Abstract
Background: Handgrip strength (HGS) is a convent measure of strength capacity and associated with several age-related health conditions such as functional disability. Asymmetric strength between limbs has been linked to diminished function. Therefore, both HGS asymmetry and weakness could be associated with functional disability. We examined the associations of HGS asymmetry and weakness on functional limitations in a nationally representative sample of older Americans. Methods: Data were analyzed from 2689 adults ≥ 60 years who participated in the 2011–2012 and 2013–2014 waves of the National Health and Nutrition Examination Survey. Weakness was defined as HGS < 26 kg for men and < 16 kg for women. Asymmetry was determined from the ratio of the dominant and non-dominant HGS. Those with HGS ratio 0.9–1.1 were considered as having HGS symmetry, and those outside this range had asymmetry. Results: Compared to those with symmetric HGS and were not weak, those with weakness alone, and both weakness and HGS asymmetry had 2.47 (95% confidence interval [CI]: 1.14–5.35) and 3.93 (CI: 1.18–13.07) greater odds for functional limitations, respectively. However, HGS asymmetry alone was not associated with functional limitations (odds ratio: 0.80; CI: 0.62–1.03). Conclusion: The use of HGS asymmetry in protocols could improve the prognostic value of handgrip dynamometers.
Keywords: aging, geriatrics, muscle strength, muscle strength dynamometer, nutrition surveys
1. Introduction
About 40% of older adults are currently living with a disability [1], and by the year 2050 it is estimated that 70 million Americans, about one fifth of the country’s population, will be aged over 65 years [2]. Moreover, those living with a functional limitation are at greater risk for additional losses in functioning [3]. If the prevalence of disability remains unchanged, in 2050, approximately 28 million Americans 65 years or older could be living with a disability [2], indicating the importance for early risk assessment and intervention. Maximal handgrip strength (HGS) serves as a convenient measure of neuromuscular integrity and function, making it a clinically-viable screening tool for several health conditions during aging [4].
Specifically, HGS is related to various functions of basic activities of daily living (BADL) [5,6] and cognitive function [7]. Previous research has found that instrumental activities of daily living (i.e., independent living tasks (IADLs)), demand high levels of cognitive function [8], and these findings suggest another pathway in which HGS may be related to different types of functional limitations. Although inabilities to perform IADLs and BADLs, and worsened physical and mental health, have been related to chronic health conditions in older adults [9], these same associations have not been made in relation to strength asymmetries.
Most research including HGS has examined either maximal or mean strength in one hand [10,11]; however, neuromuscular control during movement is proficient on the dominant side, and natural differences exist between hands depending on task complexity [12,13]. Regarding strength asymmetries, most research has focused on the lower body. Muscle activation asymmetry between dominant and non-dominant legs is linked to higher risk for falls [14] and sarcopenia [15]. Another study assessing power output and strength in younger adults, older non-fallers, and older fallers found that older fallers showed greater trends toward power asymmetry in the lower limbs and increased risk of sarcopenia [15]. Leg muscle fatigue asymmetry also increased demands on cognitive attention and ability [16], again showing a connection between asymmetry and nervous system function. Therefore, HGS asymmetry could be associated with a greater risk of functional disability.
The relationship between HGS asymmetry and functional limitations is supported by studies that have shown associations between physical decline [17], and loss of cognitive function [18] with disability, and by others who have determined that asymmetries in either lean body mass or nervous system function are related to worsened performance [17]. In support of this notion, in older Koreans, asymmetric distribution of lower-body lean mass is related to decreased gait speed [19], although lower-body strength asymmetries were not related to decreased mobility in older Americans [20].
A recent longitudinal investigation found that HGS asymmetry and weakness potentiated the risk for functional disability [21]. The findings from this study suggest that HGS asymmetry may factor into elevated functional disability risk. Given that HGS is differentially associated with limitations in individual functional tasks (e.g., grocery shopping, taking medications, preparing hot meals, etc.) [8], evaluating how HGS asymmetry is associated with other aspects of function may provide detailed insights into this association. Nonetheless, HGS asymmetry could be a more robust screening method for functional disability compared to using maximal values for a single hand. The purposes of this study were to determine the associations of HGS asymmetry and weakness on 1) functional limitations (i.e., aggregated) and 2) limitations in each aspect of function (i.e., BADL, IADL, leisure and social activities, lower extremity mobility, general physical tasks) in a nationally representative sample of older Americans. We hypothesize that those with HGS asymmetry and weakness will have greater odds for functional limitations and limitations in each aspect of function.
2. Materials and Methods
2.1. Participants
Publicly available data from 2899 adults aged at least 60 years with measures of HGS on both hands and who identified as either right- or left-handed were analyzed from the 2011–2012 and 2013–2014 waves of the National Health and Nutrition Examination Survey (NHANES). The NHANES is a program of studies designed to assess the health and nutrition status of Americans [22]. Mobile examination centers traveled to locations throughout the United States. Trained interviewers completed health interviews in the residences of participants using computer-aided interview systems and participants visited mobile examination centers for more detailed examinations [23].
Oversampling occurred for those aged at least 60 years, non-Hispanic Asians, non-Hispanic Blacks, and Hispanics to produce reliable data that better represented these demographics in the United States. Overall interview response rates were ≥68.5% for each wave of the NHANES included in our analyses [24]. The NHANES utilizes a complex, four-stage probability sampling design to generate a representative sample of non-institutionalized Americans. Sample weights were used in our analyses to account for the sampling methods and produce an unbiased national estimate [22,25]. Written informed consent was provided by participants, and NHANES protocols were approved by the National Center for Health Statistics Research Ethics Review Board (Protocol #2011-17).
