Abstract
Plant cells are frequently challenged with a wide range of adverse environmental conditions that restrict plant growth and limit the productivity of agricultural crops. Rapid development of nanotechnology and unsystematic discharge of metal containing nanoparticles (NPs) into the environment pose a serious threat to the ecological receptors including plants. Engineered nanoparticles are synthesized by physical, chemical, biological, or hybrid methods. In addition, volcanic eruption, mechanical grinding of earthquake-generating faults in Earth’s crust, ocean spray, and ultrafine cosmic dust are the natural source of NPs in the atmosphere. Untying the nature of plant interactions with NPs is fundamental for assessing their uptake and distribution, as well as evaluating phytotoxicity. Modern mass spectrometry-based proteomic techniques allow precise identification of low abundant proteins, protein–protein interactions, and in-depth analyses of cellular signaling networks. The present review highlights current understanding of plant responses to NPs exploiting high-throughput proteomics techniques. Synthesis of NPs, their morphophysiological effects on crops, and applications of proteomic techniques, are discussed in details to comprehend the underlying mechanism of NPs stress acclimation.
Keywords: nanoparticles, crop, proteomics, plant-nanoparticles interaction, nanoparticles synthesis
1. Introduction
Rapid advancement in nanotechnology has taken the food industry to a new height [1]. Nanoparticles (NPs) are ultrafine particles with a size of less than 100 nm in at least one dimension [2]. Owing to having unique physical and chemical properties, such as high surface area and nanoscale size, these microscopic particles have the potential to improve the quality of food processing, packaging, storage, transportation, functionality, and other safety aspects of food [2]. Moreover, in recent years, nanotechnology has gained tremendous attention in agriculture sector as promising agents for plant growth, fertilizers, and pesticides, ensuring sustainable crop production [3]. The engineered nanomaterials have a wide range of applications in the healthcare industry, including drug delivery [4], cellular imaging and diagnosis [5], cancer therapy [6], antimicrobials [7], biosensors [8], anti-diabetic agents [9], and cosmetics [10]. Nevertheless, unsystematic release of nano-containing biosolids and agrochemicals is a serious threat to the environment, including plants [11].
Among metal based NPs, iron NPs are widely used in environmental remediation, biomedical, diagnostic field, and drug delivery because of their unique properties, such as excellent biodegradability, low cytotoxicity, and ability to attach with multiple targeted ligands or antibodies [12,13]. Few studies have been conducted to assess the impact of iron NPs on plants [14,15]. Kim et al. [14] reported that exposure of iron NPs triggered root elongation in Arabidopsis thaliana by nZVI-mediated OH radical-induced cell wall loosening. Conversely, iron–ion/NPs did not affect physiological parameters in lettuce plant [15]. Similar to iron, copper NPs have diverse applications, such as electro metallic agent, wood preservative, bioactive, and lubricant [16]. However, unmanaged discharge of copper NPs into the environment poses an increasing threat to plants [17]. Hence, there is urgent need of in-depth research for understanding the various pathways involved in NPs stress response mechanisms in plants. Most of the phytotoxicity research so far conducted is focused on effects of NPs on seed germination and, at very early growth stages, of the plants [18]. Techniques, including cytotoxicity study [19], transcriptomics [20], and proteomics [21] have been widely used for analyzing uptake, bioaccumulation, biotransformation, and risks of NPs for food crops. Moreover, NP-mediated phytotoxicity as well as their ecotoxicity was conducted on mammalian cells [22]. These high-throughput genome-based omics techniques have been used extensively to dissect plant responses to NPs [23]. Although transcriptional analysis was performed in a variety of organisms including microbes, humans, mammalian cell lines, and other model organisms [24], information about plant–NPs interactions and NP-mediated phytotoxicity is still limited.
The high-throughput techniques used in proteomics focus on revealing structure and conformation of proteins, protein−protein, and protein−ligand interactions. Proteomics offer several advantages over the genome or transcriptome-based technologies as it directly deals with the functional molecules rather than DNA or mRNA [25]. Gel-based or gel-free proteomic techniques, protein chips/microarrays, and protein biomarkers have been widely used for reliable identification and accurate quantitation of stress responsive proteins for dissecting plant stress signaling pathways [26]. Improved protein extraction protocol and advancement in mass spectrometry have made proteomics a rapid, sensitive, and reliable technique for identification and characterization of differentially modulated proteins to assess the possible impact of NPs on crops. Alternative to single omics approach, multi-omics techniques, such as combination of transcriptomics, proteomics, and metabolomics offer more advantages in identifying the underlying response mechanisms of plants towards the environmental contaminants, including NPs [27]. This review highlights the various methods used for synthesis of NPs, their morphophysiological impact on crop plants, and applications of proteomic techniques to comprehend the underlying mechanism of NPs stress acclimation.
2. Methods for NPs Synthesis
The size, concentration, and stability of NPs primarily determine their effects on plants [23]. The characteristics of NPs largely depend on their mode of synthesis. There are various physical, chemical, and biological methods for the synthesis of economically important NPs [28]. Although the methods of NPs synthesis are diverse, there is a bare necessity to develop some ecofriendly processes so that they may be less hazardous to the environment (Table 1).
Table 1.
Mode of synthesis and characteristics of commercially important nanoparticles (NPs).
| NPs | Mode of Synthesis | Size (nm) | Characters | Ref * |
|---|---|---|---|---|
| Silver NPs | Litchi chinensis leaf extract | 41–55 | Crystalline nature | [50] |
| Tube furnace | 6.2–21.5 | Spherical shape | [29] | |
| Laser ablation | 20–50 | Pentagonal one dimensional (1-D) nanorods, nanowires, cubic/triangular-bipyramidal nanocrystals | [30] | |
| Carboxymethylated chitosan with ultraviolet light irradiation | 2–8 | Cubic crystal structure | [40] | |
| Eucalyptus macrocarpa leaf extract | 10–100 | Spherical and cubic shaped | [51] | |
| Sodium borohydride | 2–4 | Nanorods | [63] | |
| Silver nitrate with sodium borate | 20–50 | Mixture of spherical and rod NPs | [39] | |
| Wet chemical method | 20 | Nanowires | [44] | |
| Ascorbic acid as a reducing agent | 31 | Spherical shaped | [45] | |
| Silver nitrate and methanolic Rhazya stricta root extract | 20 | Spherical shaped | [52] | |
| Iron NPs | Leaf extract of barberry, Elaeagnus angustifolia, Ziziphus jujube | 40 | Spherical shaped | [53] |
| Sodium borohydride | 44.87 | Spherical shaped | [40] | |
| Ferric chloride precursor with sodium borohydride | 6 | Spherical in shape | [40] | |
| Grape tree leaf extract | 10–30 | Spherical and non-agglomerated | [54] | |
| Green tea extract | 40–60 | Amorphous in nature, chain morphology | [55] | |
| Mesoporous silica | 10–300 | Uniform pore size, large surface area, high accessible pore volume | [46] | |
| Thermal dehydration | 6–10 | globular-shape crystallites | [32] | |
| Thermal decomposition | 50 | Irregular and not spherical | [33] | |
| Zinc oxide NPs | Albizia lebbeck | 66.25 | Irregular spherical morphology | [56] |
| Chamomile flower extract | 48.2 | Pure crystalline | [64] | |
| Ricinus communis seed extract | 20 | Crystalline hexagonal | [57] | |
| Ammonium carbamate | 10–15 | Crystallite rod-shape | [47] | |
| Aloe vera leaf extract | 25–40 | Highly stable and spherical | [58] | |
| Refluxing zinc acetate precursor in diethylene/triethylene glycol | 15–100 | Oval to rod shape | [48] | |
| Copper NPs | Alcothermal method | 6 | High dispersion, narrow size distribution | [9] |
| Sodium borohydride | 17.25 | Spherical shaped | [41] | |
| Thermal decomposition | 15–30 | Nearly spherical with relatively uniform diameters | [34] | |
| Biosynthesis by Morganella | 15–20 | Crystal lattice structure | [49] | |
| Sodium borohydride | 15 | Pure crystalline metallic phase with face centered cubic, rich in dents, irregular surface | [35] | |
| Polyol method | 45 | Pure crystalline with face centered cubic structure | [36] | |
| Ocimum sanctum leaf extract | 77 | Different organic molecules, high crystallinity | [59] | |
| Wet chemical synthesis involving stoichiometric reaction | 9 | Spherical | [43] | |
| Polyol method by copper acetate hydrate in tween 80 | 580 | Crystalline nature | [37] | |
| Reduction of copper (II) acetate in water and 2-ethoxyethanol using hydrazine under reflux | 6–23 | Spherical | [40] | |
| Thermal reduction | 200–250 | Irregular particles | [42] | |
| Sonochemical reduction | 50–70 | Irregular network of small NPs | [42] | |
| Cassia alata flower extract | 110–280 | Aggregates with rough, particles, spherical | [60] | |
| Capparis zeylanica leaf extract | 50–100 | Cubical structure | [61] | |
| Syzygium aromaticum extract | 5–40 | Spherical and granular nature | [62] | |
| Titanium oxide NPs | Ytterbium fiber laser ablation | 25 | Spherical and polycrystalline | [31] |
| Taguchi method | 18.11 | Spherical | [65] | |
| Sol-gel method | 15 | Crystalline and nearly spherical | [66] |
* Ref means references.
2.1. Physical Methods for NPs Synthesis
These methods are being used for the synthesis of various economically important NPs, such as silver, copper, iron, titanium, and others. The method of tube furnace was used for the synthesis of spherical silver NPs [29]; while laser ablation resulted in the formation of triangular bipyramidal nanocrystals of silver [30]. NPs synthesized by Ytterbium fiber laser ablation were spherical in shape and polycrystalline in nature [31]. Iron NPs with the globular shape were produced using the thermal dehydration method [32]; whereas irregular shape was attained with thermal decomposition approach [33]. Furthermore, copper NPs with spherical shaped and uniform diameters were synthesized using the thermal decomposition approach [34]. The topographic map indicated that NPs synthesized through sodium borohydrate as the reducing agent produced the NPs with irregular surfaces [35], while the polyol method synthesized pure crystalline copper NPs with cubic surface [36]. When tween 80 was added as modification in the polyol method, it resulted in the formation of crystalline copper NPs [37]. The physical approaches mainly synthesized the NPs with uniform morphological characteristics, which ultimately affected their response towards the environment as well as to the living ecosystem.
