Abstract
Growing evidence highlights the endocannabinoid (EC) system involvement in cancer progression. Lipid mediators of this system are secreted by hematopoietic cells, including the ECs 2-arachidonoyl-glycerol (2AG) and arachidonoyl-ethanolamide (AEA), the 2AG metabolite 1AG, and members of N-acylethanolamine (NAE) family—palmitoyl-ethanolamide (PEA) and oleoyl-ethanolamide (OEA). However, the relevance of the EC system in myeloproliferative neoplasms (MPN) was never investigated. We explored the EC plasma profile in 55 MPN patients, including myelofibrosis (MF; n = 41), polycythemia vera (PV; n = 9), and essential thrombocythemia (ET; n = 5) subclasses and in 10 healthy controls (HC). AEA, PEA, OEA, 2AG, and 1AG plasma levels were measured by LC–MS/MS. Overall considered, MPN patients displayed similar EC and NAE levels compared to HC. Nonetheless, AEA levels in MPN were directly associated with the platelet count. MF patients showed higher levels of the sum of 2AG and 1AG compared to ET and PV patients, higher OEA/AEA ratios compared to HC and ET patients, and higher OEA/PEA ratios compared to HC. Furthermore, the sum of 2AG and 1AG positively correlated with JAK2V617F variant allele frequency and splenomegaly in MF and was elevated in high-risk PV patients compared to in low-risk PV patients. In conclusion, our work revealed specific alterations of ECs and NAE plasma profile in MPN subclasses and potentially relevant associations with disease severity.
Keywords: endocannabinoids, N-acylethanolamines, myeloproliferative neoplasms, myelofibrosis, polycythemia vera, essential thrombocythemia
1. Introduction
The endocannabinoid (EC) system is composed by the lipid endogenous compounds—N-arachidonoyl-ethanolamine (anandamide, AEA) and 2-arachidonoyl-glycerol (2AG), the enzymatic machinery responsible for ligand synthesis and degradation, and the cannabinoid receptors 1 (CB1) and 2 (CB2) [1]. AEA and 2AG are synthesized on demand from membrane phospholipids of many cell types, in the brain and peripheral tissues, as well as in blood cells. Although ECs are supposed to act via paracrine and autocrine communication, their presence in the bloodstream has been quantified and associated with multiple physiopathological conditions [2,3]. CB1 and CB2 are G-protein-coupled receptors; however, CB1 is highly expressed in the central nervous system and in nearly all peripheral tissues, and CB2 is mainly detected in immune cells [4,5]. Both receptors are involved in the regulation of cell proliferation, differentiation, apoptosis, and migration. While AEA acts as a full agonist for both CB1 and CB2, 2AG is a full agonist for CB2 [6].
As a member of the monoacyl-glycerol family, 2AG is an intermediate of diacyl- and triacyl-glycerol metabolic pathways. Due to the poor chemical stability, this compound rapidly and spontaneously isomerizes into 1AG, so that the sum of 2AG and 1AG levels (2+1AG) is often used for evaluating the 2AG biomarker potential in plasma [7,8]. In addition, although long considered inactive, the isomer 1AG was recently shown to have potential agonistic activity supporting 2AG function [9].
AEA belongs to the N-acyl-ethanolamide (NAE) family, also including oleoyl-ethanolamine (OEA) and palmitoyl-ethanolamine (PEA) [3]. These signaling lipids share the biosynthetic and degradative machinery as well as non-CB targets, such as the transient receptor potential vanilloid 1 (TRPV1), G-protein-coupled receptors GPR55 and GPR119, and peroxisome proliferator activator receptors (PPAR). NAEs were described to reciprocally influence their activity on target receptors by competing for degrading enzymes, according to an entourage effect mechanism [10,11]. Moreover, although the circulating levels of the three NAEs were found to be highly directly correlated [7,12], imbalances in their relative abundances were associated with their poor metabolic profiles [8,13]. In addition, PPAR-alpha and -gamma targets mediate NAE anti-inflammatory properties [14]. In particular, in contrast to 2AG, which is involved in immune cell recruitment, AEA suppresses pro-inflammatory cytokines production and enhances the release of anti-inflammatory cytokines regulating the immune responses [15,16,17]. Furthermore, PEA was shown to counteract systemic inflammation in mice and humans [14] and to support the increased intestinal permeability associated with inflammation along with OEA [18]. PEA also exhibited immune-modulating properties on different T-cell subsets, thereby representing a new pharmacological player for the treatment of human chronic inflammatory disorders [19].
Interestingly, ECs have been recently found to modulate hematopoiesis, including megakaryocyte maturation, thrombopoiesis, and platelet aggregation, as well as chemokine release and migration of immunocompetent cells [15,20,21,22]. Importantly, blood cells and platelets act as sources of ECs, whereas various hematopoietic cell subsets, particularly B-cells, display high levels of CB2 [23]. In addition, ECs released by platelets are involved in thrombogenic processes [24].