2.2. Measures
2.2.1. Variables of Functionality
Respondents told interviewers if they had no difficulty, some difficulty, much difficulty, or were unable to perform 19 tasks from five different aspects of function. These aspects included: (1) BADLs (“getting in and out of bed”, “using a fork, knife, and cup”, “walking between rooms on the same floor”, “dressing yourself”), (2) IADLs (“house chores”, “managing money”, “preparing meals”), (3) leisure and social activities (“going out to movies and events”, “leisure activities at home”, “attending social events”), (4) lower extremity mobility (“walking up 10 steps”, “walking for a quarter mile”), and (5) general physical tasks (“grasping or holding small objects”, “lifting or carrying”, “reaching up and overhead”, “sitting for long periods of time”, “standing for long periods of time”, “standing up from an armless chair”, “stooping, crouching, kneeling”).
Participants were considered as having a functional limitation if they reported having some difficulty, much difficulty, or were unable to perform any of the 19 tasks. Likewise, participants were considered as having a limitation in an aspect of function if they indicated having some difficulty, much difficulty, or were unable to perform any of the tasks listed within each aspect of function. Several investigations have used such criteria for defining functional limitations with NHANES data [26,27,28,29,30,31].
2.2.2. Handgrip Strength Variables
A practice trial at sub-maximal effort was completed by participants to determine if the dynamometer was fitted to their hand size and to confirm understanding of the HGS test protocol. Interviewers that administered HGS tests were trained for protocols and calibration procedures. Participants reported their hand dominance and abilities to complete HGS protocols before testing, including if they had surgery to the hands or wrists within the previous three months that prohibited them from HGS testing. Interviewers instructed participants to stand with their feet hip width apart and hold the dynamometer away from their body with arm at side with palm facing leg so that the dynamometer did not contact the body (unless physically unable). Participants were encouraged to squeeze the handle of the dynamometer hard and quickly.
The decision to start testing on the dominant or non-dominant hand was randomized. Each person squeezed the dynamometer with maximal effort, exhaling while squeezing, and then released the skeletal musculature. An HGS measurement was completed three times on each hand, alternating between hands, with a minute of rest between measures on the same hand. A digital handgrip dynamometer (Takei Dynamometer Model T.K.K.5401; Akiha-Ku, Japan) was used to measure HGS. Additional details about the NHANES HGS protocols were previously published [32,33].
The single highest value regardless of hand dominance was used for determining weakness. Men with maximal HGS < 26 kg and women with maximal HGS < 16 kg were considered weak [34]. The highest recorded handgrip values from the dominant and non-dominant hands were used to calculate HGS ratio (dominant HGS (kg) / non-dominant HGS (kg)). Although HGS may differ between hands and depend on hand dominance [35], the “10% rule” suggests that the HGS of the dominant hand is about 10% stronger than the strength of the non-dominant hand [36]. Those who had HGS ratio <0.90 or >1.10 were considered as having asymmetric HGS, while those with HGS ratio 0.90–1.10 had HGS symmetry.
2.2.3. Covariates
Age, sex, ethnicity (Hispanic, non-Hispanic Black, non-Hispanic White, other), and marital status (married, not-married) were self-reported. Standing height was measured with a fixed stadiometer, and body weight was collected with a digital scale (Mettler-Toledo International Inc.; Columbus, OH). Body mass index was calculated as body weight in kg divided by height in m2 (kg/m2). Those with a body mass index ≥ 30 kg/m2 were considered obese [37]. Respondents indicated if a healthcare provider had ever diagnosed them with diabetes, stroke, or arthritis. Moreover, respondents revealed if they experienced confusion or memory loss that was happening more often or getting worse. Participants were asked whether they had smoked at least 100 cigarettes in their lifetime (smoking history) and if they were currently smoking cigarettes. A single-item measure of self-perceived health was used wherein respondents rated their general health as either “excellent”, “very good”, “good”, “fair”, or “poor”. Depressive symptoms were assessed with the validated 9-item Patient Health Questionnaire. Each item was scored from 0–3 with higher scores indicating more severe depressive symptoms. The sum of scores across items was included in the analyses, and those with scores ≥ 10 were considered as having depression [38]. Those with missing covariates were excluded (n = 210).
2.2.4. Statistical Analyses
All analyses were conducted with SAS 9.4 software (SAS Institute; Cary, NC, USA). Participants were categorized into handgrip asymmetry and weakness groups by their HGS measurements: (1) symmetric HGS and not weak, (2) weakness alone, (3) asymmetric HGS alone, and (4) both weak and asymmetric HGS. To determine the associations of the weakness and HGS asymmetry groups with functional limitations, separate logistic regression models were run. A logistic regression model analyzed the associations of each weakness and HGS asymmetry group on functional limitations using the not weak and symmetric HGS as the reference. Similarly, individual logit regression models also evaluated the associations of each weakness and HGS asymmetry group on limitations in each aspect of function (i.e., BADLs, IADLs, leisure and social activities, lower extremity mobility activities, general physical activities) using the not weak and symmetric HGS asymmetry group as the reference. Logistic regression models produce odds ratios, and logistic models were selected for our analyses because our study design was cross-sectional and outcome variables were binary. All logit models were adjusted for sex, age, ethnicity, self-rated health, diabetes diagnosis, stroke diagnosis, arthritis diagnosis, obesity, self-perceived memory impairment, depression, current smoking status, smoking history, and marital status. A p-value of < 0.05 was used to determine statistical significance for all analyses.
