Abstract
In mammals, adaptive immunity is mediated by a broadly diverse repertoire of naive B and T lymphocytes that recirculate between secondary lymphoid organs. Initial antigen exposure promotes lymphocyte clonal expansion and differentiation, including the formation of memory cells. Antigen-specific memory cells are maintained at higher frequencies than their naive counterparts and have different functional and homing abilities. Importantly, a subset of memory cells, known as tissue-resident memory cells, is maintained without recirculating in nonlymphoid tissues, often at barrier surfaces, where they can be reactivated by antigen and rapidly perform effector functions that help protect the tissue in which they reside. Although antigen-experienced B cells are abundant at many barrier surfaces, their characterization as tissue-resident memory B (BRM) cells is not well developed. In this study, we describe the characteristics of memory B cells in various locations and discuss their possible contributions to immunity and homeostasis as bona fide BRM cells.
Keywords: memory B cell, tissue-resident memory, mucosal immunity, tissue-specific immunity
Rationale for the Existence of Tissue-Resident Memory B Cells
Primary adaptive immune responses are initiated by the activation of phagocytic antigen-presenting cells (APCs), typically dendritic cells, at the site of antigen/pathogen exposure (22,24). These APCs acquire antigen in peripheral tissues, and after activation, migrate through lymphatics to the draining lymph node (24), where they present antigen to recirculating, antigen-specific, naive T and B cells. In turn, the T and B cells respond to engagement of their T cell or B cell antigen receptors (TCRs or BCRs), combined with signals through co-stimulatory molecules and cytokine receptors (51,143,162), by proliferating and differentiating into specialized effector cell types that eventually recirculate back to the site of inflammation/infection and perform their effector functions.
In acute immune responses, the clonal expansion phase is rapidly followed by a contraction phase (87), in which most of the effector cells die by apoptosis and a much smaller population remains as memory cells (120). Despite the dramatic contraction after a primary response, however, the frequency of antigen-specific memory cells remains much higher than the initial frequency of antigen-specific naive cells (13). Moreover, memory cells are epigenetically modified to more rapidly perform their effector functions following secondary antigen encounter (77,176), thereby preventing or curtailing infections by previously encountered pathogens. Furthermore, memory lymphocytes are often imprinted by the way in which they initially encounter antigen so that they acquire homing or effector functions that are specialized to protect the site of initial antigen encounter or to protect against a particular type of pathogen (70,132,137).
Memory T cells are often broadly categorized as central memory T (TCM) cells or effector memory T (TEM) cells based on their homing properties (131). TCM cells retain expression of CCR7 and CD62L (131), which promote entry into lymph nodes through peripheral lymph node addressin (PNAd)-expressing high endothelial venules (104). In contrast, TEM cells lack CCR7 and CD62L and instead express chemokine receptors like CCR5 and CXCR3 (40,55,67), integrins like α1β1 or α4β7 (122,128), and adhesion molecules like cutaneous lymphocyte antigen (CLA) (28,104), which promote entry and recirculation through specific tissues or inflammatory sites. More recent studies show that a significant portion of memory T cells acquire properties that promote their maintenance (without recirculation), in peripheral tissues like the lung, gut, and skin (38). These cells are a subset of TEM cells and are referred to as tissue-resident memory (TRM) cells.
TRM cells include conventional CD4+ and CD8+ memory T cells, T regulatory cells (Tregs), innate-like T cells, such as invariant natural killer T (iNKT) cells, γδ T cells, and intraepithelial T cells, as well as a variety of innate lymphoid cells (ILCs) (38). In general, these cells locally sense aberrations in the local environment that might be caused by infection, stress, or damage and respond by helping to eliminate infection and restore tissue homeostasis (38). Each cell type responds to different environmental cues, including antigen, cytokines, metabolites, and even damage-associated or pathogen-associated molecular patterns (DAMPs and PAMPs) (38). As a result, these cell types are an important component of immune defense in the context of infection and are also important under homeostatic conditions, and help to maintain barrier integrity and control inflammation (38).
Although the role of B cells at mucosal surfaces is intensely studied (17,35,64,96), the formal identification of resident memory B (BRM) cells in these locations has lagged behind that of TRM cells (100). This oversight may be due, in part, to the idea that the primary function of B cells is to make long-lived antibodies that circulate throughout the body (105), whereas T cells make short-lived cytokines or cytolytic molecules that are targeted specifically to the cells that display their cognate antigens (25). Thus, one might expect that there may be little need to place memory B cells in peripheral tissues. Moreover, the activities of activated B cells are typically performed in specialized domains, such as the germinal centers (GCs) of secondary lymphoid tissues (93). Thus, memory B cells in peripheral tissue may not have access to cell types or structures that they require for their function. Despite these objections, there is now clear evidence that tissue-resident memory B cells not only exist but also perform protective functions in peripheral tissues (3). In this review, we will highlight some of the features of memory B cells in lymphoid and non-lymphoid organs that may contribute to their function as BRM cells.