EC system implication in various hematological malignancies has also been investigated [25]. In this regard, high levels of CB2 in hematopoietic precursor cells were shown to exert a role in leukemogenesis [26]. Interestingly, Jorda et al. [27] described that CB2 is expressed in acute myeloid leukemia (AML) blast cells, but not in normal myeloid cells, and that it is associated with migration of bone marrow (BM) precursors mediated by 2AG. Of interest, CB2 revealed oncogenic properties abrogating myeloid differentiation [28]. Recently, interest in the EC system emerged for another hematological malignancy, multiple myeloma (MM). Indeed, it was shown that plasma cells expressed high levels of CB2 and that cannabinoid derivatives selectively induced apoptosis in MM cell lines and primary plasma cells from MM patients [23], similarly to what previously reported for AML [29]. Hence, the EC system in these malignancies might represent a potential target for therapeutic exploitation.
At variance with the mentioned hematological malignancies, to date, no studies investigated the EC system role in myeloproliferative neoplasms (MPN). The MPN include clonal disorders of hemopoietic stem cells such as polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (MF) that are driven by mutations in Janus kinase 2 (JAK2), myeloproliferative leukemia (MPL), or calreticulin (CALR) genes [30]; however, none of these could be detected in 2–15% of the patients (triple-negative patients, TN). All MPN are characterized by an increased risk of thromboembolic complications and by the predisposition to evolve into AML. Recently, a selective JAK1/2 inhibitor, ruxolitinib, was introduced into clinical practice; however, many patients did not respond or did not tolerate this drug [31]. Therefore, more effective therapies are urgently needed.
To our best knowledge, the circulating levels of ECs and related compounds in hematological malignancies have never been reported. Here, for the first time, we investigated the circulating profile defined by levels of the sum of 2AG and 1AG and by the levels and the relative abundances of the NAE AEA, PEA, and OEA in patients affected by MPN, including ET, PV, and MF. In addition, we associated the EC and NAE profile with clinical parameters, mutational status, and disease severity to gain further insight into MPN etiology and to highlight potential disease-related biomarkers.
2. Results
2.1. Study Cohort
The cohort included 55 patients affected by MPN, recruited at the University Hospital of Bologna, and 10 healthy control (HC) volunteers from the general population. Patients were enrolled at diagnosis or after at least three months from stopping cytotoxic therapy (n = 22). MPN patients were subdivided into ET (n = 5), PV (n = 9), and MF (n = 41). Table 1 reports the clinical and laboratory parameters of each class. No differences in sex distribution (p = 0.129) were observed, whereas differences were detected in age among classes (p < 0.001). MF patients were older (median: 72 years; range: 46–89 years) compared to HC (median: 59 years; range: 31–73 years; p = 0.030), ET (median: 52; range: 42–57 years; p = 0.005) and PV (median: 57, range: 26–71 years; p = 0.002). MPN patients were further stratified into two risk categories: 11 (20%) low-risk (age of <60 years and having no thrombosis history) and 39 (70%) high-risk (age of >60 years and having thrombosis history) patients.
Table 1.
Clinical and laboratory features of patients within myeloproliferative neoplasms (MPN) subclasses (essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF)) and healthy control (HC). Data are expressed as median (range). One-way ANOVA: ET vs. MF: * p ≤ 0.050; ** p ≤ 0.010; *** p ≤ 0.001; PV vs. MF: # p ≤ 0.050; ## p ≤ 0.010; ### p ≤ 0.001; HC vs. MF: + p ≤ 0.050; ++ p ≤ 0.010; +++ p ≤ 0.001. F: female, M: male, WBC: white blood cell count, PLT: platelet count, Hgb: hemoglobin, RBC: red blood cell count, Hct: hematocrit.