2.2. Chemical Methods for NPs Synthesis
The chemical reduction using a variety of organic/inorganic reducing agents, electrochemical techniques, physicochemical reduction, and radiolysis is a well-accepted approach for the synthesis of NPs [38]. The process of reduction through various chemicals led to the synthesis of the diverse shape of properties of NPs, such as silver nitrate reduction with sodium borohydrate resulted in the mixture of spherical and rod shaped silver NPs [39]; however, iron NPs were spherical when iron salt was reduced with sodium borohydrate [40]. The reduction of copper salts with sodium borohydrate produced spherical [41] and irregular NPs [35]. Sonochemical and thermal reduction of copper hydrazine carboxylate produced a network of irregular shaped copper NPs [42]. Wet chemical synthesis involving stoichiometric reaction also produced spherical copper NPs [43]. Moreover, wet chemical method produced nanowires of silver [44]; while spherical silver NPs were produced on ascorbic acid as a reducing agent [45]. Mesoporous silica resulted in the formation of iron NPs having uniform pore size and large surface area [46]. The zinc NPs with crystalline shaped morphology were obtained using ammonium carbamate as a precipitating agent [47]; while refluxing zinc acetate precursor in diethylene and triethylene glycol synthesized oval to rod shaped NPs [48]. Due to the usage of various chemicals for NPs synthesis, there is growing concern about the possible release and effect of NPs in the surrounding environment.
2.3. Biological and Green Methods for NPs Synthesis
In biological and green methods, living organisms, such as bacteria, viruses, and plants, are used as capping and reducing agents. The crystal lattice structure of synthesized copper NPs was achieved through Morganella [49]. Silver NPs with spherical and cubic shaped having crystalline nature were synthesized using extracts of Litchi chinensis [50], Eucalyptus macrocarpa [51], and Rhazya stricta [52]. Iron NPs were synthesized using leaf extract of barberry, Elaeagnus angustifolia, saffron, Ziziphus jujube [53], grape tree [54], and green tea [55]. The involvement of Albizia lebbeck bioactive compounds in the stabilization of zincoxide NPs were confirmed through various techniques and revealed irregular spherical morphology [56]; while crystalline hexagonal stage was obtained through the seed extract of Ricinus communis [57]. Leaf extract of Aloe vera also synthesized highly stable and spherical zinc oxide NPs [58]. Copper NPs were produced using extracts of Ocimum sanctum leaf [59], Cassia alata flower [60], Capparis zelynica leaf [61], and Syzygium aromaticum solution [62]. Studies have shown that green synthesis methods exploiting plants or microorganisms are relatively safe, inexpensive, and environment-friendly.
3. Morphological and Physiological Effects of NPs on Crops
The most advanced interdisciplinary tool with the larger potential in agriculture for increased crop productivity is the nanotechnology in which NPs with varying size, concentration, and surface charge influenced the growth and development of diverse plant species [67]. A variety of NPs have been tested against germination of seeds, growth of shoot/root, and crop production [68]. NPs exert species-specific toxicity, plant organ specificity, as well as stress dependency (Table 2).
Table 2.
Mode of applications and morphophysiological responses of crops upon NPs treatments.
| NPs | Species | Mode of application | Morphophysiological responses | Ref * |
|---|---|---|---|---|
| Silver NPs | Rice | Hydroponic application | Enhanced root length | [85] |
| Wheat | Direct application | Reduced seedling growth | [86] | |
| Zucchini | Direct application | Reduced seedling biomass | [81] | |
| Wheat | Direct application | Reduced seedling biomass | [83] | |
| Mung bean | Direct application | Reduced seedling biomass | [83] | |
| Cabbage | Direct application | Decreased root length | [84] | |
| Maize | Direct application | Increased root length | [84] | |
| Eruca sativa | Direct application | Increased root length | [98] | |
| Ajwain | Direct application | Improved water use efficiency, nutrient uptake, reduced fertilizer requirement | [99] | |
| Zucchini | Hoagland solution | Reduced rate of transpiration | [81] | |
| Mung bean | Direct application | Regulated seedling growth | [83] | |
| Aluminum oxide NPs | Wheat | Direct application | Enhanced root growth | [87] |
| Maize | Hydroponic application | Reduced root elongation | [70] | |
| Soybean | Direct application | Improved survival and root growth | [92] | |
| Maize | Direct application | Increased root length | [69] | |
| Soybean | Flooding | Increased root length | [93] | |
| Radish | Aqueous suspension | Improved root growth | [69] | |
| Cucumber | Aqueous suspension | Reduced root growth | [70] | |
| Titanium oxide NPs | Wheat | Aqueous suspension | Increased root length | [71] |
| Rose | Water-agar plates with suspension | Enhanced plant resistance to fungal infection by altering endogenous hormones content | [82] | |
| Cucumber | Aqueous suspension | Restricted root growth | [69] | |
| Carrot | Aqueous suspension | Restricted root growth | [70] | |
| Wheat | Aqueous suspension | Reduced biomass | [100] | |
| Spinach | Seed treatment | Enhanced growth | [74] | |
| Spinach | Seed treatment | Significantly affected the plant growth | [94] | |
| Spinach | Foliar spray | Increased seedling growth | [95] | |
| Chickpea | Foliar spray | Improved redox status | [101] | |
| Spinach | Seed treatment | Increased dry weight and chlorophyll content | [94] | |
| Narbon bean | Seed treatment | Reduced seed germination and root length | [1] | |
| Maize | Seed treatment | Reduced seed germination and root length | [1] | |
| Wheat | Aqueous suspension | Increased shoot length | [73] | |
| Spinach | Aqueous suspension | Increased fresh and dry biomass | [74] | |
| Spinach | Aqueous suspension | Improved growth related to nitrogen fixation | [75] | |
| Spinach | Aqueous suspension | Improved light absorbance and carbon dioxide assimilation | [76] | |
| Iron NPs | Lettuce | Aqueous suspension | High concentration inhibited germination | [15] |
| Wheat | Direct application | Enhanced seed germination and plant growth | [88] | |
| Pumpkin | Direct application | No toxic effect | [102] | |
| Wheat | Direct application | Increased shoot and root biomass | [96] | |
| Wheat | Soil applied | Increased spike length, number of grains per spike, 1000 grain weight | [103] | |
| Various plants | Direct application | Development of thicker roots | [104] | |
| Copper/ Copper oxide NPs | Wheat | Direct application | Reduced root and seedling growth | [89] |
| Rose | Water-agar plates with suspension | Increased plant resistance to fungal infection by altering endogenous hormones content | [82] | |
| Pumpkin | Aqueous suspension | Reduced biomass | [81] | |
| Wheat | Direct application | Reduced seed germination | [103] | |
| Wheat | Direct application | Increased plant growth and biomass | [97] | |
| Maize | Aqueous suspension | Reduced seedling growth | [80] | |
| Mung bean | Agar culture media | Reduced seedling growth | [89] | |
| Wheat | Agar culture media | Reduced seedling growth | [89] | |
| Zucchini | Aqueous suspension | Reduced biomass and root growth | [81] | |
| Rice | Aqueous suspension | Decreased seed germination and seedlings growth | [105] | |
| Barley | Aqueous suspension | Restricted shoot and root growth | [106] | |
| Maize | Aqueous suspension | Suppressed root elongation | [80] | |
| Barley | Aqueous suspension | Decreased plasto globule and starch granule | [107] | |
| Maize | Aqueous suspension | Reduced shoot and root biomass | [108] | |
| Zinc oxide NPs | Pleuroziumschreberi | NPs suspension | Reduced L-ascorbic acid content | [109] |
| Wheat | NPs suspension | Reduced biomass | [100] | |
| Soybean | Direct application | Increased root growth | [91] | |
| Soybean | Direct application | Decreased root growth | [91] | |
| Ryegrass | Direct application | Reduced biomass, shrunken root tips, broken epidermis/root caps | [69] | |
| Soybean | Direct application | Increased root growth | [110] | |
| Maize | Aqueous suspension | Highly reduced root growth | [69] | |
| Ryegrass | Hoagland solution | Reduced biomass, shrank root tips, broken epidermis/root cap, highly vacuolated and collapsed cortical cells | [69] | |
| Carbon nanotubes | Rose | Water-agar plates with suspensions | Increased plant resistance to fungal infection by altering endogenous hormones content | [82] |
| Tomato | Aqueous suspension | Enhanced seed germination, fresh biomass, stem length | [78] | |
| Onion | Direct application | Increased root length | [79] | |
| Rice | Direct application | Delayed flowering and decreased yield | [72] | |
| Pumpkin | Aqueous suspension | Reduced biomass | [81] | |
| Wheat | Direct application | Increased root length | [80] | |
| Tomato | Aqueous suspension | Increased germination rate, fresh biomass, stem length | [78] | |
| Rice | MS medium | Delayed flowering and decreased yield | [72] | |
| Tomato | Aqueous suspension | Reduced root length | [79] | |
| Lettuce | Aqueous suspension | Reduced root length at longer exposure | [79] | |
| Cerium oxide NPs | Wheat | Direct application | Enhanced shoot growth, biomass, grain yield | [18] |
| Lettuce | Direct application | Inhibited root growth | [69] | |
| Maize | Direct application | Increased stem and root growth | [91] | |
| Maize | Aqueous suspension | Increased root and stem growth | [91] | |
| Tomato | Aqueous suspension | Reduced shoot growth | [91] | |
| Maize | Aqueous suspension | Reduced biomass | [91] | |
| Sorghum | Foliar spray | Increased leaf carbon assimilation rates, pollen germination, seed yield | [111] | |
| Rice | Direct application | Enhanced growth | [112] | |
| Onion | Foliar spray | Improved yield, plant growth, nutrient content | [113] | |
| Gold NPs | Lettuce | Aqueous suspension | Enhanced root elongation | [104] |
| Cucumber | Aqueous suspension | Improved germination | [104] | |
| Nd2O3NPs | Pumpkin | Aqueous suspension | Increased antioxidant capacity | [114] |
* Ref means references.
3.1. Plant Species Specificity of NPs
The impact of NPs depends on the type of plant species used. The aqueous suspension of aluminum oxide NPs improved the root growth of radish [69] but reduced in cucumber [70]. The aqueous suspension of titanium oxide NPs increased root length of wheat [71] but inhibited in cucumber [72]. The iron NPs aqueous suspension increased root length of Arabidopsis thaliana [14] and restricted in lettuce [15]. The aqueous suspension of titanium oxide NPs inhibited root elongation in cucumber [69] and carrot [70], but enhanced the growth of maize [1], wheat [73], and spinach [74,75,76,77]. The carbon-nanotubes suspension increased germination rate, fresh biomass, and seedling length in Solanum lycopersicum [78], Allium cepa [79], and wheat [80], while reduced in Cucurbita pepo [81], rice [72], and lettuce [79]. These studies have increased our understanding of phytotoxicity and plant responses towards NPs.