3. Results
There were 2689 (92.8%) of participants included, and their descriptive characteristics are presented in Table 1.
Table 1.
Descriptive characteristics of the participants.
| Variables | Overall (n = 2689) |
Symmetric HGS and Not Weak (n = 1427) |
Weak Only (n = 72) |
Asymmetric HGS Only (n = 1113) |
Asymmetric HGS and Weak (n = 77) |
|---|---|---|---|---|---|
| HGS (kg) | 31.7 ± 10.0 | 32.7 ± 9.6 | 18.4 ± 5.3 | 32.4 ± 9.7 | 17.2 ± 5.2 |
| HGS Ratio | 1.06 ± 0.15 | 1.01 ± 0.05 | 1.01 ± 0.05 | 1.14 ± 0.20 | 1.11 ± 0.24 |
| Age (years) | 69.6 ± 6.8 | 68.9 ± 6.7 | 75.5 ± 6.1 | 69.5 ± 6.7 | 75.8 ± 5.9 |
| Male (n (%)) | 1299 (48.2) | 711 (49.8) | 44 (61.1) | 510 (45.8) | 34 (44.1) |
| Ethnicity (n (%)) | |||||
| Hispanic | 490 (18.2) | 251 (17.6) | 10 (13.9) | 215 (19.3) | 14 (18.2) |
| Non-Hispanic Black | 638 (23.7) | 339 (23.7) | 16 (22.2) | 274 (24.6) | 9 (11.7) |
| Non-Hispanic White | 1296 (48.2) | 700 (49.1) | 34 (47.2) | 517 (46.5) | 45 (58.4) |
| Other | 265 (9.9) | 137 (9.6) | 12 (16.7) | 107 (9.6) | 9 (11.7) |
| Obese (n (%)) | 1017 (37.8) | 523 (36.6) | 31 (43.0) | 422 (40.1) | 22 (28.5) |
| Diabetes (n (%)) | 625 (23.2) | 324 (22.7) | 26 (36.1) | 252 (22.6) | 23 (29.8) |
| Arthritis (n (%)) | 1319 (49.0) | 662 (46.3) | 33 (45.8) | 575 (51.6) | 49 (63.6) |
| Stroke (n (%)) | 186 (6.9) | 78 (5.4) | 15 (20.8) | 82 (7.3) | 11 (14.2) |
| Self-Perceived Memory Impairment (n (%)) | 382 (14.2) | 188 (13.2) | 19 (26.3) | 153 (13.7) | 22 (28.5) |
| Current Smoker (n (%)) | 334 (12.4) | 183 (12.8) | 8 (11.1) | 133 (11.9) | 10 (12.9) |
| Previous Smoker (n (%)) |
1022 (37.9) | 539 (37.7) | 29 (40.2) | 426 (38.2) | 28 (36.3) |
| Currently Married (n (%)) |
1474 (54.8) | 812 (56.9) | 33 (45.8) | 601 (54.0) | 28 (36.3) |
| Depressed (n (%)) | 227 (8.4) | 107 (7.5) | 10 (13.8) | 102 (9.1) | 8 (10.3) |
| Self-Rated Health (n (%)) | |||||
| Excellent | 208 (7.7) | 118 (8.3) | 4 (5.7) | 80 (7.2) | 6 (7.8) |
| Very Good | 664 (24.7) | 355 (24.9) | 11 (15.2) | 279 (25.1) | 19 (24.7) |
| Good | 1064 (39.5) | 574 (40.2) | 27 (37.5) | 443 (39.8) | 20 (26.0) |
| Fair | 630 (23.5) | 328 (23.0) | 23 (31.9) | 254 (22.8) | 25 (32.4) |
| Poor | 123 (4.6) | 52 (3.6) | 7 (9.7) | 57 (5.1) | 7 (9.1) |
| Functional Limitation (n (%)) | 1701 (63.2) | 877 (61.4) | 62 (86.1) | 691 (62.0) | 71 (92.2) |
Note: HGS = handgrip strength.
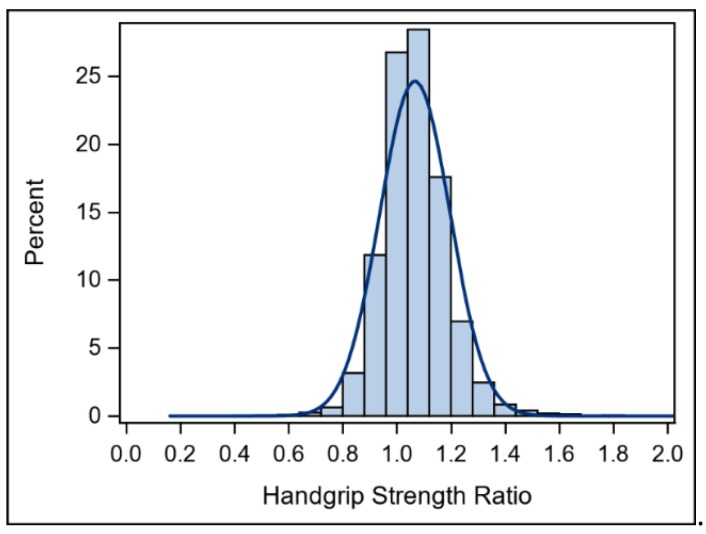
Appendix ATable A1 includes means and 95% confidence intervals for the descriptive characteristics to make comparisons across weakness and asymmetry groups. A histogram of HGS ratios is shown in Figure 1.
Figure 1.
Histogram for handgrip strength ratio.