| Descriptive Parameters | HC (n = 10) | ET (n = 5) | PV (n = 9) | MF (n = 41) |
|---|---|---|---|---|
| Sex (F/M) | 5/5 | 2/3 | 3/6 | 19/22 |
| Age (years) | 59 (31–73) | 52 (42–57) ** | 57 (26–71) ## | 72 (46–89) + |
| WBC (103/μL) | 6.1 (4.3–9.0) | 7.5 (7.4–10.3) | 8.1 (7.4–14.9) | 9.9 (1.6–38.6) |
| PLT (103/µL) | 261 (159–306) | 463 (330–656) * | 438 (229–762) | 121 (38–632) |
| Hgb (g/dL) | 14.1 (12.9–15.5) | 14.2 (14–15.4) *** | 13.9 (11–16.20) ## | 9.9 (7.2–15.28) +++ |
| RBC (106/µL) | 4.6 (4.1–5.3) | 5.59 (4.6–5.65) | 5.5 (3.38–7.4) # | 3.7(2.4–6.2) |
| Hct (%) | 41.5 (37.6–46.7) | 44.7(43–47.98) *** | 46.5 (41.8–49.5) ### | 30.87 (24.16–50.69) ++ |
2.2. EC and NAE Plasma Profile of MPN Subclasses
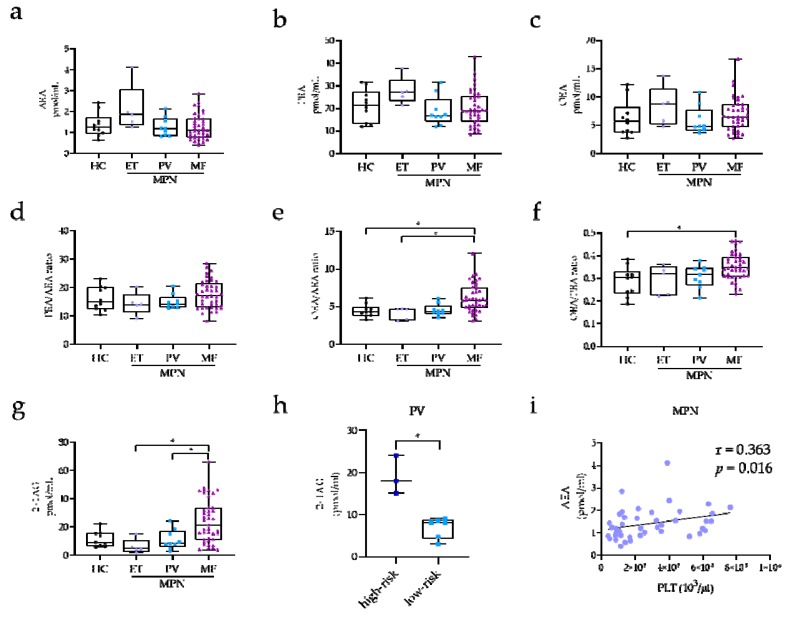
The levels of AEA, PEA, and OEA and the ratios of PEA/AEA, OEA/AEA, and OEA/PEA, along with the levels of 2+1AG for each MPN subclass and HC are reported in Figure 1. The global analysis of MPN patients showed no significant differences in EC and NAE plasma levels between patients and HC. Additionally, the concentrations of the three NAEs did not significantly vary among MPN classes and HC (AEA: p = 0.098; PEA: p = 0.203; OEA: p = 0.276; Figure 1a–c). However, when NAE ratios were evaluated, significant differences of OEA/AEA and OEA/PEA were found among classes (p = 0.001 and p = 0.005, respectively). In particular, MF patients exhibited higher OEA/AEA and OEA/PEA ratios as compared to HC (p = 0.030 and p = 0.010, respectively; Figure 1e–f) and higher OEA/AEA ratios compared to ET (p = 0.020). Furthermore, we found that 2+1AG was significantly higher in MF compared to in both ET (p = 0.001) and PV patients (p = 0.030) (Figure 1g). When PV patients were classified according to risk, high-risk PV patients showed 2-fold increase of 2+1AG levels compared to low-risk PV patients (19.0 ± 2.6 pmol/mL vs. 6.8 ± 0.9 pmol/mL; p = 0.030; Figure 1h). Notably, the overall results were not altered, when age was included as a covariate in the analysis.
Figure 1.
Box-and-whiskers plots for plasma levels of arachidonoyl-ethanolamide (AEA), (a) palmitoyl-ethanolamide (PEA); (b) oleoyl-ethanolamide (OEA); (c) PEA/AEA ratio; (d) OEA/AEA ratio; (e) OEA/PEA ratio; (f) and 1/2-arachidonoyl-glycerol (2+1AG); (g) in HC (n = 10) and ET (n = 5), PV (n = 9), and MF (n = 41) patients. One-way ANOVA: * p ≤ 0.05. (h) 2+1AG plasma levels in PV patients at high risk (age of >60 years and/or having thrombosis history; n = 3) and low risk (age of <60 years and having no history of thrombosis; n = 6). T-test: * p ≤ 0.050. (i) Pearson’s correlation results between AEA and platelet count (PLT) in MPN patients (n = 43).
Circulating levels of 2+1AG and NAEs were not associated with hematological parameters such as white blood cell count, red blood cell count, hemoglobin, platelet count, and hematocrit within MPN classes; however, we observed a direct association of AEA levels with the platelet count when the overall cohort of MPN patients was considered (r = 0.363; p = 0.016; Figure 1i).
2.3. EC and NAE Plasma Profile According to Risk Classification, Mutational Status, and Clinical Manifestations in MF Patients
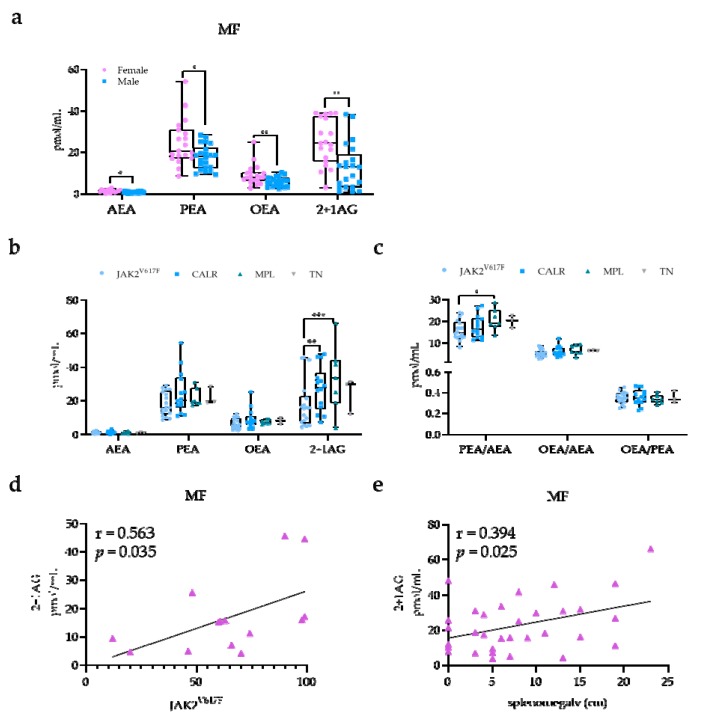
The large sample size available for the MF class (n = 41) allowed us to perform further investigations. Notably, sex differences were observed in MF, with higher NAE and 2+1AG levels in females (n = 19) compared with in males (n = 21) (AEA, p = 0.010; PEA, p = 0.022; OEA, p = 0.008; 2+1AG, p = 0.001; Figure 2a). According to the dynamic international prognostic scoring system (DIPSS) [32], MF patients displayed high risk in five cases (12.1%), intermediate-2 risk in 15 cases (36.6%), intermediate-1 risk in 13 cases (31.7%), and low risk in one case (2.4%). Besides, 15 (36.5%) patients were diagnosed as secondary MF, with five (12.1%) being post-PV MF and 10 (24.3%) being post-ET MF patients. No significant differences were detected in the EC and NAE profile among DIPSS risk categories and between primary and secondary MF.