Identifying Tissue-Resident Memory B Cells
Memory B cells are identified using a variety of markers that often differ between mice and humans. For example, IgD is expressed by naive B cells in both humans and mice and is downregulated upon activation (26). As a result, the loss of IgD is commonly used as a marker of activated/memory B cells in both species (173). IgM is also lost in isotype-switched memory B cells, but is retained in unswitched memory B cells (74). In humans, most memory B cells express CD27 (167); however, this marker is not expressed on murine memory B cells. CD38 is often used to distinguish B cell subsets: naive and memory B cells express CD38 in mice (125), whereas naive and memory B cells lack CD38 in humans (111). CD73 is also a marker of memory B cells in mice (159), at least in lymphoid tissues, although more than half of the memory B cells in the lung lack this marker (3). Thus, there is no single memory marker for B cells. Instead, multiple markers are required to identify memory B cells, in part, by eliminating non-memory cells, including naive B cells, GC B cells, and antibody secreting cells (ASCs). More importantly, we do not yet have a definitive marker of tissue-resident memory B cells in any tissue, meaning that these cells must be identified based on their functional properties.
One way that researchers can functionally distinguish tissue-resident cells from circulating cells is to use intravascular staining, in which a fluorochrome-conjugated antibody specific for the population in question is intravenously administered a few minutes before euthanasia and tissue collection (3,161). In this procedure, all cells in circulation are labeled, whereas the unlabeled cells are “protected” by their residence in a tissue (Box 1). Although this procedure clearly identifies those cells currently in circulation, it does not identify those cells that are circulating, but are currently traversing a tissue compartment (3,161). For example, all naive B cells continuously recirculate between secondary lymphoid organs. However, intravenous administration of anti-CD19 will not label those naive B cells that are currently in a B cell follicle.
To definitively show that a particular cell population resides in a tissue without recirculating, one can surgically join two animals that express distinct alleles of a protein expressed by the population of interest. This procedure, known as parabiosis (61), links the peripheral, but not the lymphatic, circulation of the two animals so that any cell in circulation will freely move between them. Some estimates suggest that, once circulatory equilibrium is established, parabionts completely exchange their entire blood supply up to ten times a day (50). As a consequence, the circulating cells in each tissue will consist of a 50:50 mix of cells from each partner, whereas the tissue-resident, noncirculating cells in each tissue will be primarily composed of endogenous cells, with little to no contribution of partner cells (Fig. 1). In many cases, the intravenous infusion of antibodies is combined with parabiosis to distinguish cells that are currently in tissues, but are actually circulating, from those that are truly tissue resident (3,151) (Box 1). Investigators also use intravital imaging to examine the short-term activities of tissue-resident memory cells in recall responses (12,76,99).
FIG. 1.
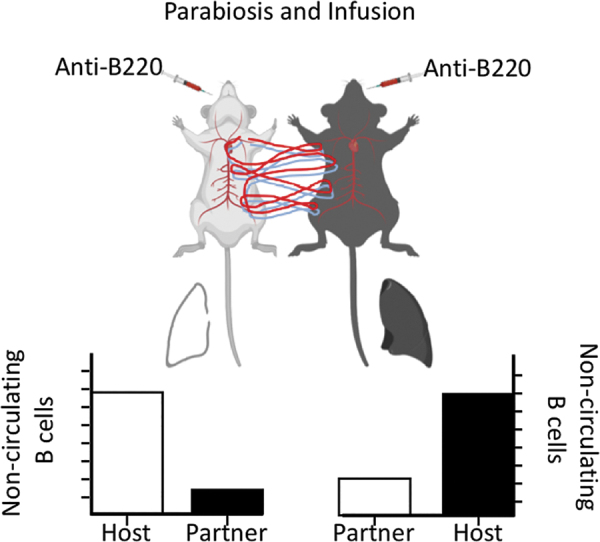
Parabiosis allows the identification of resident memory cells. Mice that can be distinguished by a congenic allele (i.e., CD45.1 and CD45.2) are initially infected/immunized to generate memory B cells. Once memory B cells are established, the mice are surgically joined at the skin and allowed to heal. Joint circulation is established in about 10 days and after that point, circulating cells will consist of a 50:50 mix of cells from each partner. Tissue-resident cells will not reach equilibrium between partner mice, resulting in a high host:partner ratio of cells.
Box 1. Features of tissue-resident memory cells.
Are not labeled with a short pulse of intravenously administered antibody.
Fail to attain equilibrium in tissues of surgically joined (parabiotic) mice.
Lack expression of lymph node homing receptors and instead express homing receptors for peripheral or inflamed tissues.
Rapidly respond to local antigen exposure by performing critical effector functions.
Are established, in part, by antigen encounter at the site of residence.