3.2. Plant Organ Specific Effects of NPs
The carbon nanotubes, copper-oxide NPs, and titanium-dioxide NPs increased resistance to fungal infection by altering the level of endogenous hormones [82]. The direct application of silver NPs reduced seedling biomass of wheat [83], zucchini [81], mung bean [83], and cabbage [84]; while it regulated the seedling growth in maize [84] and Vigna radiata [83]. The hydroponic applications of silver NPs enhanced root elongation in rice [85]; while it reduced in zucchini [81]. Changes in the morphological characteristics of treated plants depend on the types of NPs used. Silver NPs and aluminum-oxide NPs reduced [86] and improved [87], respectively, growth of wheat. The iron NPs enhanced germination ratio and plant growth [88]; while copper NPs inhibited the growth of wheat [89]. The flowering and yield of rice reduced on carbon nanotubes exposure [73]; while enhanced under cerium-oxide NPs treatment [90]. Silver NPs [84] and cerium-oxide NPs [91] improved the growth of maize; while aluminum-oxide NPs [70], titanium-oxide NPs [1], and copper NPs [80] treatments led to growth reduction. Keeping in view these studies, NPs might be involved in the alteration of growth in plants.
3.3. Stress Dependency of NPs
Various modes of applications determine the effects of NPs on growth and productivity of plants. Direct application of aluminum oxide NPs improved root length of wheat [87]; while reduced in maize in hydroponic condition [70]. Exposure of aluminum oxide NPs improved survival percentage and weight/length of root including hypocotyl of soybean under flooding stress [92,93]. There are some NPs with the capability to keep the same effects on the plant, though, applied through various ways, e.g., titanium-oxide NPs improved the growth of spinach when applied through seed treatment [94] and foliar spray [95]. Similarly, soil or direct application of iron NPs increased the growth [96] and yield [97] of wheat. The alteration in the morphology of plants is dependent on the mode of application and the type of NPs exposure is dependent on the mode of application.
4. Applications of Proteomic Techniques to Assess the Impact of NPs on Crops
With the advancements in mass spectrometry, proteomics has become a powerful technology for the identification and characterization of stress-induced proteins. Detailed proteome analysis of plant organelles generates comprehensive information about the intrinsic mechanisms of plant stress responses towards NPs. Proteomic analyses of various crops exposed to different NPs are summarized in Table 3.
Table 3.
Summary of proteomic analyses of various crops exposed to different NPs.
| NPs | Plant | Organ | Proteomic Technique | Protein Response | Ref * |
|---|---|---|---|---|---|
| Silver NPs | Soybean | Root | Gel-free (nanoLC–MS/MS) |
Decreased proteins associated with secondary metabolism, cell organization, and hormone metabolism. | [119] |
| Eruca sativa | Root | Gel-based (2-DE, nanoLC–nESI-MS/MS) |
Altered endoplasmic reticulum and vacuolar proteins involved in sulfur metabolism. | [98] | |
| Wheat | Root | Gel-based (2-DE, LC–MS/MS) |
Altered proteins involved in metabolism and cell defense. | [86] | |
| Soybean | Root | Gel-free (nanoLC–MS/MS) |
Altered proteins associated with stress, cell metabolism, signaling. | [125] | |
| Soybean | Root, Hyp ** |
Gel-free (nanoLC–MS/MS) |
Decreased protein synthesis with increased amino acid synthesis. | [93] | |
| Soybean | Root, Hyp ** |
Gel-free (nanoLC–MS/MS) |
Increased protein degradation related proteins. Decreased protein synthesis associated proteins. | [122] | |
| Wheat | Shoot | Gel-free (nanoLC–MS/MS) |
Increased proteins related to photosynthesis and protein synthesis. Decreased proteins linked to glycolysis, signaling, cell wall. | [121] | |
| Tobacco | Root, Leaf |
Gel-based (2-DE, MALDI- TOF/TOF MS) |
Altered abundance of root proteins involved in abiotic/biotic and oxidative stress responses. In leaf, proteins associated with photosynthesis markedly changed. | [131] | |
| Aluminum oxide NPs | Soybean | Root, Hyp ** |
Gel-free (nanoLC–MS/MS) |
Increased proteins related to protein synthesis, transport, and development during post- flooding recovery period. | [92] |
| Soybean | Root, Hyp ** |
Gel-free (nanoLC–MS/MS) |
Regulated the ascorbate/glutathione pathway and increased ribosomal proteins. | [120] | |
| Soybean | Root, Leaf |
Gel-free (nanoLC–MS/MS) |
Increased proteins involved in oxidation, stress signaling, and hormonal pathways. | [119] | |
| Soybean | Root, Hyp ** |
Gel-free (nanoLC–MS/MS) |
Decreased energy metabolism and changed proteins related to glycolysis compared to flooding stress. | [125] | |
| Copper NPs | Wheat | Shoot | Gel-free (nanoLC–MS/MS) |
Increased proteins related to glycolysis and tricarboxylic acid cycle. | [97] |
| Wheat | Seed | Gel-free (nanoLC–MS/MS) |
Increased proteins involved in starch degradation and glycolysis. | [103] | |
| Iron NPs | Wheat | Shoot | Gel-free (nanoLC–MS/MS) |
Decreased proteins linked to photosynthesis and protein metabolism. | [96] |
| Wheat | Seed | Gel-free (nanoLC–MS/MS) |
Increased proteins related to starch degradation, glycolysis, tricarboxylic acid cycle. | [103] | |
| Zinc oxide NPs | Soybean | Root, Leaf |
Gel-free (nanoLC–MS/MS) |
Decreased proteins involved in oxidation- reduction, stress signaling, and hormonal pathways. | [119] |
| Cerium oxide NPs | Maize | Shoot | Gel-free (nanoLC–ESI-MS/MS) |
Increased accumulation of heat shock protein. Increased ascorbate/ peroxidase/ catalase activity. | [130] |
* Ref means reference; ** Hyp stands for Hypocotyl. Abbreviations: 2-DE, two-dimensional gel electrophoresis; nESI, nanoelectro spray ionization; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight.
4.1. Proteomic Analysis of Silver NPs Challenged Crops
Silver NPs are considered as a promising antibacterial agent due to their strong biocidal effect against microorganisms [115]. These NPs are synthesized through different physical, chemical, and biological methods and well-defined parameters of size and shape [28]. The effects of silver NPs were initially analyzed using proteomic techniques in Chlamydomonas [116], Escherichia coli [117], and Bacillus thuringiensis [118]. Currently, various crop plants were exposed to silver NPs and their effects were analyzed using gel-based or gel-free proteomic techniques. Our gel-free proteomic study revealed restricted growth of soybean seedlings under silver NPs treatment [119]. Proteins related to secondary metabolism, cell organization, and hormone metabolism were mostly influenced by silver NPs exposure. In contrast, silver NPs of 15 nm in size significantly improved the soybean growth under flooding stress by enhancing proteins linked to amino acid synthesis [120]. In wheat, the accumulation of different cellular compartmental proteins on silver NPs exposure in shoot and root was mainly involved in metabolism and cell defense [86]. Silver NPs with chemical exposure increased the proteins related to photosynthesis and protein synthesis, while decreased the glycolysis, signaling, and cell wall related proteins in wheat [121]. Large numbers of proteins involved in the primary metabolism were increased in soybean [119]. Silver NPs treatment increased the proteins related to protein degradation, while decreased protein synthesis related proteins in soybean; indicating that it might improve the growth of soybean under flooding stress through protein quality control [122]. Proteins related to the oxidative stress, signaling, transcription, protein degradation, cell wall synthesis, cell division, and apoptosis were found to be increased in silver NPs exposed rice [118]. In Eruca sativa, proteins associated with the endoplasmic reticulum and vacuole were differentially modulated under silver NPs exposure [86]. These findings indicate that silver NPs primarily influence various metabolic processes in wheat and protein quality control in soybeans; thus, improving plant growth.
4.2. Proteomic Analysis of Aluminum Oxide NPs Stressed Crops
Aluminum oxide NPs are mostly used in military and commercial products [123]. Extensive usage of aluminum oxide NPs leads towards their possible leakage into environment, which ultimately interacts with living organisms including plants [124]. Proteomic analysis of soybean root treated with aluminum oxide NPs revealed an increase in the number of proteins related to protein synthesis, transport, and development during the recovery from flooding [92]. A study by Mustafa et al. [120] revealed that proteins associated with the ascorbate-glutathione cycle, as well as ribosomal proteins, were differentially influenced by aluminum oxide NPs. Moreover, high abundance of proteins involved in oxidation-reduction, stress signaling, hormonal pathways related to growth and development, were evident in aluminum oxide NPs challenged soybean [119]. A separate study has shown growth promoting effects of aluminum oxide NPs in the soybean under flooding stress by regulating energy metabolism and cell death [125].
4.3. Proteomic Analysis of Crops Exposed to Copper NPs and Iron NPs
Among the various metal-based NPs, copper NPs are by far the most well studied NPs whose toxicity has been tested in wide range of crops. They have wide applications in electronics, air/liquid filtration, ceramics, wood preservation, bioactive coatings, and films/textiles [16]. At the cellular level, copper acts as structural and catalytic component of many proteins involved in various metabolic processes. In wheat seedlings, abundance of proteins associated with glycolysis and tricarboxylic acid cycle was found to be increased; while, photosynthesis and tetrapyrrole synthesis related proteins were decreased on exposure to copper nanoparticles [97]. Wheat grains obtained after NPs exposure were analyzed through gel-free proteomic technique, which indicated an increase in proteins involved in starch degradation and glycolysis [96].
Similar to copper NPs, iron NPs have extensive industrial, commercial, and biomedical applications [12]. Because of their high reactivity and magnetic property, iron NPs have been used as remediation agents for environmental applications [13]. Iron NPs have known stimulatory effects on the seed germination and plant growth of wheat [96]. Authors exploited gel-free/label-free proteomic technique to elucidate the impact of iron NPs on shoot growth of drought tolerant and salt tolerant wheat varieties. A study revealed that differentially expressed proteins in both varieties were mainly associated with photosynthesis. Notably, proteins related to light reaction were enhanced in the salt tolerant variety compared to drought tolerant wheat on iron NPs exposure. A separate study on grain analysis of wheat indicated an increase in the number of proteins related to starch degradation, glycolysis, and the tricarboxylic acid cycle [103].
