax). The inhibition of Bad stops its activity to repress the action of anti-apoptotic B-cell lymphoma extra-large (Bcl-xL) protein, which in turn inhibits the process of apoptosis. AKT also inactivates caspases directly involved in apoptosis and forkhead box protein O1 (FOXO-1), which acts as a transcription factor and regulates the expression of proapoptotic genes, including Bcl-2-like protein 11 or Bim and Fas-ligand (FasL) [25,34,35]. AKT is also known to deregulate the activity of glycogen synthase kinase 3 β (GSK-3β) and FOXO, which ultimately leads to the upregulation of cyclin D1 [25,36,37,38,39]. Upon the activation of cyclin D1, it causes an augmentation in the expression of cyclin-dependent kinase (CDK) 4 and 6 to pass the G1-phase and enter the replication phase, i.e., the S-phase, thus enhancing proliferation. Moreover, AKT also regulates the p27 cytoplasmic localization, which plays a crucial role in tumor aggressiveness and metastasis [19,40].
One of the major downstream targets of AKT is the mTOR protein kinase. AKT phosphorylates tuberous sclerosis complex (TSC)-2 and inhibits its expression, which prevents it from activating Ras homolog enriched in brain (RHEB). As a result, the RHEB- Guanosine-5’-triphosphate (GTP) activate the mammalian target of rapamycin complex 1 (mTORC1) by binding to it [41]. Furthermore, mTORC1 triggers the activation of the eukaryotic translation initiation factor 4 (eIF4) complex, which ultimately leads to tumor progression, cell cycle progression and decreased apoptosis. The complete activation of AKT necessitates phosphorylation on serine residues in the C-terminal region by the mammalian target of rapamycin complex 2 (mTORC2). The sites of phosphorylation include serine (Ser)473 in AKT1, Ser474 in AKT2 and Ser472 in AKT 3. The reachability of the active sites governs the activity of mTORC1 and mTORC2, which are controlled by the mTOR associated proteins [42]. Such proteins, Dishevelled, Egl-10 and Pleckstrin (DEP) domain-containing mTOR-interacting protein (DEPTOR), and mammalian lethal with SEC13 protein 8 (mLST) interact with both the mTOR complexes, whereas the regulatory-associated protein of mTOR (RAPTOR), proline-rich AKT substrate of 40 kDa (PRAS40), mitogen-activated protein kinase-associated protein 1 (MAPKAP1) and the rapamycin-insensitive companion of mTOR (RICTOR) interact with mTORC1 and mTORC2, respectively. mTORC1 can regulate the different cell processes via the activation of S6. This protein also regulates translation through the 4E-binding protein 1 (4E-BP1). The PI3K protein and ribosomes with the PH-domain of its MAPKAP1 subunit play a vital role in the activation and the functioning of mTORC2 [43,44,45,46]. These events in the PI3K/AKT/mTOR signaling ultimately create an impact on the survival, protein synthesis, growth, lipid homeostasis, metabolism and cytoskeletal organization of cells.
The AKT/mTOR axis also regulates other essential processes in the cell. For instance, it regulates the deregulation of GSK-3β that is involved in the process of glycogen synthesis [47]. Besides, the components of this pathway also govern the expression of ATP citrate lyase (ACLY) that is known to be involved in fatty acid synthesis [15,48]. Furthermore, this pathway is also involved in the regulation of glucose-transport-involving proteins such as phosphatidylinositol-4-phosphate 5-kinase (PIP5K) and AS160, glycolysis-involving proteins such as hexokinase and 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase 2 (PFKFB2) and the organization of nuclear proteins via Lamin A [49,50,51,52]. Thus, the AKT/mTOR pathway is intrinsic to the regulation of several cellular processes and it plays an essential role in tumorigenesis.
3. The AKT/mTOR Pathway Activation in OC
The activation of the AKT/mTOR pathway or its components is implicated in the pathogenesis of different forms of OC, as shown in Table 1 [47,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94]. The HNSCC tissues which could not be differentiated based on the source have also been included in the table. The investigations discussed in Table 1 provide a brief idea of the association of AKT/mTOR activation with oral tumorigenesis. For example, an immunohistochemical investigation revealed a greater expression of the activated forms of key proteins of the AKT/mTOR pathway such as AKT and mTOR in oral epithelial dysplasia (OED) than OSCC and non-dysplastic oral tissues (NDOT) [70]. In addition, the immunohistochemical analysis of mTOR in both human papillomavirus (HPV) (-) and HPV-associated HNSCC lesions have revealed that it is an important molecular target in OC [95]. The clinical significance of the AKT/mTOR axis has also been evidenced in human ameloblastoma tissues that showed a higher expression of p-AKT and p-mTOR compared to normal oral mucosa [96]. In 2018, Matsuo et al. reported the pathologic significance of AKT and mTOR in OSCC patients. He also hypothesized that the downstream protein of this axis, GSK3β, drove cervical lymph node (CLN) metastasis in OSCC patients [47]. Apart from the other malignancies associated with the oral cavity, a low-grade variant of OSCC, oral verrucous carcinoma (OVC) was also reported. The AKT/mTOR signaling was reported to be involved in the development and progression of OVC [88]. In quest of identifying prognostic and predictive markers for oral cancer, in 2017, Ferreira et al. studied the expression of several proteins in OSCC tissues. This study reported that the proteins, AKT and mTOR, were abundantly found in their active forms in OSCC tissues obtained from the gingiva, hard palate and alveolar ridge, suggesting that the activation of the AKT/mTOR pathway was associated with the development of OSCC [97]. These studies showed the significance of the AKT/mTOR pathway in the development and progression of OC. Thus, the overexpression or upregulation of this pathway induces tumor growth and results in poor prognosis [98].
Table 1.
List of the studies showing the activation of AKT, mTOR or the AKT/mTOR pathway in OC.
| Type of Cancer | In vitro/ In vivo/ Ex vivo | Model | References |
|---|---|---|---|
| HNSCC | In vivo | Grhl3+/– and Grhl3∆/– /K14Cre+ (cKO) mice | [53] |
| In vitro | SCC-25, CAL-27 cells | [53] | |
| In vitro | HSC-3 cells | [54] | |
| In vitro In vitro In vitro |
CAL-27, HN30 cells CAL-27 cells HN6 cells |
[55] [56] [57] |
|
| In vivo | Tgfbr1 cKO mice | [58] | |
| In vitro | NOK-SI cells having SET protein overexpression | [59] | |
| Ex vivo | Primary tumor tissue from patients | [60] | |
| In vitro | HN5 cells | [61] | |
| In vitro | FaDu, SAS cells | [62] | |
| In vitro | IL-6 treated 686LN cells | [63] | |
| In vitro | HGF stimulated and treated UT-SCC-14, UT-SCC-15 and | [64] | |
| UT-SCC-16A cells-injected mice xenografts | |||
| In vitro In vitro In vitro |
PCI-9A, PCI-15 cells WSU-HN6, CAL27 cells Ca9-22, HSC-3 cells |
[65] [66] [67] |
|
| OED | In vitro | DOK cells | [68] |
| OED | In vitro | DOK cells | [69] |
| OED | Ex vivo | Tissue from patients | [70] |
| OED | Ex vivo | Tissue from patients | [71] |
| OED | Ex vivo | Tissue from patients | [72] |
| OED | Ex vivo | Tissue from patients | [73] |
| OEPL | Ex vivo | Tissue from patients | [74] |
| OL | Ex vivo | Tissue from patients | [72] |
| OPSCC | Ex vivo | Primary tumor tissue from patients | [75] |
| OSCC | Ex vivo | Tissue from patients | [70] |
| OSCC | Ex vivo | Tissue from patients | [47] |
| OSCC | In vivo | Keratin 17-knockout HSC3 cells injected BALB/c mice | [67] |
| OSCC | In vitro | HSC-3, HSC-4, CAL-27, UM1, UM2 cells | [71] |
| OSCC | Ex vivo | Tissue from patients | [71] |
| OSCC | Ex vivo | Tissue from patients | [76] |
| OSCC | In vitro | Tca-8113, KB cells | [77] |
| OSCC | In vitro | SCC-25, SCC-4 cells | [78] |
| OSCC | In vitro | HSC-6, CAL-33 cells | [79] |
| OSCC | In vitro | SAS, OECM-1 cells | [80] |
| OSCC | In vitro | OECM-1 cells | [72] |
| OSCC | Ex vivo | Tissue from patients | [72] |
| OSCC | In vitro | KB cells | [81] |
| OSCC OSCC OSCC |
In vitro In vitro In vitro |
SAS cells SCC-9, SCC-25 cells UM-SCC-22A cells |
[82] [68] [69] |
| OSCC | In vitro | OECM-1 cells | [83] |
| OSCC | Ex vivo | Buccal mucosa and other tissues (Stage:1-4, Grade:1, 2 or 3) | [83] |
| OSCC | In vitro | SCC-4, CAL-27 cells | [84] |
| OSCC | Ex vivo | Tissue from patients | [84] |
| OSCC | Ex vivo | Tissue from patients | [85] |
| OSCC | In vitro | AW13516 cells | [86] |
| OSCC | Ex vivo | Primary tumor tissues from patients (Stage: 1-4) | [87] |
| OSCC | Ex vivo | Tissue from patients | [88] |
| OSCC | Ex vivo | Tissue from patients | [74] |
| OVC | Ex vivo | Tissue from patients | [72] |
| OVC | Ex vivo | Tissue from patients | [88] |
| TC TD TSCC TSCC TSCC |
Ex vivo Ex vivo Ex vivo In vitro Ex vivo |
Tissue from patients (Early stage) Tissue from patients Tissue from patients SCC-4, SCC-25 Tissue from patients |
[73] [89] [89] [90] [91] |
| TSCC | In vitro | CAL-27 cells | [92] |
| TSCC | In vitro | UM1 cells | [93] |
| TSCC | In vitro | CAL-27 cells and cisplatin resistant Tca cells | [94] |
Abbreviations: cKO: Conditional knock out, Cre: Cre recombinase, DOK: Dysplastic oral keratinocyte, Grhl3: Grainyhead-like 3, HGF: Hepatocyte growth factor, HNSCC: Head and neck squamous cell carcinoma, K14: Keratin 14, NOKI-SI: Normal Oral Keratinocytes spontaneously immortalized, NOM: Normal oral mucosa, OC: Oral Cancer, OED: Oral epithelial dysplasia, OEPL: Oral epithelial precursor lesions, OL: Oral leukoplakia, OPSCC: Oropharyngeal squamous cell carcinoma, OSCC: Oral squamous cell carcinoma, OVC: Oral verrucous carcinoma, SCC: squamous cell carcinoma, SCID: Severe Combined Immunodeficiency, TC: Tongue cancer, TD: Tongue dysplasia, Tgfbr1: TGF-β receptor I, TSCC: Tongue squamous cell carcinoma.
4. Role of AKT/mTOR Pathway in Different Cellular Processes of OC Cells
The PI3K/AKT/mTOR pathway is altered in around 30.5% of HNSCC patients [99]. This pathway plays an important role in the proliferation, survival, invasion, angiogenesis, migration, protein synthesis and glucose metabolism of cells [100]. The gain of function mutations in the upstream gene, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), induces the oncogenic transformation of the AKT/mTOR signaling, leading to metabolic reprogramming in cells and subsequently causing OC development [101,102]. In 2016, Sonis and Mendes also postulated that the AKT/mTOR pathway drove the transformation of oral cells from benign to the malignant stage [103].
4.1. Proliferation and Survival
One of the most fundamental characteristics of malignant cells is the Warburg effect, which is characterized by enhanced aerobic glycolysis in cancer cells. Aberrant mTOR signaling pathway induces high glucose synthesis by lactic acid fermentation even when oxygen is sufficient (Warburg effect) which aids the survivial and progression of cancer cells [102]. For instance, the immunoregulatory protein B7-H3, also known as CD276, promoted aerobic glycolysis in OSCC cells via the AKT/mTOR aberration-induced augmented expression of hypoxia-inducible factor 1-alpha (HIF-1α), and subsequently, this led to the enhanced proliferation of OSCC cells [104]. The AKT/mTOR pathway also played a significant role in the stathmin-induced proliferation and survival of OSCC cells [105]. Similarly, a metformin derivative, HL156A, was found to inhibit OC cell viability and survival via the inhibition of insulin-like growth factor (IGF)-1/AKT/mTOR [106]. The anti-proliferative effect of Vietnamese coriander, also known as Persicaria odorata, and compound-like zerumbone was also reportedly mediated by the inhibition of the AKT/mTOR axis in OC cells [107,108]. Moreover, histone deacetylase inhibitors (HDACis) such as N-biphenyl-4-sulfonamide and 4-((biphenyl-4-ylsulfonyl) amino)-2-hydroxybenzamide, also showed antiproliferative activity in OC cells via interfering with the AKT/mTOR pathway [109]. Several studies also demonstrated that proteins such as the receptor for activated C kinase 1 (RACK1), dickkopf-related protein 3 (DKK3) and T-cadherin are crucial for the proliferation and survival of OSCC. These proteins regulate the proliferation and survival of OSCC via the activation of the AKT/mTOR pathway [71,110,111]. Besides, keratin 17 also displayed high expression in OC and this protein promoted tumorigenesis via the stimulation of AKT/mTOR signaling and glucose uptake [67].
4.2. Angiogenesis
The AKT/mTOR pathway plays an essential role in regulating the formation of blood vessels in both normal and cancer tissues. Angiogenesis is characterized by the secretion of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (b-FGF) and interleukins such as interleukin-8 (IL-8) by cancer cells [112,113]. These cytokines bind to their receptors and activate the upstream molecules of the AKT/mTOR pathway. The PI3K activates the AKT/mTOR pathway that facilitates angiogenesis and the delivery of growth factors to tumors. On the contrary, cells with aberrant mTOR signaling also adapt and survive under nutrient-deprived and hypoxic conditions via another modified cellular response to hypoxia, nutrient uptake, etc. [114,115]. The role of AKT/mTOR signaling in the regulation of angiogenesis has also been observed in a hamster model of OC [116].
4.3. Epithelial-to-Mesenchymal Transition
The AKT/mTOR pathway also plays a remarkable role in the regulation of epithelial-to-mesenchymal transition (EMT) in cancer. Loss of PTEN is known to induce PI3K oncogenic disruption, which culminates in perturbation in membrane polarity, thereby leading to EMT that further induces invasion [117]. The involvement of AKT/mTOR signaling has been observed in the p70S6K-mediated promotion of IL-6-driven EMT and the metastasis of HNSCC [63]. Recently, Liu et al. also demonstrated that bone marrow mesenchymal stem cells aggravated tumorigenesis by activating an extracellular matrix (ECM) protein, periostin, that trigerred the activation of the PI3K/AKT/mTOR pathway, culminating in enhanced EMT in HNC cells [118]. In addition, the overexpression of epithelial cell adhesion molecules (EpCAMs) has also been reported to regulate EMT in HNC cells through the PTEN/AKT/mTOR pathway [119].