Tissue-resident memory cells lose their ability to recirculate and maintain their residence in tissues primarily due to alterations in the expression of chemokine and homing receptors (117). For example, most tissue-resident memory cells, including BRM cells, reduce their expression of CCR7 and CD62L (3,104,175), thereby eliminating their ability to traverse high endothelial venules and recirculate through secondary lymphoid organs. Many memory B cells also lose their expression of CXCR5, one of the receptors that maintains B cells in lymphoid follicles (54,172). Moreover, TRM cells also poorly express the sphingosine-1-phosphate receptor (S1PR1) (142), which normally promotes the emigration of lymphocytes from tissues into blood (89). In addition, many TRM cells, as well as BRM cells, express CD69 (134), which antagonizes S1PR1 signaling (134), further impairing their ability to leave tissues and enter the circulation. Thus, tissue-resident memory cells lack homing receptors that allow re-entry into secondary lymphoid tissues or promote exit from lymphoid tissues into the blood (Box 1).
Instead, tissue-resident memory cells express a variety of homing receptors that target them to peripheral sites. For example, BRM cells in the lung express CXCR3 (3), a chemokine receptor that responds to the interferon-inducible chemokines, CXCL9, 10, and 11 (158). Memory B cells also often express CCR6 (36,148), the receptor for CCL20 (56), which is expressed by a variety of epithelial cells (56), including those in the respiratory tract, the intestinal tract, and skin—all sites where tissue-resident memory cells reside. In fact, the intestinal tract is home to more memory B cells than any other site (145) and memory B cells in the gut preferentially express CCR9 (145) and CCR10 (145), often in combination with specific integrins and adhesion molecules that promote homing to the gut as well as gut-associated lymphoid tissues (145).
Most tissue-resident memory cells in the gut express the integrin, α4β7 (57,129), which binds to mucosal addressin cell adhesion molecule (MAdCAM) and promotes homing to gut-associated lymphoid tissues (145). B cells that are primed in gut-associated lymphoid tissues are exposed to retinoic acid (RA), which is produced by retinaldehyde dehydrogenase-expressing epithelial cells and dendritic cells and triggers B cells to express α4β7 and CCR9 (145), and promotes isotype-switching to IgA (97). Similarly, the cutaneous lymphoid antigen (CLA) is commonly expressed by skin-homing lymphocytes (35). CLA binds E-selectin (35), which is upregulated on inflamed endothelial cells, and in combination with chemokine receptors like CCR4, CCR6, and CCR10, guides lymphocytes to the skin (35). Immune responses in skin-draining lymph nodes, but not mucosal lymphoid tissues, promote the expression of CLA by responding B cells (35,62). Moreover, skin-derived dendritic cells metabolize vitamin D3 into 1,25(OH)2D3, the active form of vitamin D3, which promotes CCR10 expression and suppresses α4β7 and CCR9 (35), again supporting a model in which tissue-specific priming promotes the acquisition of tissue-specific homing.
Other integrins are also involved in the homing of memory cells to peripheral tissues or in the persistence of tissue-resident memory cells in those tissues, even though they may not be tissue specific. For example, integrins like α1β1 (VLA-1), a receptor for collagen (122,124), α4β1 (VLA-4), a receptor for VCAM (102) and fibronectin, and αEβ7 (CD103), a receptor for E-cadherin, are often expressed by tissue-resident memory cells (101). CD8+ TRM cells in a variety of locations express CD103 (100), and CD103 expression is important for the maintenance of CD8+ TRM cells in those sites (78,85). However, the expression of CD103 is only reported on malignant B cells (163) and influenza-specific, lung-resident memory B cells completely lack this marker, suggesting that CD103 may not be used by tissue-resident memory B cells. In contrast, VLA-4 is highly expressed on memory B cells (20) and is required for B cells to enter some peripheral sites (79).
Where Might Tissue-Resident Memory B Cells Reside?
Tissue-resident memory T cells and ILCs often reside at barrier surfaces like the respiratory tract, the intestinal tract, and the skin (52). These same sites contain memory B cells. For example, in the upper respiratory tract, memory B cells are found in the nasal-associated lymphoid tissue (NALT) of mice (16,153) and the tonsils and adenoids of humans (75,113,138), as well as in the submucosa of the trachea and nasal passages (64,76,154). In the lower respiratory tract, memory B cells are found in the inducible bronchus-associated lymphoid tissue (iBALT) (4,118), in non-lymphoid areas underneath the airway epithelium (64), and even in the airways themselves (64), perhaps adjacent to airway-resident memory T cells (144). At least some of these memory B cells are bona fide lung-resident memory B cells as shown by antibody infusion and parabiosis experiments (3). Interestingly, the phenotype of memory B cells in the lung is distinct from those in the lymph node. Lung-resident memory B cells uniformly express CXCR3, but more than half of them lack CD73 (3), perhaps indicating a differential dependence on GCs for the generation or that they have different functional capacities to differentiate into ASCs or repopulate secondary GCs (156,177).
