Abstract
Gammaherpesviruses are highly prevalent pathogens that establish life-long infection and are associated with diverse malignancies, including lymphoproliferative diseases and B cell lymphomas. Unlike other viruses that either do not infect B cells or infect B cells transiently, gammaherpesviruses manipulate physiological B cell differentiation to establish life-long infection in memory B cells. Disruption of such viral manipulation by genetic or environmental causes is likely to seed viral lymphomagenesis. In this review, we discuss physiological and unique host and viral mechanisms usurped by gammaherpesviruses to fine tune host B cell biology for optimal infection establishment and maintenance.
Keywords: gammaherpesvirus, chronic infection, latency, germinal center response, B cell differentiation
Gammaherpesviruses as Manipulators of B Cell Differentiation
Gammaherpesviruses are ancient double-stranded DNA (dsDNA) viruses that have coevolved with their respective host and, similar to other herpesviruses, establish lifelong infection in a significant proportion of humans worldwide. All herpesviruses execute two distinct life cycles: lytic replication and latent infection. Lytic cycle is a highly immunogenic process accompanied by expression of dozens of viral genes, viral genome replication by the viral DNA synthesis machinery, and production of infectious virions. Due to its immunogenicity, lytic replication in a chronically infected host occurs at very low levels and only at limited anatomic locations.
In contrast, latency, the stealth mechanism that allows herpesviruses to maintain a lifetime infection, is the predominant herpesvirus life cycle in an immunocompetent host. Latently infected cells maintain the viral genome as an episome, which is replicated by the cellular DNA synthesis machinery during cell division. During latency, only a few, if any viral genes are expressed, with the viral transcripts acting at the RNA level or, if translated, possessing functions that attenuate proteasome-dependent degradation. Switch from latent to lytic life cycle, termed reactivation, is an important driver of herpesvirus transmission and pathogenesis.
Unlike other members of Herpesviridae family, gammaherpesviruses of many species, including the two known human gammaherpesviruses, Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), are associated with diverse cancers and lymphoproliferative diseases, including B cell lymphomas (14). In addition to cancer, EBV is the causative agent of infectious mononucleosis, a clinical entity associated with primary EBV infection and characterized by malaise, lymphadenopathy, splenomegaly and hepatomegaly, and fever (32). Intriguingly, infectious mononucleosis only manifests upon acquisition of EBV in adolescence or beyond, via saliva exchange. EBV acquisition before adolescence does not produce unique symptoms and the route of transmission is not well established.
While transmission of EBV is believed to occur, at least in adolescents, via oral shedding of the virus and subsequent exchange of saliva, prospective study of oral washes collected from initially EBV naive college students failed to detect EBV genomes above 100 copies/mL before the onset of infectious mononucleosis symptoms (31). Furthermore, in many cases, presence of EBV in oral washes coincided with the detection of the virus in blood, highlighting the fact that a better understanding of initial phases of EBV infection is needed. In contrast, chronic EBV infection can be detected at low levels (1 in 104–106 circulating memory B cells) in most healthy blood donors (81,132).
Unlike EBV that infects >95% of adults, the seroprevalence of KSHV varies from 5% to >90% depending on the geographical location, age, and HIV status; transmission occurs by both sexual and nonsexual routes (80). While KSHV tropism for B cells is clear (79,82), circulating B cells of healthy donors have undetectable levels of KSHV genome (compared to readily detectable EBV presence) (45), and manipulation of B cells during natural KSHV infection is still poorly understood.
In contrast to the transient infection of B cells by other viruses, such as lymphocytic choriomeningitis virus (LCMV) (126), Dengue virus (65), and Chandipura virus (102), latent EBV (and, likely, KSHV) is predominantly hosted by memory B cells. This presents a logistical challenge for the primary infection and the establishment of viral latency, as the memory B cell compartment represents a very small proportion of total B cells and is widely distributed across secondary lymphoid organs and tissues of the host. Therefore, several hypotheses were originally proposed to explain how EBV managed to gain access to the memory B cell compartment.
One such hypothesis put forth by David Thorley-Lawson postulated that naive B cells, that are far more abundant than memory B cells, are infected by EBV and, via expression of select latency-associated EBV genes, are driven to proliferate to eventually become long lived memory B cells (120). Thorley-Lawson proposed that a portion of EBV-infected activated B cells enters the germinal center, a site of robust B cell proliferation, somatic hypermutation, and class switching within secondary lymphoid organs. While the majority of B cells are selected against in this process, it was proposed that EBV-infected B cells expressed the two latent proteins LMP1 and LMP2A, which functionally mimic CD40 and B cell receptor (BCR) signaling to stimulate cell survival (120). This hypothesis was based on the parallels between EBV infection that ultimately leads to latent infection of memory B cells, and the physiological process of germinal center B cell differentiation whereby B cells become activated, undergo proliferation and selection, and become long-lived memory B cells.
A compelling evidence to support the germinal center hypothesis was offered in the subsequent study from the Thorley-Lawson's group showing that EBV-infected B cells in human tonsils have functional and phenotypic markers of germinal center B cells, including expression of two key germinal center proteins, BCL6 and activation-induced cytidine deaminase (AID), and were located within the germinal center follicle (100). Similarly, before the observation made by the Thorley-Lawson's group for EBV, the Efstathiou group reported expression of murine gammaherpesvirus 68 (MHV68) genes detected by in situ hybridization in germinal centers of latently infected mice (11). These observations, and the fact that many EBV-positive B cell lymphomas display phenotypic and genetic markers of germinal center progression (121), offer a strong support for the germinal center-based establishment of gammaherpesvirus latency and the germinal center origin of viral lymphomagenesis.
However, evidence supporting alternative models of EBV infection (60) questioned the importance of physiological germinal center response for the establishment of life-long latency. Unfortunately, further resolution of the competing models remains challenging due to the exquisite species specificity of human gammaherpesviruses and the inability to faithfully model germinal center responses in culture.
Recently, humanized mouse models have made it possible to study some aspects of EBV infection and lymphomagenesis in an intact host. These recent studies have offered an insight into antiviral T cell responses and have produced paradigm shifting conclusions regarding the “oncogenicity” of latency-associated EBV genes, including dispensable nature of classic viral oncogenes and important role of lytic EBV cycle during viral lymphomagenesis (70,86,99). However, the humanized mouse models do not faithfully recapitulate either the innate immune responses, due to species restriction of EBV or KSHV infection, or the physiological B cell differentiation. Furthermore, genetic manipulation of the human host is still highly challenging in the humanized mouse models.
To overcome the limitations associated with the studies of EBV and KSHV natural infection, we and other groups have utilized the MHV68 experimental system. MHV68 is a natural rodent gammaherpesvirus that is genetically and biologically related to EBV and KSHV (10,33,131), including induction of B cell lymphomas in immunocompromised mice (117). Over the past decades, the MHV68 experimental system has offered significant insights into the immunology of gammaherpesvirus infection of a natural host, insights that were possible due to the ability to genetically manipulate both the virus and the host and the power of mouse immunological tools. In this review, we will discuss current understanding of how gammaherpesvirus infection interplays with and alters physiological B cell responses and highlight key unanswered questions in the field.
B Cell Differentiation: Under the (Gammaherpesvirus) Influence
During infection by an invading pathogen, a subset of naive B cells undergoes differentiation through the germinal center response in secondary lymphoid organs to become highly antigen-specific B cells. The two resulting populations of B cells, plasma cells, and memory B cells secrete antibodies targeting the current infection or recognize future infection by the same pathogen and rapidly respond, respectively. EBV and MHV68 manipulate the physiological B cell differentiation for their own benefit.
To initiate a physiological germinal center response, B cells specific to an invading pathogen will become activated and present antigen to CD4+ T cells within the intrafollicular zone, or the border between the B and T cell zone (49). Upon establishing this antigen-specific interaction, both cell populations will express BCL6 (49,54), a key transcription factor for germinal center B cell and T follicular helper cell (Tfh) development and survival (13,27,140). Tfh will then upregulate CXCR5, PD-1, ICOS, and GL7, allowing migration into the germinal center follicle (49). B cells will also migrate into the follicle and express GL7 and CD95, becoming germinal center B cells.