4.4. Invasion and Metastasis
Tumor outgrowths, induced by alterations in the AKT/mTOR pathway, lead to invasion [120]. Upon invasion, the PI3K–PTEN crosstalk controls the chemotaxis and intravasation of cells into endothelial networks [121]. This results in the AKT/mTOR-mediated regulation of angiogenesis, EMT, migration and invasion at the same time, subsequently causing metastasis. In a particular study, a neem limonoid, gedunin, in association with epalrestat, an aldose reductase inhibitor used for the treatment of diabetic neuropathy, was found to inhibit cell migration by inhibiting AKT/mTOR signaling in squamous cell carcinoma (SCC)-131 cells [122]. In another study, C-reactive protein (CRP) increased the invasion and migration of OSCC cells plausibly via the activation of the AKT/mTOR axis [123]. In 2019, Li et al. also reported the AKT/mTOR-mediated migratory and invasive potential of OSCC cells under the Warburg effect [104]. Moreover, studies have also shown the anti-invasive and anti-migratory potential of P. odorata and zerumbone against OSCC cells, mediated by the modulation of AKT-mTOR signaling [107,108].
4.5. Autophagy
Autophagy is a process of lysosome-dependent cell degradation and the removal of damaged components of the cell along with intracellular pathogens [124,125]. It plays a significant role in cellular survival and is known to function via the modulation of the AKT/mTOR pathway in many cases. For instance, honokiol-treated OSCC cells underwent autophagy that was plausibly mediated via the downregulation of the AKT/mTORC1 signaling [126]. The involvement of AKT/mTOR signaling was also reported in Beclin-1-dependent autophagy in OSCC mediated via Tanshinone IIA, a lipophilic compound obtained from the roots of Salvia miltiorrhiza [127]. Similarly, another compound, resveratrol, was also found to exert autophagy in cisplatin-resistant CAR cells via the modulation of AKT/mTOR signaling [128]. Furthermore, the knockdown of neutrophil gelatinase-associated lipocalin (NGAL) activated mTOR and suppressed autophagy, thereby promoting the progression of OC. This study also suggested the involvement of the AKT/mTOR pathway in NGAL-mediated regulation of autophagy in OC cells [9].
4.6. Circadian Cock Signaling
The circadian clock signaling involves genes that maintain the circadian rhythm of the human body. These genes also interfere with the other cellular processes such as proliferation, apoptosis, cellular metabolism, cell cycle, immunity and endocrine signaling. Therefore, the deregulation of the clock signaling has been evidenced in various pathological conditions. The functioning of this signaling pathway requires the involvement of the AKT/mTOR pathway in OC [129,130]. For instance, the loss of circadian clock genes, Per1 and Per2, have been reported to increase the proliferation of OC cells and promote their progression by suppressing autophagy-induced apoptosis in an AKT/mTOR pathway-dependent manner [131,132]. These studies demonstrated the significance of the AKT/mTOR axis in circadian clock signaling.
4.7. Chemoresistance and Radioresistance
The increasing number of evidences suggest the pivotal role of the AKT/mTOR pathway in chemoresistance and radioresistance in cancer cells. Thus, the inhibition of this pathway might help in the reversal of chemoresistance and radioresistance, thereby making this pathway an attractive target for developing cancer therapeutics against OSCC. This pathway has been reported to be involved in chemosensitization mediated by a combination of chemotherapeutic drugs with other drugs. For example, prior treatment of chemoresistant oral epidermoid cancer cells with pantoprazole was found to chemosensitize these cells to vincristine both in vitro and in vivo via the inhibition of the AKT/mTOR pathway, among other related pathways [133]. Similarly, the anti-viral drug Ribavirin was reported to chemosensitize OSCC cells to paclitaxel via the inactivation of proteins such as AKT, mTOR, and eukaryotic translation initiation factor (eIF4E) 4E (4E-BP1) [134]. Additionally, Wang et al. also revealed that acetylshikonin considerably suppressed the growth of cisplatin-resistant OC both in in vitro cellular models and in vivo xenograft mice models by inhibiting the mTOR/PI3K/AKT signaling pathway [135]. In another preclinical study, the significant antitumor effect of a combination of mTOR inhibitor, temsirolimus and an anti-EGFR agent, cetuximab, was observed in an orthotopic model of HNSCC. The synergistic effect of this combination of drugs was also reportedly mediated via the inhibition of the PI3K/mTOR pathway [136].
Radioresistance is another phenomenon in cancer cells where the AKT/mTOR pathway plays a significant role. A study by Gu et al. indicated that tongue cancer resistance-associated protein 1 (TCRP1) mediates radioresistance in OSCC cells by elevating AKT activity and NF-κB level [137]. In 2014, Freudlsperger et al. demonstrated that the inhibition of AKT (Ser473) phosphorylation might overcome radioresistance, thereby decreasing toxicity and ameliorating the efficiency of treatment in advanced HNSCC [138]. Another study by Yu et al. evaluated the efficacy of a second generation mTOR inhibitor, AZD2014, also known as Vistusertib, as a radiosensitizing agent in primary OSCC and OSCC-derived cell lines. The co-treatment of irradiated OSCC cells with AZD2014 exhibited a synergistic dual blockade of mTORC1 and mTORC2/AKT activity and cell cycle arrest, leading to cell-growth inhibition and radiosensitization of the OSCC cells [139]. In 2017, Yu et al. demonstrated that the activation of PI3K/AKT/mTOR signaling contributed to radioresistance in OSCC. This study reported that the dual inhibition of the PI3K/mTOR axis led to the inhibition of cyclin D1/CDK4 activity, thereby inducing G1 phase arrest in OC samples [140]. Thus, the AKT/mTOR pathway is intrinsic to the regulation of chemoresistance and radioresistance in OC cells.
5. MicroRNA (miRNA), Long Noncoding RNA (lncRNA), and Circular RNA (circRNA) Regulate AKT/mTOR Pathway in OC
5.1. MicroRNAs
MicroRNAs (miRNAs) are a class of endogenous, short noncoding RNAs that are highly conserved. They regulate various critical biological processes and are known to be dysregulated in several forms of cancer [141,142]. miRNAs play a prominent role in tumor progression by directly targeting multiple genes. Thus, the investigation of aberrantly expressed miRNAs may play a crucial role in the diagnosis and treatment of OSCC or HNSCC [141,142,143]. The miRNA profiling of primary OSCC tissue specimens has revealed that 46 differentially expressed miRNAs have activated PI3K/AKT signaling genes and disrupted p53 signaling pathways [144]. Genome-wide profiling and functional assays of miRNA-99 (miR-99)-transfected HNSCC cells have revealed that mTOR is the direct target gene of miR-99 and that the increased expression of miR-99 in HNSCC downregulated the expression of mTOR genes [145]. miR-99a was also found to be associated with the enhanced tumor size in OSCC. This miRNA was also reported to be involved in AKT/mTOR signaling [146]. Moreover, miR-218 suppressed the expression of RICTOR, which further inhibited AKT S473 phosphorylation in OSCC [147]. The overexpression of miR-27a* (miR-27a-5p) in HNSCC cells has exhibited a significant cytotoxic effect compared to miR-27a-3p, and it has been found to downregulate AKT1 and mTOR by direct inhibition [148]. In another study, miR-491-3p sensitized OC cells to chemotherapy through the inhibition of mTORC2 activity by directly targeting mTORC2 component RICTOR, and it was upregulated in the drug-resistant OC cells with elevated levels of p-AKT(Ser473), p-FOXO-1(Thr24) and phospho-serum/glucocorticoid regulated kinase 1 (p-SGK1) (Ser422) [149]. Thus, miRNAs play an important role in the regulation of the AKT/mTOR pathway.
5.2. Circular RNAs
Circular RNAs (circRNAs) are another type of non-coding single-stranded closed RNA molecules that are generated as covalently closed continuous loops. Studies have reported that the activation of circRNAs induces tumor migration and invasion in several cancers. Under hypoxic conditions, circCDR1as, a circRNA antisense to the cerebellar degeneration-related protein 1 (CDR1) transcript, was found to enhance autophagy and promote the survival of OC cells by inhibiting apoptosis through the regulation of AKT/ERK1/ERK2/mTOR signaling [150]. The AKT/mTOR signaling pathway played a significant role in the hsa_circ_0007059-mediated regulation of OC cell growth. The elevated expression of hsa_circ_0007059 was found to suppress proliferation, migration and invasion, as well as induce apoptosis in OSCC cells [151].
5.3. Long Noncoding RNAs
Long noncoding RNAs (lncRNA) belong to another class of noncoding RNAs that have a length of more than 200 nucleotides [152,153,154,155]. Congregate evidence has shown that the different anomalous expression of lncRNA is closely associated with the manifestation of several diseases, including tumors [156,157,158]. In 2019, Yang et al. showed the oncogenic effect of lncRNAs in OC. This study demonstrated that the knockdown of lncRNA cancer susceptibility candidate 9 (CASC9) suppressed tumor progression by inhibiting the proliferation of OSCC cells and promoting autophagy-mediated cell apoptosis via the modulation of AKT/mTOR pathway [159]. Similarly, the silencing of the lncRNA homeobox(HOX) transcript antisense RNA (HOTAIR) induced the suppression of the autophagy in OC cells by promoting the activation of mTOR. The knockdown of this lncRNA was reported to increase the rate of apoptosis and enhance the sensitivity of OC cells to cisplatin [160].
6. Therapeutic Effect of AKT/mTOR Inhibitors in OC
The corroboration of the preclinical and clinical studies have implicated that AKT/mTOR inhibitors are emerging therapeutics for the treatment of various cancers. The encouraging outcomes of in vitro and in vivo investigations have instigated the initiation of several clinical trials of the AKT/mTOR inhibitors to determine their safety and efficacy against HNC. These inhibitors have shown remarkable prospects in the treatment of OC or HNC. The efficacy of these inhibitors in both preclinical and clinical settings is presently discussed.
6.1. Inhibitors in Preclinical Studies
It is increasingly evident from several in vitro and in vivo studies that the AKT/mTOR pathway is remarkably involved in the management of OC. Several inhibitors of this pathway, both natural and synthetic, have been identified in preclinical settings. In silico studies have also envisioned the clinical significance of natural inhibitors of AKT [161]. Huge evidence emerging from several investigations has consistently emphasized the AKT/mTOR-dependent anticancer activity of the natural compounds such as piceatannol, boswellic acid, curcumin, honokiol, magnolol, tocotrienol, capsaicin, diosgenin, garcinol, thymoquinone and gambogic acid [162,163,164,165,166,167,168,169,170,171]. Additionally, extracts from plants like Punica granatum and P. odorata have also shown to abrogate this pathway [107,172]. The inhibitors of the AKT/mTOR pathway, identified from preclinical studies, such as compounds like acetylshikonin, artesunate, arglabin, AZD2014, ellagic acid, erfosin, fenofibrate, HL156A, mecambridine, murrayanine, oleic acid, pantoprazole, vincristine, S-allylcysteine, tanshinone IIA, and ursolic acid, PI3K inhibitors such as PI-828, PI-103 and PX-866 and miRNAs such as miR-218 have been listed in Table 2 [106,107,127,133,135,139,147,173,174,175,176,177,178,179,180,181,182,183]. The artesunate-mediated suppression of mitochondrial respiration via AKT/AMP-activated protein kinase (AMPK)/mTOR inhibition was reported in in vitro and in vivo OSCC models [174]. In addition, the targeted inhibition of the AKT/mTOR axis was also obtained with PI3K inhibitors such as PI-828, PI-103 and PX-866 (Sonolisib) in OC cells SCC-4, SCC-9 and SCC-25 [181]. Furtheremore, several other compounds like honokiol, plumbagin, and small molecules such as pyrithione zinc (PYZ) have also demonstrated anti-cancer effects via the inhibition of the AKT/mTOR pathway in OC [126,184,185]. Moreover, the golden nutraceutical, curcumin, which is known to show potent anticancer effects, was found to inhibit the nicotine-induced activation of the AKT/mTOR pathway in OC models in vitro and in vivo [12]. Furthermore, other compounds like escin, zerumbone, oxymatrine and formononetin also demonstrated anti-tumor effects via the inhibition of the AKT/mTOR pathway in several preclinical models [186,187,188,189,190]. Thus, these studies led to the evaluation of AKT/mTOR inhibitors in clinical settings.
Table 2.
List of preclinical studies showing mechanism of action of several AKT/mTOR inhibitors in OC
| Inhibitor | Model | Mechanism of Action | Reference |
|---|---|---|---|
| Acetylshikonin | Both | ↓mTOR/PI3K/AKT pathway, p62, Bcl-2; ↑Beclin-1, LC3-II, Bax | [135] |
| Arglabin | Both | ↓mTOR/PI3K/AKT, Δψm; ↑ROS | [173] |
| Artesunate | Both | ↓AKT/AMPK/mTOR; ↑Mitochondrial dysfunction, ROS | [174] |
| AZD2014 | In vitro | ↓AKT, mTORC1, mTORC2, cyclin D1-CDK4, cyclin B1-CDC2; ↑caspase-3, LC3 | [139] |
| Ellagic acid | In vivo | ↓PI3K/AKT/mTOR, MAPK, VEGF/VEGFR2, HDAC6, HIF-1α | [175] |
| Erufosine | In vitro | ↓AKT/mTOR, Cyclin D1 | [176] |
| Fenofibrate | In vitro | ↓AKT, mTOR, RAPTOR; ↑AMPK | [177] |
| HL156A | Both | ↓AKT, mTOR, IGF-1, ERK1/2, NF-κB-p65, MMP-2, MMP-9↑AMPK, ROS, caspase-3 and -9, p-AMPK | [106] |
| Mecambridine | In vitro | ↓mTOR/PI3K/AKT signaling, MMP; ↑ROS | [178] |
| miR-218 | In vitro | ↓mTOR-AKT signaling pathway, RICTOR | [147] |
| Murrayanine | In vivo | ↓AKT/mTOR and Raf/MEK/ERK pathways; ↑caspase-3, Bax/Bcl-2 | [179] |
| Oleic acid | In vitro | ↓p-AKT, p-mTOR, p-S6K, p-4E-BP1, p-ERK1/2, Cyclin D1, LC3-I/ LC3-II, p62 | [180] |
| Persicaria odorata | In vitro | ↓AKT/mTOR pathway, Cyclin D1, COX-2, survivin, MMP-9, VEGF-A | [107] |
| PI-103, PI-828, PX-866 | In vitro | ↓AKT, mTOR, COX-2, Cyclin-D1, VEGF, PI3Kα, Bcl-2, NF-κB | [181] |
| PPZ + VCR | Both | ↓PI3K/AKT/mTOR pathway | [133] |
| S-Allylcysteine | In vivo | ↓pAKT, mTOR, IκBα, ERK1/2, Cyclin D1, ↓NF-κB p65,↓COX-2, ↓vimentin; ↑E-cadherin, p16 | [182] |
| Tanshinone IIA | In vitro | ↓PI3K/AKT/mTOR pathway; ↑Beclin-1/ATG7/ATG12-ATG5 | [127] |
| Ursolic acid | In vitro | ↓AKT/mTOR/NF-κB signaling, ERK, p38, MMP-2; ↑Caspases | [183] |
Abbreviations: ↑: Upregulation, ↓: Downregulation, Δψm: Mitochondrial membrane potential, 4E-BP1: Eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1, AMPK: AMP-activated protein kinase, ATG: Autophagy related, Bad: Bcl-2-associated agonist of cell death, Bax: Bcl-2-associated X protein, Bcl-2: B-cell lymphoma 2, Bcl-xL: B-cell lymphoma-extra-large, CDC2: Cell division cycle protein 2 homolog, CDK4: Cyclin-dependent kinase 4, COX-2: Cyclooxygenase 2, ERK: Extracellular-signal-regulated kinase, HDAC6: Histone deacetylase 6, HIF-1α: hypoxia-inducible factor 1-alpha, IGF-1: Insulin-like growth factor 1, IκBα: Inhibitor of nuclear factor kappa B, MAPK: Mitogen-activated protein kinase, LC3: Microtubule-associated protein 1A/1B-light chain 3, MEK: Mitogen-activated protein kinase, MMP: Matrix metalloproteinase, mTOR: Mammalian target of rapamycin, NF-κB: nuclear factor kappa-B, PI3K: Phosphoinositide 3-kinase, PPZ: Pantoprazole, pS6K: phosphorylated S6K, RAPTOR: Regulatory-associated protein of mTOR, RICTOR: Rapamycin-insensitive companion of mTOR, ROS: Reactive oxygen species, VCR: Vincristine, VEGF: Vascular endothelial growth factor.
