Abstract
Objectives:
Describe the frequency that inadequate oral feeding (IOF) is the reason why moderately preterm (MPT) infants remain hospitalized and its association with neonatal morbidities.
Study Design:
Prospective study using the NICHD Neonatal Research Network MPT Registry. Multivariable logistic regression was used to describe associations between IOF and continued hospitalization at 36 weeks postmenstrual age (PMA).
Result:
6017 MPT infants from 18 centers were included. 3376 (56%) remained hospitalized at 36 weeks PMA, of whom 1262 (37%) remained hospitalized due to IOF. IOF was associated with RDS (OR 2.02, 1.66–2.46), PDA (OR 1.86, 1.37–2.52), sepsis (OR 2.36, 95% 1.48–3.78), NEC (OR 16.14, 7.27–35.90), and BPD (OR 3.65, 2.56–5.21) compared to infants discharged and was associated with medical NEC (OR 2.06, 1.19–3.56) and BPD (OR 0.46, 0.34–0.61) compared to infants remaining hospitalized for an alternative reason.
Conclusion:
IOF is the most common barrier to discharge in MPT infants, especially among those with neonatal morbidities.
Table of Contents Summary:
Inadequate oral feedings is the primary reason moderately preterm infants are not ready for discharge at 36 weeks, contributing to lengthy and costly hospitalizations.
Introduction
Hospitalized premature infants must meet certain physiologic milestones in order to be discharged to home. These milestones relate to central nervous system maturation and include temperature control, cessation of apnea and bradycardia, and the ability to orally feed. Although there is variability in the times at which preterm infants achieve these markers of maturity, the ability to orally feed is typically the last milestone reached(1),(2). During the last weeks of hospitalization of preterm infants, the primary clinical aim is often to develop oral feeding skills(3). Essential skills needed for successful oral feeding include state regulation, motor organization, endurance, and coordination of a suck-swallow-breath pattern, and are unlikely to exist prior to 32 weeks postmenstrual age (PMA)(4). Additionally, intensive care therapeutic interventions such as endotracheal intubation, suctioning, and orogastric or nasogastric tube feeding may have a negative impact on oral sensory and oral-motor functioning of the infant(5, 6).
Risk factors that commonly delay discharge in very preterm infants are well described7. By contrast, the moderately preterm infant population (MPT, 29 0/7 to 33 6/7 weeks gestational age) is poorly studied despite accounting for five times as many NICU admissions as their very preterm counterparts(7, 8). While at less risk for major neonatal morbidities, MPT infants may experience prolonged, costly hospitalizations due to acquired illnesses or a delay in the acquisition of these essential milestones(9). The Moderate Preterm Registry of the Neonatal Research Network (NRN) collected information on common neonatal morbidities, feeding characteristics (including the dates for initiation of oral feeding and attainment of full oral feeding), and discharge information. Delayed introduction of oral feedings was identified as one of the leading reasons for a prolonged initial hospitalization(9, 10). The Registry data provide an opportunity to further examine associations between demographics, care practices, morbidities and center variations as they relate to oral feeding difficulties, and discharge after 36 weeks PMA. We hypothesized that A) morbidities associated with lower gestational age at birth, such as respiratory distress syndrome, patent ductus arteriosus, and necrotizing enterocolitis, would be strongly associated with delayed oral feeding as the primary cause of continued hospitalization after 36 weeks PMA, and B) that after adjustment for characteristics related to lower gestational age at birth, delayed oral feedings as a cause of discharge after 36 weeks PMA would vary among centers, and C) certain neonatal demographics, morbidities and complications will be associated with IOF as reason for prolonged stay even after adjustment for center.
Methods
We used prospectively collected data from the NICHD NRN MPT Registry. The MPT Registry included all live-born infants between 29 0/7 and 33 6/7 weeks gestational age (GA) who were cared for at 18 NRN sites between January 7, 2012 and October 31, 2013. Infants were excluded if they had complex congenital heart disease, gastrointestinal or upper airway malforations, chromosomal anomalies, and/or syndromes. Infants with missing data regarding 36 weeks PMA status were excluded from this analysis. All participating NRN sites obtained Institutional Review Board approval for the study, with either written parental consent or waiver of parental consent.