The germinal center is anatomically subdivided into the dark and light zones, originally based on classic histology studies that used morphological criteria, such as size and nuclear contour along with the relative abundance of T cells to show distinct zones of the germinal center (109). Germinal center B cells undergo rapid proliferation in the dark zone of the germinal center (1), with the upregulation of antiapoptotic factors such as Bcl-2, and reduced expression of cell cycle checkpoint p21 (57) and the p53 tumor suppressor (92). This rapid proliferation is coupled to mutagenesis that generates somatic hypermutation and class switching, processes that aim to increase antibody affinity and avidity. Somatic hypermutation and class switch recombination is mediated by AID (28,87). Due to the random nature of AID-mediated mutagenesis, continuous selection of germinal center B cells with antigen-specific BCR occurs via BCR-antigen interaction and costimulatory signals from Tfh in the light zone; the lack of interaction with either leads to cell death (56). Following selection, B cells can recirculate through the dark zone or differentiate into either plasma cells or memory B cells. Memory B cell differentiation is driven by continued PAX5 expression and a downregulation of BCL6 (5,16,59). To facilitate plasma cell differentiation, PAX5 must be silenced (16,89) in addition to an upregulation of BLIMP1, XBP1, and IRF-4 (57,103).
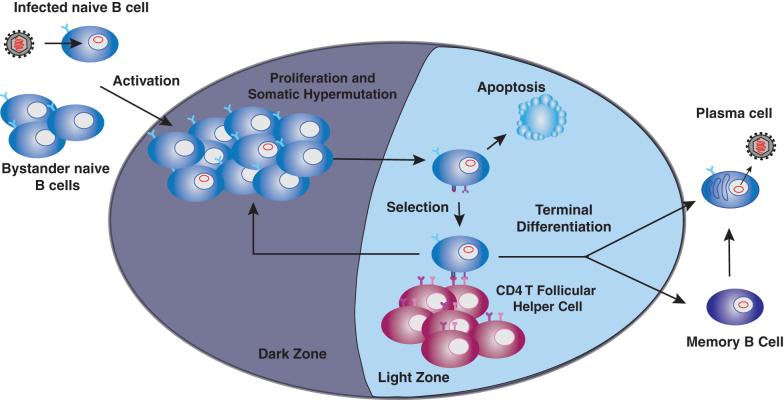
This differentiation process is usurped during gammaherpesvirus infection (Fig. 1). EBV and MHV68 infect naive B cells, with subsequent entry of both infected and uninfected B cells into the germinal center. EBV and MHV68 infection is latent in germinal center B cells (64,100), with up to 20% of germinal center B cells harboring MHV68 genome at the peak of viral latency (22,75). Due to the latent nature of infection and rapid rate of cellular proliferation, gammaherpesviruses usurp proliferation of germinal center B cells to achieve exponential increase in the viral reservoir without the need to undergo highly immunogenic lytic replication. Following further differentiation, infected B cells emerge as memory B cells that host life-long latent infection (125), or plasma cells, where EBV and MHV68 reactivate, switching from latent to lytic life cycle. Production of infectious virions by plasma cells leads to infection of naive B cells, creating a positive feedback loop that ensures the establishment of gammaherpesvirus latency.
FIG. 1.
Germinal center model of gammaherpesvirus infection. Gammaherpesviruses infect naive B cells, followed by activation and entry of both infected and bystander B cells into the germinal center reaction. Latently infected B cells (indicated by red viral episome in the nucleus) undergo cycles of proliferation, somatic hypermutation, and selection to emerge as latently infected memory B cells or plasma cells. Differentiation into plasma cells triggers viral reactivation, with subsequent production of infectious virions.
In contrast to physiological B cell differentiation, gammaherpesvirus infection elicits a rapid and robust increase in the titers of class-switched antibodies reactive against self and foreign species antigens. In contrast, titers of class-switched virus-specific antibodies arise with much slower kinetics. MHV68 induces peak titers of total class switched antibodies by about 10 days postinfection; however, MHV68-directed class switched antibodies do not peak until 3 weeks postinfection; kinetics of antiviral antibodies may be further modified by the original inoculum dose (104,111). Similarly, class-switched EBV-specific antibody responses do not peak until 6–12 weeks after EBV exposure (108).
The difference in the kinetics of overall versus virus-specific class-switched antibody induction is accounted for by the rapid increase in self-reactive antibodies. As such, levels of class-switched, self-directed antibodies, including anti-dsDNA antibodies, peak by 14 days post-MHV68 infection (40,104). Furthermore, the titers of MHV68-driven self-directed antibodies are much higher than those induced by LCMV (24), an unrelated RNA virus that infects multiple cell types, including B cells (126). Similarly, EBV infection generates robust responses against self and foreign antigens. In fact, the presence of high titer antibodies against horse antigens is diagnostic of a recent EBV infection in humans (36). Unfortunately, the mechanisms underlying this selective increase in self-directed and irrelevant antibodies driven by gammaherpesvirus infection are still poorly understood, but could potentially offer insights into the genesis of B cell-driven autoimmune disease and virus-driven lymphomas.
Because mechanisms underlying the gammaherpesvirus-driven self-directed humoral response remain enigmatic, it is difficult to test the role of nonspecific B cell differentiation in chronic infection. However, it is clear that EBV and MHV68 selectively establish infection in B cells that do not encode a virus-specific BCR (25,125). As EBV and MHV68 reactivation occurs in plasma cells (61,64) that also produce high levels of antibodies, it is tempting to speculate that intracellular virus-directed antibodies could attenuate assembly or infectivity of reactivating virus. Therefore, by preferentially establishing latency in virus-nonspecific B cells, gammaherpesviruses would ensure optimal reactivation kinetics, a feature that is particularly important in an immunocompetent, chronically infected host.
Robust, MHV68-driven germinal center response is transient in nature. Despite mild follicular hyperplasia maintained in long-term-infected animals, the magnitude of the germinal center response decreases by 42 days postinfection from its peak levels observed at 16 days postinfection (76) along with the decrease and stabilization of the frequency of latently infected splenocytes. Similarly, exuberant immune activation associated with EBV-driven infectious mononucleosis resolves over a period of weeks to months.
Interestingly, latent MHV68 genome is also present in developing and transitional B cells (17,18,116). The elegant study from the Tibbetts group demonstrated that transient treatment of long-term-infected animals with anti-IL-7, which led to depletion of transitional, but not naive or germinal center B cells, decreased the frequency of MHV68-positive naive and germinal center B cells (17). In contrast, transient depletion of IL-7 before and during early stages of MHV68 infection had no effect on the latent viral reservoir in germinal center B cells, indicating that, as the chronic infection transitions to its long-term stage, gammaherpesviruses may become more reliant on the infection of developing B cells to maintain the latent reservoir. While EBV is associated with fibrin-ring granuloma and hemophagocytic histiocytosis (15), diseases that manifest in the bone marrow, EBV infection of developing B cells in vivo has not been examined.
Living with the Enemy: Host Factors That Affect Gammaherpesvirus-Driven B Cell Differentiation
Given the limitations that are associated with the exquisite species specificity of human gammaherpesviruses, host factors that are involved in the interaction between gammaherpesviruses and B cell differentiation in vivo have been primarily defined using the MHV68 system. Intranasal inoculation of mice that lack peripheral B cells (μMT−/− mice) results in significantly attenuated establishment of MHV68 latency in the spleen (113,130,135). In contrast, lack of peripheral B cells, does not preclude long-term latent MHV68 infection in the spleen (136), likely due to ability of gammaherpesviruses to infect and establish latency in myeloid cells (41,63,95,97,105,134,137). However, long-term MHV68 reactivation is poorly controlled in μMT−/− mice, particularly in the peritoneal cavity, and μMT−/− mice receiving high-dose intraperitoneal MHV68 inoculum eventually succumb to the infection (136).
Interestingly, lack of MHV68 antibody in μMT−/− mice is not likely to be critical for the control of infection, as chronic MHV68 infection was well-controlled in mice that could only generate anti-hen egg lysozyme antibodies (77). It was proposed that, following infection, these transgenic B cells instead served as antigen presenting cells (77), facilitating anti-MHV68 T cell responses. Similarly, EBV-infected B cells can present viral antigen to T cells in culture, with subsequent inhibition of EBV-driven transformation (6). Despite well-controlled MHV68 infection in immunocompetent mice that cannot produce MHV68-specific antibodies, MHV68-specific antibody is important to attenuate increased viral reactivation and persistent replication in immunocompromised mouse models (38,53) and preexisting antiviral antibody attenuates acute replication and early latent MHV68 infection (3,123).