6.2. Inhibitors in Clinical Studies
The number of clinical trials determined to evaluate the efficacy of the inhibitors of AKT, mTOR, or the AKT/mTOR pathway in OC patients is very few. However, several clinical trials have been reported with details of such investigation in HNSCC patients (Table 3) [191,192,193,194,195,196,197,198,199,200,201,202,203,204,205]. Some of these trials have been completed or terminated, while some are still ongoing. The drugs used in clinical trials, for HNC, either as monotherapy or combinatorial therapy, are discussed below.
Table 3.
List of clinical trials showing use of inhibitors of AKT, mTOR or AKT/mTOR in HNC patients.
| Inhibitor | Combinatorial Therapy | Phase | Status | Target | Sample | NCT/REF | Reference |
|---|---|---|---|---|---|---|---|
| Bimiralisib | - | 2 | Recruiting | PI3K/mTOR | HNSCC harboring NOTCH1 LOF mutation |
NCT03740100 | - |
| CC-115 | - | 1 | Active, not recruiting |
AKT/mTOR | HNSCC | NCT01353625 | |
| Everolimus | - | 2 | Completed | mTOR | R/M HNSCC | NCT01051791 | [192] |
| Everolimus | - | 2 | Active, not recruiting | mTOR | HNC | NCT01111058 | |
| Everolimus | - | 1+2 | Active, not recruiting | mTOR | LA-HNSCC | NCT01133678 | |
| Everolimus | Cetuximab | 1 | Completed | mTOR | Recurrent HNC | NCT01637194 | |
| Everolimus | Erlotinib | 2 | Completed | mTOR | Recurrent HNSCC | NCT00942734 | [193] |
| Everolimus | Carboplatin + cetuximab | 1+2 | Completed | mTOR | Advanced HNSCC | NCT01283334 | [194] |
| Everolimus | Carboplatin + paclitaxel | 1+2 | Completed | mTOR | LA-HNSCC | NCT01333085 | [195] |
| Everolimus | Cisplatin + docetaxel | 1 | Completed | mTOR | LA-HNSCC | NCT00935961 | [196] |
| Everolimus | Cisplatin/carboplatin+ cetuximab | 1+2 | Terminated | mTOR | R/M HNSCC | NCT01009346 | |
| Everolimus | Cisplatin + radiotherapy | 1 | Terminated | mTOR | LA inoperable HNC | NCT01057277 | |
| Everolimus | Cisplatin + radiotherapy | 1 | Completed | mTOR | HNC | NCT00858663 | [197] |
| Metformin | - | - | Recruiting | PI3K/AKT | OSCC | NCT03510390 | |
| MK2206 | - | 2 | Completed | AKT | Progressive, R/M of oral cavity and SG | NCT01604772 | [198] |
| MK2206 | - | 2 | Completed | AKT | Recurrent/advanced SCC of nasopharynx | NCT01349933 | |
| Perifosine | - | 2 | Completed | AKT, PI3K | R/M HNSCC | [199] | |
| Rapamycin | - | 1+2 | Completed | mTOR | Stage II-IVA HNSCC | NCT01195922 | [200] |
| Ridaforolimus | MK-0752 | 1 | Completed | mTOR | Metastatic or LA- HNSCC | NCT01295632 | [201] |
| SF1126 | - | 2 | Terminated | PI3K, mTOR | Metastatic HNSCC | NCT02644122 | |
| Temsirolimus | - | 2 | Completed | mTOR | Platinum/cetuximab- refractory HNSCC |
NCT01172769 | [202] |
| Temsirolimus | Cetuximab | 2 | Completed | mTOR | R/M HNSCC | NCT01256385 | |
| Temsirolimus | Carboplatin + paclitaxel | 1+2 | Completed | mTOR | R/M HNSCC | NCT01016769 | [203] |
| Temsirolimus | Erlotinib | 2 | Terminated | mTOR | R/M P-R HNSCC | NCT01009203 | [204] |
| Temsirolimus | Paclitaxel+ low dose weekly carboplatin |
1+2 | Completed | mTOR | R/M HNSCC | [205] |
Abbreviations: ACC: Adenoid cyst carcinoma, HNC: Head and neck cancer, HNSCC: Head and neck squamous cell carcinoma, LA: Locally advanced, LA-SCCHN: Locall advanced squamous cell carcinoma of the head and neck, mTOR: Mammalian target of rapamycin, OSCC: Oral squamous cell carcinoma, PI3K: Phosphoinositide 3-kinase, P-R: Platinum refractory, R/M: Recurrent or metastatic, SCC: Squamous cell carcinoma, SG: Salivary gland.
6.2.1. Bimiralisib
Bimiralisib is mainly a PI3K inhibitor and to some extent, it also inhibits the mTOR kinase. The safety and efficacy of this antineoplastic compound is being evaluated in an ongoing clinical trial in patients with recurrent or metastatic (R/M) HNSCC that bear Notch homolog 1, translocation-associated (NOTCH1) loss of function (LOF) mutations (NCT03740100). The NOTCH1 protein plays a central role in the maintenance of stem cells and determination of the fate of the cells. Studies in the recent past have implicated LOF in NOTCH1 genes in HNSCC samples [206,207].
6.2.2. CC-115
CC-115 is a selective dual mTOR/DNA-dependent protein kinase (DNA-PK) inhibitor. Currently, many clinical trials are undergoing to test the safety and efficacy of this compound in various cancers. In a recent investigation involving HNSCC tumors, this compound has exhibited good tolerability and promising effects (NCT01353625) [208].
6.2.3. Everolimus
Everolimus, also known as Rad001, is an mTOR kinase inhibitor and it has been widely used in HNC patients (NCT01051791, NCT01111058; NCT01133678) [192]. Everolimus was not found to be effective in the form of monotherapy and adverse effects were observed (NCT01051791) [192]. Therefore, several clinical trials were conducted in HNC patients where everolimus was administered in combination with other drugs such as cetuximab, erlotinib, carboplatin and cetuximab, carboplatin and paclitaxel, cisplatin and docetaxel, cisplatin or carboplatin and cetuximab and cisplatin and radiotherapy. Most of these studies have been completed [193,194,195,196] (NCT01637194, NCT00942734, NCT01283334, NCT01333085, NCT00935961) while a few combinations have been discontinued due to severe side effects (NCT01009346, NCT01057277). However, the regime (everolimus, cisplatin and radiotherapy) was well tolerated in HNC patients with promising efficacy (NCT00858663) [197].
6.2.4. Metformin
The antidiabetic drug, metformin, which also possesses the ability to suppress tumor growth, is known to inhibit the PI3K/AKT signaling pathway [209]. This drug is also known to minimize oxygen consumption in cells via the suppression of mitochondrial complex I [210]. Owing to this property, the PI3K/AKT inhibitor, metformin, is being administered to HNSCC patients suffering from tissue hypoxia in order to evaluate the effect of metformin upon hypoxic conditions in tumor (NCT03510390).
6.2.5. MK2206
MK2206 is one of the few AKT inhibitors that have been used against HNC to date. This molecule is an allosteric inhibitor that inhibits the phosphorylation of AKT at sites threonine (T308) and serine (S473). This molecule has been evaluated as a drug for the treatment of R/M adenoid cyst carcinoma of oral cavity and salivary gland and recurrent/advanced SCC of the nasopharynx (NCT01604772, NCT01349933) [198]. However, these studies did not show positive outcome, and serious adverse effects such as constipation, dysphagia, gastritis, stomach ache, vertigo, vomiting, fatigue, fever, chest pain, dermal infections, increased levels of liver enzymes, hyperglycemia, flank pain, epistaxis, hiccups, dizziness and maculopapular rashes were noted.
6.2.6. Perifosine
Perifosine, an oral alkylphospholipid, is another AKT inhibitor that inhibits the phosphorylation of AKT at sites threonine (T308) and serine (S473). This molecule has been used against R/M HNSCC as monotherapy, but severe adverse effects such as abnormal bilirubin, infection, fatigue, abnormal platelet count, hyponatremia, hypercalcemia and anorexia were reported upon the use of perifosine [199].
6.2.7. Rapamycin
Rapamycin is a drug that selectively inhibits mTORC1 and impairs cancer metabolism. Phase 1 and 2 trials were carried out in stage II-IVA HNSCC patients to test the safety and efficacy of rapamycin. This compound was well tolerated and showed promising effects in HNSCC patients (NCT01195922) [200]. However, in combination with bevacizumab, rapamycin did not show any objective response (OR) in HNSCC patients [211].
6.2.8. Ridaforolimus
Ridaforolimus is a small molecule mTOR inhibitor and an analog of rapamycin with significant anticancer activity. A phase I study was conducted to evaluate the combined activity and safety profile of mTOR inhibitor, ridaforolimus, and γ-secretase inhibitor, MK-0752. This study showed that the combination of ridaforolimus and MK-0752 showed a potential antitumor effect against HNSCC. However, many adverse events were reported at the maximum tolerated dose, which would require cautious management in the course of future clinical development (NCT01295632) [201]
6.2.9. SF1126
SF1126 is an inhibitor of PI3 kinase and mTOR. It is a conjugate of LY294002 connected to an Arg-Gly-Asp-Ser (RGDS) tetrapeptide. The drug permeability of this prodrug is enhanced as it can bind to specific integrins inside the tumor. A phase 2 trial was conducted in metastatic squamous neck cancer patients with occult primary squamous cell carcinoma (SCC) to determine the efficacy of SF1126 as monotherapy, but this study was terminated due to slow enrollment (NCT02644122).
6.2.10. Temsirolimus
Temsirolimus is a water-soluble analog of rapamycin that explicitly inhibits mTOR. In patients with advanced stage HNSCC, temsirolimus suppresssed the mTOR pathway in tumors and peripheral blood mononuclear cell (PBMCs) of HNSCC with minimal side effects [212]. In 2015, Grunwald et al. assessed the efficacy of this drug in R/M HNSCC refractory to platinum and cetuximab. Of the patients involved in the study, 39.4% exhibited tumor shrinkage within the first six weeks of administration, though without any OR (NCT01172769) [202]. On the contrary, in another study, temsirolimus was used in combination with carboplatin and paclitaxel in R/M HNSCC patients and it resulted in an objective PR from 22% of the patients (NCT01016769) [203].
Temsirolimus along with erlotinib were also administered to R/M HNSCC patients, but this duo was reportedly toxic and fatal. Hence, the trial was terminated at an early stage (NCT01009203) [204]. In another trial, a combination of temsirolimus, bevacizumab and cetuximab was examined in advanced malignancies. The results showed that 25% of the patients with HNSCC achieved a PR while few patients withdrew as they started showing severe toxicities [213].
As mentioned previously, several inhibitors of the AKT/mTOR pathway have been evaluated as drugs in clinical settings. Despite a significant response in some cases, the use of these commercial synthetic inhibitors face major limitations in clinical settings [214,215,216]. For instance, the use of everolimus in cancer showed several side effects such as elevated alanine aminotransferase levels, stomatitis, hyperglycaemia, anaemia and pneumonitis [217]. In another study, temsirolimus has shown potential toxicity; however, side effects such as pneumonia, anemia and fatigue were also reported in the HNSCC patients [25]. As already mentioned, the AKT inhibitors perifosine and MK2206 also caused remarkably adverse effects. Other severe adversities caused by rapamycin, temsirolimus and everolimus included blood count abnormalities, head and neck edema, gastrointestinal disorders, increased liver enzymes, hypokalemia, hyperglycemia, hyponatremia, pruritus, mucositis, infections, nervous system disorders and fatigue [25]. Many clinical studies have been terminated due to such toxicities. To facilitate the broad-spectrum utilization of targeted inhibitors in the clinical scenario, the toxicity of these inhibitors should be minimized. Furthermore, natural inhibitors provide a safe, efficacious and an inexpensive alternative to the commercial inhibitors.
7. Conclusions
OSCC, the primary subtype of HNSCC, is a significant health issue that comprises of cancers of the oral cavity. Despite aggressive treatment methods, the five-year survival rate of OC is low. Several studies have identified major alterations in the genetic makeup of OSCC samples. However, the actual mechanism of oral tumorigenesis has not yet been deciphered. In order to alleviate the survival rate of OC patients, targeted therapy is pursued as an emerging regimen. Among several proteins and their associated signaling pathways, the AKT/mTOR pathway is one of the most altered axes reported in OC to date. Therefore, inhibitors of the AKT/mTOR pathway are sought for the prevention and treatment of OC. The preclinical studies have shown significant alteration of this axis in OC and targeting this axis by several natural and synthetic inhibitors has shown the modulation of various cellular processes such as proliferation, survival, angiogenesis, invasion, metastasis, autophagy, epithelial to mesenchymal transition, circadian clock signaling, chemoresistance and radioresistance. miRNAs, circRNAs and lncRNAs have also demonstrated an active role in the regulation of the AKT/mTOR axis in OSCC patients. Preclinical and clinical studies have illustrated the efficacy of certain inhibitors and their combination with other standard chemotherapeutic drugs such as paclitaxel, cisplatin or other inhibitors such as EGFR inhibitor or cetuximab in the prevention and treatment of OC. However, the use of these inhibitors has shown adverse effects in some cases, which need to be overcome for the persistent use of clinical inhibitors for the treatment of OC. Thus, AKT/mTOR inhibitors seem to hold great potential in the management of OC and tailoring these inhibitors for suitable use in the clinic might help to design personalized therapy for OC patients.
