Abstract
One of the most profound enigmas in B cell biology is how activation-induced deaminase (AID) is targeted to a very small region of DNA in the immunoglobulin loci. Two specific regions are singled out: the variable region of 2 kb that contains rearranged genes on the heavy, κ light, and λ light chain loci, and the switch region of ∼4 kb that contains an extensive stretch of G:C rich DNA on the heavy chain locus. Transcription is required for AID recruitment; however, many genes are also highly transcribed and do not undergo the catastrophic mutagenesis that occurs in variable and switch regions. The DNA sequences of these regions cause RNA polymerase II to accumulate for an extended distance of 2–4 kb. The stalled polymerases then recruit the transcription cofactor Spt5, and AID, which deaminates cytosines to uracils in exposed transcription bubbles. Thus, the immunoglobulin loci are unique in that a favorable combination of DNA sequences and 3′ transcription enhancers make them the perfect storm for AID-induced somatic hypermutation.
Keywords: AID, somatic hypermutation, class switch recombination, transcription
Introduction
Antibodies are encoded by immunoglobulin (Ig) heavy, κ light, and λ light chain genes located on three separate chromosomes. The two functional domains of antibodies are encoded by the variable (V) gene, which binds antigen, and the constant (C) gene, which mediates cellular interactions to eliminate antigen. V genes on the heavy chain locus are comprised of V, diversity (D), and joining (J) gene segments, and V genes on the κ and λ light chain loci are composed of V and J gene segments. The multiple, diverse copies of the gene segments are joined in pre-B cells by recombination enzymes to create a huge primary repertoire of ∼3 × 1015 unique antibodies (3) that are expressed by naive human B cells as membrane receptors. When one of these B cells encounters an antigen, such as a virus, which can bind to its receptor with an affinity of ≥Kd105, the cell is activated to divide and form a clone of daughter cells.
During clonal expansion, a separate program is activated to generate more diversity by activation-induced deaminase (AID), which initiates somatic hypermutation (SHM), and class switch recombination (CSR) (28,36). SHM produces predominantly nucleotide substitutions, and it occurs in two regions: rearranged V genes on the heavy and light chain loci, and switch (S) regions that precede most C genes on the heavy chain locus (25). The purpose of mutations in V gene exons is to change amino acid codons so Ig receptors on B cells can be selected for higher affinity. The purpose of mutations in S intron regions is to initiate double-strand breaks for CSR. During CSR, different C genes can be paired with a rearranged VDJ gene by nonhomologous recombination between S regions, for example, between Sμ and Sγ to produce IgM to IgG. Thus, in a B cell clone, one VDJ gene can associate with several C genes to generate the five main classes of antibodies: IgM, IgD, IgG, IgE, and IgA. Therefore, SHM and CSR occur using the AID enzyme to form an astounding secondary repertoire after antigen contact of >1015 antibodies (3) that are expressed by memory human B cells as membrane receptors and secreted Ig.
The AID enzyme catalyzes this amazing diversity by deaminating cytosines to uracils in single-strand DNA (2,7,8,31,34). Uracils are mutagenic in DNA and can be replicated by DNA polymerases to produce C:G to T:A transition substitutions, or they can be processed by base excision repair (BER) or mismatch repair (MMR) to produce other mutations (7,33,48). During BER, uracils are excised by uracil glycosylase (UNG), and the resulting abasic site is nicked by an endonuclease to create single-strand DNA breaks. During MMR, the U:G mismatch is recognized by MSH2-MSH6, which recruits PMS2 to make a nick and allow exonuclease to produce a gap (15). In both cases, single-strand breaks provide entry for low-fidelity DNA polymerases to synthesize mutations. Polymerase η is the major polymerase incorporating mutations opposite A:T templates (5,6,10,56,57), and polymerases κ (11), ι (26), ζ (40), and Rev1 (18) are involved less frequently. In general, mutations of G and C nucleotides are likely due to activity of AID and UNG in the BER pathway, whereas mutations of A and T nucleotides are mostly due to AID and polymerase η in the MMR pathway. In summary, AID generates uracils in the V and S regions to produce single-strand breaks for polymerases to synthesize SHM. In the S region, closely spaced uracils can be excised on both strands to produce double-strand breaks for CSR.