The status of each infant at 36 weeks PMA, including whether they were hospitalized or discharged and the reason for continued hospitalization beyond 36 weeks PMA, was documented. Reasons for continued hospitalization included a primary respiratory condition, apnea and bradycardia, inadequate oral feedings, or “other.” Full oral feeding was defined as a volume of 120 ml/kg/day. Data were collected on neonatal morbidities and therapies including severe (grade III or IV) intraventricular hemorrhage (IVH), presence of periventricular leukomalacia (PVL), patent ductus arteriosus (PDA), early-onset and late-onset culture-positive sepsis, medical and surgical necrotizing enterocolitis (NEC), respiratory distress syndrome (RDS) defined by receipt of surfactant, and bronchopulmonary dysplasia (BPD) defined as receiving supplemental oxygen at 36 weeks PMA. NEC was defined using the modified Bell’s criteria of Stage IIA or above. Early sepsis was defined as a positive blood culture at fewer than 72 hours of life. Late sepsis was defined as a positive blood culture at greater than 72 hours of life treated with five or more days of antibiotics. Additionally, data were collected regarding PMA at initiation of oral feeding and PMA at attainment of full oral feeding volume of 120 ml/kg/d, exposure to breastmilk in the first 28 days of life, and the actual diet being fed at 36 weeks PMA.
Descriptive statistics were used to compare demographic and morbidity characteristics between infants who remained hospitalized exclusively for IOF at 36 weeks PMA versus those discharged and those who remained hospitalized for other indications. Categorical outcomes were compared using Chi-square and Fisher’s exact tests. Continuous outcomes were compared using the nonparametric Wilcoxon/Kruskal Wallis test. Associations between IOF and neonatal morbidities and feeding characteristics were assessed with logistic regression after adjustment for center and these covariates: GA, small for gestational age (SGA), male sex, race, Caesarean delivery, 5 minute Apgar score, antenatal steroid use, and surfactant treatment. Adjusted odds ratios (OR) with 95% confidence intervals (CI) were reported. We used SAS 9.4 software (Cary, NC) for the statistical analyses.
Results
There were 7057 infants born between 01/07/2012 – 10/31/2013 with a gestational age of 29 to 33 weeks. Approximately 1040 were excluded due to having major syndromes or anomalies, transferring to another hospital, dying, or having missing status information at 36 weeks. The study cohort after all exclusions had 6017 infants, of whom 3376 (56%) remained hospitalized at 36 weeks PMA: 1262 (37%) due to IOF alone, 1066 (32%) due to IOF as well as another reason, and 1048 (31%) were hospitalized for a reason that did not include IOF. Infants who remained hospitalized due to IOF alone, when compared to those discharged by 36 weeks PMA, weighed less at birth (p<0.001) and were more likely to be SGA (p<0.001), male (P<0.001), and delivered by C-section (p<0.001) (Table 1). The median gestational age at birth differed statistically but not clinically among groups (32 weeks). Postnatal age at discharge (days) did differ: infants discharged by 36 weeks PMA had a median age of 20 days (IQR 15–29), while those who remained hospitalized for IOF alone were 35 days (IQR 27–46) old at discharge (P<0.001).