Those with HGS symmetry and were not weak (61.4%; 95% confidence interval (CI): 58.9%, 64.0%), and those who had HGS asymmetry only (62.0%; CI: 59.2%, 64.9%) had lower proportions of participants with functional limitations compared to those with weakness only (86.1%; CI: 78.1%, 94.1%) and both asymmetric HGS and weakness (92.2%; CI: 86.2%, 98.1%).
The results of the associations for HGS asymmetry and weakness on functional limitations are shown in Table 2.
Table 2.
Association for the handgrip strength asymmetry and weakness groups on functional limitations.
| Groups | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Weakness Alone † | 2.47 | 1.14, 5.35 |
| Handgrip Strength Asymmetry Alone † | 0.80 | 0.62, 1.03 |
| Weak and Handgrip Strength Asymmetry † | 3.93 | 1.18, 13.07 |
† Reference: Symmetric handgrip strength and not weak. Note: the model was adjusted for sex, age, ethnicity, self-rated health, diabetes diagnosis, stroke diagnosis, arthritis diagnosis, obesity, self-perceived memory impairment, depression, current smoking status, smoking history, and marital status.
Relative to those who were not weak and had HGS symmetry, those with weakness alone, and those with HGS asymmetry and weakness had differential associations for functional limitations. However, there were null findings for the association of those with HGS asymmetry alone and functional limitations.
Table 3 presents the results for the associations of the HGS asymmetry and weakness groups on limitations in each aspect of function. Those with weakness, and those with HGS asymmetry and weakness had differential associations for limitations in each aspect of function. There were no significant associations for those with HGS asymmetry alone and limitations in each aspect of function.
Table 3.
Associations for the handgrip strength asymmetry and weakness groups on limitations in each aspect of function.
| Groups | Odds Ratio | 95% Confidence Interval |
|---|---|---|
| Activities of Daily Living † | ||
| Weakness Only | 3.80 | 1.80, 8.05 |
| HGS Asymmetry Only | 1.19 | 0.88, 1.61 |
| HGS Asymmetry and Weakness | 5.28 | 2.20, 12.70 |
| Instrumental Activities of Daily Living † | ||
| Weakness Only | 2.15 | 1.14, 4.06 |
| HGS Asymmetry Only | 1.01 | 0.77, 1.32 |
| HGS Asymmetry and Weakness | 4.24 | 1.78, 10.14 |
| Leisure and Social Activities † | ||
| Weakness Only | 2.57 | 1.26, 5.24 |
| HGS Asymmetry Only | 0.80 | 0.62, 1.03 |
| HGS Asymmetry and Weakness | 3.50 | 1.26, 9.74 |
| Lower Extremity Mobility Activities † | ||
| Weakness Only | 2.44 | 1.28, 4.67 |
| HGS Asymmetry Only | 0.79 | 0.59, 1.07 |
| HGS Asymmetry and Weakness | 2.85 | 1.13, 7.19 |
| General Physical Activities † | ||
| Weakness Only | 4.25 | 2.03, 8.90 |
| HGS Asymmetry Only | 0.96 | 0.74, 1.25 |
| HGS Asymmetry and Weakness | 3.55 | 1.45, 8.68 |
† Reference: Symmetric handgrip strength and not weak. Note: the model was adjusted for sex, age, ethnicity, self-rated health, diabetes diagnosis, stroke diagnosis, arthritis diagnosis, obesity, self-perceived memory impairment, depression, current smoking status, smoking history, and marital status.
4. Discussion
The principal findings of this study were that weakness and HGS asymmetry were associated with greater odds for functional limitations in older Americans. Similar findings were observed when examining the associations between weakness and HGS asymmetry in each aspect of function; there were differential associations for those with both weakness and HGS asymmetry, and those with weakness alone on limitations in each aspect of functioning. Interestingly, those with only HGS asymmetry were not at increased odds for functional limitations. While natural strength asymmetries may exist between sides [14], the presence of both HGS asymmetry and weakness may exacerbate the odds for functional limitations. However, weakness could be driving these associations. Our findings suggest that including measures of HGS asymmetry in assessments of weakness may improve the prognostic utility of handgrip dynamometers for functional limitations.
In general, HGS has clinical value as it relates to entire body muscular strength [39], and weakness is associated with a number of important clinical health outcomes [40,41]. Due to these robust associations with clinical outcomes, HGS has been recommended as a routine vital sign for older adults [41]. However, findings of weakness and asymmetry may be more sensitive to the negative changes associated with aging than weakness alone. Measuring HGS weakness and asymmetry could also be added to other clinical screenings, such as measures of nutritional status, or physical function tests used to predict hospital stays [41]. The findings from this study indicate that handgrip asymmetry may improve a handgrip dynamometer’s ability for detecting functional declines. Similar to other elements of a comprehensive physical assessments, it would be beneficial to track weakness and asymmetry over time, giving that healthcare providers value information on patient physical trends.
In support of this notion, older Americans with both weakness and HGS asymmetry in our study were at increased odds for limitations in each aspect of functioning. Evidence suggests declines in HGS were associated with more dependence for completion of IADLs [8]. Neuropsychological and neuroanatomical changes such as decline in executive function or memory, hippocampal atrophy, and to a lesser extent white matter changes, may jeopardize one’s ability to perform IADLs [8]. The “common cause” hypothesis suggests that the same neural system deficits that could be responsible for limitations in IADLs may also be responsible for decreased muscle mass and HGS [42], which may help to explain our findings. Leisure and social activity, lower extremity mobility, and general physical activity task limitations were also more pronounced when individuals demonstrated asymmetry and weakness. Previous research is limited regarding older adults with weakness or asymmetry and the performance of physical tasks within those domains.