4.4. Proteomic Analysis of Other NPs Challenged Crops
One of the most commonly used nanomaterials in agriculture and the energy sector is titanium dioxide NPs [126]. They have diverse applications in personal skincare products, water-treatment agents, and bactericidal agents owing to their high stability and anticorrosive/photocatalytic properties [127,128]. The toxicological effects of nanometer titanium dioxide on a unicellular green alga Chlamydomonas reinhardtii were accessed by monitoring the changes in the physiology and cyto-ultrastructure [129]. Authors reported nano titanium dioxide mediated inhibition in photosynthetic efficiency and cell growth, with increased contents of carotenoids and chlorophyll b.
In addition, various NPs are being extensively utilized to improve the growth and productivity of crop plants. However, application of zinc oxide NPs had marked effects on soybean seedling growth, rigidity of roots, and root cell viability [119]. Gel-free proteomic analysis revealed down regulation oxidation-reduction cascade associated proteins, including GDSL motif lipase 5, SKU5 similar 4, galactose oxidase, and quinone reductase in zinc oxide NPs exposed roots. A separate study on cerium oxide NPs treatment in maize indicated enhanced accumulation of heat shock proteins (HSP70) and increased activity of ascorbate peroxidase and catalase [130]. This up regulated antioxidant defense system might help maize plants to overcome NPs-induced oxidative stress damages.
All of these studies indicate that NPs have the potential to modulate plant metabolic processes, and impact of NPs could be either positive or negative, depending on the plant species and type of nanoparticles used, their size, composition, concentration, and physical/chemical properties.
5. NPs Uptake and Mode of Action
The phytotoxicity of NPs largely depends on the particle size, concentration and chemistry of NPs, in addition to the chemical milieu of the subcellular sites at which the NPs are deposited [23]. Plants, being an indispensable component of terrestrial ecosystems, serve as a potential route for the factory discharged-NPs to enter the plant root system and their transportation to other parts of the plants, resulting bioaccumulation in the food chain [132]. The physico-chemical properties of soil matrix (viz. mineral composition, pH, ionic strength, dissolved organic matter, etc.) as well as the of metal based NPs (viz. size, surface charge, surface coating, etc.) are the determining factors for NPs mobility [133]. Primary-lateral root junctions are the prime sites through which NPs could enter xylem via cortex and finally reach the central cylinder [23]. Study on the uptake pathways of zinc oxide NPs by maize roots reveals that majority of the total zinc oxide NPs undergo dissolution in the exposure medium, and the released Zn2+ ions are only taken up by the roots [134]. Only a small fraction of zinc oxide NPs adsorbed on the root surface can cross the root cortex as a result of speedy cell division and root tip elongation, apart from their entry to vascular system through the gap of the Casparian strip at the sites of the primary–lateral root junction.
Once NPs enter the root cells, these ultrafine particles upon dissolution discharge metal ions that interact with the functional groups of proteins (carboxyl and sulfhydryl groups) causing altered protein activity. The released redox-active metal ions could trigger reactive oxygen species (ROS) generation through the Fenton and Haber–Weiss reactions [135]. In these reactions, the hydrogen peroxide (H2O2) is decayed by the metal ions leading to the formation of more toxic ROS, namely hydroxyl radical (•OH) and hydroxyl anion (OH−). Elevated ROS generation was documented in leaves of soybean exposed to zinc oxide NPs and silver NPs [119] as well as in copper oxide NPs challenged rice [105]. These NPs mediated excess ROS formation disturbs the cellular redox system in favor of oxidized forms, causing oxidative damage to vital cellular components including nucleic acids, lipids, and proteins [135].
Cellular compartments with extremely high oxidizing metabolic activity or with an intense rate of electron flow, such as mitochondria, chloroplasts, and peroxisomes, constitute a major source of ROS production in plants [136]. Investigations have revealed that zinc oxide NPs mediated deregulation of photosynthetic efficiency in plants is due to the down regulation of chlorophyll synthesis genes and structural genes of photosystem I [137,138]. To protect cells against such oxidative damages, plants have developed robust multi-component antioxidant defense system comprising of both enzymatic and non-enzymatic machineries [119,139]. The enzymatic antioxidant defense system chiefly includes ROS scavenging enzymes of the ascorbate–glutathione cycle, which operates in nearly all plant cell organelles [140]. The orchestrated action of key antioxidant enzymes viz. superoxide dismutase (SOD), ascorbate peroxidase (APX), catalase (CAT), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR), and glutathione reductase (GR) is an adaptive strategy of plant to cope with the NPs induced oxidative stress damages.
Moreover, NPs exposure often leads to disruption of cellular redox homeostasis and cause cell membrane damage through lipid peroxidation [105,106,108]. Among the ROS, hydroxyl radical (•OH) is known to be the most reactive, capable of stealing hydrogen atom from a methylene (-CH2-) group present in polyunsaturated fatty acid side chain of membrane lipids and, thus, initiates lipid peroxidation [141]. Since, •OH is derived from H2O2 as a consequence of one electron reduction, H2O2 scavenging peroxides play essential roles in protecting lipid membranes from NPs mediated oxidative stress. Among ROS, a recent study revealed down regulation of ascorbate peroxidase (APX1) in zinc oxide NPs challenged maize leaves with concomitant increased malondialdehyde (MDA) level, an indicative of oxidative stress induced damage to the lipid membrane [108]. The NPs-induced higher membrane damage is in accordance with the previous reports in rice [105] and Syrian barley [106].
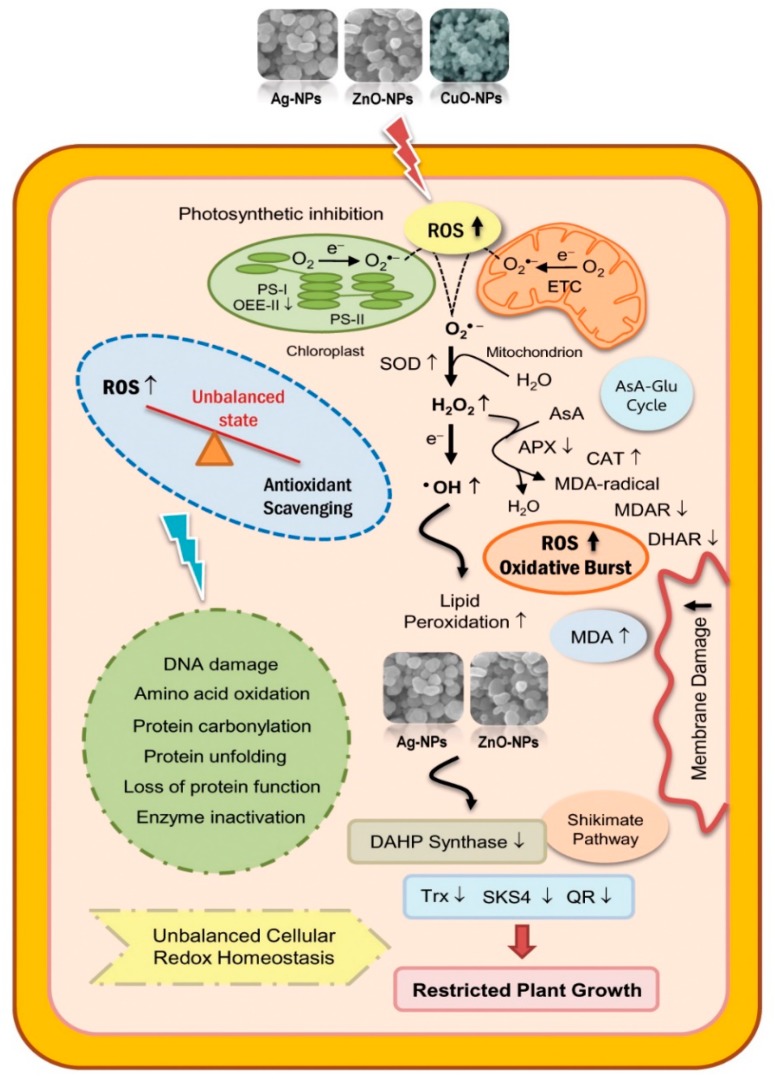
Apart from enzymatic component of ascorbate-glutathione cycle, plants have evolved a second line of defense to cope with the NPs induced oxidative stress. The thioredoxin (Trx) family protein is one of them, engage in mitigating oxidative damages by providing reducing power to reductases, detoxifying lipid hydroperoxides or repairing oxidized proteins. They also act as regulators of scavenging mechanisms and key components of signaling pathways in the plant antioxidant network [142]. In addition, these proteins are necessary for their potential roles as facilitators and regulators of protein folding and chaperone activity [143]. Furthermore, plant quinone reductases (QRs) are involved redox reactions and act as detoxification enzymes of free radicals. Soybean seedlings exposed to zinc oxide NPs and silver NPs treatments exhibited significantly declined abundance of Trx and QR proteins [119]. Severe oxidative burst evident in zinc oxide NPs and silver NPs challenged soybean might be the result of such declined protein abundance affecting optimum growth of seedlings. Enzymes of shikimate pathway involved in the synthesis of amino acids (phenylalanine, tryptophan, and tyrosine) were also found to be affected under NPs exposure. These aromatic amino acids not only act as substrates for the protein synthesis, but are also linked with formation of secondary products, including lignin, suberin, and phytoalexins. The abundance of 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) synthase, the first enzyme of the shikimate pathway, was reported to be decreased in soybean under silver NPs treatment [119]. The reduced shoot length of silver NPs exposed soybean seedlings might be the result of such marked decline in DAHP synthase level. In a nutshell, low abundance of proteins involved in oxidation-reduction, shikimate pathway might limit the growth of the silver NPs challenged soybean seedlings up to a certain level. Summarizing all these findings, a comprehensive model of cellular responses to NPs is presented in Figure 1.
Figure 1.