Figure 2.
EC and NAE plasma profile according to mutational status and clinical manifestations in MF patients. (a) Box-and-whiskers plots for plasma levels in female (n = 19) and male (n = 21) MF patients. T-test: * p ≤ 0.050; ** p ≤ 0.010. (b) AEA, PEA, OEA and 2+1AG levels; (c) PEA/AEA, OEA/AEA, and OEA/PEA ratios according to JAK2V617F (n = 17), calreticulin (CALR; n = 14), and myeloproliferative leukemia (MPL; n = 7) mutational status or triple-negative (TN; n = 3). One-way ANOVA: * p ≤ 0.050; ** p ≤ 0.010; *** p ≤ 0.001. Correlation of 2+1AG with (d) JAK2V617F variant allele frequency (VAF; n = 17) and (e) splenomegaly (n = 41). Pearson’s correlation test.
MF patients were further analyzed according to the mutational spectra as defined by JAK2V617F (n = 17), CALR (n = 14), and MPL (n = 7) mutations and by the absence of these mutations (TN; n = 3). We found that JAK2V617F-mutated MF patients had lower 2+1AG levels compared with patients carrying CALR (p = 0.006) and MPL mutations (p < 0.001) (Figure 2b), as well as lower PEA/AEA ratios compared with MPL-mutated patients (p = 0.003; Figure 2c). Data were still significant when adjusted for age as a covariate. Furthermore, 2+1AG levels were positively correlated with JAK2V617F variant allele frequency (VAF) (r = 0.563; p = 0.035; Figure 2d), but not with mutant allele burden of CALR type 1/2 (p = 0.752). Notably, 2+1AG levels were also directly associated with splenomegaly in the overall MF class (r = 0.394; p = 0.025; Figure 2e).
3. Discussion
The EC system is involved in many pathophysiological processes, and its role in cancerogenesis has been postulated [33]. The cancer-associated dysregulation of the EC system might lead to measurable changes in circulating EC levels [34]. Here, we investigated the potential role of the EC system as a disease-specific circulating hallmark of rare MPN.
MPN are known to be characterized by an increased pro-inflammatory status [30,31,35]. Although we did not detect any alteration in circulating levels of AEA, PEA, and OEA in MPN compared to in HC, we reported, for the first time, that AEA concentration was correlated with the platelet count in these patients. Of interest, it has been previously published that AEA in the blood may be one among the factors required for platelet survival [36].
Previous studies demonstrated that circulating concentrations of 2AG are increased in pro-inflammatory states [37,38,39] and are directly correlated with interleukin 6 (IL-6) levels [2,40]. Those findings, on one side, are consistent with our data showing the increased 2+1AG levels associated with the high-risk condition in PV patients. On the other side, the increased 2+1AG levels we described in MF patients could be related to the strong correlation between IL-6 levels and disease severity in MF that we described in our previous study [41]. Additionally, it has been reported that the pathogenesis of MF is linked to the altered megakaryocyte proliferation and differentiation [42,43]. Notably, Gasperi et al. [15] observed that 2AG is a regulator of megakaryocyte/platelet functions. Our findings might suggest a role of this EC in the abnormal megakaryocytopoiesis associated with MF. Furthermore, our data suggested that a potential dysregulation of NAE balance occurs in MF patients in terms of higher OEA/AEA and OEA/PEA ratios. EC and NAE dysregulation have been largely described in obesity and metabolic impairment in humans [12,37]. In previous studies performed in a cohort of healthy subjects from the general population, we reported that 2AG and OEA derangements were associated with insulin resistance and dyslipidemia independently from body mass index (BMI) [8,13]. Another study highlighted how human leukemia cells are able to induce insulin resistance as a mechanism to favor the uncontrolled growth of malignant cells [44]. Whether the dysregulation of the EC and NAE profile we described in MF is related to tumor metabolism and growth deserves further investigations.