Two Domains of SHM: Variable and Switch Regions
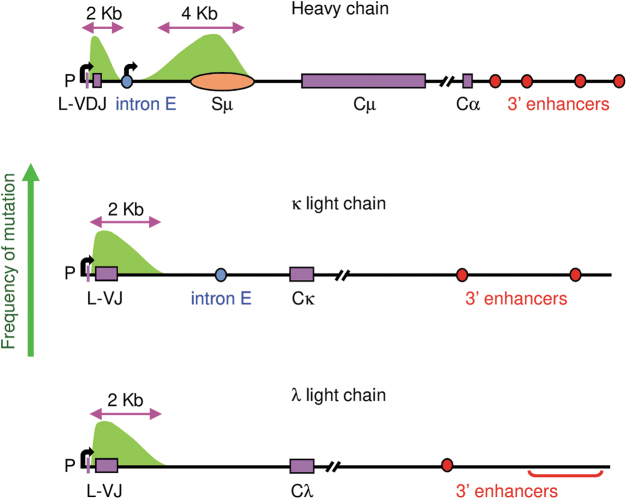
The focus of this review is to examine how AID is targeted to V and S regions on three loci using SHM as a readout for AID activity. SHM occurs at an astonishing frequency of 10−2 mutations per base pair, which is a million times higher than spontaneous mutation in other genes. Therefore, it is important to understand what DNA sequences recruit AID predominantly to the Ig loci and keep it from destroying the rest of the genome. As shown in Figure 1, the distance of mutations around rearranged VDJ and VJ genes on heavy (22), κ (14), and λ (16) loci is ∼2 kb. Mutations start downstream of the V promoter and leader (L) exon to encompass the V exon and about 1.5 kb of 3′ intron sequence, and mutations stop after 2 kb (22). In the S region in front of Cμ, SHM occurs for ∼4 kb downstream of the intron enhancer/promoter, and then drops off after the Sμ DNA sequence (35,51). All three loci have 3′ enhancers located after the C genes, which facilitate chromosomal looping to form transcriptional domains (12).
FIG. 1.
Map of SHM in immunoglobulin loci. Mutation frequency is shown by the green arrow on the y-axis, and by green peaks centered over the VDJ and VJ exons and Sμ region. For V exons, mutation starts downstream of promoter (P) at the transcription start site (bent arrow) and L exon, and proceeds for ∼2 kb. For Sμ, mutation starts downstream of the intronic transcription start site (bent arrow), and proceeds for ∼4 kb. Exons are shown as purple rectangles, and Sμ is depicted by an orange oval. Intron enhancers (E) are shown as blue circles, and 3′ enhancers are designated by red circles and brackets. SHM, somatic hypermutation.
Basically, two elements are required for SHM: transcription and 3′ enhancers. Deletion of 3′ enhancers on the heavy (38), κ (50), and λ (21) loci reduced transcription and SHM. Three sequences that are not required for SHM are V promoters, in that other promoters can be substituted (1,13,43); intron enhancers on the heavy and κ loci (17,30); and the V(D)J exon, since non-Ig sequences can be substituted to undergo SHM (52,53). Nonetheless, even though different sequences can be inserted to replace promoters and V exons, there must be additional factors that make the three Ig locus-privileged sites for high mutational activity by AID. For example, AID produces mutations at other loci, although at a reduced level, depending on the cell type and chromatin modifications marking active transcription and enhancers (47).