Schematic illustration of diverse cellular responses to nanoparticles (NPs). Exposure to metal based-NPs triggers oxidative stress through enhanced reactive oxygen species (ROS) generation, disruption of redox homeostasis, impaired photosynthetic activity, mitochondrial dysfunction, lipid peroxidation, and membrane damage. Upward arrows indicate increased and downward arrows indicate decreased protein abundance in response to NPs stress, respectively. Abbreviations: APX, ascorbate peroxidase; AsA, reduced ascorbate; CAT, catalase; DAHP, 3-deoxy-D-arabino-heptulosonate-7-phosphate; DHAR, dehydroascorbate reductase; ETC, electron transport chain; H2O2, hydrogen peroxide; MDA, malondialdehyde; MDA-radical, monodehydroascorbate radical; MDAR, monodehydroascorbate reductase; •OH, hydroxyl radical; OEE, oxygen-evolving enhancer; PS, photosystem; QR, quinone reductase; ROS, reactive oxygen species; SOD, superoxide dismutase; Trx, thioredoxin.
6. Conclusions
Nanotechnology has gained tremendous momentum in recent times because of the wide applications of NPs in agriculture, cosmetic industry, cellular imaging, medical diagnosis, biosensing, drug delivery, and cancer therapy. Nevertheless, unintended release of such commercially manufactured nanomaterials into the environment has raised global concern. Hence, considerable attention is now being paid to the methods and strategies of NPs synthesis, plant-nanomaterials interactions, and their environmental fate. As compared to traditional physical and chemical processes, green synthesis of NPs using microorganisms and plants is an environment-friendly, cost effective, safe, biocompatible, green alternative approach for large scale production of NPs. Morphophysiological as well as proteomic studies on NPs-induced phytotoxicity reveal that particle size, concentration, and chemistry of NPs, as well as the type of plant species used, are the key factors determining the type and magnitude of the cellular responses. However, more initiatives must be taken to find out whether the metal-based NPs exert phytotoxicity exclusively due to their high surface area and nanoscale size or due to the released metal ions. Moreover, there is a need for more comprehensive omics approach integrating genomics, transcriptomics, proteomics, and metabolomics, so that the impact of the applied NPs on plants can be assessed well in time.
Author Contributions
Conceptualization, S.K.; writing-original draft preparation, Z.H., F.Y., and S.K.; writing-review and editing, Z.H. and S.K.; visualization, Z.H. and S.K.; supervision, S.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grant (No. 2) from Fukui University of Technology, Japan.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Singh T., Shukla S., Kumar P., Wahla V., Bajpai V.K., Rather I.A. Application of nanotechnology in food Science: Perception and overview. Front. Microbiol. 2017;8:e1501. doi: 10.3389/fmicb.2017.01501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajpai V.K., Kamle M., Shukla S., Mahato D.K., Chandra P., Hwang S.K., Kumar P., Huh Y.S., Han Y.-K. Prospects of using nanotechnology for food preservation, safety, and security. J. Food Drug Anal. 2018;26:1201–1214. doi: 10.1016/j.jfda.2018.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khot L.R., Sankaran S., Maja J.M., Ehsani R., Schuster E.W. Applications of nanomaterials in agricultural production and crop protection: A review. Crop. Prot. 2012;35:64–70. doi: 10.1016/j.cropro.2012.01.007. [DOI] [Google Scholar]
- 4.Albrecht M.A., Evans C.W., Raston C.L. Green chemistry and the health implications of nanoparticles. Green Chem. 2006;8:417–432. doi: 10.1039/b517131h. [DOI] [Google Scholar]
- 5.Xu D., Liu M., Zou H. A new strategy for fabrication of water dispersible and biodegradable fluorescent organic nanoparticles with AIE and ESIPT characteristics and their utilization for bioimaging. Talanta. 2017;174:803–808. doi: 10.1016/j.talanta.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 6.Hassan H.F., Mansour A.M., Abo-Youssef A.M., Elsadek B.E., Messiha B.A. Zinc oxide nanoparticles as a novel anticancer approach; in vitro and in vivo evidence. Clin. Exp. Pharmacol. Physiol. 2017;44:235–243. doi: 10.1111/1440-1681.12681. [DOI] [PubMed] [Google Scholar]
- 7.Nirmala M., Anukaliani A. Synthesis and characterization of undoped and TM (Co, Mn) doped ZnO nanoparticles. Mater. Lett. 2011;65:2645–2648. doi: 10.1016/j.matlet.2011.06.029. [DOI] [Google Scholar]
- 8.Thapa A., Soares A.C., Soares J.C. Carbon nanotube matrix for highly sensitive biosensors to detect pancreatic cancer biomarker CA19-9. ACS Appl. Mater. Interfaces. 2017;9:25878–25886. doi: 10.1021/acsami.7b07384. [DOI] [PubMed] [Google Scholar]
- 9.El-Gharbawy R.M., Emara A.M., Abu-Risha S.E. Zinc oxide nanoparticles and a standard antidiabetic drug restore the function and structure of beta cells in Type-2 diabetes. Biomed. Pharmacother. 2016;84:810–820. doi: 10.1016/j.biopha.2016.09.068. [DOI] [PubMed] [Google Scholar]
- 10.Rosi N.L., Mirkin C.A. Nanostructures in biodiagnostics. Chem. Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 11.Auffan M., Rose J., Bottero J.Y., Lowry G.V., Jolivet J.P., Wiesner M.R. Towards a definition of inorganic nanoparticles from an environmental, health and safety perspective. Nat. Nanotechnol. 2009;4:4–41. doi: 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- 12.Teja A.S., Koh P.Y. Synthesis, properties, and applications of magnetic iron oxide nanoparticles. Prog. Cryst. Growth Charact. 2009;55:22–45. doi: 10.1016/j.pcrysgrow.2008.08.003. [DOI] [Google Scholar]
- 13.Yan W., Lien H.I., Koel B.E., Zhang W. Iron nanoparticles for environmental clean-up: Recent development and future outlook. Environ. Sci. Processes Impacts. 2013;15:63–77. doi: 10.1039/C2EM30691C. [DOI] [PubMed] [Google Scholar]
- 14.Kim J., Lee Y., Kim E., Gu S., Sohn E.J., Soe Y.S., An H.J., Chang Y.S. Exposure of iron nanoparticles to Arabidopsis thaliana enhances root elongation by triggering cell wall loosening. Environ. Sci. Technol. 2014;48:3477–3485. doi: 10.1021/es4043462. [DOI] [PubMed] [Google Scholar]
- 15.Trujillo-Reyes J., Majumdar S., Botez C.E., Peralta-Videa J.R., Gardea-Torresdey J.L. Exposure studies of core–shell Fe/Fe3O4 and Cu/CuO NPs to lettuce (Lactuca sativa) plants: Are they a potential physiological and nutritional hazard? J. Hazard. Mater. 2014;267:255–263. doi: 10.1016/j.jhazmat.2013.11.067. [DOI] [PubMed] [Google Scholar]
- 16.Yang C.W., Zen J.M., Kao Y.L., Hsu C.T., Chung T.C., Chang C.C., Chou C.C. Multiple screening of urolithic organic acids with copper nanoparticle-plated electrode: Potential assessment of urolithic risks. Anal. Biochem. 2009;395:224–230. doi: 10.1016/j.ab.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 17.Jia H., Chen S., Wang X., Shi C., Liu K., Zhang S., Li J. Copper oxide nanoparticles alter cellular morphology via disturbing the actin cytoskeleton dynamics in Arabidopsis roots. Nanotoxicology. 2020;14:127–144. doi: 10.1080/17435390.2019.1678693. [DOI] [PubMed] [Google Scholar]
- 18.Rico C.M., Majumdar S., Duarte-Gardea M., Peralta-Videa J.R., Gardea-Torresdey J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011;59:3485–3498. doi: 10.1021/jf104517j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patlolla A.K., Berry A., May L., Tchounwou P.B. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles. Int. J. Environ. Res. Public Health. 2012;9:1649–1662. doi: 10.3390/ijerph9051649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaveh R., Li S., Ranjbar S., Tehrani R., Brueck C.L., Aken B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013;47:10637–10644. doi: 10.1021/es402209w. [DOI] [PubMed] [Google Scholar]
- 21.Hossain Z., Mustafa G., Komatsu S. Plant responses to nanoparticle stress. Int. J. Mol. Sci. 2015;16:26644–52663. doi: 10.3390/ijms161125980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee S., Kim S., Kim S., Lee I. Assessment of phytotoxicity of ZnO NPs on a medicinal plant, Fagopyrum esculentum. Environ. Sci. Pollut. Res. 2013;20:848–854. doi: 10.1007/s11356-012-1069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dietz K.J., Herth S. Plant nanotoxicology. Trends Plant Sci. 2011;16:582–589. doi: 10.1016/j.tplants.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Asharani P.V., Mun G.L.K., Hande M.P., Valiyaveettil S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2009;3:279–290. doi: 10.1021/nn800596w. [DOI] [PubMed] [Google Scholar]
- 25.Barkla B.J., Vera-Estrella R., Pantoja O. Progress and challenges for abiotic stress proteomics of crop plants. Proteomics. 2013;13:1801–1815. doi: 10.1002/pmic.201200401. [DOI] [PubMed] [Google Scholar]
- 26.Abdelhamid H.N., Wu H.F. Proteomics analysis of the mode of antibacterial action of nanoparticles and their interactions with proteins. Trends Anal. Chem. 2015;65:30–46. doi: 10.1016/j.trac.2014.09.010. [DOI] [Google Scholar]
- 27.Wang W., Luo M., Fu Y., Wang S., Efferth T., Zu Y. Glycyrrhizic acid nanoparticles inhibit LPS-induced inflammatory mediators in 264.7 mouse macrophages compared with unprocessed glycyrrhizic acid. Int. J. Nanomed. 2013;8:1377. doi: 10.2147/IJN.S37788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iravani S., Golghar B. Green synthesis of silver nanoparticles using Pinus ldarica bark extract. Biol. Med. Res. Int. 2014;2013:1–5. doi: 10.1155/2013/639725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung J., Oh H., Noh H., Ji J., Kim S. Metal nanoparticle generation using a small ceramic heater with a local heating area. J. Aerosol. Sci. 2006;37:1662–1670. doi: 10.1016/j.jaerosci.2006.09.002. [DOI] [Google Scholar]