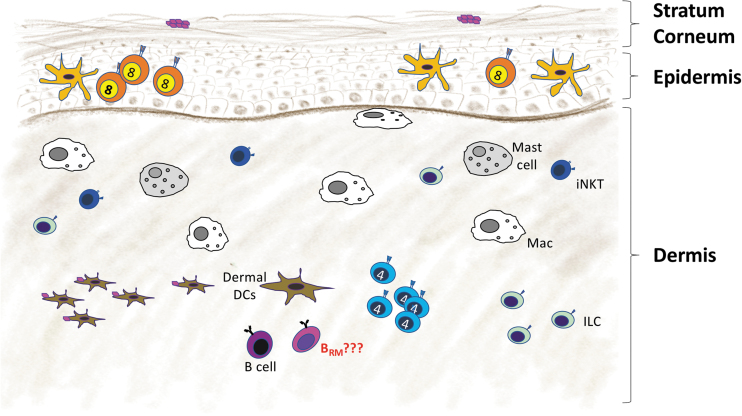
Memory B cells are also found in the skin, one of the largest barrier surfaces and an important site for immunological defense [reviewed in Egbuniwe et al. (35)]. Skin is stratified into an outer layer, the epidermis, a middle layer, the dermis, and a lower layer, the hypodermis (114). Specialized skin dendritic cells (Langerhans cells) and skin-resident memory CD8+ T cells reside in the epidermis (28), whereas macrophages, mast cells, NK cells, skin-resident memory CD4+ T cells (28), dermal dendritic cells, ILCs, and B cells are found in the dermis (Fig. 2) (112). Memory B cells in the skin express E selectin ligand (ESL-1) (95,146) as well as α4 and β1 integrins (41), similar to skin-resident memory CD4+ T cells, which use ESL-1 for homing to inflamed skin (157). Memory B cells in the skin also express CCR6 and migrate toward its ligand, CCL20, which is expressed by skin epithelial cells (41).
FIG. 2.
Resident memory B cells in the skin. The immune cells enter the dermis of the skin through capillary venules and exit through lymphatic vessels. Langerhans cells and resident memory CD8+ T cells are maintained in the epidermis. However, most other immune cells, including macrophages (Macs), ILCs, iNKT cells, mast cells, dermal DCs, and helper CD4+ T cells, are found in the dermis. Memory B cells reside in the dermis and can be reactivated locally by antigen. DCs, dendritic cells; ILCs, innate lymphoid cells; iNKT, invariant natural killer T.
Both B2 and B1 B cells are found in skin, with the B2 population more prevalent than the B1 cells (42). Interestingly, α4β1 integrin-expressing B1 B cells from the peritoneal cavity can migrate to inflamed skin (41) and secrete the anti-inflammatory cytokine, IL-10 (42). Clonal expansions of particular BCRs are observed in both normal skin and melanoma (103,133), suggesting that skin-resident B cells are responding to local antigens. Moreover, activation-induced cytidine deaminase (AID) is expressed by B cells in normal skin and in melanoma lesions (133), suggesting that isotype switching may occur locally in the skin. In melanoma, skin-resident IgG1-expressing B cells inhibit disease (71), whereas IgG4-expressing B cells exacerbate disease (63). Pemphigus patients also experience an isotype-specific activation of their disease (141). Patients with skin allergies or parasitic infection of the skin often have increased frequencies of IgE-expressing B cells in their skin (116). Long-term IgM, IgA, and IgG responses to tumor antigens and autoantigens are observed in the skin, but the origin of these cells and the role of resident memory cells in the maintenance of these cells need further scrutiny (30).
The intestinal tract, another barrier surface, contains more B cells and produces more immunoglobulin than any other location in the body due to continuous exposure to antigens from food and commensal organisms [reviewed in Cerf-Bensussan and Gaboriau-Routhiau (23)]. Memory B cells reside in numerous locations in the gut, including lymphoid structures such as Peyer's patches and isolated lymphoid follicles in the small intestine (83,123), colonic patches in the large intestine (1), and non-lymphoid areas in the lamina propria beneath the mucosal epithelium. The lymphoid tissues of the gut are classic mucosal structures, with a dome epithelium containing M cells that transport antigens from the gut lumen to the underlying immune cells (106). Gut-associated lymphoid tissues contain large B cell follicles, many with GCs, but most of the memory B cells are layered beneath the dome epithelium and some even reside in the basolateral pocket of M cells (43,86), where they are poised to receive incoming antigen. Although the lamina propria of the intestine is not an organized lymphoid tissue, it nonetheless contains a wide variety of tissue-resident cells (150). Intraepithelial lymphocytes are embedded in the intestinal epithelium and consist of both αβ and γδ T cells as well as ILCs (14,44,45,152), but not B cells. Instead, IgM+ and IgA+ memory B cells as well as IgA-secreting plasmablasts reside underneath the epithelium (86). Many memory B cells in the lamina propria and Peyer's patches are long lived (83), perhaps resident. These cells typically express the mucosal homing receptors α4β7 and CCR9 (43), and upon adoptive transfer, recolonize the lamina propria and Peyer's Patches, where they are maintained for extended periods (84).