Acknowledgments
This work was supported by the grant RBMH/PNH/SB/12/2015-1438 awarded to Ajaikumar B. Kunnumakkara by Indian Council of Medical Research, Government of India. Kishore Banik acknowledges UGC, New Delhi, India for providing the fellowship. Alan Prem Kumar is supported by a grant from the National Medical Research Council of Singapore. Alan Prem Kumar is also supported by the National Medical Research Council of Singapore and the Singapore Ministry of Education under its Research Centres of Excellence initiative to Cancer Science Institute of Singapore, National University of Singapore.
Conflicts of Interest
The authors express no conflict of interest.
References
- 1.Roy N.K., Monisha J., Padmavathi G., Lalhruaitluanga H., Kumar N.S., Singh A.K., Bordoloi D., Baruah M.N., Ahmed G.N., Longkumar I., et al. Isoform-Specific Role of Akt in Oral Squamous Cell Carcinoma. Biomolecules. 2019;9:253. doi: 10.3390/biom9070253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Naggar M.H., Abdel Bar F.M., Choudhary H., Javadi M., Shimizu K., Kunnumakkara A.B., Badria F.A. Synthesis of new selective cytotoxic ricinine analogues against oral squamous cell carcinoma. Nat. Prod. Res. 2019:1–12. doi: 10.1080/14786419.2019.1663513. [DOI] [PubMed] [Google Scholar]
- 3.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 4.Hashim D., Genden E., Posner M., Hashibe M., Boffetta P. Head and neck cancer prevention: From primary prevention to impact of clinicians on reducing burden. Ann. Oncol. 2019;30:744–756. doi: 10.1093/annonc/mdz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behera A.K., Kumar M., Shanmugam M.K., Bhattacharya A., Rao V.J., Bhat A., Vasudevan M., Gopinath K.S., Mohiyuddin A., Chatterjee A., et al. Functional interplay between YY1 and CARM1 promotes oral carcinogenesis. Oncotarget. 2019;10:3709–3724. doi: 10.18632/oncotarget.26984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinha N., Panda P.K., Naik P.P., Das D.N., Mukhopadhyay S., Maiti T.K., Shanmugam M.K., Chinnathambi A., Zayed M.E., Alharbi S.A., et al. Abrus agglutinin promotes irreparable DNA damage by triggering ROS generation followed by ATM-p73 mediated apoptosis in oral squamous cell carcinoma. Mol. Carcinog. 2017;56:2400–2413. doi: 10.1002/mc.22679. [DOI] [PubMed] [Google Scholar]
- 7.Sawhney M., Rohatgi N., Kaur J., Shishodia S., Sethi G., Gupta S.D., Deo S.V., Shukla N.K., Aggarwal B.B., Ralhan R. Expression of NF-kappaB parallels COX-2 expression in oral precancer and cancer: Association with smokeless tobacco. Int. J. Cancer. 2007;120:2545–2556. doi: 10.1002/ijc.22657. [DOI] [PubMed] [Google Scholar]
- 8.Baek S.H., Ko J.H., Lee H., Jung J., Kong M., Lee J.W., Lee J., Chinnathambi A., Zayed M.E., Alharbi S.A., et al. Resveratrol inhibits STAT3 signaling pathway through the induction of SOCS-1: Role in apoptosis induction and radiosensitization in head and neck tumor cells. Phytomedicine Int. J. Phytother. Phytopharm. 2016;23:566–577. doi: 10.1016/j.phymed.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Monisha J., Roy N.K., Padmavathi G., Banik K., Bordoloi D., Khwairakpam A.D., Arfuso F., Chinnathambi A., Alahmadi T.A., Alharbi S.A., et al. NGAL is Downregulated in Oral Squamous Cell Carcinoma and Leads to Increased Survival, Proliferation, Migration and Chemoresistance. Cancers. 2018;10:228. doi: 10.3390/cancers10070228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bordoloi D., Monisha J., Roy N.K., Padmavathi G., Banik K., Harsha C., Wang H., Kumar A.P., Arfuso F., Kunnumakkara A.B. An Investigation on the Therapeutic Potential of Butein, A Tretrahydroxychalcone Against Human Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. Apjcp. 2019;20:3437–3446. doi: 10.31557/APJCP.2019.20.11.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vander Broek R., Mohan S., Eytan D.F., Chen Z., Van Waes C. The PI3K/Akt/mTOR axis in head and neck cancer: Functions, aberrations, cross-talk, and therapies. Oral Dis. 2015;21:815–825. doi: 10.1111/odi.12206. [DOI] [PubMed] [Google Scholar]
- 12.Clark C.A., McEachern M.D., Shah S.H., Rong Y., Rong X., Smelley C.L., Caldito G.C., Abreo F.W., Nathan C.O. Curcumin inhibits carcinogen and nicotine-induced Mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prev. Res. (Phila) 2010;3:1586–1595. doi: 10.1158/1940-6207.CAPR-09-0244. [DOI] [PubMed] [Google Scholar]
- 13.Roy N.K., Bordoloi D., Monisha J., Padmavathi G., Kotoky J., Golla R., Kunnumakkara A.B. Specific Targeting of Akt Kinase Isoforms: Taking the Precise Path for Prevention and Treatment of Cancer. Curr. Drug Targets. 2017;18:421–435. doi: 10.2174/1389450117666160307145236. [DOI] [PubMed] [Google Scholar]
- 14.Bordoloi D., Banik K., Padmavathi G., Vikkurthi R., Harsha C., Roy N.K., Singh A.K., Monisha J., Wang H., Kumar A.P., et al. TIPE2 Induced the Proliferation, Survival, and Migration of Lung Cancer Cells Through Modulation of Akt/mTOR/NF-kappaB Signaling Cascade. Biomolecules. 2019;9:836. doi: 10.3390/biom9120836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khwairakpam A.D., Banik K., Girisa S., Shabnam B., Shakibaei M., Fan L., Arfuso F., Monisha J., Wang H., Mao X., et al. The vital role of ATP citrate lyase in chronic diseases. J. Mol. Med. (Berl) 2020;98:71–95. doi: 10.1007/s00109-019-01863-0. [DOI] [PubMed] [Google Scholar]
- 16.Sailo B.L., Banik K., Girisa S., Bordoloi D., Fan L., Halim C.E., Wang H., Kumar A.P., Zheng D., Mao X., et al. FBXW7 in Cancer: What Has Been Unraveled Thus Far? Cancers. 2019;11:246. doi: 10.3390/cancers11020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabnam B., Padmavathi G., Banik K., Girisa S., Monisha J., Sethi G., Fan L., Wang L., Mao X., Kunnumakkara A.B. Sorcin a Potential Molecular Target for Cancer Therapy. Transl. Oncol. 2018;11:1379–1389. doi: 10.1016/j.tranon.2018.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J.S., Cui W. Proliferation, survival and metabolism: The role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050–3060. doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- 19.Wee P., Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers. 2017;9:52. doi: 10.3390/cancers9050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh S.S., Yap W.N., Arfuso F., Kar S., Wang C., Cai W., Dharmarajan A.M., Sethi G., Kumar A.P. Targeting the PI3K/Akt signaling pathway in gastric carcinoma: A reality for personalized medicine? World J. Gastroenterol. 2015;21:12261–12273. doi: 10.3748/wjg.v21.i43.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siveen K.S., Ahn K.S., Ong T.H., Shanmugam M.K., Li F., Yap W.N., Kumar A.P., Fong C.W., Tergaonkar V., Hui K.M., et al. Y-tocotrienol inhibits angiogenesis-dependent growth of human hepatocellular carcinoma through abrogation of AKT/mTOR pathway in an orthotopic mouse model. Oncotarget. 2014;5:1897–1911. doi: 10.18632/oncotarget.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ong P.S., Wang L.Z., Dai X., Tseng S.H., Loo S.J., Sethi G. Judicious Toggling of mTOR Activity to Combat Insulin Resistance and Cancer: Current Evidence and Perspectives. Front. Pharmacol. 2016;7:395. doi: 10.3389/fphar.2016.00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baek S.H., Ko J.H., Lee J.H., Kim C., Lee H., Nam D., Lee J., Lee S.G., Yang W.M., Um J.Y., et al. Ginkgolic Acid Inhibits Invasion and Migration and TGF-beta-Induced EMT of Lung Cancer Cells Through PI3K/Akt/mTOR Inactivation. J. Cell. Physiol. 2017;232:346–354. doi: 10.1002/jcp.25426. [DOI] [PubMed] [Google Scholar]
- 24.Cintas C., Guillermet-Guibert J. Heterogeneity of Phosphatidylinositol-3-Kinase (PI3K)/AKT/Mammalian Target of Rapamycin Activation in Cancer: Is PI3K Isoform Specificity Important? Front. Oncol. 2017;7:330. doi: 10.3389/fonc.2017.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marquard F.E., Jucker M. PI3K/AKT/mTOR signaling as a molecular target in head and neck cancer. Biochem. Pharmacol. 2020;172:113729. doi: 10.1016/j.bcp.2019.113729. [DOI] [PubMed] [Google Scholar]
- 26.Wadhwa B., Makhdoomi U., Vishwakarma R., Malik F. Protein kinase B: Emerging mechanisms of isoform-specific regulation of cellular signaling in cancer. Anticancer Drugs. 2017;28:569–580. doi: 10.1097/CAD.0000000000000496. [DOI] [PubMed] [Google Scholar]
- 27.Chan T.O., Rittenhouse S.E., Tsichlis P.N. AKT/PKB and other D3 phosphoinositide-regulated kinases: Kinase activation by phosphoinositide-dependent phosphorylation. Annu. Rev. Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 28.Murthy S.S., Tosolini A., Taguchi T., Testa J.R. Mapping of AKT3, encoding a member of the Akt/protein kinase B family, to human and rodent chromosomes by fluorescence in situ hybridization. Cytogenet Cell Genet. 2000;88:38–40. doi: 10.1159/000015481. [DOI] [PubMed] [Google Scholar]
- 29.Green B.D., Jabbour A.M., Sandow J.J., Riffkin C.D., Masouras D., Daunt C.P., Salmanidis M., Brumatti G., Hemmings B.A., Guthridge M.A., et al. Akt1 is the principal Akt isoform regulating apoptosis in limiting cytokine concentrations. Cell Death Differ. 2013;20:1341–1349. doi: 10.1038/cdd.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chong Z.Z., Li F., Maiese K. Activating Akt and the brain’s resources to drive cellular survival and prevent inflammatory injury. Histol. Histopathol. 2005;20:299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohan C.D., Srinivasa V., Rangappa S., Mervin L., Mohan S., Paricharak S., Baday S., Li F., Shanmugam M.K., Chinnathambi A., et al. Trisubstituted-Imidazoles Induce Apoptosis in Human Breast Cancer Cells by Targeting the Oncogenic PI3K/Akt/mTOR Signaling Pathway. PLoS ONE. 2016;11:e0153155. doi: 10.1371/journal.pone.0153155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J.H., Kim C., Um J.Y., Sethi G., Ahn K.S. Casticin-Induced Inhibition of Cell Growth and Survival Are Mediated through the Dual Modulation of Akt/mTOR Signaling Cascade. Cancers. 2019;11:254. doi: 10.3390/cancers11020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang M.H., Lee J.H., Ko J.H., Jung S.H., Sethi G., Ahn K.S. Brassinin Represses Invasive Potential of Lung Carcinoma Cells through Deactivation of PI3K/Akt/mTOR Signaling Cascade. Molecules. 2019;24:1584. doi: 10.3390/molecules24081584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinz N., Jucker M. Distinct functions of AKT isoforms in breast cancer: A comprehensive review. Cell Commun. Signal. 2019;17:154. doi: 10.1186/s12964-019-0450-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavalcante G.C., Schaan A.P., Cabral G.F., Santana-da-Silva M.N., Pinto P., Vidal A.F., Ribeiro-Dos-Santos A. A Cell’s Fate: An Overview of the Molecular Biology and Genetics of Apoptosis. Int. J. Mol. Sci. 2019;20:4133. doi: 10.3390/ijms20174133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang K., Chen Z., Gao J., Shi W., Li L., Jiang S., Hu H., Liu Z., Xu D., Wu L. The Key Roles of GSK-3beta in Regulating Mitochondrial Activity. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017;44:1445–1459. doi: 10.1159/000485580. [DOI] [PubMed] [Google Scholar]
- 37.Lee J.H., Chinnathambi A., Alharbi S.A., Shair O.H.M., Sethi G., Ahn K.S. Farnesol abrogates epithelial to mesenchymal transition process through regulating Akt/mTOR pathway. Pharmacol. Res. 2019;150:104504. doi: 10.1016/j.phrs.2019.104504. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.W., Kim S.M., Bae H., Nam D., Lee J.H., Lee S.G., Shim B.S., Kim S.H., Ahn K.S., Choi S.H., et al. Embelin inhibits growth and induces apoptosis through the suppression of Akt/mTOR/S6K1 signaling cascades. Prostate. 2013;73:296–305. doi: 10.1002/pros.22574. [DOI] [PubMed] [Google Scholar]
- 39.Park K.R., Nam D., Yun H.M., Lee S.G., Jang H.J., Sethi G., Cho S.K., Ahn K.S. beta-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011;312:178–188. doi: 10.1016/j.canlet.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L., Wu J., Ling M.T., Zhao L., Zhao K.N. The role of the PI3K/Akt/mTOR signalling pathway in human cancers induced by infection with human papillomaviruses. Mol. Cancer. 2015;14:87. doi: 10.1186/s12943-015-0361-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y., Kwok-Shing Ng P., Kucherlapati M., Chen F., Liu Y., Tsang Y.H., de Velasco G., Jeong K.J., Akbani R., Hadjipanayis A., et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell. 2017;31:820–832. doi: 10.1016/j.ccell.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roux P.P., Topisirovic I. Signaling Pathways Involved in the Regulation of mRNA Translation. Mol. Cell. Biol. 2018;38:e00070-18. doi: 10.1128/MCB.00070-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guertin D.A., Sabatini D.M. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 44.Peterson T.R., Laplante M., Thoreen C.C., Sancak Y., Kang S.A., Kuehl W.M., Gray N.S., Sabatini D.M. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sethi G., Ahn K.S., Sung B., Kunnumakkara A.B., Chaturvedi M.M., Aggarwal B.B. SH-5, an AKT inhibitor potentiates apoptosis and inhibits invasion through the suppression of anti-apoptotic, proliferative and metastatic gene products regulated by IkappaBalpha kinase activation. Biochem. Pharmacol. 2008;76:1404–1416. doi: 10.1016/j.bcp.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 46.Nair A.S., Shishodia S., Ahn K.S., Kunnumakkara A.B., Sethi G., Aggarwal B.B. Deguelin, an Akt inhibitor, suppresses IkappaBalpha kinase activation leading to suppression of NF-kappaB-regulated gene expression, potentiation of apoptosis, and inhibition of cellular invasion. J. Immunol. 2006;177:5612–5622. doi: 10.4049/jimmunol.177.8.5612. [DOI] [PubMed] [Google Scholar]