- 30.Tsuji T., Kakita T., Tsuji M. Preparation of nano-size particle of silver with femtosecond laser ablation in water. Appl. Surface Sci. 2003;206:314–320. doi: 10.1016/S0169-4332(02)01230-8. [DOI] [Google Scholar]
- 31.Boutinguiza M., Comesaña R., Lusquiños F., Riveiro A., Pou J. Production of nanoparticles from natural hydroxylapatite by laser ablation. Nanoscale Res. Lett. 2011;6:255. doi: 10.1186/1556-276X-6-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zboril R., Mashlan M., Barcova K., Vujtek M. Thermally induced solid-state syntheses of γ-Fe2O3 nanoparticles and their transformation to α-Fe2O3 via ε-Fe2O3. Hyperfine Interact. 2002;139:597. doi: 10.1023/A:1021226929237. [DOI] [Google Scholar]
- 33.Chin S.F., Pang S.C., Tan C.H. Green synthesis of magnetite nanoparticles (via thermal decomposition method) with controllable size and shape. J. Mater. Environ. Sci. 2011;3:299–302. [Google Scholar]
- 34.Das D., Nathb B.C., Phukonc P., Dolui S.K. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Colloids Surf. B Biointerfaces. 2013;101:430–433. doi: 10.1016/j.colsurfb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Soomro R.A., Sherazi S.T., Sirajuddin H., Memon N., Shah M.R., Kalwar N.H., Hallam K.R., Shah A. Synthesis of air stable copper nanoparticles and their use in catalysis. Adv. Mat. Lett. 2014;5:191–198. doi: 10.5185/amlett.2013.8541. [DOI] [Google Scholar]
- 36.Park B.K., Jeong S., Kim D., Moon J., Lim S., Kim J.S. Synthesis and size control of monodisperse copper nanoparticles by polyol method. J. Colloid Interface Sci. 2007;311:417–424. doi: 10.1016/j.jcis.2007.03.039. [DOI] [PubMed] [Google Scholar]
- 37.Ramyadevi J., Jeyasubramanian K., Marikani A., Rajakumar G., Rahuman A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012;71:114–116. doi: 10.1016/j.matlet.2011.12.055. [DOI] [Google Scholar]
- 38.Peyser L.A., Vinson A.E., Bartko A.P., Dickson R.M. Photoactivated fluorescence from individual silver nanoclusters. Science. 2001;291:103–106. doi: 10.1126/science.291.5501.103. [DOI] [PubMed] [Google Scholar]
- 39.Orendoff C.J., Gearheart L., Jana N.R., Murphy C.J. Aspect ratio dependence on surface enhanced Raman scattering using silver and gold nanorod substrates. Phys. Chem. Chem. Phys. 2006;8:165–170. doi: 10.1039/B512573A. [DOI] [PubMed] [Google Scholar]
- 40.Huang K., Ehrman S.H. Synthesis of iron nanoparticles via chemical reduction with palladium ion seeds. Langmuir. 2007;23:1419–1426. doi: 10.1021/la0618364. [DOI] [PubMed] [Google Scholar]
- 41.Selvarani M., Prema P. Evaluation of antibacterial efficacy of chemically synthesized copper and zerovalent iron nanoparticles. Asian J. Pharm. Clin. Res. 2013;6:223–227. [Google Scholar]
- 42.Dhas N.A.C., Raj P., Gedanken A. Synthesis, characterization, and properties of metallic copper nanoparticles. Chem. Mater. 1998;10:1446–1452. doi: 10.1021/cm9708269. [DOI] [Google Scholar]
- 43.Ruparelia J.P., Chatterjee A.K., Duttagupta S.P., Mukherji S. Strain specificity in antimicrobial activity of silver and copper nanoparticles. Acta Biomater. 2008;4:707–716. doi: 10.1016/j.actbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Fu H., Yang X., Jiang X., Yu A. Bimetallic Ag–Au nanowires: Synthesis, growth mechanism, and catalytic properties. Langmuir. 2013;29:7134–7142. doi: 10.1021/la400753q. [DOI] [PubMed] [Google Scholar]
- 45.Qin Y., Ji X., Jing J., Liu H., Wu H., Yang W. Size control over spherical silver nanoparticles by ascorbic acid reduction. Colloids Surf. A. 2010;372:172–176. doi: 10.1016/j.colsurfa.2010.10.013. [DOI] [Google Scholar]
- 46.Kim J., Kim H.S., Lee N., Kim T., Kim H., Yu T., Song I.C., Moon W.K., Hyeon T. Multifunctional uniform nanoparticles composed of a magnetite nanocrystal core and a mesoporous silica shell for magnetic resonance and fluorescence imaging and for drug delivery, Angewandte Chemie. Int. Ed. 2008;47:8438–8441. doi: 10.1002/anie.200802469. [DOI] [PubMed] [Google Scholar]
- 47.Wang L., Muhammed M. Synthesis of zinc oxide nanoparticles with controlled morphology. J. Mater. Chem. 1999;9:2871–2878. doi: 10.1039/a907098b. [DOI] [Google Scholar]
- 48.Pranjali P.M., Patil P.M., Dhanavade M.J., Badiger M.V., Shadija P.G., Lokhande A.C., Bohara R.A. Synthesis and characterization of zinc oxide nanoparticles by using polyol chemistry for their antimicrobial and antibiofilm activity. Biochem. Biophys. Rep. 2019;17:71–80. doi: 10.1016/j.bbrep.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramanathan R., Field M.R., O’Mullane A.P., Smooker P.M., Bhargava S.K., Bansal V. Aqueous phase synthesis of copper nanoparticles: A link between heavy metal resistance and nanoparticle synthesis ability in bacterial systems. Nanoscale. 2013;5:2300–2306. doi: 10.1039/C2NR32887A. [DOI] [PubMed] [Google Scholar]
- 50.Iqbal M.J., Ali S., Rashid S., Kamran M., Malik M.F., Sughra K., Zeeshan N., Afroz A., Saleem J., Saghir M. Biosynthesis of silver nanoparticles from leaf extract of Litchi chinensis and its dynamic biological impact on microbial cells and human cancer cell lines. Cell Mol. Biol. 2018;64:42–47. doi: 10.14715/cmb/2018.64.13.9. [DOI] [PubMed] [Google Scholar]
- 51.Poinern G.E.J., Chapman P., Shah M., Fawcett D. Green biosynthesis of silver nanocubes using the leaf extracts from Eucalyptus macrocarpa. Nano Bull. 2013;2:1–7. [Google Scholar]
- 52.Shehzad A., Qureshi M., Jabeen S., Ahmad R., Alabdalall A.H., Aljafary M.A., Al-Suhaimi E. Synthesis, characterization and antibacterial activity of silver nanoparticles using Rhazya stricta. Peer J. 2018;6:60–86. doi: 10.7717/peerj.6086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gholami A., Khosravi R., Khosravi A., Samadi Z. Data on the optimization of the synthesis of green iron nanoparticles using plants indigenous to South Khorasan. Data Brief. 2018;21:1779–1783. doi: 10.1016/j.dib.2018.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Machado S., Pinto S.L., Grosso J.P., Nouws H.P.A., Albergaria J.T., Delerue-Matos C. Green production of zero-valent iron nanoparticles using tree leaf extracts. Sci. Total Environ. 2013;445–446:1–8. doi: 10.1016/j.scitotenv.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 55.Shahwana T., Abu Sirriah S., Nairata M., Boyacıb E., Eroglub A.E., Scottc T.B., Hallam K.R. Green synthesis of iron nanoparticles and their application as a fentonlike catalyst for the degradation of aqueous cationic and anionic dyes. Chem. Eng. J. 2011;172:258–266. [Google Scholar]
- 56.Umar H., Kavaz D., Rizaner N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomedicine. 2018;14:87–100. doi: 10.2147/IJN.S186888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shobha N., Nanda N., Giresha A.S., Manjappa P., Dharmappa K.K., Nagabhushana B.M. Synthesis and characterization of Zinc oxide nanoparticles utilizing seed source of Ricinus communis and study of its antioxidant, antifungal and anticancer activity. Mater. Sci. Eng. C. Mater. Biol. Appl. 2019;97:842–850. doi: 10.1016/j.msec.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 58.Sangeetha G., Rajeshwari S., Venckatesh R. Green synthesis of zinc oxide nanoparticles by aloe barbadensis miller leaf extract: Structure and optical properties. Mater. Res. Bull. 2011;46:2560–2566. doi: 10.1016/j.materresbull.2011.07.046. [DOI] [Google Scholar]
- 59.Kulkarni V.D., Kulkarni P.S. Green synthesis of copper nanoparticles using Ocimum sanctum leaf extract. Int. J. Chem. Stud. 2013;1:1–4. [Google Scholar]
- 60.Jayalakshmi Y.A. Green synthesis of copper oxide nanoparticles using aqueous extract of flowers of cassia alata and particles characterisation. Int. J. Nanomater. Biostruct. 2014;4:66–71. [Google Scholar]
- 61.Saranyaadevi K., Subha V., Ravindran R.S., Renganathan S. Synthesis and characterization of copper nanoparticle using Capparis zeylanica leaf extract. Int. J.Chem.Tech. Res. 2014;6:4533–4541. [Google Scholar]
- 62.Subhankari I., Nayak P.L. Synthesis of copper nanoparticles using Syzygium aromaticum (Cloves) aqueous extract by using green chemistry. World J. Nano Sci. Technol. 2013;2:14–17. [Google Scholar]
- 63.Aslani F., Bagheri S., Julkapli N.M., Juraimi A.S., Hashemi F.S.G., Baghdadi A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014;2014:641759. doi: 10.1155/2014/641759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ogunyemi S.O., Abdallah Y., Zhang M., Fouad H., Hong X., Ibrahim E., Masum M.M.I., Hossain A., Mo J., Li B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019;47:341–352. doi: 10.1080/21691401.2018.1557671. [DOI] [PubMed] [Google Scholar]