The formation of IgA-secreting cells in the gut can occur in a T cell-dependent and T cell-independent manner (37); however, the generation of long-lived memory B cells only occurs in T-dependent responses (48,139,145). Interestingly, the sequential introduction of commensals into the gut promotes a series of IgA responses, with IgA-secreting cells of a prior specificity being replaced by those of a newer specificity, suggesting that most IgA-secreting cells are short lived and that they are out-competed by cells responding to more recent antigens (48). However, long-lived memory B cells and IgA production are observed in strong T-dependent responses against pathogens like rotavirus (139,145) or antigens like cholera toxin (6,83). IgM+ memory B cells responding to commensal antigens can persist in gut-associated lymphoid tissues and continuously produce IgM- and IgA-secreting cells (86). However, other studies suggest that oral immunization elicits distinct populations of IgA-secreting cells and memory B cells (10). Taken together, these data suggest that the establishment of memory B cells and perhaps resident memory B cells in the gut is dependent on both the type of antigen and its persistence.
Although gut-associated responses are generally thought to occur in gut-associated lymphoid tissues, a significant portion of IgA is derived from B cells in the peritoneal cavity and Omentum (46,69). The omentum is an adipose tissue that connects the stomach, spleen, pancreas, and colon and contains organized lymphoid structures called milky spots (94). Like the B cells in the peritoneal cavity, many of the B cells in the omental milky spots are B1 cells (119), which are dependent on CXCL13 made by local macrophages (5). Interestingly, peritoneal B1 and B2 cells often home to the small intestine and produce IgA (11), likely due to the activity of GATA6-dependent macrophages in the peritoneal cavity that convert vitamin A to RA (107), which promotes isotype switching to IgA and imprints the expression of gut-homing receptors (98,130). Both B1 and B2 cells in the peritoneal cavity express the S1PR1 (73), which promotes their egress into the circulation. Not surprisingly, treatment with the S1PR1 modulator, FTY-720, leads to the rapid disappearance of peritoneal B cells and reduces secretory IgA production in the gut (73). These data suggest that peritoneal and omental B cells are circulatory rather than resident. However, parabiosis experiments show that both B1 and B2 cells in the peritoneal cavity do not reach equilibrium—even after 8 weeks (5). Moreover, peritoneal infection with Ehrlichia muris elicits IgM+ memory cells and IgM-secreting cells that persist in the peritoneal cavity, even in mice lacking conventional secondary lymphoid organs (59), suggesting that at least some memory B cells maintain residency in the peritoneal cavity and omentum.
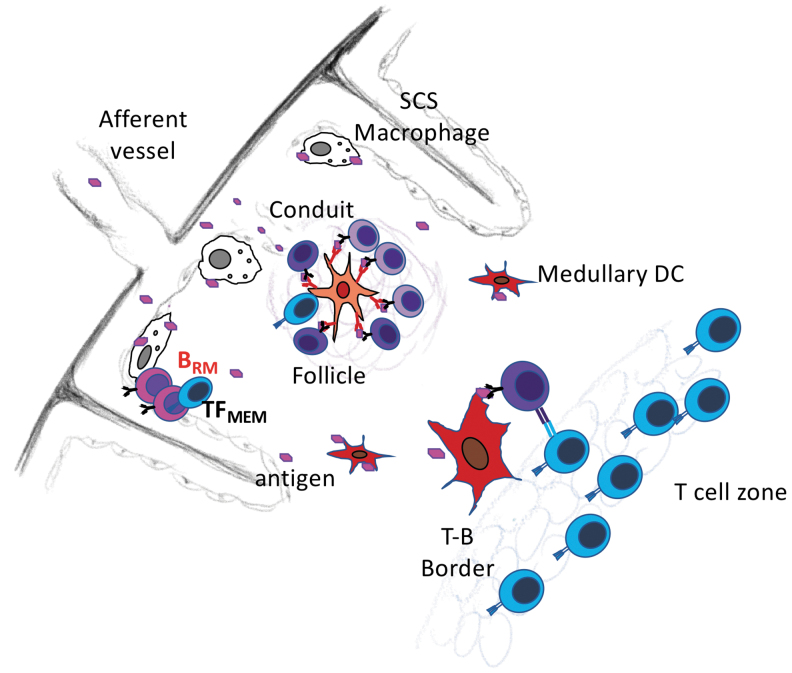
Although tissue-resident memory cells are most often associated with peripheral, non-lymphoid tissues and barrier sites, some tissue-resident memory cells reside in secondary lymphoid organs (2,99). For example, memory B cells reside just beneath the subcapsular sinus of the lymph node (Fig. 3), adjacent to subcapsular macrophages (60,99). Interestingly, CD169+ subcapsular macrophages capture large antigens from the incoming lymph and transfer them to naive B cells, which carry those antigens to the B cell follicle and present them to T cells (21,60,115). However, naive B cells pass through the subcapsular sinus in less than 24 h, whereas memory B cells persist in this location nearly three times as long (99). Upon secondary antigen exposure, memory B cells in the sinus become activated, rapidly proliferate, and generate foci of short-lived antibody-secreting cells (99). Memory B cells also accumulate in the B cell follicles of lymph nodes (39), where they are situated in close proximity to memory T follicular helper cells (8). Again, secondary antigen encounter leads to local proliferation and differentiation (8). Although these studies do not demonstrate tissue residency, other experiments using antibody infusion and parabiosis show that some influenza-specific memory B cells are retained in the lung-draining lymph node and do not recirculate (3). Thus, at least some memory B cells are lymph node-resident memory cells and are positioned for secondary antigen encounter and rapid recall responses.