Another relevant finding of our study relies on the association of the EC system with the mutational spectrum of MF. For instance, JAK2V617F-mutated patients displayed lower mean levels of 2+1AG compared to CALR and MPL mutation carriers. As 2AG is rapidly released in response to pro-inflammatory stimulation of immune cells [45], our results seem to suggest that specific alterations of the immune system depend on the mutational status, as previously reported by our group [46]. On the other hand, 2+1AG was directly correlated with JAK2V617F VAF. Most importantly, increasing levels of this EC were associated, in the overall MF cohort, with splenomegaly, a marker of disease severity. These results led us to hypothesize that 2AG levels might be differentially regulated by the three driver-mutated genes in MF and that increasing levels are closely related to disease severity.
Despite the fact that the role of gender in the symptomatology of MPN is still undefined [47,48], Barraco et al. [49] observed that female patients had a specific phenotype with slower disease progression and better prognosis. Here, for the first time, we found higher PEA and 2+1AG plasma levels in MPN female compared to in male patients (data not shown), which was particularly evident in MF subclass, showing a similar trend also for AEA and OEA plasma levels. These features supported the need for gender-specific analysis to better interpret experimental results in MPN.
Altogether, our work highlights the potential involvement of the EC system in the pathophysiology of MPN, further revealing specific associations with features of MPN subclasses. Circulating levels of ECs and related compounds are part of the complex immune-neuro-endocrine system [1,17,34,50], and the present investigation might suggest that this cross-talk is deranged in MPN. Indeed, the depicted alteration in EC and NAE plasma profile might represent a putative biomarker to monitor disease onset and progression in hematological malignancies. Nevertheless, the observations that we reported need to be substantiated in further studies involving larger cohorts of patients and other hematological malignancies, taking into account sex specificities.
In conclusion, our work involving severe and rare hematological malignancies, overall referred to as MPN, revealed for the first time disease-specific alterations of EC and NAE plasma profile, which could help in elucidating the impact of the EC and NAE systems in the pathogenesis, progression, and identification of novel therapeutic strategies.
4. Materials and Methods
4.1. Study Cohort
All patients and HC gave written informed consent under the approval of the local medical ethical committee of the University Hospital of Bologna (Code 7/2019/Sper/AOUBO of 01/23/2019-Comitato Etico di Area Vasta Emilia Centro), and the study was conducted in accordance with the Declaration of Helsinki. Ten HC from the general population and 55 patients affected by MPN were recruited at the University Hospital of Bologna.
4.2. Blood Sampling
Patients and HC gave blood between 8 and 10 a.m,. after overnight fasting. Blood was collected into K2 EDTA-containing tubes (Vacutainer® tubes, Becton Dickinson, Franklin Lakes, NJ, USA) and processed within 1 h from withdrawal. Tubes were centrifuged for 15 min at 3000× g to obtain platelet-poor plasma, and the derivative was aliquoted and stored at −80 °C.
4.3. Mutation Analysis
JAK2V617F allele-burden was assessed in granulocyte DNA with the ipsogen JAK2 MutaQuant Kit (Qiagen, Marseille, France) 505 on the 7900 HT Fast Real-Time PCR System (Applied Biosystem, Monza, Italy). CALR exon 9 sequencing was performed by the next-generation sequencing (NGS) approach with GS Junior (Roche-454 platform; Roche Diagnostics, Monza, Italy); analysis was performed with AVA Software (GRCh38 as referenced). Rare CALR mutations identified by NGS were confirmed by Sanger sequencing. MPL mutations were investigated by the ipsogen MPLW515K/L MutaScreen Kit (Qiagen) and by Sanger sequencing (for MPLS505N and other secondary exon 10 mutations).
4.4. EC and NAE Measurements
AEA, PEA, OEA, 1AG, and 2AG plasma levels were measured by a validated in-house assay [7]. Briefly, 0.5 mL of plasma underwent liquid–liquid extraction with 2 mL toluene after the addition of isotopic internal standards. Extracts were injected into the LC–MS/MS platform (HPLC Series200, PerkinElmer, Waltham, Massachusetts; API4000 QTrap, Sciex, Toronto, ON, Canada), separated on a Discovery HS C18 column (7.5 cm × 4.6 mm; particle size: 3 µm), ionized in positive mode by atmospheric pressure chemical ionization and detected by multiple reaction monitoring of both quantitative and confirmation transitions. Baseline separation between 2AG and 1AG isomers was achieved. Functional sensitivities were 0.02 for AEA, 0.20 for PEA and OEA, 0.16 for 2AG, and 0.08 pmol/mL for 1AG.
4.5. Statistical Analysis
Mean, SD, frequency, median and range were used as descriptive statistics. PEA/AEA, OEA/AEA, and OEA/PEA molar ratios and the sum of 2AG and 1AG were computed. The normality of variable distribution was analyzed by the Kolmogorov–Smirnov test. All significantly skewed variables showing a positive skewness were transformed according to the equation written as log10(x + k), whereas those showing a negative skewness were transformed according to the equation described as the squared root of (x + k). k values resulting in zero skewness after transformations were chosen. Differences in age, sex, and study-specific variables among classes were tested by T-test, ANOVA, and ANCOVA. Specifically, for MF disease, factors other than gender as platelet count (<100 × 109/L), anemia (hemoglobin: <10), peripheral blasts (≥1%), marrow fibrosis grade, large splenomegaly (palpable, ≥10 cm below the left costal margin), and MPN-10 total symptoms score (TSS) (≥20) were considered in univariate analysis. Regression analysis was performed according to the Pearson’s correlation test. The outliers were detected using the Grubbs’s test and excluded from the analyses. p-values <0.050 were considered significant. Statistical analyses were performed by Graphpad (Graphpad Software Inc., La Jolla, CA, USA) and by Medcalc version 18.2.1 (MedCalc Software bvba, Ostend, Belgium).