- 65.Taran M., Rad M., Alayi M. Biosynthesis of TiO2 and ZnO nanoparticles by Halomonas elongata IBRC-M 10214 in different conditions of medium. Bioimpacts. 2017;8:81–89. doi: 10.15171/bi.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dodoo-Arhin D., Buabeng F.P., Mwabora J.M., Amaniampong P.N., Agbe H., Nyankson E., Obada D.O., Asiedu N.Y. The effect of titanium dioxide synthesis technique and its photocatalytic degradation of organic dye pollutants. Heliyon. 2018;4:e00681. doi: 10.1016/j.heliyon.2018.e00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma X., Geiser-Lee J., Deng Y., Kolmakov A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010;408:3053–3061. doi: 10.1016/j.scitotenv.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 68.Budhani S., Egboluche N.P., Arslan Z., Yu H., Deng H. Phytotoxic effect of silver nanoparticles on seed germination and growth of terrestrial plants. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2019;37:330–355. doi: 10.1080/10590501.2019.1676600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin D., Xing B. Phytotoxicity of nanoparticles: Inhibition of seed germination and root growth. Environ. Pollut. 2007;150:243–250. doi: 10.1016/j.envpol.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 70.Yang L., Watts D.J. Particles surface characteristics may play an important role in phytotoxicity of alumina nanoparticles. Toxicol. Lett. 2005;158:122–132. doi: 10.1016/j.toxlet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Larue C., Laurette J., Herlin-Boime N., Khodja H., Fayard B., Flank A., Brisset F., Brisset M. Accumulation, translocation, and impact of TiO2 nanoparticles in wheat (Triticum aestivum spp.): Influence of diameter and crystal phase. Sci. Total Environ. 2012;43:197–208. doi: 10.1016/j.scitotenv.2012.04.073. [DOI] [PubMed] [Google Scholar]
- 72.Lin S., Reppert J., Hu Q., Hudson J.S., Reid M.L., Ratnikova T.A., Rao A.M., Luo H., Key P.C. Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small. 2009;5:1128–1132. doi: 10.1002/smll.200801556. [DOI] [PubMed] [Google Scholar]
- 73.Rafique C., Arshad N., Khakhar M.F., Qazi I.A., Hamza A., Vivic N. Growth response of wheat to titania nanoparticles application. NJES. 2014;7:42–46. [Google Scholar]
- 74.Hong F.H., Zhou J., Liu C., Yang F., Wu C., Zheng L., Yang P. Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol. Trace Elem. Res. 2005;105:269–279. doi: 10.1385/BTER:105:1-3:269. [DOI] [PubMed] [Google Scholar]
- 75.Yang F., Liu C., Gao F., Su M., Wu X., Zheng L., Hong F., Yang P. The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol. Trace Elem. Res. 2007;119:77–88. doi: 10.1007/s12011-007-0046-4. [DOI] [PubMed] [Google Scholar]
- 76.Linglan M., Chao L., Chunxiang Q., Sitao Y., Jie L., Fengqing G., Fashui H. Rubisco activase mRNA expression in spinach: Modulation by nanoanatase treatment. Biol. Trace Elem. Res. 2008;122:168–178. doi: 10.1007/s12011-007-8069-4. [DOI] [PubMed] [Google Scholar]
- 77.Lu C.M., Zhang C.Y., Wen J.Q., Wu G.R., Tao M.X. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2010;21:168–172. [Google Scholar]
- 78.Khodakovskaya M., Dervishi E., Mahmood M., Xu Y., Li Z., Watanabe F., Biris A.S. Carbon nanotubes are able to penetrate plant seed coat and dramatically affect seed germination and plant growth. ACS Nano. 2009;3:3221–3227. doi: 10.1021/nn900887m. [DOI] [PubMed] [Google Scholar]
- 79.Canas J.E., Long M., Nations S., Vadan R., Dai L., Luo M. Effects of functionalized and nonfunctionalized single-walled carbon-nanotubes on root elongation of select crop species. Nanomater. Environ. 2008;27:1922–1931. doi: 10.1897/08-117.1. [DOI] [PubMed] [Google Scholar]
- 80.Wang X., Han H., Liu X., Gu X., Chen K., Lu D. Multi-walled carbon nanotubes can enhance root elongation of wheat (Triticum aestivum) plants. J. Nanopart. Res. 2012;14:841. doi: 10.1007/s11051-012-0841-5. [DOI] [Google Scholar]
- 81.Stampoulis D., Sinha S.K., White J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009;43:9473–9479. doi: 10.1021/es901695c. [DOI] [PubMed] [Google Scholar]
- 82.Hao Y., Fang P., Ma C., White J.C., Xiang Z., Wang H., Zhang Z., Rui Y., Xing B. Engineered nanomaterials inhibit Podosphaera pannosa infection on rose leaves by regulating phytohormones. Environ. Res. 2018;170:1–6. doi: 10.1016/j.envres.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 83.Singh D., Kumar A. Effects of nano silver oxide and silver ions on growth of Vigna radiata. Bull. Environ. Contam. Toxicol. 2015;95:379–384. doi: 10.1007/s00128-015-1595-4. [DOI] [PubMed] [Google Scholar]
- 84.Pokhrel L.R., Dubey B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013;45:321–332. doi: 10.1016/j.scitotenv.2013.02.059. [DOI] [PubMed] [Google Scholar]
- 85.Yang R., Diao Y., Abayneh B. Removal of Hg(0) from simulated flue gas over silver-loaded rice husk gasification char. R. Soc. Open Sci. 2018;5:180248. doi: 10.1098/rsos.180248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vannini C., Domingo G., Onelli E., Mattia F.D., Bruni I., Marsoni M., Bracale M. Phytotoxicand genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014;171:1142–1148. doi: 10.1016/j.jplph.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Riahi-Madvar A., Rezaee F., Jalali V. Effects of alumina nanoparticles on morphological properties and antioxidant system of Triticum aestivum. Iran. J. Plant Physiol. 2012;3:595–603. [Google Scholar]
- 88.Feizi H., Moghaddam P.R., Shahtahmassebi N., Fotovot A. Assessment of concentration of nano and bulk iron oxide particles on early growth of wheat (Triticum aestivum L.) Annu. Rev. Res. Biol. 2013;3:752–761. [Google Scholar]
- 89.Lee W.M., An Y.J., Yoon H., Kweon H.S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Nanomater. Environ. 2008;27:1915–1921. doi: 10.1897/07-481.1. [DOI] [PubMed] [Google Scholar]
- 90.Divva K., Vijayan S., Nair S.J., Jisha M.S. Optimization of chitosan nanoparticle synthesis and its potential application as germination elicitor of Oryza sativa L. Int. J. Biol. Macromol. 2018;124:1053–1059. doi: 10.1016/j.ijbiomac.2018.11.185. [DOI] [PubMed] [Google Scholar]
- 91.López-Moreno M., De la Rosa G., Hernandez-Viezcas J., Castillo-Michel H., Botez C., Peralta-Videa J., Gardea-Torresdey J. Evidence of the differential biotransformation and genotoxicity of ZnO and CeO nanoparticles on soybean (Glycine max) plants. Environ. Sci. Technol. 2010;44:7315–7320. doi: 10.1021/es903891g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yasmeen F., Raja N.I., Mustafa G., Sakata K., Komatsu S. Quantitative proteomic analysis of post flooding recovery in soybean roots exposed to aluminum oxide NPs. J. Proteom. 2016;143:136–150. doi: 10.1016/j.jprot.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 93.Mustafa G., Komatsu S. Insights into the response of soybean mitochondrial proteins to various sizes of aluminum oxide nanoparticles under flooding stress. J. Proteome Res. 2016;15:4464–4475. doi: 10.1021/acs.jproteome.6b00572. [DOI] [PubMed] [Google Scholar]
- 94.Zheng L., Hong F., Lu S., Liu C. Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 2005;104:83–91. doi: 10.1385/BTER:104:1:083. [DOI] [PubMed] [Google Scholar]
- 95.Gao F.Q., Hong F.H., Liu C., Zheng L., Su M.Y., Wu X., Yang F., Wu C., Yang P. Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach e inducing complex of Rubisco activase. Biol. Trace Elem. Res. 2006;111:239–253. doi: 10.1385/BTER:111:1:239. [DOI] [PubMed] [Google Scholar]
- 96.Yasmeen F., Raja N.I., Razzaq A., Komatsu S. Gel-free/label-free proteomic analysis of wheat shoot in stress tolerant varieties under iron nanoparticles exposure. Biochim. Biophys. Acta. 2016;1864:1586–1598. doi: 10.1016/j.bbapap.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 97.Yasmeen F., Raja N.I., Ilyas N., Komatsu S. Quantitative proteomic analysis of shoot in stress tolerant wheat varieties on copper nanoparticle exposure. Plant Mol. Biol. Rep. 2018;36:326–340. doi: 10.1007/s11105-018-1082-2. [DOI] [Google Scholar]
- 98.Vannini C., Domingo G., Onelli E., Prinsi B., Marsoni M., Espen L., Bracale M. Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS ONE. 2013;8:e68752. doi: 10.1371/journal.pone.0068752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seghatoleslami M., Faiza H., Mousav G., Berahmand A. Effect of magnetic field and silver nanoparticles on yield and water use efficiency of Carum copticum under water stress conditions. Pol. J. Chem. Technol. 2015;17:110–114. doi: 10.1515/pjct-2015-0016. [DOI] [Google Scholar]
- 100.Jacob D.L., Borchardt J.D., Navaratnam L., Otte M.L., Bezbaruah A.N. Uptake and translocation of Ti from nanoparticles in crops and wetland plants. Int. J. Phytoremediation. 2013;15:142–153. doi: 10.1080/15226514.2012.683209. [DOI] [PubMed] [Google Scholar]
- 101.Mohammadi R., Maali-Amiri R., Abbasi A. Effect of TiO2 nanoparticles on chickpea response to cold stress. Biol. Trace Elem. Res. 2013;152:403–410. doi: 10.1007/s12011-013-9631-x. [DOI] [PubMed] [Google Scholar]
- 102.Zhu H., Han J., Xiao J.Q., Jin Y. Uptake, translocation, and accumulation of manufactured iron oxide by pumpkin plants. J. Environ. Monit. 2008;10:713–717. doi: 10.1039/b805998e. [DOI] [PubMed] [Google Scholar]
- 103.Yasmeen F., Raja N.I., Razzaq A., Komatsu S. Proteomic and physiological analyses of wheat seeds exposed to copper and iron nanoparticles. Biochim. Biophys. Acta Proteins Proteom. 2017;1865:28–42. doi: 10.1016/j.bbapap.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 104.Barrena R., Casals E., Colón J., Font X., Sánchez A., Puntes V. Evaluation of the ecotoxicity of model nanoparticles. Chemosphere. 2009;75:850–857. doi: 10.1016/j.chemosphere.2009.01.078. [DOI] [PubMed] [Google Scholar]
- 105.Shaw A.K., Hossain Z. Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere. 2013;93:906–915. doi: 10.1016/j.chemosphere.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 106.Shaw A.K., Ghosh S., Kalaji H.M., Bosa K., Brestic M., Zivcak M., Hossain Z. Nano-CuO stress induced modulation of antioxidative defense and photosynthetic performance of Syrian barley (Hordeum vulgare L.) Environ. Exp. Bot. 2014;102:37–47. doi: 10.1016/j.envexpbot.2014.02.016. [DOI] [Google Scholar]