Table 1:
Demographic and Perinatal Characteristics
| IOF* (n=1262) | IOF + Other Reason (n=1066) | Alternative Reason** (n=1048) | Discharged (n=2641) | P-value IOF v. Alternative | P-value IOF v. Discharged | |
|---|---|---|---|---|---|---|
| Gestational age (weeks), median (IQR) | 32 (30, 33) | 31 (30, 32) | 31 (30, 33) | 32 (31, 33) | 0.002 | <0.001 |
| Birth weight (g), median (IQR) | 1620 (1349,1900) | 1497 (1240,1790) | 1580 (1320,1880) | 1810 (1560, 2061) | 0.03 | <0.001 |
| Age at 36 weeks (days), median (IQR) | 28 (21, 37) | 33 (24, 41) | 30 (22, 39) | 26 (20, 34) | <0.001 | <0.001 |
| Age at discharge (days), median (IQR) | 35 (27, 46) | 46 (36, 56) | 38 (29, 48) | 20 (15, 29) | 0.004 | <0.001 |
| SGA, n (%) | 270 (21) | 271/1065 (25) | 216/1046 (21) | 209/2639 (8) | 0.66 | <0.001 |
| Male sex, n (%) | 680 (54) | 581/1065 (55) | 539/1047 (52) | 1298/2639 (49) | 0.25 | 0.006 |
| Race, n (%) | ||||||
| Black | 383/1258 (30) | 255/1061 (24) | 356/1046 (34) | 1017/2629 (39) | 0.07 | <0.001 |
| White | 728/1258 (58) | 646/1061 (61) | 599/1046 (57) | 1365/2629 (52) | 0.77 | <0.001 |
| Other | 147/1258 (12) | 160/1061 (15) | 91/1046 (9) | 247/2629 (9) | 0.02 | 0.03 |
| Antenatal steroid use, n (%) | 1061/1253 (85) | 881/1058 (83) | 889/1038 (86) | 2273/2621 (87) | 0.52 | 0.09 |
| Antenatal antibiotic use, n (%) | 881/1243 (71) | 739/1045 (71) | 758/1030 (74) | 1876/2612 (72) | 0.15 | 0.54 |
| Multiple birth, n (%) | 388 (31) | 319 (30) | 337 (32) | 753 (29) | 0.47 | 0.15 |
| Rupture of membranes >18 h, n (%) | 207/1189 (17) | 136/1019 (13) | 184/984 (19) | 554/2461 (23) | 0.44 | <0.001 |
| Cesarean delivery, n (%) | 818 (65) | 776 (73) | 691 (66) | 1456/2639 (55) | 0.57 | <0.001 |
| 5-min Apgar score <5, n (%) | 56/1256 (4) | 82/1058 (8) | 46/1039 (4) | 60/2629 (2) | 0.97 | <0.001 |
| Intubation in the delivery room, n (%) | 181 (14) | 260 (24) | 165/1047 (16) | 237/2638 (9) | 0.34 | <0.001 |
IOF: Inadequate oral feeds
Alternative Reason group does not include any infant who was designated to be IOF
Of the 2114 infants who remained hospitalized for a reason other than IOF, or IOF plus another reason, 515 (15%) were due to apnea/bradycardia alone, 119 (4%) due to another primary respiratory issue, 326 (10%) due to ”other” reasons, and 1151 (33%) for more than one reason. One-thousand sixty-six (93%) of these infants remaining hospitalized beyond 36 weeks PMA for more than one reason that included inadequate oral feeding. Infants who remained hospitalized for an alternative reason had lower GA at birth (p=0.002) and weighed less at birth (p=0.03) compared to those remaining hospitalized for IOF alone and were older at both 36 weeks PMA (p<0.001) and at discharge (p=0.004).