In addition to the mere presence of peripheral B cells, it is clear that the induction of germinal center response is critical for the peak levels of MHV68 latency. The first studies to offer evidence supporting the importance of T cell-dependent B cell differentiation during MHV68 infection demonstrated decreased splenomegaly and MHV68 reactivation in CD4 T cell depleted mice and an important role of CD4 T cells in MHV68-driven nonspecific B cell differentiation (112,129). Subsequently, it was demonstrated that the cognate T-B cell interaction via CD40/CD40L and MHC class II expression on B cells was required for the stimulation of non-MHV68-specific antibodies (104). These early studies led to elegant publications from the Speck group cementing the critical importance of Tfh and IL-21 in MHV68-driven germinal center response and the establishment of viral latency (20,21).
B cell activation is important before the entry into the germinal center response. Not surprisingly, infected cell-intrinsic NF-κB signaling supported the efficient establishment and maintenance of MHV68 latent infection, as demonstrated using an MHV68 mutant expressing dominant negative IκBα (58). The importance of NF-κB has been further demonstrated by the fact that EBV and KSHV encode viral proteins that modulate NF-κB activity. For example, EBV encodes LMP1, which activates NF-κB signaling by acting as functional mimic of CD40 receptor (55,122,127). KSHV encodes vFLIP, which drives NF-κB activation via TRAF2 and TRAF3 interactions (43,74). Importantly, both of these viral proteins facilitate cellular transformation (42,48).
Consistent with the importance of B cell activation for the establishment of gammaherpesvirus latency, MyD88−/− mice demonstrate decreased B cell activation, MHV68-driven germinal center response, and establishment and maintenance of latent infection, with the phenotypes likely driven by a B cell-intrinsic MyD88 deficiency (39). MyD88 is involved in a number of signaling pathways, including IL-1 signaling. We have recently shown that IL-1R1−/− mice demonstrate attenuated germinal center response following MHV68 infection, with attenuated induction of virus-specific and self-directed antibodies (24). However, frequency of MHV68 DNA-positive splenocytes was comparable in control and IL-1R1−/− mice, with most of infected cells representing germinal center B cells ((24) and unpublished observations), suggesting that several MyD88-dependent pathways function to support the establishment of MHV68 latency.
Given the role of B cell activation in the gammaherpesvirus-driven germinal center response and the establishment of latent infection, we hypothesized that host factors restricting B cell activation would be deleterious for the virus. SHP1 (encoded by PTPN6), a tyrosine phosphatase expressed in hematopoietic cells, is a negative regulator of immune cell activation (50,66). In B cells, SHP1 is a cytoplasmic protein that localizes to the BCR to dephosphorylate several substrates, including Igα-Igβ subunits, Syk, and BLNK, ultimately attenuating BCR-proximal signaling [reviewed in Tamir et al. (115)]. Correspondingly, SHP1 expression is significantly decreased in EBV or KSHV positive B cell lymphomas, including Burkitt's, Diffuse Large B cell, Monomorphic Posttransplant Lymphoproliferative Disease, and Primary Effusion lymphomas (26,85,91,133). Surprisingly, we found that B cell-, but not T cell-intrinsic SHP1 expression supported efficient germinal center B cell expansion and latency establishment during MHV68 infection (46). These findings are consistent with the role of SHP1 expression in germinal center B cells during the physiological germinal center response (51), supporting the concept that gammaherpesviruses usurp some physiological B cell differentiation mechanisms to achieve their goal of establishing life-long infection.
However, it is also clear that the mechanisms governing gammaherpesvirus-driven germinal center response do not fully overlap with those guiding physiological germinal center reactions. In addition to the robust gammaherpesvirus-driven induction of self- but not virus-directed class-switched antibodies, specific host factors seem to be selectively important or dispensable for the gammaherpesvirus-induced B cell differentiation. We showed that MHV68-driven germinal center response was significantly exaggerated and failed to contract in IRF-1−/− mice (75) along with a significant elevation in the frequency of MHV68 DNA-positive splenocytes. In contrast, IRF-1−/− mice immunized with sheep red blood cells or infected with LCMV, displayed germinal center responses equivalent to those observed in wild-type mice (75). Thus, IRF-1 selectively attenuates gammaherpesvirus-driven germinal center response, with little effect on B cell differentiation induced by immunization or an RNA virus infection.
Similarly, while B cell intrinsic STAT3 expression is required for germinal center response induction and maintenance following several physiological stimuli, it is entirely dispensable for the MHV68-driven germinal center response (29,96). It is likely that many more mechanisms unique to gammaherpesvirus-driven B cell differentiation await to be discovered in the future studies.
Beyond the germinal center response, expression of BLIMP1, XBP1, and IRF-4 facilitate differentiation of germinal center B cells into plasma cells (57,103). This differentiation triggers reactivation, a switch from latent to lytic viral cycle. The question of how these viruses “know” that the B cell has differentiated has been approached from the perspective of gene expression regulation. Specifically, gammaherpesvirus reactivation is initiated by the expression of viral transcription factor(s) that are critical for the induction of downstream lytic viral genes. Promoters of such lytic cycle masterminds in EBV, KSHV, and MHV68 include elements bound by XBP1, such that expression of XBP1 in vitro induces viral reactivation (9,23,73,114,139,141). Intriguingly, B cell-specific XBP-1 deficiency had no effect on MHV68 reactivation in vivo (73), indicating that there is a redundancy among plasma cell transcription factors in mediating gammaherpesvirus reactivation. In contrast, B cell-intrinsic deficiency of IRF-4 resulted in attenuated generation of plasma cells following MHV68 infection along with a dramatic decrease in viral reactivation (73).
Gammaherpesvirus Proteins and B Cell Differentiation: When Extra Convincing of the Host Is Needed
In contrast to host factors that are likely to be expressed in a majority of cells within the relevant B cell subpopulation, only a small proportion of any B cell population will be infected. For example, up to 20% of germinal center B cells are MHV68 positive at the peak of viral latency, with the frequency of virus-positive cells decreasing as long-term infection is established; the virus is even less abundant in other B cell subpopulations (19,35). Thus, gammaherpesviruses are likely to trigger a combination of infected cell intrinsic and extrinsic mechanisms to drive germinal center response and differentiation of both infected and uninfected B cells.
MHV68 latency-associated nuclear antigen (LANA, encoded by orf73) is a latent gammaherpesvirus protein, with genetic and functional homologs present in KSHV and EBV (37,84,128). MHV68 LANA, while mostly dispensable for acute infection in vivo, is critical for the efficient establishment of latency and viral reactivation, a devastating defect that cannot be rescued by increasing the infectious dose of LANA null MHV68 mutant (37,84). LANA is expressed during MHV68 latent infection (88) and, similar to its counterparts in human viruses, is responsible for tethering the viral episome to the cellular chromosomes upon division of the latently infected cell, ensuring the “inheritance” of the viral plasmid by the daughter cells.
Despite similar acute infection parameters, mice infected with LANA-deficient MHV68 mutant display decreased expansion of Tfh population and complete lack of induction of anti-dsDNA antibodies (40), suggesting that efficient establishment of latency and/or other LANA functions are critical to drive self-reactive B cell response. Interestingly, Sh2d1a deficient mice that have significantly attenuated Tfh responses also fail to increase levels of anti-dsDNA antibodies following MHV68 infection (40), highlighting the important role of germinal center response in the gammaherpesvirus-driven differentiation of self-reactive B cells.
Consistent with observations during the MHV68 infection, KSHV LANA transgene driven by the endogenous viral promoter induces follicular hyperplasia, germinal center responses, and B cell lymphomas in transgenic mice (34). Thus, the manipulation of B cell differentiation by gammaherpesvirus LANA proteins is conserved across host species and goes beyond the maintenance of the viral episome, as the B cell phenotypes in LANA transgenic mice occur in the absence of the viral genome.