Acknowledgments
The authors acknowledge the supports from AIL Bologna ODV.
Abbreviations
| MPN | myeloproliferative neoplasms |
| MF | myelofibrosis |
| ET | essential thrombocythemia |
| PV | polycythemia vera |
| HC | healthy control |
| EC | endocannabinoid |
| NAE | N-acylethanolamide |
| AEA | N-arachidonoyl-ethanolamine (anandamide) |
| 1AG | 1-arachidonoyl-glycerol |
| 2AG | 2-arachidonoyl-glycerol |
| 2+1AG | 1/2-arachidonoyl-glycerol |
| PEA | palmitoyl-ethanolamide |
| OEA | oleoyl-ethanolamide |
| LC–MS/MS | liquid chromatography–tandem mass spectrometry |
| CB1/2 | cannabinoid receptors ½ |
| WBC | white blood cell |
| PLT | platelet count |
| Hct | hematocrit |
| RBC | red blood cell |
| Hgb | hemoglobin |
| TSS | total symptoms score |
| DIPSS | dynamic international prognostic scoring system |
| JAK2 | janus kinase 2 |
| CALR | calreticulin |
| MPL | myeloproliferative leukemia protein |
| TN | triple-negative |
| VAF | variant allele frequency |
| BMI | body mass index |
Author Contributions
D.F., F.F., and L.C. contributed to the conception, research design, drafting of the manuscript, and conceptualization methodology; D.F., M.M. (Marina Martello), M.B., G.C., and C.T. collected human samples; F.F. and M.M. (Marco Mezzullo) performed the LC–MS/MS measurements in study samples; D.F., F.F., and D.B. performed data curation; E.O. performed the mutational molecular analysis; G.A., F.P., and A.C. provided clinical data and enrolled patients for the study; U.P., A.C., F.P., M.C., and L.C. reviewed and edited the manuscript; D.F. acquired the funding; L.C., U.P., M.C., and F.P. provided the supervision of the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SIE-Società Italiana di Ematologia e Associazione “Amici di Beat Leukemia Alessandro Cevenini ONLUS”. In addition, the study was partially supported by a fellowship from Associazione Italiana Ricerca sul Cancro (AIRC-Fellowship Abroad, Rif. 20930).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Hillard C.J. The Endocannabinoid Signaling System in the CNS: A Primer. Int. Rev. Neurobiol. 2015;125:1–47. doi: 10.1016/bs.irn.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knight J.M., Szabo A., Zhao S. Circulating endocannabinoids during hematopoietic stem cell transplantation: A pilot study. Neurobiol. Stress. 2015;2:44–50. doi: 10.1016/j.ynstr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsuboi K., Uyama T., Okamoto Y., Ueda N. Endocannabinoids and related N-acylethanolamines: Biological activities and metabolism. Inflamm. Regen. 2018;38:28. doi: 10.1186/s41232-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Howlett A.C., Blume L.C., Dalton G.D. CB(1) cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010;17:1382–1393. doi: 10.2174/092986710790980023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lakiotaki E., Giaginis C., Tolia M. Clinical Significance of Cannabinoid Receptors CB1 and CB2 Expression in Human Malignant and Benign Thyroid Lesions. Biomed. Res. Int. 2015;2015:839403. doi: 10.1155/2015/839403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kose S., Aerts-Kaya F., Kopru C.Z. Human bone marrow mesenchymal stem cells secrete endocannabinoids that stimulate in vitro hematopoietic stem cell migration effectively comparable to beta-adrenergic stimulation. Exp. Hematol. 2018;57:30–41. doi: 10.1016/j.exphem.2017.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Fanelli F., Di Lallo V.D., Belluomo I. Estimation of reference intervals of five endocannabinoids and endocannabinoid related compounds in human plasma by two dimensional- LC/MS/MS. J. Lipid Res. 2012;53:481–493. doi: 10.1194/jlr.M021378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fanelli F., Mezzullo M., Belluomo I. Plasma 2-arachidonoylglycerol is a biomarker of age and menopause related insulin resistance and dyslipidemia in lean but not in obese men and women. Mol. Metab. 2017;6:406–415. doi: 10.1016/j.molmet.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docs K., Meszar Z., Gonda S. The Ratio of 2-AG to Its Isomer 1-AG as an Intrinsic Fine Tuning Mechanism of CB1 Receptor Activation. Front Cell Neurosci. 2017;11:39. doi: 10.3389/fncel.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balvers M.G., Wortelboer H.M., Witkamp R.F., Verhoeckx K.C. Liquid chromatography-tandem mass spectrometry analysis of free and esterified fatty acid N-acyl ethanolamines in plasma and blood cells. Anal. Biochem. 2013;434:275–283. doi: 10.1016/j.ab.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Borrelli F., Romano B., Petrosino S. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br. J. Pharmacol. 2015;172:142–158. doi: 10.1111/bph.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jumpertz R., Guijarro A., Pratley R.E., Piomelli D., Krakoff J. Central and peripheral endocannabinoids and cognate acylethanolamides in humans: Association with race, adiposity, and energy expenditure. J. Clin. Endocrinol. Metab. 