The proportion of infants by center who remained hospitalized due to IOF alone ranged from 6% to 66%. After adjustment for covariates, there remained significant center variation among the 18 centers included in the registry with regard to the proportion of infants who remained hospitalized at 36 weeks PMA for IOF alone (Figure 1). Associations between IOF and neonatal morbidities were assessed with logistic regression after adjustment for center and the aforementioned covariates. In comparison to the infants who were discharged by 36 weeks PMA, those who remained hospitalized for IOF alone were more likely to have had RDS (OR 2.02, 1.66–2.46), a PDA treated medically (OR 1.86, 1.37–2.52) or surgically (OR 2.69, 1.36–5.32), sepsis (OR 2.36, 1.48–3.78), NEC (OR 16.14, 7.27–35.90), and BPD (OR 3.65, 2.56–5.21) (Table 2). When compared to infants who remained hospitalized for an alternative reason, those who remained hospitalized for IOF alone were more likely to have had medical NEC (OR 2.06, 1.19–3.56) and were less likely to have BPD (OR 0.46, 0.34–0.61).
Figure 1:
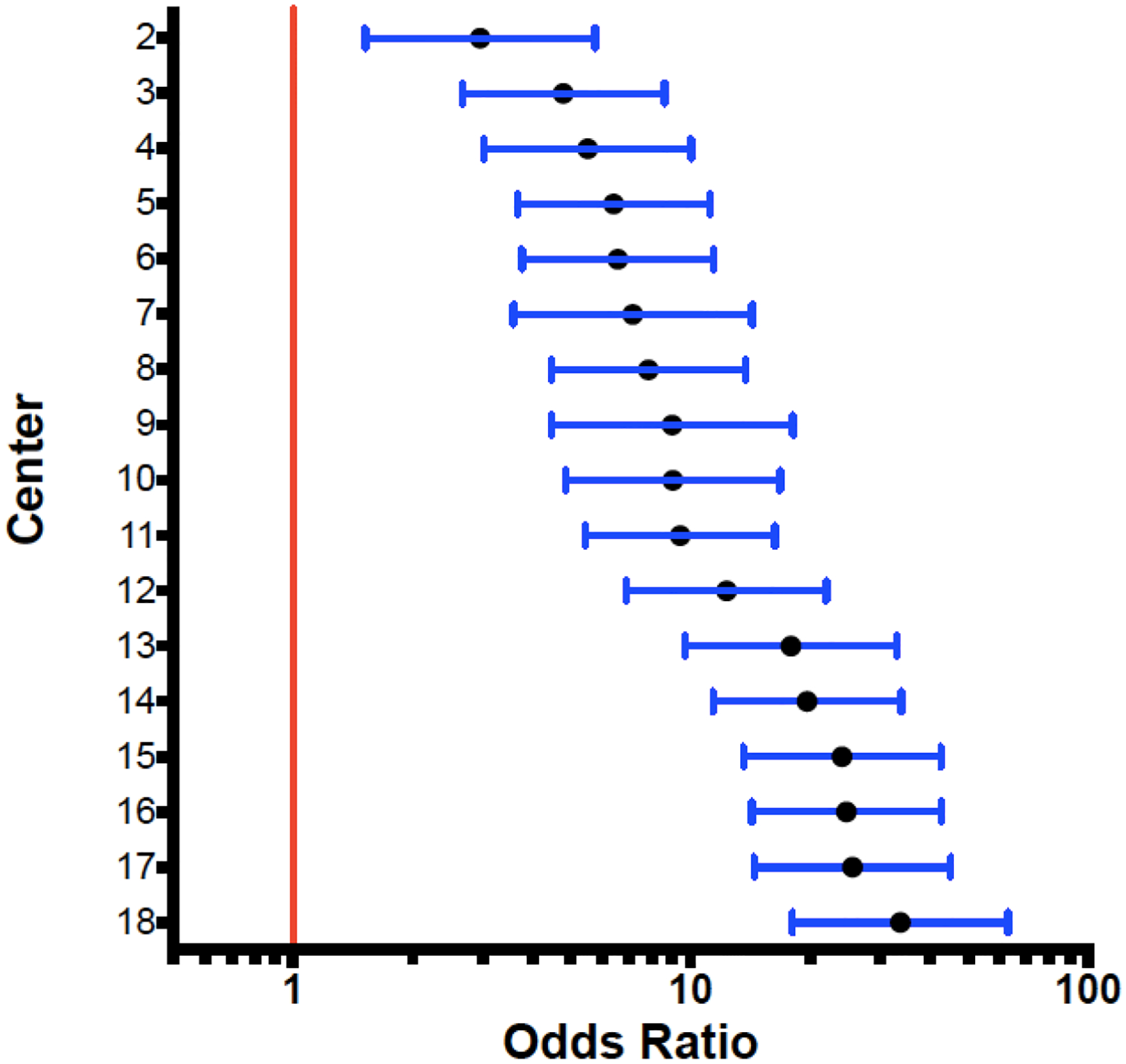
Comparison of Center Effect on IOF as a Reason for Hospitalization at 36 weeks PMA
The red line represents the reference center, which had the fewest patients hospitalized for IOF at 36 weeks PMA
Table 2:
In-Hospital Morbidities
| IOF* (n=1262) | Alternative Reason (n=1048) | Discharged by 36 wks PMA (n=2641) | Adjusted OR (95%CI) IOF v. Alternativeb | Adjusted OR (95%CI) IOF v. Dischargedb | |
|---|---|---|---|---|---|
| Severe IVH, n (%) | 5/751 (1) | 6/701 (1) | 12/1153 (1) | 0.37 (0.09, 1.54) | 0.34 (0.09, 1.25) |
| Any IVH | 90/751 (12) | 90/701 (13) | 142/1153 (12) | 0.95 (0.67, 1.32) | 1.20 (0.88, 1.63) |
| PVL (at 28 days or 36 wks), n (%) | 19/428 (4) | 11/415 (3) | 13/342 (4) | 1.67 (0.72, 3.89) | 1.04 (0.47, 2.29) |
| Any Sepsis, n (%) | 46 (4) | 45 (4) | 38/2639 (1) | 0.93 (0.58, 1.47) | 2.36 (1.48, 3.78) |
| Early Sepsis | 11 (1) | 10 (1) | 8 (0) | 0.98 (0.38, 2.55) | 3.39 (1.18, 9.72) |
| Late Sepsis | 37 (3) | 36 (3) | 30 (1) | 0.94 (0.57, 1.56) | 2.28 (1.36, 3.81) |
| Proven NEC, n (%)^ | 51 (4) | 28 (3) | 7 (0) | 1.75 (1.07, 2.83) | 16.14 (7.27, 35.90) |
| Medical | 44/51 (86) | 21/28 (7) | 7/7 (100) | 2.06 (1.19, 3.56) | 13.61 (6.07, 30.54) |
| Surgical | 7/51 (14) | 7/28 (25) | 0/7 (0) | 0.84 (0.29, 2.41) | - |
| PDA, n (%) | 104 (8) | 99 (9) | 98/2640 (4) | 0.97 (0.71, 1.33) | 1.86 (1.37, 2.52) |
| Treated medically | 30/103 (29) | 24/99 (24) | 13/98 (13) | 0.94 (0.52, 1.68) | 2.69 (1.36, 5.32) |
| Treated surgically | 2/103 (2) | 0/99 (0) | 0/98 (0) | - | - |
| Surfactant treatment, n (%) | 335 (27) | 306 (29) | 366/2640 (14) | 0.89 (0.72, 1.11) | 2.02 (1.66, 2.46) |
| Bronchopulmonary dysplasia, n (%) | 132/1229 (11) | 183/1028 (18) | 56/2530 (2) | 0.46 (0.34, 0.61) | 3.65 (2.56, 5.21) |
| Postnatal steroids, n (%)^ | 2 (0) | 9 (1) | 1 (0) | 0.21 (0.04, 1.00) | 2.02 (0.18, 22.88) |
IOF: Inadequate oral feeds
Alternative Reason group does not include any infant who was designated to be IOF alone or in combination with another reason for discharge after 36 weeks PMA.