- 47.Matsuo F.S., Andrade M.F., Loyola A.M., da Silva S.J., Silva M.J.B., Cardoso S.V., de Faria P.R. Pathologic significance of AKT, mTOR, and GSK3beta proteins in oral squamous cell carcinoma-affected patients. Virchows Arch. Int. J. Pathol. 2018;472:983–997. doi: 10.1007/s00428-018-2318-0. [DOI] [PubMed] [Google Scholar]
- 48.Khwairakpam A.D., Shyamananda M.S., Sailo B.L., Rathnakaram S.R., Padmavathi G., Kotoky J., Kunnumakkara A.B. ATP citrate lyase (ACLY): A promising target for cancer prevention and treatment. Curr. Drug Targets. 2015;16:156–163. doi: 10.2174/1389450115666141224125117. [DOI] [PubMed] [Google Scholar]
- 49.Xie Y., Shi X., Sheng K., Han G., Li W., Zhao Q., Jiang B., Feng J., Li J., Gu Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review) Mol. Med. Rep. 2019;19:783–791. doi: 10.3892/mmr.2018.9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cartee G.D., Wojtaszewski J.F. Role of Akt substrate of 160 kDa in insulin-stimulated and contraction-stimulated glucose transport. Appl. Physiol. Nutr. Metab. = Physiol. Appl. Nutr. Et Metab. 2007;32:557–566. doi: 10.1139/H07-026. [DOI] [PubMed] [Google Scholar]
- 51.Barron C.C., Bilan P.J., Tsakiridis T., Tsiani E. Facilitative glucose transporters: Implications for cancer detection, prognosis and treatment. Metab. Clin. Exp. 2016;65:124–139. doi: 10.1016/j.metabol.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Chiarini F., Evangelisti C., Cenni V., Fazio A., Paganelli F., Martelli A.M., Lattanzi G. The Cutting Edge: The Role of mTOR Signaling in Laminopathies. Int. J. Mol. Sci. 2019;20:847. doi: 10.3390/ijms20040847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Georgy S.R., Cangkrama M., Srivastava S., Partridge D., Auden A., Dworkin S., McLean C.A., Jane S.M., Darido C. Identification of a Novel Proto-oncogenic Network in Head and Neck Squamous Cell Carcinoma. J. Natl. Cancer Inst. 2015;107:djv152. doi: 10.1093/jnci/djv152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Katase N., Nishimatsu S.I., Yamauchi A., Yamamura M., Terada K., Itadani M., Okada N., Hassan N.M.M., Nagatsuka H., Ikeda T., et al. DKK3 Overexpression Increases the Malignant Properties of Head and Neck Squamous Cell Carcinoma Cells. Oncol. Res. 2018;26:45–58. doi: 10.3727/096504017X14926874596386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu D.W., Yuan Y.X., Qiao J.K., Yu C., Yang X., Wang L.Z., Zhang Z.Y., Zhong L.P. Enhanced anticancer activity of a protein phosphatase 2A inhibitor on chemotherapy and radiation in head and neck squamous cell carcinoma. Cancer Lett. 2015;356:773–780. doi: 10.1016/j.canlet.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 56.Hu J., He Y., Yan M., Zhu C., Ye W., Zhu H., Chen W., Zhang C., Zhang Z. Dose dependent activation of retinoic acid-inducible gene-I promotes both proliferation and apoptosis signals in human head and neck squamous cell carcinoma. PLoS ONE. 2013;8:e58273. doi: 10.1371/journal.pone.0058273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patel V., Ramesh A., Traicoff J.L., Baibakov G., Emmert-Buck M.R., Gutkind J.S., Knezevic V. Profiling EGFR activity in head and neck squamous cell carcinoma by using a novel layered membrane Western blot technology. Oral Oncol. 2005;41:503–508. doi: 10.1016/j.oraloncology.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Bian Y., Terse A., Du J., Hall B., Molinolo A., Zhang P., Chen W., Flanders K.C., Gutkind J.S., Wakefield L.M., et al. Progressive tumor formation in mice with conditional deletion of TGF-beta signaling in head and neck epithelia is associated with activation of the PI3K/Akt pathway. Cancer Res. 2009;69:5918–5926. doi: 10.1158/0008-5472.CAN-08-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sobral L.M., Coletta R.D., Alberici L.C., Curti C., Leopoldino A.M. SET/I2PP2A overexpression induces phenotypic, molecular, and metabolic alterations in an oral keratinocyte cell line. Febs J. 2017;284:2774–2785. doi: 10.1111/febs.14148. [DOI] [PubMed] [Google Scholar]
- 60.Szczepanski M.J., Czystowska M., Szajnik M., Harasymczuk M., Boyiadzis M., Kruk-Zagajewska A., Szyfter W., Zeromski J., Whiteside T.L. Triggering of Toll-like receptor 4 expressed on human head and neck squamous cell carcinoma promotes tumor development and protects the tumor from immune attack. Cancer Res. 2009;69:3105–3113. doi: 10.1158/0008-5472.CAN-08-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raulf N., Lucarelli P., Thavaraj S., Brown S., Vicencio J.M., Sauter T., Tavassoli M. Annexin A1 regulates EGFR activity and alters EGFR-containing tumour-derived exosomes in head and neck cancers. Eur. J. Cancer. 2018;102:52–68. doi: 10.1016/j.ejca.2018.07.123. [DOI] [PubMed] [Google Scholar]
- 62.Liu C.J., Chang W.J., Chen C.Y., Sun F.J., Cheng H.W., Chen T.Y., Lin S.C., Li W.C. Dynamic cellular and molecular modulations of diabetes mediated head and neck carcinogenesis. Oncotarget. 2015;6:29268–29284. doi: 10.18632/oncotarget.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu D., Cheng J., Sun G., Wu S., Li M., Gao Z., Zhai S., Li P., Su D., Wang X. p70S6K promotes IL-6-induced epithelial-mesenchymal transition and metastasis of head and neck squamous cell carcinoma. Oncotarget. 2016;7:36539–36550. doi: 10.18632/oncotarget.9282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baschnagel A.M., Galoforo S., Thibodeau B.J., Ahmed S., Nirmal S., Akervall J., Wilson G.D. Crizotinib Fails to Enhance the Effect of Radiation in Head and Neck Squamous Cell Carcinoma Xenografts. Anticancer Res. 2015;35:5973–5982. [PubMed] [Google Scholar]
- 65.Ettl T., Viale-Bouroncle S., Hautmann M.G., Gosau M., Kolbl O., Reichert T.E., Morsczeck C. AKT and MET signalling mediates antiapoptotic radioresistance in head neck cancer cell lines. Oral Oncol. 2015;51:158–163. doi: 10.1016/j.oraloncology.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 66.Zhang S., Bian H., Li X., Wu H., Bi Q., Yan Y., Wang Y. Hydrogen sulfide promotes cell proliferation of oral cancer through activation of the COX2/AKT/ERK1/2 axis. Oncol. Rep. 2016;35:2825–2832. doi: 10.3892/or.2016.4691. [DOI] [PubMed] [Google Scholar]
- 67.Khanom R., Nguyen C.T., Kayamori K., Zhao X., Morita K., Miki Y., Katsube K., Yamaguchi A., Sakamoto K. Keratin 17 Is Induced in Oral Cancer and Facilitates Tumor Growth. PLoS ONE. 2016;11:e0161163. doi: 10.1371/journal.pone.0161163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sperandio F.F., Giudice F.S., Correa L., Pinto D.S., Jr., Hamblin M.R., de Sousa S.C. Low-level laser therapy can produce increased aggressiveness of dysplastic and oral cancer cell lines by modulation of Akt/mTOR signaling pathway. J. Biophotonics. 2013;6:839–847. doi: 10.1002/jbio.201300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lysne D., Johns J., Walker A., Ecker R., Fowler C., Lawson K.R. P-cadherin potentiates ligand-dependent EGFR and IGF-1R signaling in dysplastic and malignant oral keratinocytes. Oncol. Rep. 2014;32:2541–2548. doi: 10.3892/or.2014.3545. [DOI] [PubMed] [Google Scholar]
- 70.Martins F., de Sousa S.C., Dos Santos E., Woo S.B., Gallottini M. PI3K-AKT-mTOR pathway proteins are differently expressed in oral carcinogenesis. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2016;45:746–752. doi: 10.1111/jop.12440. [DOI] [PubMed] [Google Scholar]
- 71.Zhang X., Liu N., Ma D., Liu L., Jiang L., Zhou Y., Zeng X., Li J., Chen Q. Receptor for activated C kinase 1 (RACK1) promotes the progression of OSCC via the AKT/mTOR pathway. Int. J. Oncol. 2016;49:539–548. doi: 10.3892/ijo.2016.3562. [DOI] [PubMed] [Google Scholar]
- 72.Wu H.T., Ko S.Y., Fong J.H., Chang K.W., Liu T.Y., Kao S.Y. Expression of phosphorylated Akt in oral carcinogenesis and its induction by nicotine and alkaline stimulation. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2009;38:206–213. doi: 10.1111/j.1600-0714.2008.00659.x. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe S., Sato K., Okazaki Y., Tonogi M., Tanaka Y., Yamane G.Y. Activation of PI3K-AKT pathway in oral epithelial dysplasia and early cancer of tongue. Bull. Tokyo Dent. Coll. 2009;50:125–133. doi: 10.2209/tdcpublication.50.125. [DOI] [PubMed] [Google Scholar]
- 74.Tashiro K., Oikawa M., Miki Y., Takahashi T., Kumamoto H. Immunohistochemical assessment of growth factor signaling molecules: MAPK, Akt, and STAT3 pathways in oral epithelial precursor lesions and squamous cell carcinoma. Odontology. 2020;108:91–101. doi: 10.1007/s10266-019-00428-4. [DOI] [PubMed] [Google Scholar]
- 75.Rong C., Muller M., Flechtenmacher C., Holzinger D., Dyckhoff G., Bulut O.C., Horn D., Plinkert P., Hess J., Affolter A. Differential Activation of ERK Signaling in HPV-Related Oropharyngeal Squamous Cell Carcinoma. Cancers. 2019;11:584. doi: 10.3390/cancers11040584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jieru J., Shuang L., Jiyuan L., Jun Z., Tang X. [Expression and clinical significance of serine-threonine kinase/mammalian target of rapamycin/p70 S6K signal path- way in oral squamous cell carcinoma] Hua Xi Kou Qiang Yi Xue Za Zhi = Huaxi Kouqiang Yixue Zazhi = West China J. Stomatol. 2014;32:504–508. doi: 10.7518/hxkq.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang H., Deng X., Zhang J., Ou Z., Mai J., Ding S., Huo S. Elevated Expression of Zinc Finger Protein 703 Promotes Cell Proliferation and Metastasis through PI3K/AKT/GSK-3beta Signalling in Oral Squamous Cell Carcinoma. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017;44:920–934. doi: 10.1159/000485360. [DOI] [PubMed] [Google Scholar]
- 78.Liu M., Gao X., Liu C.L. Increased expression of lncRNA FTH1P3 promotes oral squamous cell carcinoma cells migration and invasion by enhancing PI3K/Akt/GSK3b/ Wnt/beta-catenin signaling. Eur. Rev. Med Pharmacol. Sci. 2018;22:8306–8314. doi: 10.26355/eurrev_201812_16528. [DOI] [PubMed] [Google Scholar]
- 79.Jiang X., Wang J., Chen X., Hong Y., Wu T., Chen X., Xia J., Cheng B. Elevated autocrine chemokine ligand 18 expression promotes oral cancer cell growth and invasion via Akt activation. Oncotarget. 2016;7:16262–16272. doi: 10.18632/oncotarget.7585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsieh M.J., Chien S.Y., Lin J.T., Yang S.F., Chen M.K. Polyphyllin G induces apoptosis and autophagy cell death in human oral cancer cells. Phytomedicine Int. J. Phytother. Phytopharm. 2016;23:1545–1554. doi: 10.1016/j.phymed.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 81.Wang H., Wu Q., Liu Z., Luo X., Fan Y., Liu Y., Zhang Y., Hua S., Fu Q., Zhao M., et al. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis. 2014;5:e1155. doi: 10.1038/cddis.2014.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohnishi Y., Yasui H., Kakudo K., Nozaki M. Cetuximab-resistant oral squamous cell carcinoma cells become sensitive in anchorage-independent culture conditions through the activation of the EGFR/AKT pathway. Int. J. Oncol. 2015;47:2165–2172. doi: 10.3892/ijo.2015.3215. [DOI] [PubMed] [Google Scholar]
- 83.Tseng Y.H., Yang C.C., Lin S.C., Cheng C.C., Lin S.H., Liu C.J., Chang K.W. Areca nut extract upregulates vimentin by activating PI3K/AKT signaling in oral carcinoma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2011;40:160–166. doi: 10.1111/j.1600-0714.2010.00978.x. [DOI] [PubMed] [Google Scholar]
- 84.Jing Y., Jin Y., Wang Y., Chen S., Zhang X., Song Y., Wang Z., Pu Y., Ni Y., Hu Q. SPARC promotes the proliferation and metastasis of oral squamous cell carcinoma by PI3K/AKT/PDGFB/PDGFRbeta axis. J. Cell. Physiol. 2019 doi: 10.1002/jcp.28205. [DOI] [PubMed] [Google Scholar]
- 85.Nakayama H., Ikebe T., Beppu M., Shirasuna K. High expression levels of nuclear factor kappaB, IkappaB kinase alpha and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. 2001;92:3037–3044. doi: 10.1002/1097-0142(20011215)92:12<3037::AID-CNCR10171>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 86.Alam H., Bhate A.V., Gangadaran P., Sawant S.S., Salot S., Sehgal L., Dange P.P., Chaukar D.A., D’Cruz A.K., Kannanl S., et al. Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma. BMC Cancer. 2012;12:32. doi: 10.1186/1471-2407-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim J., Kim J.H., Paeng J.Y., Kim M.J., Hong S.D., Lee J.I., Hong S.P. Prognostic value of activated Akt expression in oral squamous cell carcinoma. J. Clin. Pathol. 2005;58:1199–1205. doi: 10.1136/jcp.2004.024786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chaisuparat R., Limpiwatana S., Kongpanitkul S., Yodsanga S., Jham B.C. The Akt/mTOR pathway is activated in verrucous carcinoma of the oral cavity. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2016;45:581–585. doi: 10.1111/jop.12422. [DOI] [PubMed] [Google Scholar]