2011;96:787–791. doi: 10.1210/jc.2010-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fanelli F., Mezzullo M., Repaci A. Profiling plasma N-Acylethanolamine levels and their ratios as a biomarker of obesity and dysmetabolism. Mol. Metab. 2018;14:82–94. doi: 10.1016/j.molmet.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esposito G., Capoccia E., Turco F. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-alpha activation. Gut. 2014;63:1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- 15.Gasperi V., Avigliano L., Evangelista D. 2-Arachidonoylglycerol enhances platelet formation from human megakaryoblasts. Cell Cycle. 2014;13:3938–3947. doi: 10.4161/15384101.2014.982941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cabral G.A., Griffin-Thomas L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert Rev. Mol. Med. 2009;11:e3. doi: 10.1017/S1462399409000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiurchiu V., Battistini L., Maccarrone M. Endocannabinoid signalling in innate and adaptive immunity. Immunology. 2015;144:352–364. doi: 10.1111/imm.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karwad M.A., Macpherson T., Wang B. Oleoylethanolamine and palmitoylethanolamine modulate intestinal permeability in vitro via TRPV1 and PPARalpha. Faseb J. 2017;31:469–481. doi: 10.1096/fj.201500132. [DOI] [PubMed] [Google Scholar]
- 19.Chiurchiu V., Leuti A., Smoum R., Mechoulam R., Maccarrone M. Bioactive lipids ALIAmides differentially modulate inflammatory responses of distinct subsets of primary human T lymphocytes. Faseb J. 2018;32:5716–5723. doi: 10.1096/fj.201800107R. [DOI] [PubMed] [Google Scholar]
- 20.Jiang S., Fu Y., Avraham H.K. Regulation of hematopoietic stem cell trafficking and mobilization by the endocannabinoid system. Transfusion. 2011;51:65s–71s. doi: 10.1111/j.1537-2995.2011.03368.x. [DOI] [PubMed] [Google Scholar]
- 21.Patinkin D., Milman G., Breuer A., Fride E., Mechoulam R. Endocannabinoids as positive or negative factors in hematopoietic cell migration and differentiation. Eur. J. Pharmacol. 2008;595:1–6. doi: 10.1016/j.ejphar.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Galve-Roperh I., Chiurchiu V., Diaz-Alonso J., Bari M., Guzman M., Maccarrone M. Cannabinoid receptor signaling in progenitor/stem cell proliferation and differentiation. Prog. Lipid Res. 2013;52:633–650. doi: 10.1016/j.plipres.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Barbado M.V., Medrano M., Caballero-Velazquez T. Cannabinoid derivatives exert a potent anti-myeloma activity both in vitro and in vivo. Int. J. Cancer. 2017;140:674–685. doi: 10.1002/ijc.30483. [DOI] [PubMed] [Google Scholar]
- 24.Randall M.D. Endocannabinoids and the haematological system. Br. J. Pharmacol. 2007;152:671–675. doi: 10.1038/sj.bjp.0707420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giaginis C., Lakiotaki E., Korkolopoulou P., Konstantopoulos K., Patsouris E., Theocharis S. Endocannabinoid System: A Promising Therapeutic Target for the Treatment of Haematological Malignancies? Curr. Med. Chem. 2016;23:2350–2362. doi: 10.2174/0929867323666160530144934. [DOI] [PubMed] [Google Scholar]
- 26.Joosten M., Valk P.J., Jorda M.A. Leukemic predisposition of pSca-1/Cb2 transgenic mice. Exp. Hematol. 2002;30:142–149. doi: 10.1016/S0301-472X(01)00779-2. [DOI] [PubMed] [Google Scholar]
- 27.Jorda M.A., Rayman N., Tas M. The peripheral cannabinoid receptor Cb2, frequently expressed on AML blasts, either induces a neutrophilic differentiation block or confers abnormal migration properties in a ligand-dependent manner. Blood. 2004;104:526–534. doi: 10.1182/blood-2003-12-4357. [DOI] [PubMed] [Google Scholar]
- 28.Jorda M.A., Rayman N., Valk P., De Wee E., Delwel R. Identification, characterization, and function of a novel oncogene: The peripheral cannabinoid receptor Cb2. Ann. N. Y. Acad. Sci. 2003;996:10–16. doi: 10.1111/j.1749-6632.2003.tb03227.x. [DOI] [PubMed] [Google Scholar]
- 29.Kampa-Schittenhelm K.M., Salitzky O., Akmut F., Illing B., Kanz L., Salih H.R., Schittenhelm M.M. Dronabinol has preferential antileukemic activity in acute lymphoblastic and myeloid leukemia with lymphoid differentiation patterns. BMC Cancer. 2016;16:25. doi: 10.1186/s12885-015-2029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tefferi A., Vainchenker W. Myeloproliferative Neoplasms: Molecular Pathophysiology, Essential Clinical Understanding, and Treatment Strategies. J. Clin. Oncol. 2011;29:573–582. doi: 10.1200/JCO.2010.29.8711. [DOI] [PubMed] [Google Scholar]
- 31.Palandri F., Breccia M., Bonifacio M. Life after ruxolitinib: Reasons for discontinuation, impact of disease phase, and outcomes in 218 patients with myelofibrosis. Cancer. 2020;126:1243–1252. doi: 10.1002/cncr.32664. [DOI] [PubMed] [Google Scholar]