Adjusted odds ratio were obtained using Logistic regression analysis adjusting for covariates gestational age, SGA, gender, race, mode of delivery, antenatal steroids, surfactant, 5 minute APGAR scores, and center
Models not adjusted for center variations due to convergence issues of the model
Infants in the IOF group were more likely to be exposed to parenteral nutrition (OR 1.88, 1.45–2.43) when compared to the discharged to home group. Additionally, infants who remained hospitalized for IOF were more likely to be fed human milk in the first 28 days of life (OR 1.30, 1.05–1.61) than their earlier discharged counterparts. Both the IOF and alternative reason for hospitalization groups had similar human milk exposure and caloric density of feeds at 36 weeks PMA with the exception that those in the IOF group were more likely to have received human milk with fortifier (OR 1.28, 1.05–1.57). Both the alternative reason for hospitalization and discharged groups started oral feeding sooner than the IOF group: 335 and 334 weeks compared to 341 weeks (OR 0.11, 0.03–0.19 and OR 0.38, 0.31–0.44) respectively. Feeding characteristics of the IOF, alternative reason, and discharged groups with adjustment for center effect and covariates are noted in Table 3.
Table 3:
Feeding Characteristics
| IOF* (n=1262) | Alternative Reason** (n=1048) | Discharged at 36 wks PMA (n=2641) | Adjusted OR(95%CI) IOF v. Alternativeb | Adjusted OR (95%CI) IOF v. Dischargedb | |
|---|---|---|---|---|---|
| Exposure to parental nutrition, n (%) | 1059 (84) | 853/1047 (82) | 2005/2639 (76) | 0.97 (0.71, 1.34) | 1.88 (1.45, 2.43) |
| OG/NG feeds after 24 hours, n (%) | 1238/1259 (98) | 1000/1044 (96) | 2255/2628 (86) | 2.97 (1.69, 5.22) | 7.25 (4.53, 11.61) |
| Fed human milk in the first 28 days, n (%) | 1108/1261 (88) | 908 (87) | 2172/2638 (82) | 1.09 (0.83, 1.42) | 1.30 (1.05, 1.61) |
| PMA of first oral feed (weeks)c, median (IQR) | 341/7 (334/7, 344/7) | 336/7 (331/7, 342/7) | 334/7 (33, 34) | 0.11 (0.03, 0.19) | 0.38 (0.31, 0.44) |
| PMA of full (120 ml/kg) oral feeds (weeks)c, median (IQR) | 363/7 (36, 371/7) | 354/7 (346/7, 36) | 345/7(341/7, 352/7) | 0.87 (0.78, 0.96) | 1.53 (1.46, 1.60) |
| At status, Human milk, n (%) | 418 (33) | 383 (37) | 1038/2636 (39) | 1.65 (0.87, 1.29) | 0.85 (0.72, 1.00) |
| At status, Human milk with fortifier, n (%) | 446 (35) | 262 (25) | 816/2636 (31) | 1.28 (1.05, 1.57) | 0.91 (0.77, 1.06) |
| At status, Formula 22 kcal, n (%) | 520 (41) | 481 (46) | 1241/2636 (47) | 0.88 (0.72, 1.07) | 0.88 (0.75, 1.03) |
| At status, Formula 24 kcal, n (%) | 365 (29) | 245 (23) | 696/2636 (26) | 1.05 (0.85, 1.30) | 1.02 (0.86, 1.22) |
| At status, Other feed, n (%) | 92 (7) | 116 (11) | 121/2636 (5) | 0.83 (0.61, 1.14) | 1.90 (1.40, 2.57) |
| At status, No enteral feeds, n (%) | 7 (1) | 6 (1) | 28/2636 (1) | 0.90 (0.30, 2.72) | 0.48 (0.20, 1.12) |
IOF: Inadequate oral feeds
Alternative Reason group does not include any infant who was designated to be IOF
At status: At 36 weeks PMA or at discharge if before 36 weeks PMA
Adjusted odds ratio were obtained using Logistic regression analysis adjusting for covariates gestational age, SGA, sex, race, mode of delivery, antenatal steroids, surfactant, 5 minute APGAR scores and center
Adjusted estimates were obtained using Median regression analysis adjusting for covariates gestational age, SGA, sex, race, mode of delivery, antenatal steroids, surfactant, 5 minute APGAR scores and center
There were significant differences between the IOF group and both the alternative reason and discharged groups in terms of birth weight (p=0.03 and p<0.001 respectively) (Table 4). Additionally, the IOF group weighed more at 36 weeks PMA than infants in the alternative reason group (p=0.006), but there was no significant weight difference at discharge. Those discharged prior to 36 weeks PMA weighed significantly less than the infants who remained hospitalized for IOF alone (p<0.001).