FIG. 3.
Resident memory B cells are located under the subcapsular sinus in lymph nodes. Circulating and resident memory B cells are found in lymph nodes. At least some of the resident memory B cells reside below the subcapsular sinus. As a result, they are poised to encounter antigens that are delivered from the afferent lymphatic vessels to the subcapsular sinus. Antigens are often captured by subcapsular sinus macrophages, which present antigens to B cells. Memory TFH-like cells also remain in this location and provide help to reactivating memory B cells.
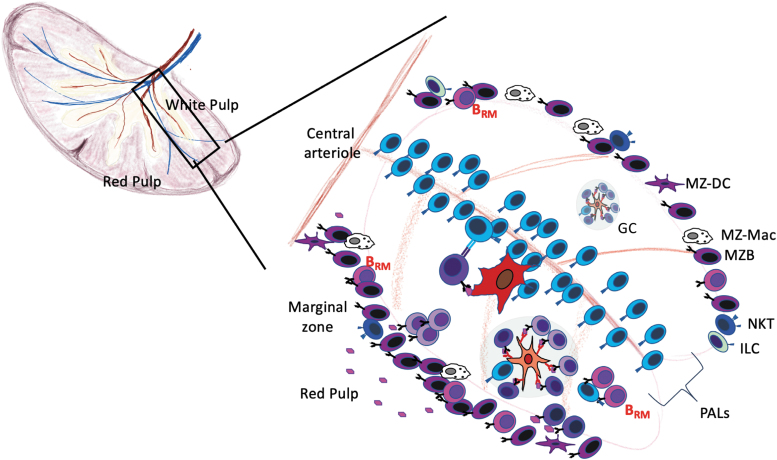
Memory B cells also reside in the spleen (Fig. 4). Many of these memory B cells express CCR6 and are found in the marginal zone and perifollicular areas of the spleen (36,82). Moreover, CCR6 is required for their placement and secondary responsiveness (36). Interestingly, the splenic marginal zone is functionally analogous to the subcapsular area of lymph nodes (92,147). In mice, blood is delivered to the marginal sinus, whereas in humans, the blood is delivered to the red pulp and perifollicular zone (92,147). Like the subcapsular area of lymph nodes, the marginal zone contains B cells and macrophages and collects incoming antigens and pathogens (88). Although most marginal zone B cells are not bona fide memory cells, they poorly migrate in parabiosis experiments (27,160), suggesting that they are primarily a resident population. Marginal zone B cells are phenotypically and functionally distinct from follicular B cells and respond more efficiently to innate signals like LPS (160), although it is not clear whether memory B cells in the marginal zone share these properties. Despite the tissue-resident properties of marginal zone B cells in mice, IgM+CD27+ memory B cells are found in the peripheral blood of humans and have a gene expression profile similar to splenic marginal zone B cells of the same phenotype (169). These cells also have highly mutated BCRs, suggesting that they are selected in GCs (82). Moreover, these cells do not appear in the circulation until marginal zone B cells develop in the spleen and, in splenectomized individuals, the frequency of IgM+CD27+ marginal zone memory cells is reduced (53), suggesting that the circulating cells are directly related to those in the marginal zone.
FIG. 4.
Resident memory B cells in the spleen. The open circulation into the spleen allows antigen entry into the red pulp, which is separated from the white pulp or the PALS by the MZ. Some IgM+ memory B cells reside for extended periods in the MZ, where they are poised to encounter blood-borne antigens. Memory B cells also reside in the B cell follicles of the spleen, just outside GCs near memory TFH cells. IgM+ memory B cells are more scattered throughout the B cell follicle. GCs, germinal centers; MZ, marginal zone; PALS, periarteriolar sheath.
In addition to the memory B cells in the marginal zone, splenic memory B cells are also found in B cell follicles adjacent to GCs and close to CD4 T cells (2), and upon challenge immunization, present antigen to CD4 cells and rapidly differentiate into antibody-secreting cells (2). Moreover, experiments using antibody infusion and parabiosis show that, although most influenza-specific memory B cells in the spleen are recirculating, the memory population does not completely reach equilibrium (3). Thus, at least some memory B cells in the spleen and lymph nodes have properties of tissue-resident cells. However, it is not yet clear what subsets of memory B cells are tissue resident (or not) or whether tissue residency impacts their functional activities.