Indeed, the driving factors behind weakness and strength asymmetry are unclear. HGS asymmetries with weakness could be simply due to disuse of the non-dominant limb [43]. Alternatively, declines in grasping ability are reflective of reduced neural and motor system function [4,7]. For example, shifts in hand dominance and motor control could reflect changes in brain hemisphere functioning during aging [7]. When such shifts occur, muscle activation could also decline during a grip force task [44]. Therefore, difficulties in modulating the appropriate motor networks and decreasing motor performance exist in older adults [44]. Additionally, because the primary motor cortex in each hemisphere of the brain controls movement on the opposite side of the body, asymmetries between arms should be affected by asymmetries in the brain, and, in fact, asymmetries in somatosensory cortex have been reported [45].
Fortunately, various forms of exercise including strength and balance training, have been successful in mitigating some of the declines associated with aging [46,47,48]. While not the main outcome variable, a number of training studies have assessed HGS, with mixed results [46]. One study investigated the differences in high-speed versus low-speed resistance training in healthy older women and found that HGS improved [47]. Similarly, a 10-week study assessed the effects of aerobic and resistance exercise order in older men. Irrespective of exercise order, both groups saw increases in HGS and other measures of strength, walking ability, and flexibility [49]. Therefore, weakness could be reversible with exercise, but training just HGS may not address the root causation of asymmetry. In addition, a meta-analytical review found that a majority of training studies finding large improvements in HGS did so with multiple whole-body exercise modes such as strength, endurance, flexibility, and balance training [46].
The strength of this work includes the use of a nationally-representative sample and the statistical control of important covariates, yet we must acknowledge some of its limitations. Some self-report bias may have occurred within participant responses. For example, indication of hand dominance for each participant had to be established in order to calculate HGS ratio. In addition, levels of difficulty in the five aspects of function were self-reported by participants. However, self-reports are common in large epidemiological studies. NHANES data are cross-sectional, and protocols used for measuring HGS may be inconsistent with protocols used in similar investigations. Moreover, specific details in protocol manuals could be lacking. Longitudinal data for occupational history was unavailable. The null associations and wider confidence intervals could be attributed to lower sample sizes in the HGS asymmetry and weakness groups. Additionally, the “10% rule” was used to determine HGS asymmetry in participants; however, HGS ratios may vary between hands at an individual level [34]. Different cut-off points would likely affect the strength of the relationship between HGS asymmetry and weakness for functional limitations. More research is needed to determine if asymmetry is associated with function beyond weakness, and to decipher the difference between loss of muscle strength or sensorimotor ability. Further development of intervention strategies to combat functional decline and loss of HGS in older adults is merited.
5. Conclusions
Older adults who have both weakness and asymmetric HGS have greater odds for functional limitations. Similarly, those who were both weak had asymmetric HGS had greater odds for limitations in each aspect of function. Weakness could be driving these associations. Including HGS asymmetry in HGS protocols may help to improve the prognostic value of handgrip dynamometers and operationalization of strength capacity. Measures of HGS asymmetry will also not compromise the feasibility of HGS assessments because hand dominance and multiple measures of HGS are recorded in most HGS protocols.
Appendix A
Table A1.
Descriptive characteristics of the participants.
| Variables | Overall | Group 1 | Group 2 | Group 3 | Group 4 |
|---|---|---|---|---|---|
| HGS (kg) | 31.7 (31.4, 32.1) | 32.7 (32.2, 33.2) | 18.4 (16.9, 19.4) | 32.4 (31.8, 33.0) | 17.2 (16.2, 18.6) |
| HGS Ratio | 1.06 (1.06, 1.07) | 1.01 (1.01, 1.02) | 1.01 (1.00, 1.02) | 1.14 (1.12, 1.15) | 1.11 (1.05, 1.16) |
| Age (years) | 69.6 (69.3, 69.8) | 68.9 (68.6, 69.3) | 75.5 (74.1, 77.0) | 69.5 (69.1, 69.9) | 75.8 (74.5, 77.2) |