Table 4:
Anthropometric Data
| IOF (n=1262) | Alternative Reason (n=1048) | Discharged at 36 wks PMA (n=2641) | P-value IOF v. Alternativea | P-value IOF v. Dischargeda | |
|---|---|---|---|---|---|
| Birth weight (grams), median (IQR) | 1620 (1349,1900) | 1580 (1320, 1880) | 1810 (1560, 2061) | 0.03 | <0.001 |
| Weight (grams) at status (36 wks,), median (IQR) | 2249 (2010, 2470) | 2205 (1978, 2430) | - | 0.006 | - |
| Weight at discharge (grams), median (IQR) | 2468 (2210, 2757) | 2475 (2180, 2760) | 2170 (2000, 2366) | 0.60 | <0.001 |
P-values were obtained using using Chi-square tests and Fisher EXACT test for categorical variables and nonparametric Wilcoxon test for continuous variables
Discussion
During the last weeks of hospitalization of preterm infants, the primary clinical aim is often to develop oral feeding skills. Previous studies have found that feeding behaviors of premature infants matured between 33 and 36 weeks PMA, with swallowing infrequently interrupting respiration during feeding after 35 weeks PMA (4). Developmental delays in oral feeding are common. Prolonged use of endotracheal tubes for respiratory support and nasogastric or orogastric tubes for gavage feeding may cause sensory problems that affect feeding development of the preterm infant (5, 11). As previously reported in this cohort, inadequate oral feeding was the most common barrier to discharge in MPT infants(9). Our study showed that these infants who remained hospitalized at 36 weeks PMA had more morbidities related to prematurity than their discharged counterparts, with a higher likelihood of medical and surgical NEC and lower likelihood of BPD in the IOF group as compared to infants with other reasons for remaining hospitalized. A possible explanation for these associated morbidities is that by having NEC, premature infants may have had feeds withheld and missed the PMA critical window for acquiring oral feeding skills; or, they may have just been “sicker” babies with more procedures and negative experiences impacting their oral skills. Infants with BPD likely remained hospitalized for several reasons beyond having oral feeding difficulties alone.
Little is known about how variation in management or discharge policies among practitioners affects length of stay. A previous study found that inter-NICU variation in recorded attainment of maturational milestones was the most significant influence on length of stay (1). The study suggests that the timing of apnea resolution could be influenced by differences in monitoring methods between units. For example, longer use of pulse oximetry was associated with later documentation of apnea resolution(1). Similarly, variation in feeding practices may delay the recognition of mature feeding behavior. Unfortunately, we did not have information about the feeding protocols used in each of the 18 centers included in our study to speculate why there was such variation in the proportion of infants who remained hospitalized for IOF at 36 weeks PMA. While feeding protocols are commonly used in the NICU setting, there is no standard practice, and large variations in practice exist. Our data showed a significant difference in the PMA of the first oral feed attempts, with the IOF group introduced to oral feeding later than the infants in the alternative reason and discharged groups. Additionally, those in the discharged group attained full oral feeding 13 days earlier than those in the IOF group.
A previous study found significant inter-unit variation in growth rates and feeding practices in 450 infants born between 30 and 35 weeks gestation among 15 Massachusetts-based neonatal intensive care units (12). Infants born before 33 weeks GA received oral feeds sooner than those in our study at an average of 32.9 weeks PMA, and later initiation of oral feedings was linked with prolonged gavage feeding. Unlike our study, however, this study was limited to a homogenous group of healthy premature infants in an effort to minimize the confounding effects of illness. We included infants who developed in-hospital morbidities related to their prematurity in an effort to elucidate why a MPT infant may remain hospitalized at 36 weeks PMA when theoretically he/she could be physiologically ready to be discharged. Infants with RDS, PDA, sepsis, NEC, and BPD were more likely to remain hospitalized at 36 weeks PMA due to IOF after adjusted analyses when compared to infants who were discharged to home by 36 weeks PMA. Furthermore, gastrointestinal-specific morbidities such as medical and surgical NEC were associated with delays in oral feeding when comparing the IOF group to infants who remained hospitalized for an alternative reason.