Targeting to the Switch Region
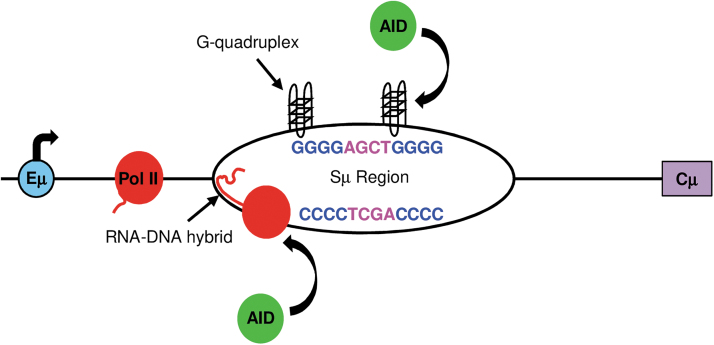
Mutations in intronic S regions are clearly due to unique DNA sequences that promote transcriptional pausing of RNA polymerase II by forming single-strand structures, and that encode multiple AID hotspots. S regions are located upstream of each C gene, except Cδ, and extend for 3–9 kb (41,49). They contain repetitive sequences of clusters of 3–4 G nucleotides on the nontranscribed strand, and they are interspersed with multiple AGCT motifs that are favored to bind AID (20,46). Importantly, these G:C rich sequences have been shown to form stable structures such as R-loops (39,54) and G quadruplexes (9). As shown in Figure 2, transcription by RNA polymerase II creates an RNA-DNA hybrid with G-rich RNA bound to C-rich DNA. The strong hydrogen bonding between the riboguanosines and deoxycytosines on the transcribed strand allows the RNA:DNA hybrid to pair for a long distance. On the nontranscribed strand, G quadruplexes form and have been shown to bind AID (32,37). RNA polymerases slow down when trying to transcribe through these structures in the S region (35,42,45). The Spt5 cofactor binds to stalled polymerases, and in turn, AID binds to Spt5 (29).
FIG. 2.
Secondary DNA structures promote AID activity in S regions. Transcription (bent arrow) starts in the intron E (blue). DNA sequences of the top nontranscribed strand and bottom transcribed strand are shown, with AGCT or TCGA hotspots shown in pink. As RNA polymerase II (red) transcribes through the S region, the transcript bonds with the C-rich transcribed DNA strand, creating an RNA-DNA hybrid or R-loop, and G quadruplexes form on the nontranscribed DNA strand. These structures slow transcription, and recruit AID (green). AID then deaminates C to U in hotspots on both strands. AID, activation-induced deaminase.
In this manner, AID is recruited to the paused polymerases to deaminate C in the plethora of AGCT hotspots on both DNA strands. Furthermore, multiple AGCT hotspots that are closely spaced will allow single-strand breaks on both strands to be processed into blunt double-strand breaks for nonhomologous end joining to another S region for CSR. When the Sμ sequence is removed, there is no SHM and no CSR (19,35). When Sμ sequences are inserted in V exons on the heavy chain, SHM is abundant and double-strand breaks form (52). In summary, the DNA sequences of S regions have clusters of G:C bp to form secondary structures, which slow transcription and enhance AID activity on multiple hotspots.
Targeting to the Variable Region
While G:C rich DNA promotes SHM in the S regions, the V region is more enigmatic because there are no G:C repeats in either the V(D)J exons or flanking sequences to form R-loop or G quadruplex structures, and there are fewer AGCT hotspots. Nonetheless, the frequency of SHM in V regions is equal to that in S regions, implying that AID must be efficiently recruited by some other mechanism. Applying what is known about proteins associated with stalled RNA polymerases in the S region, Maul et al. (24) showed that RNA polymerase II enzymes accumulated in V regions from germinal center B cells for an extended distance of 1.2 kb from the promoter, and they generated abundant single-strand DNA regions. Spt5 and AID also bound to DNA in V regions, suggesting that they associated with RNA polymerase II. Furthermore, single-strand DNA breaks occur in V regions, which would allow entry for low-fidelity DNA polymerases to generate SHM (55). The occurrence of mutations on both strands means that both strands become single stranded at some point during transcription (27). Thus, for both V and S regions, there is an accumulation of RNA polymerase II, Spt5, and AID, which is correlated with SHM frequency (Fig. 3). However, the mechanism that causes RNA polymerase II to pause in V regions is currently unknown and of great interest.
FIG. 3.
Correlation between RNA polymerase II, Spt5, AID, and SHM in V and S regions. RNA polymerase II is paused in the S region because of the G:C rich DNA sequence; however, it is not known why it is paused in the V region. RNA polymerase II, green line; AID, turquoise line; Spt5, solid red; and mutations, solid gold.