- 89.Massarelli E., Liu D.D., Lee J.J., El-Naggar A.K., Lo Muzio L., Staibano S., De Placido S., Myers J.N., Papadimitrakopoulou V.A. Akt activation correlates with adverse outcome in tongue cancer. Cancer. 2005;104:2430–2436. doi: 10.1002/cncr.21476. [DOI] [PubMed] [Google Scholar]
- 90.Principe S., Mejia-Guerrero S., Ignatchenko V., Sinha A., Ignatchenko A., Shi W., Pereira K., Su S., Huang S.H., O’Sullivan B., et al. Proteomic Analysis of Cancer-Associated Fibroblasts Reveals a Paracrine Role for MFAP5 in Human Oral Tongue Squamous Cell Carcinoma. J. Proteome Res. 2018;17:2045–2059. doi: 10.1021/acs.jproteome.7b00925. [DOI] [PubMed] [Google Scholar]
- 91.Zhang J., Wen H.J., Guo Z.M., Zeng M.S., Li M.Z., Jiang Y.E., He X.G., Sun C.Z. TRB3 overexpression due to endoplasmic reticulum stress inhibits AKT kinase activation of tongue squamous cell carcinoma. Oral Oncol. 2011;47:934–939. doi: 10.1016/j.oraloncology.2011.06.512. [DOI] [PubMed] [Google Scholar]
- 92.Li Z., Liu J., Li L., Shao S., Wu J., Bian L., He Y. Epithelial mesenchymal transition induced by the CXCL9/CXCR3 axis through AKT activation promotes invasion and metastasis in tongue squamous cell carcinoma. Oncol. Rep. 2018;39:1356–1368. doi: 10.3892/or.2017.6169. [DOI] [PubMed] [Google Scholar]
- 93.Zhang H., Sun J.D., Yan L.J., Zhao X.P. PDGF-D/PDGFRbeta promotes tongue squamous carcinoma cell (TSCC) progression via activating p38/AKT/ERK/EMT signal pathway. Biochem. Biophys. Res. Commun. 2016;478:845–851. doi: 10.1016/j.bbrc.2016.08.035. [DOI] [PubMed] [Google Scholar]
- 94.Zheng X., Li J., Peng C., Zhao J., Chi J., Meng X., Yun X., Li D., Yu Y., Gao M., et al. MicroRNA-24 induces cisplatin resistance by targeting PTEN in human tongue squamous cell carcinoma. Oral Oncol. 2015;51:998–1003. doi: 10.1016/j.oraloncology.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 95.Molinolo A.A., Marsh C., Dinali M.E., Gangane N., Jennison K., Hewitt S., Patel V., Seiwert T.Y., Gutkind J.S. mTOR as a molecular target in HPV-associated oral and cervical squamous carcinomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012;18:2558–2568. doi: 10.1158/1078-0432.CCR-11-2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li N., Sui J., Liu H., Zhong M., Zhang M., Wang Y., Hao F. Expression of phosphorylated Akt/mTOR and clinical significance in human ameloblastoma. Int. J. Clin. Exp. Med. 2015;8:5236–5244. [PMC free article] [PubMed] [Google Scholar]
- 97.Ferreira D.M., Neves T.J., Lima L.G.C.A., Alves F.A., Begnami M.D. Prognostic implications of the phosphatidylinositol 3-kinase/Akt signaling pathway in oral squamous cell carcinoma: Overexpression of p-mTOR indicates an adverse prognosis. Appl. Cancer Res. 2017;37:41. doi: 10.1186/s41241-017-0046-4. [DOI] [Google Scholar]
- 98.Lakshminarayana S., Augustine D., Rao R.S., Patil S., Awan K.H., Venkatesiah S.S., Haragannavar V.C., Nambiar S., Prasad K. Molecular pathways of oral cancer that predict prognosis and survival: A systematic review. J. Carcinog. 2018;17:7. doi: 10.4103/jcar.JCar_17_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gong W., Xiao Y., Wei Z., Yuan Y., Qiu M., Sun C., Zeng X., Liang X., Feng M., Chen Q. Toward the use of precision medicine for the treatment of head and neck squamous cell carcinoma. Oncotarget. 2017;8:2141–2152. doi: 10.18632/oncotarget.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Reviews. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang S., Bader A.G., Vogt P.K. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc. Natl. Acad. Sci. USA. 2005;102:802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tan F.H., Bai Y., Saintigny P., Darido C. mTOR Signalling in Head and Neck Cancer: Heads Up. Cells. 2019;8:333. doi: 10.3390/cells8040333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sonis S.T., Amaral Mendes R. Could the PI3K canonical pathway be a common link between chronic inflammatory conditions and oral carcinogenesis? J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2016;45:469–474. doi: 10.1111/jop.12436. [DOI] [PubMed] [Google Scholar]
- 104.Li Z., Liu J., Que L., Tang X. The immunoregulatory protein B7-H3 promotes aerobic glycolysis in oral squamous carcinoma via PI3K/Akt/mTOR pathway. J. Cancer. 2019;10:5770–5784. doi: 10.7150/jca.29838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ju W.T., Ma H.L., Zhao T.C., Liang S.Y., Zhu D.W., Wang L.Z., Li J., Zhang Z.Y., Zhou G., Zhong L.P. Stathmin guides personalized therapy in oral squamous cell carcinoma. Cancer Sci. 2020;111:1303–1313. doi: 10.1111/cas.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lam T.G., Jeong Y.S., Kim S.A., Ahn S.G. New metformin derivative HL156A prevents oral cancer progression by inhibiting the insulin-like growth factor/AKT/mammalian target of rapamycin pathways. Cancer Sci. 2018;109:699–709. doi: 10.1111/cas.13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Devi Khwairakpam A., Monisha J., Roy N.K., Bordoloi D., Padmavathi G., Banik K., Khatoon E., Kunnumakkara A.B. Vietnamese coriander inhibits cell proliferation, survival and migration via suppression of Akt/mTOR pathway in oral squamous cell carcinoma. J. Basic Clin. Physiol. Pharmacol. 2019 doi: 10.1515/jbcpp-2019-0162. [DOI] [PubMed] [Google Scholar]
- 108.Zainal N.S., Gan C.P., Lau B.F., Yee P.S., Tiong K.H., Abdul Rahman Z.A., Patel V., Cheong S.C. Zerumbone targets the CXCR4-RhoA and PI3K-mTOR signaling axis to reduce motility and proliferation of oral cancer cells. Phytomedicine Int. J. Phytother. Phytopharm. 2018;39:33–41. doi: 10.1016/j.phymed.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 109.Zagni C., Pistara V., Oliveira L.A., Castilho R.M., Romeo G., Chiacchio U., Rescifina A. Serendipitous discovery of potent human head and neck squamous cell carcinoma anti-cancer molecules: A fortunate failure of a rational molecular design. Eur. J. Med. Chem. 2017;141:188–196. doi: 10.1016/j.ejmech.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 110.Katase N., Nishimatsu S.I., Yamauchi A., Yamamura M., Fujita S. DKK3 knockdown confers negative effects on the malignant potency of head and neck squamous cell carcinoma cells via the PI3K/Akt and MAPK signaling pathways. Int. J. Oncol. 2019;54:1021–1032. doi: 10.3892/ijo.2018.4667. [DOI] [PubMed] [Google Scholar]
- 111.Wang Q., Zhang X., Song X., Zhang L. Overexpression of T-cadherin inhibits the proliferation of oral squamous cell carcinoma through the PI3K/AKT/mTOR intracellular signalling pathway. Arch. Oral Biol. 2018;96:74–79. doi: 10.1016/j.archoralbio.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 112.Shanmugam M.K., Warrier S., Kumar A.P., Sethi G., Arfuso F. Potential Role of Natural Compounds as Anti-Angiogenic Agents in Cancer. Curr. Vasc. Pharmacol. 2017;15:503–519. doi: 10.2174/1570161115666170713094319. [DOI] [PubMed] [Google Scholar]
- 113.Sethi G., Chatterjee S., Rajendran P., Li F., Shanmugam M.K., Wong K.F., Kumar A.P., Senapati P., Behera A.K., Hui K.M., et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma In vitro and in vivo. Mol. Cancer. 2014;13:66. doi: 10.1186/1476-4598-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abraham R.T. mTOR as a positive regulator of tumor cell responses to hypoxia. Curr. Top. Microbiol. Immunol. 2004;279:299–319. doi: 10.1007/978-3-642-18930-2_18. [DOI] [PubMed] [Google Scholar]
- 115.Kalaany N.Y., Sabatini D.M. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–731. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kishore T.K., Ganugula R., Gade D.R., Reddy G.B., Nagini S. Gedunin abrogates aldose reductase, PI3K/Akt/mToR, and NF-kappaB signaling pathways to inhibit angiogenesis in a hamster model of oral carcinogenesis. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016;37:2083–2093. doi: 10.1007/s13277-015-4003-0. [DOI] [PubMed] [Google Scholar]
- 117.Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu C., Feng X., Wang B., Wang X., Wang C., Yu M., Cao G., Wang H. Bone marrow mesenchymal stem cells promote head and neck cancer progression through Periostin-mediated phosphoinositide 3-kinase/Akt/mammalian target of rapamycin. Cancer Sci. 2018;109:688–698. doi: 10.1111/cas.13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang M.H., Sun R., Zhou X.M., Zhang M.Y., Lu J.B., Yang Y., Zeng L.S., Yang X.Z., Shi L., Xiao R.W., et al. Epithelial cell adhesion molecule overexpression regulates epithelial-mesenchymal transition, stemness and metastasis of nasopharyngeal carcinoma cells via the PTEN/AKT/mTOR pathway. Cell Death Dis. 2018;9:2. doi: 10.1038/s41419-017-0013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Samuels Y., Diaz L.A., Jr., Schmidt-Kittler O., Cummins J.M., Delong L., Cheong I., Rago C., Huso D.L., Lengauer C., Kinzler K.W., et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 121.Kolsch V., Charest P.G., Firtel R.A. The regulation of cell motility and chemotaxis by phospholipid signaling. J. Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tanagala K.K.K., Baba A.B., Kowshik J., Reddy G.B., Nagini S. Gedunin, A Neem Limonoid in Combination with Epalrestat Inhibits Cancer Hallmarks by Attenuating Aldose Reductase-Driven Oncogenic Signaling in SCC131 Oral Cancer Cells. Anti-Cancer Agents Med. Chem. 2018;18:2042–2052. doi: 10.2174/1871520618666180731093433. [DOI] [PubMed] [Google Scholar]
- 123.Du J., Hu W., Yang C., Wang Y., Wang X., Yang P. C-reactive protein is associated with the development of tongue squamous cell carcinoma. Acta Biochim. Et Biophys. Sin. 2018;50:238–245. doi: 10.1093/abbs/gmy004. [DOI] [PubMed] [Google Scholar]
- 124.Deng S., Shanmugam M.K., Kumar A.P., Yap C.T., Sethi G., Bishayee A. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125:1228–1246. doi: 10.1002/cncr.31978. [DOI] [PubMed] [Google Scholar]
- 125.Hwang S.T., Kim C., Lee J.H., Chinnathambi A., Alharbi S.A., Shair O.H.M., Sethi G., Ahn K.S. Cycloastragenol can negate constitutive STAT3 activation and promote paclitaxel-induced apoptosis in human gastric cancer cells. Phytomedicine Int. J. Phytother. Phytopharm. 2019;59:152907. doi: 10.1016/j.phymed.2019.152907. [DOI] [PubMed] [Google Scholar]
- 126.Huang K.J., Kuo C.H., Chen S.H., Lin C.Y., Lee Y.R. Honokiol inhibits In vitro and in vivo growth of oral squamous cell carcinoma through induction of apoptosis, cell cycle arrest and autophagy. J. Cell. Mol. Med. 2018;22:1894–1908. doi: 10.1111/jcmm.13474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Qiu Y., Li C., Wang Q., Zeng X., Ji P. Tanshinone IIA induces cell death via Beclin-1-dependent autophagy in oral squamous cell carcinoma SCC-9 cell line. Cancer Med. 2018;7:397–407. doi: 10.1002/cam4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang C.H., Lee C.Y., Lu C.C., Tsai F.J., Hsu Y.M., Tsao J.W., Juan Y.N., Chiu H.Y., Yang J.S., Wang C.C. Resveratrol-induced autophagy and apoptosis in cisplatin-resistant human oral cancer CAR cells: A key role of AMPK and Akt/mTOR signaling. Int. J. Oncol. 2017;50:873–882. doi: 10.3892/ijo.2017.3866. [DOI] [PubMed] [Google Scholar]
- 129.Xiong H., Yang Y., Yang K., Zhao D., Tang H., Ran X. Loss of the clock gene PER2 is associated with cancer development and altered expression of important tumor-related genes in oral cancer. Int. J. Of Oncol. 2018;52:279–287. doi: 10.3892/ijo.2017.4180. [DOI] [PubMed] [Google Scholar]
- 130.Su X., Chen D., Yang K., Zhao Q., Zhao D., Lv X., Ao Y. The circadian clock gene PER2 plays an important role in tumor suppression through regulating tumor-associated genes in human oral squamous cell carcinoma. Oncol. Rep. 2017;38:472–480. doi: 10.3892/or.2017.5653. [DOI] [PubMed] [Google Scholar]
- 131.Yang G., Yang Y., Tang H., Yang K. Loss of the clock gene Per1 promotes oral squamous cell carcinoma progression via the AKT/mTOR pathway. Cancer Sci. 2020 doi: 10.1111/cas.14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu H., Gong X., Yang K. Overexpression of the clock gene Per2 suppresses oral squamous cell carcinoma progression by activating autophagy via the PI3K/AKT/mTOR pathway. J. Cancer. 2020;11:3655–3666. doi: 10.7150/jca.42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lu Z.N., Shi Z.Y., Dang Y.F., Cheng Y.N., Guan Y.H., Hao Z.J., Tian B., He H.W., Guo X.L. Pantoprazole pretreatment elevates sensitivity to vincristine in drug-resistant oral epidermoid carcinoma In vitro and in vivo. Biomed. Pharmacother. = Biomed. Pharmacother. 2019;120:109478. doi: 10.1016/j.biopha.2019.109478. [DOI] [PubMed] [Google Scholar]