Infants who remained hospitalized for IOF actually weighed more on average at 36 weeks PMA than those hospitalized from an alternative reason despite feeding difficulties being the primary reason for which they remained hospitalized. A previous study demonstrated significant inter-NICU variation regarding the time to regain birthweight and net growth velocity(12). The study notes that gavage feeding likely enhanced growth velocity by both increasing energy intake and minimizing energy consumption, which could help explain why the IOF group in our study had greater weight at 36 weeks PMA.
Prolonged hospitalization has been shown to correlate with poorer parent-infant relationships, failure to thrive, child abuse, and abandonment (13). Additionally, parents struggle to cope with feeding difficulties in preterm infants, with feeding issues being a primary concern for families post-discharge(14, 15). A Cochrane review comparing early discharge home with gavage feeds and health care support to later discharge home when full oral feeds have been established concluded that there were insufficient numbers of quality trials to make a practice recommendation (16). Several small studies suggest not only a reduced length of stay in the intensive care unit, but also a reduction in infection and improved breastfeeding in the infants sent home with nasogastric (NG) gavage feedings (17). Data from a single center demonstrated that home NG feedings as an option for infants remaining in the NICU with ongoing oral feeding problems may lead to improved feeding outcomes. The study included 9 preterm and 8 full-term infants enrolled in their medical home program who were discharged home with supplemental NG feeds with the expectation that they would be able to attain full oral feeds. Infants were followed closely in their NICU follow-up clinic after discharge and no infants were re-hospitalized due to complications such as tube dislodgement, oropharyngeal trauma, and aspiration event. NG feeds were used on average for 8 weeks after discharge, and only 1 infant required a gastrostomy tube. This suggests that home nasogastric supplemental tube feeding may be an option to reduce hospital length of stay and decrease the need for surgical placement of a gastrostomy tube.
There are some limitations to our study. Very little data was collected with regards to initiation of enteral feedings and feeding intolerance. The feeding protocols and detailed explanatory data were not available, so we were unable to examine how and when providers determined oral feeding readiness, recognized feeding cues and initiated oral feeds, and their progression to full oral feeds. There was no data collected regarding infants who were both breast and bottle feeding. Some units prioritize the establishment of breastfeeding prior to introducing bottle feeding, which may reflect a delay in oral feeding. Many units have feeding teams comprised of speech pathologists and/or occupational therapists; however, information was not collected on whether such a team was used at each of the centers and the level of involvement of feeding therapists in the decision-making of oral feeding initiation and advancement. Furthermore, discharge criteria across centers was not determined. Consequently, we were unable to decipher inter-unit variations that could explain why one center had 6% of infants who remained hospitalized at 36 weeks PMA for IOF and another had 66%. Similarly, decisions as to when to fortify feeds with additional calories could not be extrapolated from the data available, although many intensive care units have protocols that help guide such decision-making. Because of limited anthropometric data collection, we could not assess growth velocity or other growth parameters. There also was not any post-discharge feeding and growth data collected to assess the ongoing success of the infant’s feeding. Lastly, our assessment of the relationship between IOF and intracranial pathology was limited by the fact that cranial imaging was not universally obtained in this population.
Despite these limitations, our study provides valuable information about a relatively understudied population, their feeding competencies, and potential reasons for prolonged hospitalization. We confirmed that IOF was the most common reason MPT infants remained hospitalized at 36 weeks PMA. We also demonstrated that several morbidities of prematurity are associated with inadequate oral feeding. The significant center difference observed suggests that greater standardization may improve recognition of oral feeding readiness and maturation. The data from this study may lead to the design of future prospective studies that aim to reduce the length of stay of MPT infants who remain hospitalized for feeding immaturity.
Conclusion:
IOF is the most common barrier to discharge in MPT infants, and there is wide center variation with regard to this discharge competency. Infants hospitalized at 36 weeks PMA for IOF had more morbidities associated with prematurity than those discharged by 36 weeks PMA; however, those who remained hospitalized for IOF were separated from their counterparts who remained hospitalized for another reason by only gastrointestinal morbidities. Further studies are needed to determine therapies and modalities to overcome feeding as a barrier to discharge in premature infants.
Supplementary Material
Acknowledgements:
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network’s Moderate Preterm Registry through cooperative agreements. While NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations:
- IOF
inadequate oral feeding
- MPT
moderately preterm
- PMA
postmenstrual age
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- OR
odds ratios
- CI
confidence intervals
- GA
gestational age
- ml/kg/d
milliliters per kilogram per day
- IVH
intraventricular hemorrhage
- PVL
periventricular leukomalacia
- PDA
patent ductus arteriosus
- NEC
necrotizing enterocolitis
- RDS
respiratory distress syndrome
- BPD
bronchopulmonary dysplasia
- SGA
small for gestational age
- NG
nasogastric
Footnotes
Financial Disclosure Statement for all authors: the authors have no financial relationships relevant to this article to disclose
Potential Conflicts of Interest: the authors have no conflicts of interest relevant to this article to disclose.