As detailed in the examples above, tissue-resident memory T and B cells persist at sites of previous antigen encounter, which allows them to rapidly detect incoming antigen and respond accordingly. In part, their tropism to these sites may be due to the expression of homing receptors that were imprinted by location-specific factors. However, the most relevant location-specific factor is likely to be antigen itself. For example, responding T cells must re-encounter antigen in a peripheral site to become tissue-resident memory cells in that tissue (65,150). Similarly, responding B cells must re-encounter antigen in a peripheral tissue to establish tissue residency in that location (3). The requirement for prospective tissue-resident memory cells to re-encounter antigen ensures that they return to the site of antigen exposure, independent of factors like homing receptor expression. This re-encounter with antigen likely occurs very early in a primary response, consistent with the early formation of memory cells in general (168). However, persistent antigen, such as that in oil-emulsion vaccines (18,47), tumors (110,135), or tissue-specific target autoantigens (126), may drive continued recruitment or local expansion of recruited memory cells and maintain their residency at that site.
Functions of Tissue-Resident Memory B Cells
Tissue-resident cells like T cells or ILCs have a variety of functions, most notably the rapid production of effector molecules following infection or local inflammation (72,108). In this regard, tissue-resident memory B cells are likely poised to make antibody following a secondary encounter with antigen. For example, influenza-specific, lung-resident memory B cells rapidly differentiate in situ into antibody-secreting cells following a challenge infection (3), and BRM cells in the subcapsular sinus of lymph nodes rapidly differentiate following recall immunization (99). In the context of infection, rapid antibody production at a barrier surface will likely accelerate pathogen clearance either directly, by neutralizing activity or complement activation, or indirectly, through the FcR-dependent functions of phagocytic or cytolytic cell types (31,149,164).
The current paradigm suggests that reactivated memory B cells either differentiate into antibody-secreting cells or re-enter the GC for continued expansion and affinity maturation (32,91,109). One might expect that tissue-resident memory B cells, particularly those in non-lymphoid compartments like the skin, lung, or gut, might be exclusively biased toward ASC differentiation, as these cells would not reside in (or even near) a B cell follicle and would probably not encounter T follicular helper cells. Moreover, as tissue-resident cells, they have likely lost the homing receptors to return to lymphoid structures. Consequently, resident memory cells in peripheral tissues may be restricted to a single fate—rapid differentiation into an antibody-secreting cell. In contrast, those memory B cells in follicular areas are more likely to encounter TFH cells and may be destined to re-enter the GC. Given that subsets of memory B cells that are more prone to become antibody-secreting cells or re-enter the GC can be defined based on cell surface phenotype (177), it seems likely that these same markers (and perhaps additional markers like CD73) (3,159) may distinguish tissue-resident memory B cells in different locations and with different functions. Another interesting question is whether tissue-resident memory cells are destined to terminally differentiate into antibody-secreting cells or whether they also self-renew in the periphery, outside of a GC.
Recall responses of tissue-resident memory B cells are almost certainly dependent on interactions with T cells. Although dendritic cells are often described as the most potent APCs for priming T cells, B cells can be particularly effective APCs due to their ability to selectively (and with high affinity) acquire cognate antigen through their BCR. As a result, tissue-resident memory B cells may be particularly potent APCs for T cells of the same specificity, perhaps themselves tissue-resident memory cells, thereby promoting the production of cytokines or cytolytic activity. This enhanced antigen-presenting function may be beneficial when re-encountering pathogens (155), but may be detrimental when re-encountering allergens or autoantigens, due to the inappropriate production of cytokines that recruit inflammatory cells or cause tissue damage (126).
Of course, cytokines are also produced by activated B cells, and like T cells, the way in which B cells are initially activated determines what cytokines they are likely to make (33,34,49). Notably, B cells can make inflammatory cytokines like IFNγ and GM-CSF (9,121,166), which along with antibodies, may help clear infection, but may also promote pathological responses and tissue damage. For example, memory B cells are associated with autoimmune diseases of the skin, including pemphigus vulgaris (174), atopic dermatitis (29), scleroderma (15), and Sjogren's syndrome (127). Given that pemphigus is mediated by autoantibodies against desmoglein 1 and 3 (174) and that lesions return at the same sites in the skin, one might expect that tissue-resident memory B cells are, in part, responsible for this pathology, perhaps by making cytokines and by locally stimulating antigen-specific memory T cells. Conversely, B cells can also produce anti-inflammatory cytokines, including IL-10 and IL-35 (19,136), which reduce inflammation and help restore tissue homeostasis. In fact, IL-10-expressing B cells reside in normal skin (42) and suppress inflammation-driven fibrosis (90), psoriasis (7), and SLE (171). These cells may also promote wound healing (58), as the introduction of splenic B cells to wounds increased healing (140). One might imagine that pathogen-specific memory B cells in the lung or gut may perform similar functions.