- 107.Rajput V.D., Minkina T., Fedorenko A., Mandzhieva S., Sushkova S., Lysenko V., Duplii N., Azarov A., Aleksandrovich V. Destructive effect of copper oxide nanoparticles on ultrastructure of chloroplast, plastoglobules and starch grains in spring barley (Hordeum sativum) Int. J. Agric. Biol. 2019;21:171–174. [Google Scholar]
- 108.Adhikari S., Adhikari A., Ghosh S., Roy D., Azahar I., Basuli D., Hossain Z. Assessment of ZnO-NPs toxicity in maize: An integrative microRNAomic approach. Chemosphere. 2020;249:126197. doi: 10.1016/j.chemosphere.2020.126197. [DOI] [PubMed] [Google Scholar]
- 109.Motyka O., Štrbová K., Olšovská E., Seidlerová J. Influence of nano-ZnO exposure to plants on l-ascorbic acid levels: Indication of nanoparticle-induced oxidative stress. J. Nanosci. Nanotechnol. 2019;19:3019–3023. doi: 10.1166/jnn.2019.15862. [DOI] [PubMed] [Google Scholar]
- 110.López-Moreno M.L., De la Rosa G., Hernández-Viezcas J.A., Peralta-Videa J.R., Gardea-Torresdey J.L. X-ray absorption spectroscopy (XAS) corroboration of the uptake and storage of CeO(2) nanoparticles and assessment of their differential toxicity in four edible plant species. J. Agric. Food Chem. 2010;58:3689–3693. doi: 10.1021/jf904472e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Djanaguiraman M., Belliraj N., Bossmann S.H., Prasad P.V.V. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega. 2018;3:2479–2491. doi: 10.1021/acsomega.7b01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Divya K., Smitha V., Jisha M.S. Antifungal, antioxidant and cytotoxic activities of chitosan nanoparticles and its use as an edible coating on vegetables. Int. J.Biol. Macromol. 2018;114:572–577. doi: 10.1016/j.ijbiomac.2018.03.130. [DOI] [PubMed] [Google Scholar]
- 113.Abd El-Aziz M.E., Morsi S.M.M., Salama D.M., Abdel-Aziz M.S., AbdElwahed M.S., Shaaban E.A., Youssef A.M. Preparation and characterization of chitosan/polyacrylicacid/copper nanocomposites and their impact on onion production. Int. J. Biol. Macromol. 2019;123:856–865. doi: 10.1016/j.ijbiomac.2018.11.155. [DOI] [PubMed] [Google Scholar]
- 114.Chen G., Ma C., Mukherjee A., Musante C., Zhang J., White J.C., Dhankher O.P., Xing B. Tannic acid alleviates bulk and nanoparticle Nd2O3 toxicity in pumpkin: A physiological and molecular response. Nanotoxicology. 2016;10:1243–1253. doi: 10.1080/17435390.2016.1202349. [DOI] [PubMed] [Google Scholar]
- 115.Weir E., Lawlor A., Whelan A., Regan F. The use of nanoparticles in antimicrobial materials and their characterization. Analyst. 2008;133:835–845. doi: 10.1039/b715532h. [DOI] [PubMed] [Google Scholar]
- 116.Simon D.F., Domingos R.F., Hauser C., Hutchins C.M., Zerges W., Wilkinson K.J. Transcriptome sequencing (RNA-seq) analysis of the effects of metal nanoparticle exposure on the transcriptome of Chlamydomonas reinhardtii. Appl. Environ. Microbiol. 2013;79:4774–4785. doi: 10.1128/AEM.00998-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lok C.N., Ho C.M., Chen R., He Q.Y., Yu W.Y., Sun H., Tam P.K., Chiu J.F., Che C.M. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J. Proteome Res. 2006;5:916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 118.Mirzajani F., Askari H., Hamzelou S., Schober Y., Rompp A., Ghassempour A., Spengler B. Proteomics study of silver nanoparticles toxicity on Bacillus thuringiensis. Ecotoxicol. Environ. Saf. 2014;100:122–130. doi: 10.1016/j.ecoenv.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 119.Hossain Z., Mustafa G., Sakata K., Komatsu S. Insights into the proteomic response of soybean towards Al2O3, ZnO, and Ag nanoparticles stress. J. Hazard. Mater. 2016;304:291–305. doi: 10.1016/j.jhazmat.2015.10.071. [DOI] [PubMed] [Google Scholar]
- 120.Mustafa G., Sakata K., Komatsu S. Proteomic analysis of soybean root exposed to varying sizes of silver nanoparticles under flooding stress. J. Proteom. 2016;148:113–125. doi: 10.1016/j.jprot.2016.07.027. [DOI] [PubMed] [Google Scholar]
- 121.Jhanzab H.M., Razzaq A., Bibi Y., Yasmeen F., Yamaguchi H., Hitachi K., Tsuchida K., Komatsu K. Proteomic analysis of the effect of inorganic and organic chemicals on silver nanoparticles in wheat. Int. J. Mol. Sci. 2019;20:825. doi: 10.3390/ijms20040825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hashimoto T., Mustafa G., Nishiuchi T., Komatsu S. Comparative analysis of the effect of inorganic and organic chemicals with silver nanoparticles on soybean under flooding stress. Int. J. Mol. Sci. 2020;21:1300. doi: 10.3390/ijms21041300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Handy R.D., Kamme F., Lead J.R., Hasselov M., Owen R., Crane M. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 2008;17:287–314. doi: 10.1007/s10646-008-0199-8. [DOI] [PubMed] [Google Scholar]
- 124.Shabnam N., Kim H. Non-toxicity of nano alumina: A case on mung bean seedlings. Ecotoxicol. Environ. Saf. 2018;165:423–433. doi: 10.1016/j.ecoenv.2018.09.033. [DOI] [PubMed] [Google Scholar]
- 125.Mustafa G., Sakata K., Komatsu S. Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J. Proteom. 2015;128:280–297. doi: 10.1016/j.jprot.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 126.Hou J., Wang L., Wang C., Zhang S., Liu H., Li S., Wang X. Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms. J. Environ. Sci. 2019;75:40–53. doi: 10.1016/j.jes.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 127.Riu J., Maroto A., Rius F.X. Nanosensors in environmental analysis. Talanta. 2006;69:288–301. doi: 10.1016/j.talanta.2005.09.045. [DOI] [PubMed] [Google Scholar]
- 128.Tan X., Wang X., Chen C., Sun A. Effect of soil humic and fulvic acids, pH and ionic strength on Th(IV) sorption to TiO2 nanoparticles. Appl. Radiat. Isot. 2007;65:375–381. doi: 10.1016/j.apradiso.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 129.Chen L.Z., Zhou L.N., Liu Y.D., Deng S.Q., Wu H., Wang G.H. Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii. Ecotoxicol. Environ. Saf. 2012;84:155–162. doi: 10.1016/j.ecoenv.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 130.Zhao L., Peng B., Hernandez-Viezcas J.A., Rico C., Sun Y., Peralta-Videa J.R., Tang X., Niu G., Jin L., Varela-Ramirez A. Stress response and tolerance of Zea mays to CeO2 nanoparticles: Cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano. 2012;6:9615–9622. doi: 10.1021/nn302975u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.PeharecŠtefanić P., Jarnević M., Cvjetko P., Biba R., Šikić S., Tkalec M., Cindrić M., Letofsky-Papst I., Balen B. Comparative proteomic study of phytotoxic effects of silver nanoparticles and silver ions on tobacco plants. Environ. Sci. Pollut. Res. Int. 2019;26:22529–22550. doi: 10.1007/s11356-019-05552-w. [DOI] [PubMed] [Google Scholar]
- 132.Du W., Sun Y., Ji R., Zhu J., Wu J., Guo H. TiO2 and ZnO nanoparticles negatively affect wheat growth and soil enzyme activities in agricultural soil. J. Environ. Monit. 2011;13:822e828. doi: 10.1039/c0em00611d. [DOI] [PubMed] [Google Scholar]
- 133.Chen H. Metal based nanoparticles in agricultural system: Behavior, transport, and interaction with plants. Chem. Spec. Bioavailab. 2018;30:123–134. doi: 10.1080/09542299.2018.1520050. [DOI] [Google Scholar]
- 134.Lv J., Zhang S., Luo L., Zhang J., Yang K., Christie P. Accumulation, speciation and uptake pathway of ZnO nanoparticles in maize. Environ. Sci. Nano. 2015;2:68–77. doi: 10.1039/C4EN00064A. [DOI] [Google Scholar]
- 135.Halliwell B., Gutteridge J.M.C. Free Radicals in Biology and Medicine. 2nd ed. Clarendon Press; Oxford, UK: 1989. [Google Scholar]
- 136.Tripathy B.C., Oelmüller R. Reactive oxygen species generation and signaling in plants. Plant Signal Behav. 2012;7:1621–1633. doi: 10.4161/psb.22455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang X., Yang X., Chen S., Li Q., Wang W., Hou C., Gao X., Wang L., Wang S. Zinc oxide nanoparticles affect biomass accumulation and photosynthesis in Arabidopsis. Front. Plant Sci. 2016;6:1243. doi: 10.3389/fpls.2015.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wang X.P., Li Q.Q., Pei Z.M., Wang S.C. Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol. Plant. 2018;62:801e808. doi: 10.1007/s10535-018-0813-4. [DOI] [Google Scholar]
- 139.Hossain Z., Nouri M.Z., Komatsu S. Plant cell organelle proteomics in response to abiotic stress. J. Prot. Res. 2012;11:37–48. doi: 10.1021/pr200863r. [DOI] [PubMed] [Google Scholar]
- 140.Mittova V., Volokita M., Guy M., Tal M. Activities of SOD and the ascorbate–glutathione cycle enzymes in subcellular compartments in leaves and roots of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennelli. Physiol. Plant. 2000;110:42–51. doi: 10.1034/j.1399-3054.2000.110106.x. [DOI] [Google Scholar]
- 141.Barber D.J.W., Thomas J.K. Reactions of radicals with lecithin bilayers. Radiat. Res. 1978;74:51–58. doi: 10.2307/3574756. [DOI] [Google Scholar]
- 142.Dos Santos C.V., Rey P. Plant thioredoxins are key actors in oxidative stress response. Trends Plant Sci. 2006;11:329–334. doi: 10.1016/j.tplants.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 143.Berndt C., Lillig C.H., Holmgren A. Thioredoxins and glutaredoxins as facilitators of protein folding. Biochim. Biophys. Acta. 2008;1783:641–650. doi: 10.1016/j.bbamcr.2008.02.003. [DOI] [PubMed] [Google Scholar]