In addition to LANA and its functional homologs, several unique (at least sequence-wise) EBV proteins expressed in latently infected B cells are thought to directly affect the germinal center stage of infection. EBV LMP1 is expressed in infected germinal center B cells (4) and is a powerful, constitutively active functional homolog of CD40 that is postulated to support proliferation and survival of EBV-infected germinal center B cells [reviewed in Kieser and Sterz (52)]. Interestingly, EBV-driven lymphomagenesis in humanized mice is independent of LMP1 in the presence of CD4 T cells. In contrast, depletion of CD4 or blocking CD40 signaling completely abolishes lymphomagenic ability of LMP1-deficient EBV mutant, highlighting the functional redundancy of LMP1 and CD4 T cell help/CD40 signaling (70).
LMP1 is coexpressed in infected germinal center B cells with LMP2A, with the latter being a functional mimic of BCR signaling. EBV infection of “crippled” germinal center B cells that no longer express BCR due to debilitating heavy-chain mutations can rescue the survival and proliferation of these cells in vitro and yield clonal lymphoblastoid cell lines, with LMP2A presumably playing a role in this process (71). LMP2A expression driven by highly active immunoglobulin heavy-chain promoter and intronic enhancer in transgenic mice resulted in attenuated heavy-chain rearrangement and increased numbers of IgM-negative circulating B cells (12).
Similar to LMP1, deletion of LMP2A has minimal effect on EBV-induced lymphomagenesis in humanized mice (69). However, simultaneous deficiency of LMP1 and LMP2A results in fewer tumors with delayed onset, highlighting biologically redundant functions of these two EBV proteins with respect to viral lymphomagenesis, at least in the context of humanized mouse model (69). The role of LMP1 and LMP2 in the EBV-driven germinal center response remains unclear due to the limitations of humanized mouse models. However, expression of these two EBV genes gives rise to CD8 T cell epitopes that may be important for the contraction of the EBV-driven germinal center response and control of latent viral reservoir (78).
While genes homologous to EBV LMP1 and LMP2A are not present in other gammaherpesviruses, functional homologs certainly exist. Expression of KSHV-encoded K1 in place of LMP2A prevents apoptosis of primary BCR negative human B cells infected with a chimeric EBV mutant in vitro (110). High levels of K1 expression as a SV40 promoter-driven transgene in mice led to lymphoid hyperplasia with a small proportion of transgenic mice developing frank B cell lymphomas at 18 months of age (8). The role of endogenous levels of KSHV K1 expression in B cell differentiation during chronic infection remains unclear.
MHV68 M2 is a multifunctional latent protein that is expressed in infected germinal center B cells (118). M2 has several B cell-specific functions: at low doses of infection, it facilitates MHV68 infection of germinal center B cells (44), at high doses of infection M2 promotes differentiation of MHV68-infected germinal center B cells to plasma cells and subsequent viral reactivation (118). Importantly, M2 expression by B cells, in the absence of infection, was sufficient to increase expression of IL-2, IL-6, MIP1α, and IL-10, with IL-10 supporting the expansion of M2 expressing B cells (106). The induction of IL-10 by M2 was later shown to be IRF-4-dependent (94). The ability of MHV68 M2 to stimulate cytokine production by B cells is particularly interesting, because KSHV encodes viral IL-6 and MIP-1α (90), while EBV encodes viral IL-10 (83,101), indicating overlapping functions between M2 and human gammaherpesvirus proteins. Finally, adoptive transfer of naive B cells that express MHV68 M2 drives their differentiation into germinal center B cells or plasma cells without any additional stimulation of the recipient host (118), phenotypes that mimic those attributed to LMP1 and/or LMP2A expression.
All gammaherpesviruses encode a single conserved protein kinase. Despite the conserved nature of this viral protein, the role of the gammaherpesvirus kinase during chronic infection remains poorly understood. Our group was the first to demonstrate that both expression and enzymatic activity of the MHV68 protein kinase (orf36) support the establishment of chronic infection, especially under conditions of low infectious dose (24,116), however, the underlying mechanism remained unclear.
Recently, we demonstrated that expression and enzymatic activity of MHV68 orf36 promotes MHV68-driven germinal center response and generation of self-reactive, but not MHV68-specific antibodies, for the first time genetically separating physiological, virus-directed, and abnormal, self-reactive B cell differentiation induced by gammaherpesvirus infection (24). This observation was particularly unexpected, as known gammaherpesvirus “manipulators” of germinal center response (discussed above) are expressed in latently infected cells, whereas gammaherpesvirus protein kinases are classically associated with the lytic viral cycle. Recently, we have detected MHV68 orf36 expression in splenocytes harvested from latently infected mice (Paul Sylvester and Vera Tarakanova, unpublished observations), with ongoing studies defining the subsets of B cells expressing MHV68 protein kinase during chronic infection.
Similar to the functional conservation of latent gammaherpesvirus proteins observed across species, expression of KSHV protein kinase orf36 as a transgene (under the control of endogenous viral promoter) resulted in expression of viral kinase in B cells, increased germinal center response and class-switching, and eventual generation of B cell lymphomas that continued to express the KSHV protein kinase (2). This important study was not only the first to demonstrate the oncogenic role of the gammaherpesvirus gene classically associated with the lytic infection but also highlighted the functional conservation of gammaherpesvirus protein kinases across different host species. Because gammaherpesvirus protein kinases are multifunctional proteins that interact with numerous systems of the host, future studies need to define the specific viral kinase–host interactions that are responsible for the manipulation of B cell differentiation and lymphomagenesis.
Gammaherpesvirus Pathogenesis: The Case of Deranged B Cells
Similar to other oncogenic viruses, gammaherpesvirus-driven lymphomagenesis is almost certainly an accidental outcome of well-evolved virus–host interactions, an accident that is driven by changes in host genetics and/or environmental influence. The accidental nature of gammaherpesvirus lymphomagenesis is best illustrated by the challenges that have to be overcome to faithfully model such lymphomagenesis in vivo. With a few exceptions (i.e., KSHV orf36 transgenic mouse model that generated lymphomas in 66% of immunocompetent mice) (2), expression of a single gammaherpesvirus gene or even the entire KSHV latency locus under the endogenous transcriptional control produces lymphomas in only a limited number of immunocompetent animals (11–16%) (34,107), consistent with the low frequency of gammaherpesvirus lymphomagenesis in the human population. The incidence of lymphomas typically can be increased by placing the viral gene under the control of a robust constitutively active promoter, introduction of other oncogenic host mutations, or using severely immunocompromised animals; in other words, creating conditions that disrupt the physiological virus–host interactions.
Unfortunately, because the risk factors underlying gammaherpesvirus lymphomagenesis remain poorly defined and the low frequency of infected individuals that develop cancer, it is currently highly challenging to gain any insight into the mechanism of “natural” gammaherpesvirus lymphomagenesis in humans. Signature genetic mutations have not been reported in gammaherpesvirus-driven lymphomas, with an exception of EBV-positive Burkitt's lymphomas that are characterized by the chromosomal translocation that stimulates expression of c-myc. What is clear is that many gammaherpesvirus-positive lymphomas have evidence of germinal center origin, whether by retaining phenotypic markers of germinal center B cells and/or genetic signatures consistent with somatic hypermutation (121).
In support of the germinal center origin of viral lymphomagenesis, germinal center stage of B cell differentiation is, arguably, the most prone to transformation due to rapid cellular proliferation, downregulation of tumor suppressors, and expression of mutagenic AID. Finally, expression of EBV or KSHV proteins that are expressed in latently infected B cells, including germinal center B cells, is in many cases sufficient, especially at high levels of expression, to generate lymphomas or lymphoproliferative disease in transgenic animals; the role of these viral proteins during lymphomagenesis in humans is difficult to define.