| Male (n (%)) | 48.2 (46.4, 50.1) | 49.8 (47.2, 52.3) | 61.1 (49.8, 72.3) | 45.8 (42.8, 48.7) | 44.1 (33.0, 55.2) |
| Ethnicity (n (%)) | |||||
| Hispanic | 18.2 (16.7, 19.7) | 17.6 (15.6, 19.6) | 13.9 (5.9, 21.8) | 19.3 (17.0, 21.6) | 18.2 (9.5, 26.8) |
| Non-Hispanic Black | 23.7 (22.1, 25.3) | 23.7 (21.5, 25.9) | 22.2 (12.6, 31.8) | 24.6 (22.0, 27.1) | 11.7 (4.5, 18.8) |
| Non-Hispanic White | 48.2 (46.2, 50.0) | 49.1 (46.4, 51.6) | 47.2 (35.6, 58.7) | 46.5 (43.5, 49.3) | 58.4 (47.4, 69.4) |
| Other | 9.9 (8.7, 10.9) | 9.6 (8.0, 11.1) | 16.7 (8.0, 25.2) | 9.6 (7.8, 11.3) | 11.7 (4.5, 18.8) |
| Obese (n (%)) | 37.8 (35.9, 39.6) | 36.6 (34.1, 39.1) | 43.0 (31.6, 54.4) | 40.1 (36.7, 42.5) | 28.5 (18.4, 38.6) |
| Diabetes (n (%)) | 23.2 (21.6, 24.8) | 22.7 (20.5, 24.8) | 36.1 (25.0, 47.2) | 22.6 (20.1, 25.1) | 29.8 (19.6, 40.0) |
| Arthritis (n (%)) | 49.0 (47.1, 50.9) | 46.3 (43.7, 48.9) | 45.8 (34.3, 57.3) | 51.6 (48.7, 54.6) | 63.6 52.8, 74.3) |
| Stroke (n (%)) | 6.9 (5.9, 7.8) | 5.4 (4.2, 6.6) | 20.8 (11.4, 30.2) | 7.3 (5.8, 8.9) | 14.2 (6.4, 22.1) |
| Self-Perceived Memory Impairment (n (%)) | 14.2 (12.9, 15.5) | 13.2 (11.4, 14.9) | 26.3 (16.2, 36.5) | 13.7 (11.7, 15.7) | 28.5 (18.4, 38.6) |
| Current Smoker (n (%)) |
12.4 (11.1, 13.6) | 12.8 (11.0, 14.5) | 11.1 (3.8, 18.3) | 11.9 (10.0, 13.8) | 12.9 (5.4, 20.5) |
| Previous Smoker (n (%)) |
37.9 (36.1, 39.8) | 37.7 (35.2, 40.2) | 40.2 (28.9, 51.6) | 38.2 (35.4, 41.1) | 36.3 (25.6, 47.1) |
| Currently Married (n (%)) |
54.8 (52.9, 56.6) | 56.9 (54.2, 59.4) | 45.8 (34.3, 57.3) | 54.0 (51.0, 56.9) | 36.3 (25.6, 47.1) |
| Depressed (n (%)) | 8.4 (7.4, 9.5) | 7.5 (6.1, 8.9) | 13.8 (5.9, 21.8) | 9.1 (7.4, 10.8) | 10.3 (3.5, 17.2) |
| Self-Rated Health (n (%)) | |||||
| Excellent | 7.7 (6.7, 8.7) | 8.3 (6.8, 9.6) | 5.7 (0.2, 10.8) | 7.2 (5.6, 8.7) | 7.8 (1.8, 13.7) |
| Very Good | 24.7 (23.0, 26.3) | 24.9 (22.6, 27.1) | 15.2 (6.9, 23.5) | 25.1 (22.5, 27.6) | 24.7 (15.0, 34.3) |
| Good | 39.5 (37.7, 41.4) | 40.2 (37.6, 42.7) | 37.5 (26.3, 48.6) | 39.8 (36.9, 42.6) | 26.0 (16.1, 35.7) |
| Fair | 23.5 (21.8, 25.0) | 23.0 (20.8, 25.2) | 31.9 (21.1, 42.7) | 22.8 (20.3, 25.2) | 32.4 (22.0, 42.9) |
| Poor | 4.6 (3.7, 5.3) | 3.6 (2.6, 4.6) | 9.7 (2.8, 16.5) | 5.1 (3.8, 6.4) | 9.1 (2.6, 15.5) |
| Functional Limitation (n (%)) | 63.2 (61.4, 65.0) | 61.4 (58.9, 64.0) | 86.1 (78.1, 94.1) | 62.0 (59.2, 64.9) | 92.2 (86.2, 98.1) |
Note: Group 1 = symmetric HGS and not weak; Group 2 = weakness alone; Group 3 = HGS asymmetry alone; Group 4 = HGS asymmetry and weakness; HGS = handgrip strength.
Author Contributions
Conceptualization, K.C., N.J., L.K., R.W., R.M.; Methodology, K.C., N.J., L.K., R.W., S.S., W.J.K., B.C., R.M.; Software, K.C., N.J., L.K., R.W., S.S., W.J.K., B.C., R.M.; Validation, R.M.; Formal analysis, R.M.; Investigation, K.C., N.J., L.K., R.W., S.S., W.J.K., B.C., R.M.; Resources, K.C., N.J., L.K., R.W., S.S., W.J.K., B.C., R.M.; Writing—original draft preparation, K.C., N.J., L.K., R.W., R.M.; Writing—review and editing, K.C., N.J., L.K., R.W., S.S., W.J.K., B.C., R.M.; Visualization, R.M.; Supervision, R.M.; Project administration, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Okoro C.A., Hollis N.D., Cyrus A.C., Griffin-Blake S. Prevalence of disabilities and health care access by disability status and type among adults—United States, 2016. Morb. Mortal Wkly Rep. 2018;67:882. doi: 10.15585/mmwr.mm6732a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortman J.M., Velkoff V., Hogan H. An aging nation: The older population in the United States. Econ. Stat. Adm. US Dep. Commer. 2014;1964:1–28. [Google Scholar]
- 3.McGrath R.P., Clark B., Erlandson K. Impairments in individual autonomous living tasks and time to self-care disability in middle-aged and older adults. J. Am. Med. Dir. Assoc. 2019;20:730–735.e3. doi: 10.1016/j.jamda.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson R.G. Get a grip: Individual variations in grip strength are a marker of brain health. Neurobiol. Aging. 2018 doi: 10.1016/j.neurobiolaging.2018.07.023. [DOI] [PubMed] [Google Scholar]
- 5.Al Snih S., Markides K., Ottenbacher K., Raji M. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin. Exp. Res. 2004;16:481–486. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 6.Taekema D., Gussekloo J., Maier A., Westendorp R., de Craen A. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing. 2010;39:331–337. doi: 10.1093/ageing/afq022. [DOI] [PubMed] [Google Scholar]