- 134.Dai D., Chen H., Tang J., Tang Y. Inhibition of mTOR/eIF4E by anti-viral drug ribavirin effectively enhances the effects of paclitaxel in oral tongue squamous cell carcinoma. Biochem. Biophys. Res. Commun. 2017;482:1259–1264. doi: 10.1016/j.bbrc.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 135.Wang P., Gao W., Wang Y., Wang J. Acetylshikonin inhibits In vitro and in vivo tumorigenesis in cisplatin-resistant oral cancer cells by inducing autophagy, programmed cell death and targeting m-TOR/PI3K/Akt signalling pathway. J. BUON Off. J. Balk. Union Oncol. 2019;24:2062–2067. [PubMed] [Google Scholar]
- 136.Bozec A., Ebran N., Radosevic-Robin N., Sudaka A., Monteverde M., Toussan N., Etienne-Grimaldi M.C., Nigro C.L., Merlano M., Penault-Llorca F., et al. Combination of mTOR and EGFR targeting in an orthotopic xenograft model of head and neck cancer. Laryngoscope. 2016;126:E156–E163. doi: 10.1002/lary.25754. [DOI] [PubMed] [Google Scholar]
- 137.Gu Y., Fan S., Liu B., Zheng G., Yu Y., Ouyang Y., He Z. TCRP1 promotes radioresistance of oral squamous cell carcinoma cells via Akt signal pathway. Mol. Cell. Biochem. 2011;357:107–113. doi: 10.1007/s11010-011-0880-8. [DOI] [PubMed] [Google Scholar]
- 138.Freudlsperger C., Horn D., Weissfuss S., Weichert W., Weber K.J., Saure D., Sharma S., Dyckhoff G., Grabe N., Plinkert P., et al. Phosphorylation of AKT(Ser473) serves as an independent prognostic marker for radiosensitivity in advanced head and neck squamous cell carcinoma. Int. J. Cancer. 2015;136:2775–2785. doi: 10.1002/ijc.29328. [DOI] [PubMed] [Google Scholar]
- 139.Yu C.C., Huang H.B., Hung S.K., Liao H.F., Lee C.C., Lin H.Y., Li S.C., Ho H.C., Hung C.L., Su Y.C. AZD2014 Radiosensitizes Oral Squamous Cell Carcinoma by Inhibiting AKT/mTOR Axis and Inducing G1/G2/M Cell Cycle Arrest. PLoS ONE. 2016;11:e0151942. doi: 10.1371/journal.pone.0151942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yu C.C., Hung S.K., Lin H.Y., Chiou W.Y., Lee M.S., Liao H.F., Huang H.B., Ho H.C., Su Y.C. Targeting the PI3K/AKT/mTOR signaling pathway as an effectively radiosensitizing strategy for treating human oral squamous cell carcinoma In vitro and in vivo. Oncotarget. 2017;8:68641–68653. doi: 10.18632/oncotarget.19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang Q., Lv L., Li Y., Ji H. MicroRNA655 suppresses cell proliferation and invasion in oral squamous cell carcinoma by directly targeting metadherin and regulating the PTEN/AKT pathway. Mol. Med. Rep. 2018;18:3106–3114. doi: 10.3892/mmr.2018.9292. [DOI] [PubMed] [Google Scholar]
- 142.Selvi R.B., Swaminathan A., Chatterjee S., Shanmugam M.K., Li F., Ramakrishnan G.B., Siveen K.S., Chinnathambi A., Zayed M.E., Alharbi S.A., et al. Inhibition of p300 lysine acetyltransferase activity by luteolin reduces tumor growth in head and neck squamous cell carcinoma (HNSCC) xenograft mouse model. Oncotarget. 2015;6:43806–43818. doi: 10.18632/oncotarget.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chi H. miR-194 regulated AGK and inhibited cell proliferation of oral squamous cell carcinoma by reducing PI3K-Akt-FoxO3a signaling. Biomed. Pharmacother. = Biomed. Pharmacother. 2015;71:53–57. doi: 10.1016/j.biopha.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 144.Manikandan M., Deva Magendhra Rao A.K., Arunkumar G., Manickavasagam M., Rajkumar K.S., Rajaraman R., Munirajan A.K. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol. Cancer. 2016;15:28. doi: 10.1186/s12943-016-0512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen D., Chen Z., Jin Y., Dragas D., Zhang L., Adjei B.S., Wang A., Dai Y., Zhou X. MicroRNA-99 family members suppress Homeobox A1 expression in epithelial cells. PLoS ONE. 2013;8:e80625. doi: 10.1371/journal.pone.0080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Manikandan M., Deva Magendhra Rao A.K., Arunkumar G., Rajkumar K.S., Rajaraman R., Munirajan A.K. Down Regulation of miR-34a and miR-143 May Indirectly Inhibit p53 in Oral Squamous Cell Carcinoma: A Pilot Study. Asian Pac. J. Cancer Prev. Apjcp. 2015;16:7619–7625. doi: 10.7314/APJCP.2015.16.17.7619. [DOI] [PubMed] [Google Scholar]
- 147.Uesugi A., Kozaki K., Tsuruta T., Furuta M., Morita K., Imoto I., Omura K., Inazawa J. The tumor suppressive microRNA miR-218 targets the mTOR component Rictor and inhibits AKT phosphorylation in oral cancer. Cancer Res. 2011;71:5765–5778. doi: 10.1158/0008-5472.CAN-11-0368. [DOI] [PubMed] [Google Scholar]
- 148.Wu X., Bhayani M.K., Dodge C.T., Nicoloso M.S., Chen Y., Yan X., Adachi M., Thomas L., Galer C.E., Jiffar T., et al. Coordinated targeting of the EGFR signaling axis by microRNA-27a*. Oncotarget. 2013;4:1388–1398. doi: 10.18632/oncotarget.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zheng G., Jia X., Peng C., Deng Y., Yin J., Zhang Z., Li N., Deng M., Liu X., Liu H., et al. The miR-491-3p/mTORC2/FOXO1 regulatory loop modulates chemo-sensitivity in human tongue cancer. Oncotarget. 2015;6:6931–6943. doi: 10.18632/oncotarget.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gao L., Dou Z.C., Ren W.H., Li S.M., Liang X., Zhi K.Q. CircCDR1as upregulates autophagy under hypoxia to promote tumor cell survival via AKT/ERK(1/2)/mTOR signaling pathways in oral squamous cell carcinomas. Cell Death Dis. 2019;10:745. doi: 10.1038/s41419-019-1971-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Su W., Wang Y., Wang F., Zhang B., Zhang H., Shen Y., Yang H. Circular RNA hsa_circ_0007059 indicates prognosis and influences malignant behavior via AKT/mTOR in oral squamous cell carcinoma. J. Cell. Physiol. 2019 doi: 10.1002/jcp.28156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen X., Tang F.R., Arfuso F., Cai W.Q., Ma Z., Yang J., Sethi G. The Emerging Role of Long Non-Coding RNAs in the Metastasis of Hepatocellular Carcinoma. Biomolecules. 2019;10:66. doi: 10.3390/biom10010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cheng J.T., Wang L., Wang H., Tang F.R., Cai W.Q., Sethi G., Xin H.W., Ma Z. Insights into Biological Role of LncRNAs in Epithelial-Mesenchymal Transition. Cells. 2019;8:1178. doi: 10.3390/cells8101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mishra S., Verma S.S., Rai V., Awasthee N., Chava S., Hui K.M., Kumar A.P., Challagundla K.B., Sethi G., Gupta S.C. Long non-coding RNAs are emerging targets of phytochemicals for cancer and other chronic diseases. Cell. Mol. Life Sci. Cmls. 2019;76:1947–1966. doi: 10.1007/s00018-019-03053-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ma Z., Wang Y.Y., Xin H.W., Wang L., Arfuso F., Dharmarajan A., Kumar A.P., Wang H., Tang F.R., Warrier S., et al. The expanding roles of long non-coding RNAs in the regulation of cancer stem cells. Int. J. Biochem. Cell Biol. 2019;108:17–20. doi: 10.1016/j.biocel.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 156.Peng W.X., Koirala P., Mo Y.Y. LncRNA-mediated regulation of cell signaling in cancer. Oncogene. 2017;36:5661–5667. doi: 10.1038/onc.2017.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Gao P., Wei G.H. Genomic Insight into the Role of lncRNA in Cancer Susceptibility. Int. J. Mol. Sci. 2017;18:1239. doi: 10.3390/ijms18061239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun M., Kraus W.L. From discovery to function: The expanding roles of long noncoding RNAs in physiology and disease. Endocr. Rev. 2015;36:25–64. doi: 10.1210/er.2014-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang Y., Chen D., Liu H., Yang K. Increased expression of lncRNA CASC9 promotes tumor progression by suppressing autophagy-mediated cell apoptosis via the AKT/mTOR pathway in oral squamous cell carcinoma. Cell Death Dis. 2019;10:41. doi: 10.1038/s41419-018-1280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang X., Liu W., Wang P., Li S. RNA interference of long noncoding RNA HOTAIR suppresses autophagy and promotes apoptosis and sensitivity to cisplatin in oral squamous cell carcinoma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2018;47:930–937. doi: 10.1111/jop.12769. [DOI] [PubMed] [Google Scholar]
- 161.Roy N.K., Monisha J., Singh A., Padmavathi G., Kunnumakkara A.B. Isoform-specific Role of Akt Kinase in Cancer and its Selective Targeting by Potential Anticancer Natural Agents. Nat. Prod. J. 2019;9:1. doi: 10.2174/2210315509666190314145257. [DOI] [Google Scholar]
- 162.Banik K., Ranaware A.M., Harsha C., Nitesh T., Girisa S., Deshpande V., Fan L., Nalawade S.P., Sethi G., Kunnumakkara A.B. Piceatannol: A natural stilbene for the prevention and treatment of cancer. Pharmacol. Res. 2020;153:104635. doi: 10.1016/j.phrs.2020.104635. [DOI] [PubMed] [Google Scholar]
- 163.Roy N.K., Parama D., Banik K., Bordoloi D., Devi A.K., Thakur K.K., Padmavathi G., Shakibaei M., Fan L., Sethi G., et al. An Update on Pharmacological Potential of Boswellic Acids against Chronic Diseases. Int. J. Mol. Sci. 2019;20:4101. doi: 10.3390/ijms20174101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kunnumakkara A.B., Banik K., Bordoloi D., Harsha C., Sailo B.L., Padmavathi G., Roy N.K., Gupta S.C., Aggarwal B.B. Googling the Guggul (Commiphora and Boswellia) for Prevention of Chronic Diseases. Front. Pharmacol. 2018;9:686. doi: 10.3389/fphar.2018.00686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kunnumakkara A.B., Harsha C., Banik K., Vikkurthi R., Sailo B.L., Bordoloi D., Gupta S.C., Aggarwal B.B. Is curcumin bioavailability a problem in humans: Lessons from clinical trials. Expert Opin. Drug Metab. Toxicol. 2019;15:705–733. doi: 10.1080/17425255.2019.1650914. [DOI] [PubMed] [Google Scholar]
- 166.Kunnumakkara A.B., Bordoloi D., Harsha C., Banik K., Gupta S.C., Aggarwal B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017;131:1781–1799. doi: 10.1042/CS20160935. [DOI] [PubMed] [Google Scholar]
- 167.Banik K., Ranaware A.M., Deshpande V., Nalawade S.P., Padmavathi G., Bordoloi D., Sailo B.L., Shanmugam M.K., Fan L., Arfuso F., et al. Honokiol for cancer therapeutics: A traditional medicine that can modulate multiple oncogenic targets. Pharmacol. Res. 2019;144:192–209. doi: 10.1016/j.phrs.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 168.Ranaware A.M., Banik K., Deshpande V., Padmavathi G., Roy N.K., Sethi G., Fan L., Kumar A.P., Kunnumakkara A.B. Magnolol: A Neolignan from the Magnolia Family for the Prevention and Treatment of Cancer. Int. J. Mol. Sci. 2018;19:2362. doi: 10.3390/ijms19082362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sailo B.L., Banik K., Padmavathi G., Javadi M., Bordoloi D., Kunnumakkara A.B. Tocotrienols: The promising analogues of vitamin E for cancer therapeutics. Pharmacol. Res. 2018;130:259–272. doi: 10.1016/j.phrs.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 170.Kunnumakkara A.B., Sailo B.L., Banik K., Harsha C., Prasad S., Gupta S.C., Bharti A.C., Aggarwal B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018;16:14. doi: 10.1186/s12967-018-1381-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Banik K., Harsha C., Bordoloi D., Lalduhsaki Sailo B., Sethi G., Leong H.C., Arfuso F., Mishra S., Wang L., Kumar A.P., et al. Therapeutic potential of gambogic acid, a caged xanthone, to target cancer. Cancer Lett. 2018;416:75–86. doi: 10.1016/j.canlet.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 172.Khwairakpam A.D., Bordoloi D., Thakur K.K., Monisha J., Arfuso F., Sethi G., Mishra S., Kumar A.P., Kunnumakkara A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018;133:53–64. doi: 10.1016/j.phrs.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 173.He W., Lai R., Lin Q., Huang Y., Wang L. Arglabin is a plant sesquiterpene lactone that exerts potent anticancer effects on human oral squamous cancer cells via mitochondrial apoptosis and downregulation of the mTOR/PI3K/Akt signaling pathway to inhibit tumor growth in vivo. J. BUON Off. J. Balk. Union Oncol. 2018;23:1679–1685. [PubMed] [Google Scholar]
- 174.Xiao Q., Yang L., Hu H., Ke Y. Artesunate targets oral tongue squamous cell carcinoma via mitochondrial dysfunction-dependent oxidative damage and Akt/AMPK/mTOR inhibition. J. Bioenerg. Biomembr. 2020 doi: 10.1007/s10863-020-09823-x. [DOI] [PubMed] [Google Scholar]
- 175.Kowshik J., Giri H., Kishore T.K., Kesavan R., Vankudavath R.N., Reddy G.B., Dixit M., Nagini S. Ellagic acid inhibits VEGF/VEGFR2, PI3K/Akt and MAPK signaling cascades in the hamster cheek pouch carcinogenesis model. Anti-Cancer Agents Med. Chem. 2014;14:1249–1260. doi: 10.2174/1871520614666140723114217. [DOI] [PubMed] [Google Scholar]
- 176.Kapoor V., Zaharieva M.M., Das S.N., Berger M.R. Erufosine simultaneously induces apoptosis and autophagy by modulating the Akt-mTOR signaling pathway in oral squamous cell carcinoma. Cancer Lett. 2012;319:39–48. doi: 10.1016/j.canlet.2011.12.032. [DOI] [PubMed] [Google Scholar]