Intriguingly, an emerging area of research has established an important role for lytic gammaherpesvirus cycle and/or proteins in viral lymphomagenesis. Infection of humanized mice with lytic replication deficient EBV mutant resulted in a five-fold decrease in the incidence of lymphomas compared to wild-type EBV, despite equivalent establishment of latent infection (68). Furthermore, infection with a “superlytic” EBV mutant had given rise to the same incidence of lymphomas in humanized mice as infection with wild-type EBV (67). Similarly, expression of KSHV protein kinase, a classical lytic viral gene, was sufficient to drive B cell lymphomas in a majority of transgenic mice (2). Given this emerging connection, it is critical to revisit the traditional dogmas sorting gammaherpesvirus genes into latent and lytic “boxes” and comprehensively redefine viral gene expression in the relevant B cell subpopulations, including germinal center B cells in vivo, during natural infection.
In a counterargument of the germinal center origin of gammaherpesvirus positive lymphomas, AID expression in B cells can be induced by EBV and KSHV infection (7,47), potentially stimulating genetic changes associated with germinal center response without the actual experience of progressing through this stage of differentiation. Furthermore, KSHV infection of mature B cells also activates expression of RAG, a mutagenic V(D)J recombinase normally limited in expression to early stages of B cell development (124). This KSHV-driven reexpression of RAG induces further V(D)J recombination, offering an explanation for a well-known bias of KSHV infection for Igλ B cells.
It is possible that virus-induced expression of mutagenic enzymes cooperates with physiological and environmental stimuli of AID expression to promote lymphomagenesis. One such environmental stimulus is Plasmodium infection, which leads to prolonged expansion of germinal center response along with sustained expression of AID (98), including in nongerminal center B cells (138), and is a well-established risk factor for endemic EBV-driven Burkitt's lymphoma. Thus, it is important to continue questioning and refining the germinal center model to gain insight into the mechanisms underlying gammaherpesvirus lymphomagenesis.
While the association of gammaherpesvirus infection with cancer is well-established, there is an additional, although much more controversial, association between EBV infection and autoimmune disease. Despite controversy, this association seems intuitively plausible, given the unique ability of gammaherpesviruses to induce robust nonvirus-specific B cell differentiation, especially during the early stages of chronic infection, a process that could synergize with host genetic or environmental susceptibility to autoimmune disease. However, causative association between autoimmune disease has been challenging to demonstrate given the high prevalence and life-long nature of EBV infection.
EBV infectious mononucleosis is the least controversial risk factor for multiple sclerosis (MS), with infectious mononucleosis occurrence shown to temporally precede and greatly increase the risk of the subsequent MS development (72,119). While the causative link between EBV infection and the development of systemic lupus erythematosus (SLE) is more debatable, buffy coat EBV viral loads are significantly increased in SLE patients with active compared to inactive disease (93) along with impaired EBV-, but not HCMV-specific CD8 T cell responses (62). This increased EBV activity is thought to exacerbate severity of already established SLE, including nephritis [discussed in Draborg et al. (30)]. The role of EBV in other autoimmune diseases remains a subject of debate and it is likely that, similar to other disease models, the infection synergizes with other host susceptibilities (genetic, environmental, etc.) to promote disease.
Conclusion and Future Directions
The fascinating relationship between gammaherpesviruses and B cell differentiation lies at the heart of chronic infection and viral pathogenesis. While a number of host and viral factors involved in this relationship have been identified, we still know surprisingly little regarding this relationship. This is an unacceptable knowledge gap considering the fact that there are currently no approaches to generate sterilizing immunity, clear existing gammaherpesvirus infection, or to precisely identify and manage susceptibility to gammaherpesvirus-driven disease in humans. Because many aspects of B cell differentiation only occur in the context of an intact host, animal models are critical to define the relevant molecular and cellular mechanisms that selectively govern the intricate dance between the gammaherpesvirus infection and B cell differentiation, especially for human gammaherpesviruses. Defining unique mechanisms that regulate gammaherpesvirus-driven B cell differentiation will lay the foundation for targeted preventative and therapeutic approaches against gammaherpesvirus-driven disease.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by 1F31CA243364 (K.E.J.), CA183593, and CA203923 (V.L.T.).
References
- 1. Allen CD, Okada T, Tang HL, and Cyster JG. Imaging of germinal center selection events during affinity maturation. Science 2007;315:528–531 [DOI] [PubMed] [Google Scholar]
- 2. Anders PM, Montgomery ND, Montgomery SA, Bhatt AP, Dittmer DP, and Damania B. Human herpesvirus-encoded kinase induces B cell lymphomas in vivo. J Clin Invest 2018;128:2519–2534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arico E, Robertson KA, Belardelli F, Ferrantini M, and Nash AA. Vaccination with inactivated murine gammaherpesvirus 68 strongly limits viral replication and latency and protects type I IFN receptor knockout mice from a lethal infection. Vaccine 2004;22:1433–1440 [DOI] [PubMed] [Google Scholar]
- 4. Babcock GJ, Hochberg D, and Thorley-Lawson DA. The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 2000;13:497–506 [DOI] [PubMed] [Google Scholar]
- 5. Basso K, Klein U, Niu H, et al. Tracking CD40 signaling during germinal center development. Blood 2004;104:4088–4096 [DOI] [PubMed] [Google Scholar]
- 6. Bejarano MT, Masucci MG, Morgan A, Morein B, Klein G, and Klein E. Epstein-Barr virus (EBV) antigens processed and presented by B cells, B blasts, and macrophages trigger T-cell-mediated inhibition of EBV-induced B-cell transformation. J Virol 1990;64:1398–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bekerman E, Jeon D, Ardolino M, and Coscoy L. A role for host activation-induced cytidine deaminase in innate immune defense against KSHV. PLoS Pathog 2013;9:e1003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berkova Z, Wang S, Sehgal L, Patel KP, Prakash O, and Samaniego F. Lymphoid hyperplasia and lymphoma in KSHV K1 transgenic mice. Histol Histopathol 2015;30:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhende PM, Dickerson SJ, Sun X, Feng WH, and Kenney SC. X-box-binding protein 1 activates lytic Epstein-Barr virus gene expression in combination with protein kinase D. J Virol 2007;81:7363–7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blaskovic D, Stancekova M, Svobodova J, and Mistrikova J. Isolation of five strains of herpesviruses from two species of free living small rodents. Acta Virol 1980;24:468. [PubMed] [Google Scholar]
- 11. Bowden RJ, Simas JP, Davis AJ, and Efstathiou S. Murine gammaherpesvirus 68 encodes tRNA-like sequences which are expressed during latency. J Gen Virol 1997;78:1675–1687 [DOI] [PubMed] [Google Scholar]
- 12. Caldwell RG, Wilson JB, Anderson SJ, and Longnecker R. Epstein-Barr virus LMP2A drives B cell development and survival in the absence of normal B cell receptor signals. Immunity 1998;9:405–411 [DOI] [PubMed] [Google Scholar]
- 13. Cattoretti G, Pasqualucci L, Ballon G, et al. Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell 2005;7:445–455 [DOI] [PubMed] [Google Scholar]
- 14. Cesarman E. Gammaherpesviruses and lymphoproliferative disorders. Annu Rev Pathol 2014;9:349–372 [DOI] [PubMed] [Google Scholar]
- 15. Chung HJ, Chi HS, Jang S, and Park CJ. Epstein-Barr virus infection associated with bone marrow fibrin-ring granuloma. Am J Clin Pathol 2010;133:300–304 [DOI] [PubMed] [Google Scholar]
- 16. Cobaleda C, Schebesta A, Delogu A, and Busslinger M. Pax5: the guardian of B cell identity and function. Nat Immunol 2007;8:463. [DOI] [PubMed] [Google Scholar]