- 7.McGrath R., Robinson-Lane S.G., Cook S. Handgrip strength is associated with poorer cognitive functioning in aging americans. J. Alzheimer’s Dis. 2019;70:1187–1196. doi: 10.3233/JAD-190042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGrath R.P., Erlandson K., Vincent B. Decreased handgrip strength is associated with impairments in each autonomous living task for aging adults in the United States. J. Frailty Aging. 2019;8:141–145. doi: 10.14283/jfa.2018.47. [DOI] [PubMed] [Google Scholar]
- 9.Mueller-Schotte S., Zuithoff N.P.A., Van der Schouw Y.T., Schuurmans M.J., Bleijenberg N. Trends in Risk of Limitations in Instrumental Activities of Daily Living Over Age in Older Persons With and Without Multiple Chronic Conditions. J. Gerontol. Ser. A. 2019;75:197–203. doi: 10.1093/gerona/glz049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts H.C., Denison H.J., Martin H.J. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing. 2011;40:423–429. doi: 10.1093/ageing/afr051. [DOI] [PubMed] [Google Scholar]
- 11.Schaap L.A., Fox B., Henwood T. Grip strength measurement: Towards a standardized approach in sarcopenia research and practice. Eur. Geriatr. Med. 2016;7:247–255. doi: 10.1016/j.eurger.2015.11.012. [DOI] [Google Scholar]
- 12.Provins K.A. The specificity of motor skill and manual asymmetry: A review of the evidence and its implications. J. Mot. Behav. 1997;29:183–192. doi: 10.1080/00222899709600832. [DOI] [PubMed] [Google Scholar]
- 13.Provins K.A., Cunliffe P. The reliability of some motor performance tests of handedness. Neuropsychologia. 1972 doi: 10.1016/0028-3932(72)90060-7. [DOI] [PubMed] [Google Scholar]
- 14.Kim S., Lockhart T. Gait asymmetry: Factors influencing slip severity and tendency among older adults. Proc. Hum. Factors Ergon. Soc. 2006:1332–1335. doi: 10.1177/154193120605001316. [DOI] [Google Scholar]
- 15.Christopher I., Smith H. Strength, power output and symmetry of leg muscles: Effect of age and history of falling. Artic. Eur. J. Appl. Physiol. 2007 doi: 10.1007/s00421-006-0247-0. [DOI] [PubMed] [Google Scholar]
- 16.Yogev G., Plotnik M., Peretz C., Giladi N., Hausdorff J.M. Gait asymmetry in patients with Parkinson’s disease and elderly fallers: When does the bilateral coordination of gait require attention? Exp. Brain. Res. 2007;177:336–346. doi: 10.1007/s00221-006-0676-3. [DOI] [PubMed] [Google Scholar]
- 17.Kelley G.A., Kelley K.S. Is sarcopenia associated with an increased risk of all-cause mortality and functional disability? Exp. Gerontol. 2017;96:100–103. doi: 10.1016/j.exger.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee Y., Kim J.H., Lee K.J., Han G., Kim J.L. Association of cognitive status with functional limitation and disability in older adults. Aging Clin. Exp. Res. 2005;17:20–28. doi: 10.1007/BF03337716. [DOI] [PubMed] [Google Scholar]
- 19.Lee E.J., Lee S.A., Soh Y., Kim Y., Won C.W., Chon J. Association between asymmetry in lower extremity lean mass and functional mobility in older adults living in the community: Results from the Korean Frailty and Aging Cohort Study. Medicine. 2019;98:e17882. doi: 10.1097/MD.0000000000017882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ward R.E., Beauchamp M.K., Latham N.K. Neuromuscular impairments contributing to persistently poor and declining lower-extremity mobility among older adults: New findings informing geriatric rehabilitation. Arch. Phys. Med. Rehabil. 2016;97:1316–1322. doi: 10.1016/j.apmr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGrath R., Vincent B., Jurivich D. Handgrip strength asymmetry and weakness together are associated with functional disability in aging Americans. J. Gerontol. A Biol. Sci. Med. Sci. 2020 doi: 10.1093/gerona/glaa100. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics National Health and Nutrition Examination Survey, 2013–2014. [(accessed on 4 May 2020)]; Available online: https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/NHANES_Overview_Brochure.pdf.
- 23.National Center for Health Statistics National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010. [(accessed on 9 March 2020)]; Available online: https://www.cdc.gov/nchs/data/series/sr_01/sr01_056.pdf.
- 24.National Health and Nutrition Examination Survey (NHANES) Response Rates and Population Totals. [(accessed on 10 March 2020)]; Available online: https://wwwn.cdc.gov/nchs/nhanes/ResponseRates.aspx.
- 25.National Center for Health Statistics National Health and Nutrition Examination Survey: Analytic Guidelines, 1999–2010. [(accessed on 4 May 2020)]; Available online: https://www.cdc.gov/nchs/data/series/sr_02/sr02_161.pdf.