Conclusion
Several factors have been identified that promote SHM in V and S regions, such as DNA sequences that cause RNA polymerases to stall and recruit Spt5 and AID. Therefore, transcription is essential for AID activity. The loss of the promoter results in loss of SHM; however, alternate promoters, such as the β-globin promoter, suffice to initiate transcription and SHM (53). Consistent with the requirement for transcription, the 3′ enhancers on the heavy chain locus, comprising hs3a, hs1,2, hs3b, and hs4 enhancers, are also required for SHM and CSR. Deletion of the entire 3′ enhancer region reduced accumulation of RNA polymerase II, Spt5, and AID at the V and S regions, and decreased SHM and CSR (24,44). Similar results of reduced SHM in V regions occurred when the 3′ enhancers were deleted on the κ and λ light chain loci. Thus, the 3′ enhancers control transcription levels and correspondingly SHM. The Ig heavy and κ loci also contain a second enhancer called the intron enhancer. Unlike the 3′ enhancers, the intron enhancers do not promote SHM in the V region (17,23).
In addition to promoters and enhancers, cis DNA sequences in the V region have been examined for their role in stimulating SHM. Surprisingly, the V(D)J exon itself is not involved in recruiting AID, in that other sequences can be substituted to undergo SHM (52,53). A likely candidate for a sequence that could be involved in AID activity is the human JH6 intronic sequence, since every rearranged VDJ allele contains this sequence. However, deletion of this intron sequence had no effect on SHM (4). Thus, AID recruitment to V regions is a mystery, since other heavily transcribed genes do not sustain this abnormally high level of SHM. This question eclipses our fundamental knowledge of DNA repair, error-prone DNA polymerases, and transcription, and puts AID targeting in the realm of one of the last frontiers to be solved in B cell biology.
Acknowledgments
We thank Robert Maul and Mark Hutchinson for help with the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported entirely through the Intramural Research Program at the National Institutes of Health, National Institute on Aging (AG000714).
References
- 1. Betz AG, Milstein C, Gonzalez-Fernandez A, et al. Elements regulating somatic hypermutation of an immunoglobulin kappa gene: critical role for the intron enhancer/matrix attachment region. Cell 1994;77:239–248 [DOI] [PubMed] [Google Scholar]
- 2. Bransteitter R, Pham P, Scharff MD, et al. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc Natl Acad Sci U S A 2003;100:4102–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Briney B, Inderbitzin A, Joyce C, et al. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature 2019;566:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Castiblanco DP, Norton DD, Maul RW, et al. JH6 downstream intronic sequence is dispensable for RNA polymerase II accumulation and somatic hypermutation of the variable gene in Ramos cells. Mol Immunol 2018;97:101–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delbos F, Aoufouchi S, Faili A, et al. DNA polymerase eta is the sole contributor of A/T modifications during immunoglobulin gene hypermutation in the mouse. J Exp Med 2007;204:17–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delbos F, De Smet A, Faili A, et al. Contribution of DNA polymerase eta to immunoglobulin gene hypermutation in the mouse. J Exp Med 2005;201:1191–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Noia J, and Neuberger MS. Altering the pathway of immunoglobulin hypermutation by inhibiting uracil-DNA glycosylase. Nature 2002;419:43–48 [DOI] [PubMed] [Google Scholar]
- 8. Dickerson SK, Market E, Besmer E, et al. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med 2003;197:1291–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Duquette ML, Handa P, Vincent JA, et al. Intracellular transcription of G-rich DNAs induces formation of G-loops, novel structures containing G4 DNA. Genes Dev 2004;18:1618–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faili A, Aoufouchi S, Weller S, et al. DNA polymerase eta is involved in hypermutation occurring during immunoglobulin class switch recombination. J Exp Med 2004;199:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faili A, Stary A, Delbos F, et al. A backup role of DNA polymerase kappa in Ig gene hypermutation only takes place in the complete absence of DNA polymerase eta. J Immunol 2009;182:6353–6359 [DOI] [PubMed] [Google Scholar]