- 17. Coleman CB, McGraw JE, Feldman ER, et al. A gammaherpesvirus Bcl-2 ortholog blocks B cell receptor-mediated apoptosis and promotes the survival of developing B cells in vivo. PLoS Pathog 2014;10:e1003916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Coleman CB, Nealy MS, and Tibbetts SA. Immature and transitional B cells are latency reservoirs for a gammaherpesvirus. J Virol 2010;84:13045–13052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Collins CM, Boss JM, and Speck SH. Identification of infected B-cell populations by using a recombinant murine gammaherpesvirus 68 expressing a fluorescent protein. J Virol 2009;83:6484–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collins CM, and Speck SH. Expansion of murine gammaherpesvirus latently infected B cells requires T follicular help. PLoS Pathog 2014;10:e1004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Collins CM, and Speck SH. Interleukin 21 signaling in B cells is required for efficient establishment of murine gammaherpesvirus latency. PLoS Pathog 2015;11:e1004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins CM, and Speck SH. Tracking murine gammaherpesvirus 68 infection of germinal center B cells in vivo. PLoS One 2012;7:e33230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dalton-Griffin L, Wilson SJ, and Kellam P. X-box binding protein 1 contributes to induction of the Kaposi's sarcoma-associated herpesvirus lytic cycle under hypoxic conditions. J Virol 2009;83:7202–7209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Darrah EJ, Jondle CN, Johnson KE, et al. Conserved gammaherpesvirus protein kinase selectively promotes irrelevant B cell responses. J Virol 2019;93:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Decalf J, Godinho-Silva C, Fontinha D, Marques S, and Simas JP. Establishment of murine gammaherpesvirus latency in B cells is not a stochastic event. PLoS Pathog 2014;10:e1004269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Delibrias CC, Floettmann JE, Rowe M, and Fearon DT. Downregulated expression of SHP-1 in Burkitt lymphomas and germinal center B lymphocytes. J Exp Med 1997;186:1575–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dent AL, Shaffer AL, Yu X, Allman D, and Staudt LM. Control of inflammation, cytokine expression, and germinal center formation by BCL-6. Science 1997;276:589–592 [DOI] [PubMed] [Google Scholar]
- 28. Dickerson SK, Market E, Besmer E, and Papavasiliou FN. AID mediates hypermutation by deaminating single stranded DNA. J Exp Med 2003;197:1291–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ding C, Chen X, Dascani P, et al. STAT3 signaling in B cells is critical for germinal center maintenance and contributes to the pathogenesis of murine models of lupus. J Immunol 2016;196:4477–4486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Draborg A, Izarzugaza JM, and Houen G. How compelling are the data for Epstein-Barr virus being a trigger for systemic lupus and other autoimmune diseases? Curr Opin Rheumatol 2016;28:398–404 [DOI] [PubMed] [Google Scholar]
- 31. Dunmire SK, Grimm JM, Schmeling DO, Balfour HH Jr, and Hogquist KA. The incubation period of primary Epstein-Barr virus infection: viral dynamics and immunologic events. PLoS Pathog 2015;11:e1005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dunmire SK, Hogquist KA, and Balfour HH. Infectious mononucleosis. Curr Top Microbiol Immunol 2015;390:211–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Efstathiou S, Ho YM, Hall S, Styles CJ, Scott SD, and Gompels UA. Murine herpesvirus 68 is genetically related to the gammaherpesviruses Epstein-Barr virus and herpesvirus saimiri. J Gen Virol 1990;71:1365–1372 [DOI] [PubMed] [Google Scholar]
- 34. Fakhari FD, Jeong JH, Kanan Y, and Dittmer DP. The latency-associated nuclear antigen of Kaposi sarcoma-associated herpesvirus induces B cell hyperplasia and lymphoma. J Clin Invest 2006;116:735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Flano E, Kim IJ, Moore J, Woodland DL, and Blackman MA. Differential gamma-herpesvirus distribution in distinct anatomical locations and cell subsets during persistent infection in mice. J Immunol 2003;70:3828–3834 [DOI] [PubMed] [Google Scholar]
- 36. Fleisher GR, Collins M, and Fager S. Limitations of available tests for diagnosis of infectious mononucleosis. J Clin Microbiol 1983;17:619–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fowler P, Marques S, Simas JP, and Efstathiou S. ORF73 of murine herpesvirus-68 is critical for the establishment and maintenance of latency. J Gen Virol 2003;84:3405–3416 [DOI] [PubMed] [Google Scholar]
- 38. Gangappa S, Kapadia SB, Speck SH, and Virgin HW. Antibody to a lytic cycle viral protein decreases gammaherpesvirus latency in B-cell-deficient mice. J Virol 2002;76:11460–11468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gargano LM, Moser JM, and Speck SH. Role for MyD88 signaling in murine gammaherpesvirus 68 latency. J Virol 2008;82:3853–3863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gauld SB, De Santis JL, Kulinski JM, et al. Modulation of B-cell tolerance by murine gammaherpesvirus 68 infection: requirement for Orf73 viral gene expression and follicular helper T cells. Immunology 2013;139:197–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gregory SM, Wang L, West JA, Dittmer DP, and Damania B. Latent Kaposi's sarcoma-associated herpesvirus infection of monocytes downregulates expression of adaptive immune response costimulatory receptors and proinflammatory cytokines. J Virol 2012;86:3916–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Guasparri I, Keller SA, and Cesarman E. KSHV vFLIP is essential for the survival of infected lymphoma cells. J Exp Med 2004;199:993–1003 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Guasparri I, Wu H, and Cesarman E. The KSHV oncoprotein vFLIP contains a TRAF-interacting motif and requires TRAF2 and TRAF3 for signalling. EMBO Rep 2006;7:114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Herskowitz J, Jacoby MA, and Speck SH. The murine gammaherpesvirus 68 M2 gene is required for efficient reactivation from latently infected B cells. J Virol 2005;79:2261–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hudnall SD, Chen T, Allison P, Tyring SK, and Heath A. Herpesvirus prevalence and viral load in healthy blood donors by quantitative real-time polymerase chain reaction. Transfusion 2008;48:1180–1187 [DOI] [PubMed] [Google Scholar]
- 46. Johnson KE, Lange PT, Jondle CN, et al. B cell-intrinsic SHP1 expression promotes gammaherpesvirus-driven germinal center response and the establishment of chronic infection. J Virol 2019. [Epub ahead of print]. doi: 10.1128/JVI.01232-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kalchschmidt JS, Bashford-Rogers R, Paschos K, et al. Epstein-Barr virus nuclear protein EBNA3C directly induces expression of AID and somatic mutations in B cells. J Exp Med 2016;213:921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kaye KM, Izumi KM, and Kieff E. Epstein-Barr virus latent membrane protein 1 is essential for B-lymphocyte growth transformation. Proc Natl Acad Sci U S A 1993;90:9150–9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kerfoot SM, Yaari G, Patel JR, et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 2011;34:947–960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Khalil AM, Cambier JC, and Shlomchik MJ. B cell receptor signal transduction in the GC is short-circuited by high phosphatase activity. Science 2012;336:1178–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khalil AM, Cambier JC, and Shlomchik MJ. B cell signal transduction in germinal center B cells is short-circuited by increased phosphatase activity. Science 2012;336:1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kieser A, and Sterz KR. The latent membrane protein 1 (LMP1). Curr Top Microbiol Immunol 2015;391:119–149 [DOI] [PubMed] [Google Scholar]
- 53. Kim IJ, Flano E, Woodland DL, and Blackman MA. Antibody-mediated control of persistent g-herpesvirus infection. J Immunol 2002;168:3958–3964 [DOI] [PubMed] [Google Scholar]
- 54. Kitano M, Moriyama S, Ando Y, et al. Bcl6 protein expression shapes pre-germinal center B cell dynamics and follicular helper T cell heterogeneity. Immunity 2011;34:961–972 [DOI] [PubMed] [Google Scholar]
- 55. Klein E, Teramoto N, Gogolák P, Nagy N, and Björkholm M. LMP-1, the Epstein–Barr virus-encoded oncogene with a B cell activating mechanism similar to CD40. Immunol Lett 1999;68:147–154 [DOI] [PubMed] [Google Scholar]
- 56. Klein U, and Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol 2008;8:22. [DOI] [PubMed] [Google Scholar]