- 32.Passamonti F., Cervantes F., Vannucchi A.M. A dynamic prognostic model to predict survival in primary myelofibrosis: A study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment) Blood. 2009;115:1703–1708. doi: 10.1182/blood-2009-09-245837. [DOI] [PubMed] [Google Scholar]
- 33.Moreno E., Cavic M., Krivokuca A., Casado V., Canela E. The Endocannabinoid System as a Target in Cancer Diseases: Are We There Yet? Front Pharmacol. 2019;10:339. doi: 10.3389/fphar.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sailler S., Schmitz K., Jäger E. Regulation of circulating endocannabinoids associated with cancer and metastases in mice and humans. Oncoscience. 2014;1:272–282. doi: 10.18632/oncoscience.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sollazzo D., Forte D., Polverelli N. Crucial factors of the inflammatory microenvironment (IL-1beta/TNF-alpha/TIMP-1) promote the maintenance of the malignant hemopoietic clone of myelofibrosis: An in vitro study. Oncotarget. 2016;7:43974–43988. doi: 10.18632/oncotarget.9949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Catani M.V., Gasperi V., Evangelista D., Finazzi Agro A., Avigliano L., Maccarrone M. Anandamide extends platelets survival through CB(1)-dependent Akt signaling. Cell Mol. Life Sci. 2010;67:601–610. doi: 10.1007/s00018-009-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bluher M., Engeli S., Kloting N. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes. 2006;55:3053–3060. doi: 10.2337/db06-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Turcotte C., Chouinard F., Lefebvre J.S., Flamand N. Regulation of inflammation by cannabinoids, the endocannabinoids 2-arachidonoyl-glycerol and arachidonoyl-ethanolamide, and their metabolites. J. Leukoc. Biol. 2015;97:1049–1070. doi: 10.1189/jlb.3RU0115-021R. [DOI] [PubMed] [Google Scholar]
- 39.Turcotte C., Blanchet M.R., Laviolette M., Flamand N. The CB2 receptor and its role as a regulator of inflammation. Cell Mol. Life Sci. 2016;73:4449–4470. doi: 10.1007/s00018-016-2300-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hillard C.J. Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology. 2018;43:155–172. doi: 10.1038/npp.2017.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sollazzo D., Forte D., Polverelli N. Circulating Calreticulin Is Increased in Myelofibrosis: Correlation with Interleukin-6 Plasma Levels, Bone Marrow Fibrosis, and Splenomegaly. Mediat. Inflamm. 2016;2016:5860657. doi: 10.1155/2016/5860657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ciurea S.O., Merchant D., Mahmud N. Pivotal contributions of megakaryocytes to the biology of idiopathic myelofibrosis. Blood. 2007;110:986–993. doi: 10.1182/blood-2006-12-064626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malara A., Abbonante V., Zingariello M., Migliaccio A., Balduini A. Megakaryocyte Contribution to Bone Marrow Fibrosis: Many Arrows in the Quiver. Mediterr. J. Hematol. Infect Dis. 2018;10:e2018068. doi: 10.4084/mjhid.2018.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ye H., Adane B., Khan N. Subversion of Systemic Glucose Metabolism as a Mechanism to Support the Growth of Leukemia Cells. Cancer Cell. 2018;34:659–673.e6. doi: 10.1016/j.ccell.2018.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berdyshev E.V., Schmid P.C., Krebsbach R.J., Schmid H.H. Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. Faseb J. 2001;15:2171–2178. doi: 10.1096/fj.01-0181com. [DOI] [PubMed] [Google Scholar]
- 46.Romano M., Sollazzo D., Trabanelli S. Mutations in JAK2 and Calreticulin genes are associated with specific alterations of the immune system in myelofibrosis. Oncoimmunology. 2017;6:e1345402. doi: 10.1080/2162402X.2017.1345402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Geyer H.L., Dueck A.C., Scherber R.M., Mesa R.A. Impact of Inflammation on Myeloproliferative Neoplasm Symptom Development. Mediat. Inflamm. 2015;2015:1–9. doi: 10.1155/2015/284706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geyer H.L., Kosiorek H., Dueck A.C. Associations between gender, disease features and symptom burden in patients with myeloproliferative neoplasms: An analysis by the MPN QOL International Working Group. Haematologica. 2017;102:85–93. doi: 10.3324/haematol.2016.149559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barraco D., Mora B., Guglielmelli P. Gender effect on phenotype and genotype in patients with post- polycythemia vera and post-essential thrombocythemia myelofibrosis: Results from the MYSEC project. Blood Cancer J. 2018;8:89. doi: 10.1038/s41408-018-0128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gasperi V., Evangelista D., Savini I., Del Principe D., Avigliano L., Maccarrone M., Catani M.V. Downstream effects of endocannabinoid on blood cells: Implications for health and disease. Cell Mol. Life Sci. 2015;72:3235–3252. doi: 10.1007/s00018-015-1924-0. [DOI] [PMC free article] [PubMed] [Google Scholar]