- 12. Feldman S, Achour I, Wuerffel R, et al. Constraints contributed by chromatin looping limit recombination targeting during Ig class switch recombination. J Immunol 2015;194:2380–2389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fukita Y, Jacobs H, and Rajewsky K. Somatic hypermutation in the heavy chain locus correlates with transcription. Immunity 1998;9:105–114 [DOI] [PubMed] [Google Scholar]
- 14. Gearhart PJ, and Bogenhagen DF. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A 1983;80:3439–3443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Girelli Zubani G, Zivojnovic M, De Smet A, et al. Pms2 and uracil-DNA glycosylases act jointly in the mismatch repair pathway to generate Ig gene mutations at A-T base pairs. J Exp Med 2017;214:1169–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez-Fernandez A, Gupta SK, Pannell R, et al. Somatic mutation of immunoglobulin lambda chains: a segment of the major intron hypermutates as much as the complementarity-determining regions. Proc Natl Acad Sci U S A 1994;91:12614–12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Inlay MA, Gao HH, Odegard VH, et al. Roles of the Ig kappa light chain intronic and 3′ enhancers in Igk somatic hypermutation. J Immunol 2006;177:1146–1151 [DOI] [PubMed] [Google Scholar]
- 18. Jansen JG, Langerak P, Tsaalbi-Shtylik A, et al. Strand-biased defect in C/G transversions in hypermutating immunoglobulin genes in Rev1-deficient mice. J Exp Med 2006;203:319–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khamlichi AA, Glaudet F, Oruc Z, et al. Immunoglobulin class-switch recombination in mice devoid of any S mu tandem repeat. Blood 2004;103:3828–3836 [DOI] [PubMed] [Google Scholar]
- 20. Kohli RM, Maul RW, Guminski AF, et al. Local sequence targeting in the AID/APOBEC family differentially impacts retroviral restriction and antibody diversification. J Biol Chem 2010;285:40956–40964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kothapalli N, Norton DD, and Fugmann SD. Cutting edge: a cis-acting DNA element targets AID-mediated sequence diversification to the chicken Ig light chain gene locus. J Immunol 2008;180:2019–2023 [DOI] [PubMed] [Google Scholar]
- 22. Lebecque SG, and Gearhart PJ. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5′ boundary is near the promoter, and 3′ boundary is approximately 1 kb from V(D)J gene. J Exp Med 1990;172:1717–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li F, Yan Y, Pieretti J, et al. Comparison of identical and functional Igh alleles reveals a nonessential role for Emu in somatic hypermutation and class-switch recombination. J Immunol 2010;185:6049–6057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maul RW, Cao Z, Venkataraman L, et al. Spt5 accumulation at variable genes distinguishes somatic hypermutation in germinal center B cells from ex vivo-activated cells. J Exp Med 2014;211:2297–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maul RW, and Gearhart PJ. AID and somatic hypermutation. Adv Immunol 2010;105:159–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maul RW, MacCarthy T, Frank EG, et al. DNA polymerase iota functions in the generation of tandem mutations during somatic hypermutation of antibody genes. J Exp Med 2016;213:1675–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Milstein C, Neuberger MS, and Staden R. Both DNA strands of antibody genes are hypermutation targets. Proc Natl Acad Sci U S A 1998;95:8791–8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Muramatsu M, Kinoshita K, Fagarasan S, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000;102:553–563 [DOI] [PubMed] [Google Scholar]
- 29. Pavri R, Gazumyan A, Jankovic M, et al. Activation-induced cytidine deaminase targets DNA at sites of RNA polymerase II stalling by interaction with Spt5. Cell 2010;143:122–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Perlot T, Alt FW, Bassing CH, et al. Elucidation of IgH intronic enhancer functions via germ-line deletion. Proc Natl Acad Sci U S A 2005;102:14362–14367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Petersen-Mahrt SK, Harris RS, and Neuberger MS. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 2002;418:99–103 [DOI] [PubMed] [Google Scholar]
- 32. Qiao Q, Wang L, Meng FL, et al. AID recognizes structured DNA for class switch recombination. Mol Cell 2017;67:361–373.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rada C, Di Noia JM, and Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol Cell 2004;16:163–171 [DOI] [PubMed] [Google Scholar]
- 34. Rada C, Williams GT, Nilsen H, et al. Immunoglobulin isotype switching is inhibited and somatic hypermutation perturbed in UNG-deficient mice. Curr Biol 2002;12:1748–1755 [DOI] [PubMed] [Google Scholar]