- 57. Klein U, Tu Y, Stolovitzky GA, et al. Transcriptional analysis of the B cell germinal center reaction. Proc Natl Acad Sci U S A 2003;100:2639–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Krug LT, Moser JM, Dickerson SM, and Speck SH. Inhibition of NF-κB activation in vivo impairs establishment of gammaherpesvirus latency. PLoS Pathog 2007;3:97–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kuo TC, Shaffer AL, Haddad J, Choi YS, Staudt LM, and Calame K. Repression of BCL-6 is required for the formation of human memory B cells in vitro. J Exp Med 2007;204:819–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kurth J, Hansmann ML, Rajewsky K, and Kuppers R. Epstein-Barr virus-infected B cells expanding in germinal centers of infectious mononucleosis patients do not participate in the germinal center reaction. Proc Natl Acad Sci U S A 2003;100:4730–4735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Laichalk LL, and Thorley-Lawson DA. Terminal differentiation into plasma cells initiates the replicative cycle of Epstein-Barr virus in vivo. J Virol 2005;79:1296–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Larsen M, Sauce D, Deback C, et al. Exhausted cytotoxic control of Epstein-Barr virus in human lupus. PLoS Pathog 2011;7:e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li H, Ikuta K, Sixbey JW, and Tibbetts SA. A replication-defective {gamma}-herpesvirus efficiently establishes long-term latency in macrophages but not B cells in vivo. J Virol 2008;82: 8500–8508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Liang X, Collins CM, Mendel JB, Iwakoshi NN, and Speck SH. Gammaherpesvirus-driven plasma cell differentiation regulates virus reactivation from latently infected B lymphocytes. PLoS Pathog 2002;5:e1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lin YW, Wang KJ, Lei HY, et al. Virus replication and cytokine production in dengue virus-infected human B lymphocytes. J Virol 2002;76:12242–12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev 2009;228:342–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ma S.-D., Yu X, Mertz JE, et al. An Epstein-Barr virus (EBV) mutant with enhanced BZLF1 expression causes lymphomas with abortive lytic EBV infection in a humanized mouse model. J Virol 2012;86:7976–7987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ma SD, Hegde S, Young KH, et al. A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas. J Virol 2011;85:165–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ma SD, Tsai MH, Romero-Masters JC, et al. Latent membrane protein 1 (LMP1) and LMP2A collaborate to promote Epstein-Barr virus-induced B cell lymphomas in a cord blood-humanized mouse model but are not essential. J Virol 2017;91:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ma SD, Xu X, Plowshay J, et al. LMP1-deficient Epstein-Barr virus mutant requires T cells for lymphomagenesis. J Clin Invest 2015;125:304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Mancao C, Altmann M, Jungnickel B, and Hammerschmidt W. Rescue of “crippled” germinal center B cells from apoptosis by Epstein-Barr virus. Blood 2005;106:4339–4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Martyn CN, Cruddas M, and Compston DA. Symptomatic Epstein-Barr virus infection and multiple sclerosis. J Neurol Neurosurg Psychiatry 1993;56:167–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Matar CG, Rangaswamy US, Wakeman BS, Iwakoshi N, and Speck SH. Murine gammaherpesvirus 68 reactivation from B cells requires IRF4 but not XBP-1. J Virol 2014;88:11600–11610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Matta H, and Chaudhary PM. Activation of alternative NF-κB pathway by human herpes virus 8-encoded Fas-associated death domain-like IL-1β-converting enzyme inhibitory protein (vFLIP). Proc Natl Acad Sci U S A 2004;101:9399–9404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Mboko WP, Olteanu H, Ray A, et al. Tumor suppressor interferon-regulatory factor 1 counteracts the germinal center reaction driven by a cancer-associated gammaherpesvirus. J Virol 2016;90:2818–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mboko WP, Olteanu H, Ray A, et al. Tumor suppressor IRF-1 counteracts germinal center reaction driven by a cancer-associated gammaherpesvirus. J Virol 2015;90:2818–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. McClellan KB, Gangappa S, Speck SH, and Virgin HW. Antibody-independent control of gamma-herpesvirus latency via B cell induction of anti-viral T cell responses. PLoS Pathog 2006;2:e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Meij P, Leen A, Rickinson AB, et al. Identification and prevalence of CD8(+) T-cell responses directed against Epstein-Barr virus-encoded latent membrane protein 1 and latent membrane protein 2. Int J Cancer 2002;99:93–99 [DOI] [PubMed] [Google Scholar]
- 79. Mesri EA, Cesarman E, Arvanitakis L, et al. Human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus is a new transmissable virus that infects B cells. J Exp Med 1996;183:2385–2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Minhas V, and Wood C. Epidemiology and transmission of Kaposi's sarcoma-associated herpesvirus. Viruses 2014;6:4178–4194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Miyashita EM, Yang B, Lam KM, Crawford DH, and Thorley-Lawson DA. A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell 1995;80:593–601 [DOI] [PubMed] [Google Scholar]
- 82. Monini P, Colombini S, Sturzl M, et al. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood 1999;93:4044–4058 [PubMed] [Google Scholar]
- 83. Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, and Mosmann TR. Homology of cytokine synthesis inhibitory factor (il-10) to the Epstein-Barr virus gene BCRFI. Science 1990;248:1230–1234 [DOI] [PubMed] [Google Scholar]
- 84. Moorman NJ, Willer DO, and Speck SH. The gammaherpesvirus 68 latency-associated nuclear antigen homolog is critical for the establishment of splenic latency. J Virol 2003;77:10295–10303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Morscio J, Dierickx D, and Tousseyn T. Molecular pathogenesis of B-cell posttransplant lymphoproliferative disorder: what do we know so far? Clin Dev Immunol 2013;2013:150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Munz C. Humanized mouse models for Epstein Barr virus infection. Curr Opin Virol 2017;25:113–118 [DOI] [PubMed] [Google Scholar]
- 87. Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, and Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000;102:553–563 [DOI] [PubMed] [Google Scholar]
- 88. Nealy MS, Coleman CB, Li H, and Tibbetts SA. Use of a virus-encoded enzymatic marker reveals that a stable fraction of memory B cells expresses latency-associated nuclear antigen throughout chronic gammaherpesvirus infection. J Virol 2010;84:7523–7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nera K.-P., Kohonen P, Narvi E, et al. Loss of Pax5 promotes plasma cell differentiation. Immunity 2006;24:283–293 [DOI] [PubMed] [Google Scholar]
- 90. Nicholas J, Ruvolo VR, Burns WH, et al. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med 1997;3:287–292 [DOI] [PubMed] [Google Scholar]
- 91. Ok CY, Li L, Xu-Monette ZY, et al. Prevalence and clinical implications of Epstein-Barr virus infection in de novo diffuse large B-cell lymphoma in Western countries. Clin Cancer Res 2014;20:2338–2349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Phan RT, and Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature 2004;432:635–639 [DOI] [PubMed] [Google Scholar]
- 93. Piroozmand A, Haddad Kashani H, and Zamani B. Correlation between Epstein-Barr virus infection and disease activity of systemic lupus erythematosus: a cross-sectional study. Asian Pac J Cancer Prev 2017;18:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rangaswamy US, and Speck SH. Murine gammaherpesvirus M2 protein induction of IRF4 via the NFAT pathway leads to IL-10 expression in B cells. PLoS Pathog 2014;10:e1003858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rappocciolo G, Jenkins FJ, Hensler HR, et al. DC-SIGN is a receptor for human herpesvirus 8 on dendritic cells and macrophages. J Immunol 2006;176:1741–1749 [DOI] [PubMed] [Google Scholar]
- 96. Reddy SS, Foreman HC, Sioux TO, et al. Ablation of STAT3 in the B cell compartment restricts gammaherpesvirus latency in vivo. MBio 2016;7:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Rekow MM, Darrah EJ, Mboko WP, Lange PT, and Tarakanova VL. Gammaherpesvirus targets peritoneal B-1 B cells for long-term latency. Virology 2016;492:140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Robbiani DF, Deroubaix S, Feldhahn N, et al. Plasmodium infection promotes genomic instability and AID-dependent B cell lymphoma. Cell 2015;162:727–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Romero-Masters JC, Ohashi M, Djavadian R, et al. An EBNA3C-deleted Epstein-Barr virus (EBV) mutant causes B-cell lymphomas with delayed onset in a cord blood-humanized mouse model. PLoS Pathog 2018;14:e1007221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Roughan JE, and Thorley-Lawson DA. The intersection of Epstein-Barr virus with the germinal center. J Virol 2009;83:3968–3976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Rousset F, Garcia E, Defrance T, et al. Interleukin 10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci U S A 1992;89:1890–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Roy S, Pavitrakar D, Gunjikar R, Ayachit VM, Bondre VP, and Sapkal GN. Monocytes and B cells support active replication of Chandipura virus. BMC Infect Dis 2016;16:487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Saito M, Gao J, Basso K, et al. A signaling pathway mediating downregulation of BCL6 in germinal center B cells is blocked by BCL6 gene alterations in B cell lymphoma. Cancer Cell 2007;12:280–292 [DOI] [PubMed] [Google Scholar]