- 26.McGrath R.P., Ottenbacher K.J., Vincent B.M., Kraemer W.J., Peterson M.D. Muscle weakness and functional limitations in an ethnically diverse sample of older adults. Ethn. Heal. 2017:1–12. doi: 10.1080/13557858.2017.1418301. [DOI] [PubMed] [Google Scholar]
- 27.Hajjar I., Wharton W., Mack W.J., Levey A.I., Goldstein F.C. Racial disparity in cognitive and functional disability in hypertension and all-cause mortality. Am. J. Hypertens. 2015;29:185–193. doi: 10.1093/ajh/hpv084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jindai K., Nielson C.M., Vorderstrasse B.A., Quiñones A.R. Peer Reviewed: Multimorbidity and Functional Limitations Among Adults 65 or Older, NHANES 2005–2012. Prev. Chronic. Dis. 2016;13:1–11. doi: 10.5888/pcd13.160174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krok-Schoen J.L., Price A.A., Luo M., Kelly O.J., Taylor C.A. Low dietary protein intakes and associated dietary patterns and functional limitations in an aging population: A NHANES analysis. J. Nutr. Health Aging. 2019;23:338–347. doi: 10.1007/s12603-019-1174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L.-W., Chen W.-L., Peng T.-C. All-cause mortality risk in elderly individuals with disabilities: A retrospective observational study. BMJ Open. 2016;6:e011164. doi: 10.1136/bmjopen-2016-011164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McGrath R., Stastny S., Casperson S., Jahns L., Roemmich J., Hackney K.J. Daily Protein Intake and Distribution of Daily Protein Consumed Decreases Odds for Functional Disability in Older Americans. J. Aging Health. 2019 doi: 10.1177/0898264319881864. (Online ahead of print) [DOI] [PubMed] [Google Scholar]
- 32.National Health and Nutrition Examination Survey (NHANES) Muscle Strength Procedures Manual. [(accessed on 10 March 2020)]; Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2011-2012/manuals/Muscle_Strength_Proc_Manual.pdf.
- 33.National Health and Nutrition Examination Survey (NHANES) Muscle Strength Procedures Manual. [(accessed on 10 March 2020)];2013 Available online: https://wwwn.cdc.gov/nchs/data/nhanes/2013-2014/manuals/Muscle_Strength_2013.pdf.
- 34.Alley D.E., Shardell M.D., Peters K.W. Grip strength cutpoints for the identification of clinically relevant weakness. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2014:559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersen P., Petrick M., Connor H., Conklin D.J. Grip strength and hand dominance: Challenging the 10% rule. Am. J. Occup. Ther. 1989;43:444–447. doi: 10.5014/ajot.43.7.444. [DOI] [PubMed] [Google Scholar]
- 36.Armstrong C.A., Oldham J.A. A comparison of dominant and non-dominant hand strengths. J. Hand. Surg. 1999;24:421–425. doi: 10.1054/JHSB.1999.0236. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention Defining Adult Overweight and Obesity. [(accessed on 10 March 2020)]; Available online: https://www.cdc.gov/obesity/adult/defining.html.
- 38.Kroenke K., Spitzer R.L., Williams J.B.W. The PHQ-9: Validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Engelbert R., Wind A.E., Takken T., Helders P.J.M., Engelbert R.H.H. Is grip strength a predictor for total muscle strength in healthy children, adolescents, and young adults? Eur. J. Pediatr. 2009 doi: 10.1007/s00431-009-1010-4. [DOI] [PubMed] [Google Scholar]
- 40.Yorke A.M., Curtis A.B., Shoemaker M., Vangsnes E. Grip strength values stratified by age, gender, and chronic disease status in adults aged 50 years and older. J. Geriatr. Phys. Ther. 2015;38:115–121. doi: 10.1519/JPT.0000000000000037. [DOI] [PubMed] [Google Scholar]
- 41.Bohannon R.W. Muscle strength: Clinical and prognostic value of hand-grip dynamometry. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:465–470. doi: 10.1097/MCO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 42.Christensen H., Mackinnon A., Korten A., Jorm A. The “common cause hypothesis” of cognitive aging: Evidence for not only a common factor, but also specific associations of age with vision and grip strength in a cross-sectional analysis. Psychol. Aging. 2001;16:588–599. doi: 10.1037/0882-7974.16.4.588. [DOI] [PubMed] [Google Scholar]
- 43.Straight C.R., Brady A.O., Evans E.M. Asymmetry in leg extension power impacts physical function in community-dwelling older women. Menopause. 2016;23:410–416. doi: 10.1097/GME.0000000000000543. [DOI] [PubMed] [Google Scholar]
- 44.Ward N., Swayne O., Newton J. Age-dependent changes in the neural correlates of force modulation: An fMRI study. Neurobiol. Aging. 2008;29:1434–1446. doi: 10.1016/j.neurobiolaging.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung P., Baumgärtner U., Bauermann T. Asymmetry in the human primary somatosensory cortex and handedness. Neuroimage. 2003;19:913–923. doi: 10.1016/S1053-8119(03)00164-2. [DOI] [PubMed] [Google Scholar]
- 46.Labott B.K., Bucht H., Morat M., Morat T., Donath L. Effects of Exercise Training on Handgrip Strength in Older Adults: A Meta-Analytical Review. Gerontology. 2019;65:686–698. doi: 10.1159/000501203. [DOI] [PubMed] [Google Scholar]
- 47.Ramírez-Campillo R., Castillo A., de la Fuente C.I. High-speed resistance training is more effective than low-speed resistance training to increase functional capacity and muscle performance in older women. Exp. Gerontol. 2014;58:51–57. doi: 10.1016/j.exger.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 48.Milanović Z., Pantelić S., Trajković N., Sporiš G., Kostić R., James N. Clinical Interventions in Aging Age-related decrease in physical activity and functional fitness among elderly men and women. Clin. Interv. Aging. 2013;8:549–556. doi: 10.2147/CIA.S44112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiotsu Y., Watanabe Y., Tujii S., Yanagita M. Effect of exercise order of combined aerobic and resistance training on arterial stiffness in older men. Exp. Gerontol. 2018;111:27–34. doi: 10.1016/j.exger.2018.06.020. [DOI] [PubMed] [Google Scholar]