- 35. Rajagopal D, Maul RW, Ghosh A, et al. Immunoglobulin switch mu sequence causes RNA polymerase II accumulation and reduces dA hypermutation. J Exp Med 2009;206:1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Revy P, Muto T, Levy Y, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2). Cell 2000;102:565–575 [DOI] [PubMed] [Google Scholar]
- 37. Ribeiro de Almeida C, Dhir S, Dhir A, et al. RNA helicase DDX1 converts RNA G-quadruplex structures into R-loops to promote IgH class switch recombination. Mol Cell 2018;70:650–662.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rouaud P, Vincent-Fabert C, Saintamand A, et al. The IgH 3′ regulatory region controls somatic hypermutation in germinal center B cells. J Exp Med 2013;210:1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roy D, and Lieber MR. G clustering is important for the initiation of transcription-induced R-loops in vitro, whereas high G density without clustering is sufficient thereafter. Mol Cell Biol 2009;29:3124–3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saribasak H, Maul RW, Cao Z, et al. DNA polymerase zeta generates tandem mutations in immunoglobulin variable regions. J Exp Med 2012;209:1075–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stavnezer J, Guikema JE, and Schrader CE. Mechanism and regulation of class switch recombination. Annu Rev Immunol 2008;26:261–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tornaletti S, Park-Snyder S, and Hanawalt PC. G4-forming sequences in the non-transcribed DNA strand pose blocks to T7 RNA polymerase and mammalian RNA polymerase II. J Biol Chem 2008;283:12756–12762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tumas-Brundage K, and Manser T. The transcriptional promoter regulates hypermutation of the antibody heavy chain locus. J Exp Med 1997;185:239–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Vincent-Fabert C, Fiancette R, Pinaud E, et al. Genomic deletion of the whole IgH 3′ regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood 2010;116:1895–1898 [DOI] [PubMed] [Google Scholar]
- 45. Wang L, Wuerffel R, Feldman S, et al. S region sequence, RNA polymerase II, and histone modifications create chromatin accessibility during class switch recombination. J Exp Med 2009;206:1817–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang M, Rada C, and Neuberger MS. Altering the spectrum of immunoglobulin V gene somatic hypermutation by modifying the active site of AID. J Exp Med 2010;207:141–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang Q, Oliveira TY, Jankovic M, et al. Epigenetic targeting of activation-induced cytidine deaminase. Proc Natl Acad Sci U S A 2014;111:18667–18672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wiesendanger M, Kneitz B, Edelmann W, et al. Somatic hypermutation in MutS homologue (MSH)3-, MSH6-, and MSH3/MSH6-deficient mice reveals a role for the MSH2-MSH6 heterodimer in modulating the base substitution pattern. J Exp Med 2000;191:579–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu X, and Stavnezer J. DNA polymerase beta is able to repair breaks in switch regions and plays an inhibitory role during immunoglobulin class switch recombination. J Exp Med 2007;204:1677–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xiang Y, and Garrard WT. The downstream transcriptional enhancer, Ed, positively regulates mouse Ig kappa gene expression and somatic hypermutation. J Immunol 2008;180:6725–6732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Xue K, Rada C, and Neuberger MS. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2-/- ung-/- mice. J Exp Med 2006;203:2085–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeap LS, Hwang JK, Du Z, et al. Sequence-intrinsic mechanisms that target AID mutational outcomes on antibody genes. Cell 2015;163:1124–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Yelamos J, Klix N, Goyenechea B, et al. Targeting of non-Ig sequences in place of the V segment by somatic hypermutation. Nature 1995;376:225–229 [DOI] [PubMed] [Google Scholar]
- 54. Yu K, Chedin F, Hsieh CL, et al. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nat Immunol 2003;4:442–451 [DOI] [PubMed] [Google Scholar]
- 55. Zanotti KJ, Maul RW, Yang W, et al. DNA breaks in Ig V regions are predominantly single stranded and are generated by UNG and MSH6 DNA repair pathways. J Immunol 2019;202:1573–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zeng X, Negrete GA, Kasmer C, et al. Absence of DNA polymerase eta reveals targeting of C mutations on the nontranscribed strand in immunoglobulin switch regions. J Exp Med 2004;199:917–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zeng X, Winter DB, Kasmer C, et al. DNA polymerase eta is an A-T mutator in somatic hypermutation of immunoglobulin variable genes. Nat Immunol 2001;2:537–541 [DOI] [PubMed] [Google Scholar]