- 104. Sangster MY, Topham DJ, D'Costa S, et al. Analysis of the virus-specific and nonspecific B cell response to a persistent B-lymphotropic gammaherpesvirus. J Immunol 2000;164:1820–1828 [DOI] [PubMed] [Google Scholar]
- 105. Shimakage M, Kimura M, Yanoma S, et al. Expression of latent and replicative-infection genes of Epstein-Barr virus in macrophage. Arch Virol 1999;144:157–166 [DOI] [PubMed] [Google Scholar]
- 106. Siegel AM, Herskowitz JH, and Speck SH. The MHV68 M2 protein drives IL-10 dependent B cell proliferation and differentiation. PLoS Pathog 2008;4:e1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sin SH, and Dittmer DP. Viral latency locus augments B-cell response in vivo to induce chronic marginal zone enlargement, plasma cell hyperplasia, and lymphoma. Blood 2013;121:2952–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H, and Nasrallah GK. Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: an update. Front Oncol 2018;8:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Stein H, Bonk A, Tolksdorf G, Lennert K, Rodt H, and Gerdes J. Immunohistologic analysis of the organization of normal lymphoid tissue and non-Hodgkin's lymphomas. J Histochem Cytochem 1980;28:746–760 [DOI] [PubMed] [Google Scholar]
- 110. Steinbruck L, Gustems M, Medele S, Schulz TF, Lutter D, and Hammerschmidt W. K1 and K15 of Kaposi's sarcoma-associated herpesvirus are partial functional homologues of latent membrane protein 2A of Epstein-Barr virus. J Virol 2015;89:7248–7261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Stevenson PG, and Doherty PC. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J Virol 1998;72:943–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stevenson PG, and Doherty PC. Non-antigen-specific B-cell activation following murine gammaherpesvirus infection is CD4 independent in vitro but CD4 dependent in vivo. J Virol 1999;73:1075–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stewart JP, Usherwood EJ, Ross A, Dyson H, and Nash T. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J Exp Med 1998;187:1941–1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sun CC, and Thorley-Lawson DA. Plasma cell-specific transcription factor XBP-1s binds to and transactivates the Epstein-Barr virus BZLF1 promoter. J Virol 2007;81:13566–13577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Tamir I, Dal Porto JM, and Cambier JC. Cytoplasmic protein tyrosine phosphatases SHP-1 and SHP-2: regulators of B cell signal transduction. Curr Opin Immunol 2000;12:307–315 [DOI] [PubMed] [Google Scholar]
- 116. Tarakanova VL, Stanitsa E, Leonardo SM, Bigley TM, and Gauld SB. Conserved gammaherpesvirus kinase and histone variant H2AX facilitate gammaherpesvirus latency in vivo. Virology 2010;405:50–61 [DOI] [PubMed] [Google Scholar]
- 117. Tarakanova VL, Suarez FS, Tibbetts SA, et al. Murine gammaherpesvirus 68 infection induces lymphoproliferative disease and lymphoma in BALB á2 microglobulin deficient mice. J Virol 2005;79:14668–14679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Terrell S, and Speck SH. Murine gammaherpesvirus M2 antigen modulates splenic B cell activation and terminal differentiation in vivo. PLoS Pathog 2017;13:e1006543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Thacker EL, Mirzaei F, and Ascherio A. Infectious mononucleosis and risk for multiple sclerosis: a meta-analysis. Ann Neurol 2006;59:499–503 [DOI] [PubMed] [Google Scholar]
- 120. Thorley-Lawson DA. Epstein-Barr virus: exploiting the immune system. Nat Rev Immunol 2001;1:75–82 [DOI] [PubMed] [Google Scholar]
- 121. Thorley-Lawson DA, and Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med 2004;350:1328–1337 [DOI] [PubMed] [Google Scholar]
- 122. Thornburg N, Kulwichit W, Edwards R, Shair K, Bendt K, and Raab-Traub N. LMP1 signaling and activation of NF-κB in LMP1 transgenic mice. Oncogene 2006;25:288. [DOI] [PubMed] [Google Scholar]
- 123. Tibbetts SA, McClellan JS, Gangappa S, Speck SH, and Virgin HW. Effective vaccination against long-term gammaherpesvirus latency. J Virol 2003;77:2522–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Totonchy J, Osborn JM, Chadburn A, et al. KSHV induces immunoglobulin rearrangements in mature B lymphocytes. PLoS Pathog 2018;14:e1006967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tracy SI, Kakalacheva K, Lunemann JD, Luzuriaga K, Middeldorp J, and Thorley-Lawson DA. Persistence of Epstein-Barr virus in self-reactive memory B cells. J Virol 2012;86:12330–12340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Trapecar M, Khan S, Cohn BL, Wu F, and Sanjabi S. B cells are the predominant mediators of early systemic viral dissemination during rectal LCMV infection. Mucosal Immunol 2018;11:1158–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Uchida J, Yasui T, Takaoka-Shichijo Y, et al. Mimicry of CD40 signals by Epstein-Barr virus LMP1 in B lymphocyte responses. Science 1999;286:300–303 [DOI] [PubMed] [Google Scholar]
- 128. Uppal T, Banerjee S, Sun Z, Verma S, and Robertson E. KSHV LANA—the master regulator of KSHV latency. Viruses 2014;6:4961–4998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Usherwood EJ, Ross AJ, Allen DJ, and Nash AA. Murine gammaherpesvirus-induced splenomegaly: a critical role for CD4 T cells. J Gen Virol 1996;77:627–630 [DOI] [PubMed] [Google Scholar]
- 130. Usherwood EJ, Stewart JP, Robertson K, Allen DJ, and Nash AA. Absence of splenic latency in murine gammaherpesvirus 68-infected B cell-deficient mice. J Gen Virol 1996;77:2819–2825 [DOI] [PubMed] [Google Scholar]
- 131. Virgin HW, Latreille P, Wamsley P, et al. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J Virol 1997;71:5894–5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wagner H, Bein G, Bitsch A, and Kirchner H. Detection and quantification of latently infected B lymphocytes in Epstein-Barr virus-seropositive, healthy individuals by polymerase chain reaction. J Clin Microbiol 1992;30:2826–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wang C, Zhu C, Wei F, et al. Constitutive activation of interleukin-13/STAT6 contributes to Kaposi's sarcoma-associated herpesvirus-related primary effusion lymphoma cell proliferation and survival. J Virol 2015;89:10416–10426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Wang LX, Kang G, Kumar P, et al. Humanized-BLT mouse model of Kaposi's sarcoma-associated herpesvirus infection. Proc Natl Acad Sci U S A 2014;111:3146–3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Weck KE, Kim SS, Virgin HI, and Speck SH. B cells regulate murine gammaherpesvirus 68 latency. J Virol 1999;73:4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Weck KE, Kim SS, Virgin HW, and Speck SH. B cells regulate murine gammaherpesvirus 68 latency. J Virol 1999;73:4651–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Weck KE, Kim SS, Virgin HW, and Speck SH. Macrophages are the major reservoir of latent murine gammaherpesvirus 68 in peritoneal cells. J Virol 1999;73:3273–3283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Wilmore JR, Maue AC, and Rochford R. Plasmodium chabaudi infection induces AID expression in transitional and marginal zone B cells. Immun Inflamm Dis 2016;4:497–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Wilson SJ, Tsao EH, Webb BL, et al. X box binding protein XBP-1s transactivates the Kaposi's sarcoma-associated herpesvirus (KSHV) ORF50 promoter, linking plasma cell differentiation to KSHV reactivation from latency. J Virol 2007;81:13578–13586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Ye B, Cattoretti G, Shen Q, et al. The BCL-6 proto-oncogene controls germinal-centre formation and Th2-type inflammation. Nat Genet 1997;16:161–170 [DOI] [PubMed] [Google Scholar]
- 141. Yu F, Harada JN, Brown HJ, et al. Systematic identification of cellular signals reactivating Kaposi sarcoma-associated herpesvirus. PLoS Pathog 2007;3:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]