References:
- 1.Eichenwald EC, Blackwell M, Lloyd JS, Tran T, Wilker RE, Richardson DK. Inter-neonatal intensive care unit variation in discharge timing: influence of apnea and feeding management. Pediatrics. 2001;108(4):928–33. [DOI] [PubMed] [Google Scholar]
- 2.Bakewell-Sachs S, Medoff-Cooper B, Escobar GJ, Silber JH, Lorch SA. Infant functional status: the timing of physiologic maturation of premature infants. Pediatrics. 2009;123(5):e878–86. [DOI] [PubMed] [Google Scholar]
- 3.Lau C Oral Feeding in the Preterm Infant. NeoReviews. 2006;7(1):e19–e27. [Google Scholar]
- 4.Mizuno K, Ueda A. Neonatal feeding performance as a predictor of neurodevelopmental outcome at 18 months. Developmental medicine and child neurology. 2005;47(5):299–304. [DOI] [PubMed] [Google Scholar]
- 5.Torola H, Lehtihalmes M, Yliherva A, Olsen P. Feeding skill milestones of preterm infants born with extremely low birth weight (ELBW). Infant behavior & development. 2012;35(2):187–94. [DOI] [PubMed] [Google Scholar]
- 6.Arvedson J, Clark H, Lazarus C, Schooling T, Frymark T. Evidence-based systematic review: effects of oral motor interventions on feeding and swallowing in preterm infants. American journal of speech-language pathology / American Speech-Language-Hearing Association. 2010;19(4):321–40. [DOI] [PubMed] [Google Scholar]
- 7.Cotten CM, Oh W, McDonald S, Carlo W, Fanaroff AA, Duara S, et al. Prolonged hospital stay for extremely premature infants: risk factors, center differences, and the impact of mortality on selecting a best-performing center. Journal of perinatology : official journal of the California Perinatal Association. 2005;25(10):650–5. [DOI] [PubMed] [Google Scholar]
- 8.March of Dimes. Available from: http://marchofdimes.org. [August 17, 2018].
- 9.Walsh MC, Bell EF, Kandefer S, Saha S, Carlo WA, D’Angio CT, et al. Neonatal outcomes of moderately preterm infants compared to extremely preterm infants. Pediatric research. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brumbaugh JE, Colaizy TT, Saha S, Van Meurs KP, Das A, Walsh MC, et al. Oral feeding practices and discharge timing for moderately preterm infants. Early human development. 2018;120:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pridham K, Steward D, Thoyre S, Brown R, Brown L. Feeding skill performance in premature infants during the first year. Early human development. 2007;83(5):293–305. [DOI] [PubMed] [Google Scholar]
- 12.Blackwell MT, Eichenwald EC, McAlmon K, Petit K, Linton PT, McCormick MC, et al. Interneonatal intensive care unit variation in growth rates and feeding practices in healthy moderately premature infants. Journal of perinatology : official journal of the California Perinatal Association. 2005;25(7):478–85. [DOI] [PubMed] [Google Scholar]
- 13.Merritt TA, Pillers D, Prows SL. Early NICU discharge of very low birth weight infants: a critical review and analysis. Seminars in neonatology : SN. 2003;8(2):95–115. [DOI] [PubMed] [Google Scholar]
- 14.Pridham K, Saxe R, Limbo R. Feeding issues for mothers of very low-birth-weight, premature infants through the first year. The Journal of perinatal & neonatal nursing. 2004;18(2):161–9. [DOI] [PubMed] [Google Scholar]
- 15.DeMauro SB, Patel PR, Medoff-Cooper B, Posencheg M, Abbasi S. Postdischarge feeding patterns in early- and late-preterm infants. Clinical pediatrics. 2011;50(10):957–62. [DOI] [PubMed] [Google Scholar]
- 16.Collins CT, Makrides M, McPhee AJ. Early discharge with home support of gavage feeding for stable preterm infants who have not established full oral feeds. The Cochrane database of systematic reviews. 2003(4):Cd003743. [DOI] [PubMed] [Google Scholar]
- 17.Meerlo-Habing ZE, Kosters-Boes EA, Klip H, Brand PL. Early discharge with tube feeding at home for preterm infants is associated with longer duration of breast feeding. Archives of disease in childhood Fetal and neonatal edition. 2009;94(4):F294–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