- 177.Jan C.I., Tsai M.H., Chiu C.F., Huang Y.P., Liu C.J., Chang N.W. Fenofibrate Suppresses Oral Tumorigenesis via Reprogramming Metabolic Processes: Potential Drug Repurposing for Oral Cancer. Int. J. Biol. Sci. 2016;12:786–798. doi: 10.7150/ijbs.13851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lin N., Li Z., Wang D., Zheng K., Wu Y., Wang H. Mecambridine induces potent cytotoxic effects, autophagic cell death and modulation of the mTOR/PI3K/Akt signaling pathway in HSC-3 oral squamous cell carcinoma cells. Oncol. Lett. 2018;15:292–296. doi: 10.3892/ol.2017.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhou H., Li H., Cao Y., Sang X., Liu X. Murrayanine exerts antiproliferative effects on human oral cancer cells through inhibition of AKT/mTOR and Raf/MEK/ERK signalling pathways In vitro and inhibits tumor growth in vivo. J. BUON Off. J. Balk. Union Oncol. 2019;24:2423–2428. [PubMed] [Google Scholar]
- 180.Jiang L., Wang W., He Q., Wu Y., Lu Z., Sun J., Liu Z., Shao Y., Wang A. Oleic acid induces apoptosis and autophagy in the treatment of Tongue Squamous cell carcinomas. Sci. Rep. 2017;7:11277. doi: 10.1038/s41598-017-11842-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Aggarwal S., John S., Sapra L., Sharma S.C., Das S.N. Targeted disruption of PI3K/Akt/mTOR signaling pathway, via PI3K inhibitors, promotes growth inhibitory effects in oral cancer cells. Cancer Chemother. Pharmacol. 2019;83:451–461. doi: 10.1007/s00280-018-3746-x. [DOI] [PubMed] [Google Scholar]
- 182.Pai M.H., Kuo Y.H., Chiang E.P., Tang F.Y. S-Allylcysteine inhibits tumour progression and the epithelial-mesenchymal transition in a mouse xenograft model of oral cancer. Br. J. Nutr. 2012;108:28–38. doi: 10.1017/S0007114511005307. [DOI] [PubMed] [Google Scholar]
- 183.Lin C.W., Chin H.K., Lee S.L., Chiu C.F., Chung J.G., Lin Z.Y., Wu C.Y., Liu Y.C., Hsiao Y.T., Feng C.H., et al. Ursolic acid induces apoptosis and autophagy in oral cancer cells. Environ. Toxicol. 2019;34:983–991. doi: 10.1002/tox.22769. [DOI] [PubMed] [Google Scholar]
- 184.Pan S.T., Qin Y., Zhou Z.W., He Z.X., Zhang X., Yang T., Yang Y.X., Wang D., Qiu J.X., Zhou S.F. Plumbagin induces G2/M arrest, apoptosis, and autophagy via p38 MAPK- and PI3K/Akt/mTOR-mediated pathways in human tongue squamous cell carcinoma cells. Drug Des. Dev. Ther. 2015;9:1601–1626. doi: 10.2147/DDDT.S76057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Srivastava G., Matta A., Fu G., Somasundaram R.T., Datti A., Walfish P.G., Ralhan R. Anticancer activity of pyrithione zinc in oral cancer cells identified in small molecule screens and xenograft model: Implications for oral cancer therapy. Mol. Oncol. 2015;9:1720–1735. doi: 10.1016/j.molonc.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Girisa S., Shabnam B., Monisha J., Fan L., Halim C.E., Arfuso F., Ahn K.S., Sethi G., Kunnumakkara A.B. Potential of Zerumbone as an Anti-Cancer Agent. Molecules. 2019;24:734. doi: 10.3390/molecules24040734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Prasannan R., Kalesh K.A., Shanmugam M.K., Nachiyappan A., Ramachandran L., Nguyen A.H., Kumar A.P., Lakshmanan M., Ahn K.S., Sethi G. Key cell signaling pathways modulated by zerumbone: Role in the prevention and treatment of cancer. Biochem. Pharmacol. 2012;84:1268–1276. doi: 10.1016/j.bcp.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 188.Cheong D.H.J., Arfuso F., Sethi G., Wang L., Hui K.M., Kumar A.P., Tran T. Molecular targets and anti-cancer potential of escin. Cancer Lett. 2018;422:1–8. doi: 10.1016/j.canlet.2018.02.027. [DOI] [PubMed] [Google Scholar]
- 189.Halim C.E., Xinjing S.L., Fan L., Bailey Vitarbo J., Arfuso F., Tan C.H., Narula A.S., Kumar A.P., Sethi G., Ahn K.S. Anti-cancer effects of oxymatrine are mediated through multiple molecular mechanism(s) in tumor models. Pharmacol. Res. 2019;147:104327. doi: 10.1016/j.phrs.2019.104327. [DOI] [PubMed] [Google Scholar]
- 190.Ong S.K.L., Shanmugam M.K., Fan L., Fraser S.E., Arfuso F., Ahn K.S., Sethi G., Bishayee A. Focus on Formononetin: Anticancer Potential and Molecular Targets. Cancers. 2019;11:611. doi: 10.3390/cancers11050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.ClinicalTrials.gov is a Resource Provided by the U.S. National Library of Medicine. [(accessed on 16 April 2020)]; Available online: https://clinicaltrials.gov/
- 192.Geiger J.L., Bauman J.E., Gibson M.K., Gooding W.E., Varadarajan P., Kotsakis A., Martin D., Gutkind J.S., Hedberg M.L., Grandis J.R., et al. Phase II trial of everolimus in patients with previously treated recurrent or metastatic head and neck squamous cell carcinoma. Head Neck. 2016;38:1759–1764. doi: 10.1002/hed.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Massarelli E., Lin H., Ginsberg L.E., Tran H.T., Lee J.J., Canales J.R., Williams M.D., Blumenschein G.R., Jr., Lu C., Heymach J.V., et al. Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2015;26:1476–1480. doi: 10.1093/annonc/mdv194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Saba N.F., Hurwitz S.J., Magliocca K., Kim S., Owonikoko T.K., Harvey D., Ramalingam S.S., Chen Z., Rojerio J., Mendel J., et al. Phase 1 and pharmacokinetic study of everolimus in combination with cetuximab and carboplatin for recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer. 2014;120:3940–3951. doi: 10.1002/cncr.28965. [DOI] [PubMed] [Google Scholar]
- 195.Raymond E., Tourneau C.L., Gatineau M., Delord J.-P., Fayette J., Tijeras-Raballand C.D.A., Albert S., Granier M., Chibaudel B., Hadengue A., et al. CAPRA: Safety, efficacy, and translational biomarkers of weekly everolimus, carboplatin, and paclitaxel as induction therapy for locally advanced head and neck squamous cell carcinoma (HNSCC) J. Clin. Oncol. 2017;31:6036. doi: 10.1200/jco.2013.31.15_suppl.6036. [DOI] [Google Scholar]
- 196.Fury M.G., Sherman E., Ho A.L., Xiao H., Tsai F., Nwankwo O., Sima C., Heguy A., Katabi N., Haque S., et al. A phase 1 study of everolimus plus docetaxel plus cisplatin as induction chemotherapy for patients with locally and/or regionally advanced head and neck cancer. Cancer. 2013;119:1823–1831. doi: 10.1002/cncr.27986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Fury M.G., Lee N.Y., Sherman E., Ho A.L., Rao S., Heguy A., Shen R., Korte S., Lisa D., Ganly I., et al. A phase 1 study of everolimus + weekly cisplatin + intensity modulated radiation therapy in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013;87:479–486. doi: 10.1016/j.ijrobp.2013.06.2043. [DOI] [PubMed] [Google Scholar]
- 198.Ho A.L., Foster N.R., Meyers J.P., Vasudeva S.D., Katabi N., Pfiser C.R.A.D.G., Horvath L.E., Erlichman C., Schwartz G.K. Alliance A091104: A phase II trial of MK-2206 in patients (pts) with progressive, recurrent/metastatic adenoid cystic carcinoma. J. Clin. Oncol. 2015;33:6039. doi: 10.1200/jco.2015.33.15_suppl.6039. [DOI] [Google Scholar]
- 199.Argiris A., Cohen E., Karrison T., Esparaz B., Mauer A., Ansari R., Wong S., Lu Y., Pins M., Dancey J., et al. A phase II trial of perifosine, an oral alkylphospholipid, in recurrent or metastatic head and neck cancer. Cancer Biol. Ther. 2006;5:766–770. doi: 10.4161/cbt.5.7.2874. [DOI] [PubMed] [Google Scholar]
- 200.Day T.A., Shirai K., O’Brien P.E., Matheus M.G., Godwin K., Sood A.J., Kompelli A., Vick J.A., Martin D., Vitale-Cross L., et al. Inhibition of mTOR Signaling and Clinical Activity of Rapamycin in Head and Neck Cancer in a Window of Opportunity Trial. Clin. Cancer Res. 2019;25:1156–1164. doi: 10.1158/1078-0432.CCR-18-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Piha-Paul S.A., Munster P.N., Hollebecque A., Argiles G., Dajani O., Cheng J.D., Wang R., Swift A., Tosolini A., Gupta S. Results of a phase 1 trial combining ridaforolimus and MK-0752 in patients with advanced solid tumours. Eur. J. Cancer. 2015;51:1865–1873. doi: 10.1016/j.ejca.2015.06.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Grünwald V., Keilholz U., Boehm A., Guntinas-Lichius O., Hennemann B., Schmoll H.J., Ivanyi P., Abbas M., Lehmann U., Koch A., et al. TEMHEAD: A single-arm multicentre phase II study of temsirolimus in platin- and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN Group (AIO) Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2015;26:561–567. doi: 10.1093/annonc/mdu571. [DOI] [PubMed] [Google Scholar]
- 203.Fury M.G., Sherman E., Ho A., Katabi N., Sima C., Kelly K.W., Nwankwo O., Haque S., Pfister D.G. A phase I study of temsirolimus plus carboplatin plus paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC) Cancer Chemother. Pharmacol. 2012;70:121–128. doi: 10.1007/s00280-012-1894-y. [DOI] [PubMed] [Google Scholar]
- 204.Bauman J.E., Arias-Pulido H., Lee S.J., Fekrazad M.H., Ozawa H., Fertig E., Howard J., Bishop J., Wang H., Olson G.T., et al. A phase II study of temsirolimus and erlotinib in patients with recurrent and/or metastatic, platinum- refractory head and neck squamous cell carcinoma. Oral Oncol. 2013;49:461–467. doi: 10.1016/j.oraloncology.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dunn L.A., Fury M.G., Xiao H., Baxi S.S., Sherman E.J., Korte S., Pfister C., Haque S., Katabi N., Ho A.L., et al. A phase II study of temsirolimus added to low-dose weekly carboplatin and paclitaxel for patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) Ann. Oncol. Off. J. Eur. Soc. Med Oncol. 2017;28:2533–2538. doi: 10.1093/annonc/mdx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Agrawal N., Frederick M.J., Pickering C.R., Bettegowda C., Chang K., Li R.J., Fakhry C., Xie T.X., Zhang J., Wang J., et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science. 2011;333:1154–1157. doi: 10.1126/science.1206923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Stransky N., Egloff A.M., Tward A.D., Kostic A.D., Cibulskis K., Sivachenko A., Kryukov G.V., Lawrence M.S., Sougnez C., McKenna A., et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Munster P., Mita M., Mahipal A., Nemunaitis J., Massard C., Mikkelsen T., Cruz C., Paz-Ares L., Hidalgo M., Rathkopf D., et al. First-In-Human Phase I Study of A Dual mTOR Kinase And DNA-PK Inhibitor (CC-115) In Advanced Malignancy. Cancer Manag. Res. 2019;11:10463–10476. doi: 10.2147/CMAR.S208720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu Y., Zhang Y., Jia K., Dong Y., Ma W. Metformin inhibits the proliferation of A431 cells by modulating the PI3K/Akt signaling pathway. Exp. Ther. Med. 2015;9:1401–1406. doi: 10.3892/etm.2015.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Zannella V.E., Dal Pra A., Muaddi H., McKee T.D., Stapleton S., Sykes J., Glicksman R., Chaib S., Zamiara P., Milosevic M., et al. Reprogramming metabolism with metformin improves tumor oxygenation and radiotherapy response. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013;19:6741–6750. doi: 10.1158/1078-0432.CCR-13-1787. [DOI] [PubMed] [Google Scholar]
- 211.Cohen E.E., Sharma M.R., Janisch L., Llobrera M., House L., Wu K., Ramirez J., Fleming G.F., Stadler W.M., Ratain M.J. A phase I study of sirolimus and bevacizumab in patients with advanced malignancies. Eur. J. Cancer. 2011;47:1484–1489. doi: 10.1016/j.ejca.2011.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ekshyyan O., Mills G.M., Lian T., Amirghahari N., Rong X., Lowery-Nordberg M., Abreo F., Veillon D.M., Caldito G., Speicher L., et al. Pharmacodynamic evaluation of temsirolimus in patients with newly diagnosed advanced-stage head and neck squamous cell carcinoma. Head Neck. 2010;32:1619–1628. doi: 10.1002/hed.21374. [DOI] [PubMed] [Google Scholar]
- 213.Liu X., Kambrick S., Fu S., Naing A., Subbiah V., Blumenschein G.R., Glisson B.S., Kies M.S., Tsimberidou A.M., Wheler J.J., et al. Advanced malignancies treated with a combination of the VEGF inhibitor bevacizumab, anti-EGFR antibody cetuximab, and the mTOR inhibitor temsirolimus. Oncotarget. 2016;7:23227–23238. doi: 10.18632/oncotarget.7594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Harsha C., Banik K., Bordoloi D., Kunnumakkara A.B. Antiulcer properties of fruits and vegetables: A mechanism based perspective. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2017;108:104–119. doi: 10.1016/j.fct.2017.07.023. [DOI] [PubMed] [Google Scholar]
- 215.Monisha J., Padmavathi G., Roy N.K., Deka A., Bordoloi D., Anip A., Kunnumakkara A.B. NF-kappaB Blockers Gifted by Mother Nature: Prospectives in Cancer Cell Chemosensitization. Curr. Pharm. Des. 2016;22:4173–4200. doi: 10.2174/1381612822666160609110231. [DOI] [PubMed] [Google Scholar]
- 216.Kunnumakkara A.B., Bordoloi D., Sailo B.L., Roy N.K., Thakur K.K., Banik K., Shakibaei M., Gupta S.C., Aggarwal B.B. Cancer drug development: The missing links. Exp. Biol. Med. (Maywoodn. J.) 2019;244:663–689. doi: 10.1177/1535370219839163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yamanaka K., Petrulionis M., Lin S., Gao C., Galli U., Richter S., Winkler S., Houben P., Schultze D., Hatano E., et al. Therapeutic potential and adverse events of everolimus for treatment of hepatocellular carcinoma - systematic review and meta-analysis. Cancer Med. 2013;2:862–871. doi: 10.1002/cam4.150. [DOI] [PMC free article] [PubMed] [Google Scholar]