B cells have a particular association with Th2 cells. B cells are required to maintain Th2 memory cells (81) and to eliminate helminths by enhancing Th2 responses (170). B cells are also required to generate and maintain allergic Th2 responses in the airways (68,80). Along with Th2 cells, B cells can also promote the progression of tumors (63). These responses all involve the antigen-presenting functions of B cells (80,170), most likely to TFH-like cells that are the precursors to Th2 cells (80). These same functions are likely to exacerbate Th2-driven responses like atopic dermatitis (66), perhaps through antigen-presenting skin-resident memory B cells. In contrast, B cells reduce local inflammation in contact hypersensitivity by acting as regulatory B cells (165). Given that many of these activities occur in non-lymphoid tissues, it seems likely that BRM cells are contributing to these processes.
Conclusions
The focus of many researchers on B cell activities in specialized domains of secondary lymphoid organs and the idea that the function of B cells is limited to antibody production have delayed the identification and functional characterization of tissue-resident memory B cells. However, recent advances in our ability to identify antigen-specific B cells using labeled antigens and “B cell tetramers” combined with techniques like parabiosis, antibody infusion, and intravital microscopy are now making it possible to characterize antigen-specific memory B cells in peripheral tissues. In addition, the appreciation that B cells have antibody-independent functions that allow them to regulate immune responses both positively and negatively provides an incentive to better understand how B cells participate in health and disease. As a result, we are now looking forward to new studies demonstrating the functional contribution of tissue-resident memory B cells in resistance to infection, the progression and resolution of inflammation, as well as tissue homeostasis and repair.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was funded by grants, AI097357, AI109962, and AI142737, from the National Institute for Allergy and Infectious Diseases.
References
- 1. Agace WW, and McCoy KD. Regionalized development and maintenance of the intestinal adaptive immune landscape. Immunity 2017;46:532–548 [DOI] [PubMed] [Google Scholar]
- 2. Aiba Y, Kometani K, Hamadate M, et al. Preferential localization of IgG memory B cells adjacent to contracted germinal centers. Proc Natl Acad Sci U S A 2010;107:12192–12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allie SR, Bradley JE, Mudunuru U, et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol 2019;20:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Allie SR, and Randall TD. Pulmonary immunity to viruses. Clin Sci (Lond) 2017;131:1737–1762 [DOI] [PubMed] [Google Scholar]
- 5. Ansel KM, Harris RBS, and Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 2002;16:67–76 [DOI] [PubMed] [Google Scholar]
- 6. Apter FM, Lencer WI, Finkelstein RA, et al. Monoclonal immunoglobulin A antibodies directed against cholera toxin prevent the toxin-induced chloride secretory response and block toxin binding to intestinal epithelial cells in vitro. Infect Immun 1993;61:5271–5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asadullah K, Sterry W, Stephanek K, et al. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J Clin Invest 1998;101:783–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asrir A, Aloulou M, Gador M, et al. Interconnected subsets of memory follicular helper T cells have different effector functions. Nat Commun 2017;8:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ballesteros-Tato A, Stone SL, and Lund FE. Innate IFNγ-producing B cells. Cell Res 2014;24:135–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bemark M, Hazanov H, Strömberg A, et al. Limited clonal relatedness between gut IgA plasma cells and memory B cells after oral immunization. Nat Commun 2016;7:12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berberich S, Förster R, and Pabst O. The peritoneal micromilieu commits B cells to home to body cavities and the small intestine. Blood 2007;109:4627–4634 [DOI] [PubMed] [Google Scholar]
- 12. Beura LK, Mitchell JS, Thompson EA, et al. Intravital mucosal imaging of CD8+ resident memory T cells shows tissue-autonomous recall responses that amplify secondary memory. Nat Immunol 2018;19:173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Blattman JN, Antia R, Sourdive DJD, et al. Estimating the precursor frequency of naive antigen-specific CD8 T cells. J Exp Med 2002;195:657–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonneville M, Janeway CA, Ito K, et al. Intestinal intraepithelial lymphocytes are a distinct set of γδ T cells. Nature 1988;336:479–481 [DOI] [PubMed] [Google Scholar]
- 15. Bosello S, Angelucci C, Lama G, et al. Characterization of inflammatory cell infiltrate of scleroderma skin: B cells and skin score progression. Arthritis Res Ther 2018;20:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boyden AW, Legge KL, and Waldschmidt TJ. Pulmonary infection with influenza A virus induces site-specific germinal center and T follicular helper cell responses. PLoS One 2012;7:e40733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brandtzaeg P, and Johansen F-E. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev 2005;206:32–63 [DOI] [PubMed] [Google Scholar]
- 18. Brewer KD, Weir GM, Dude I, et al. Unique depot formed by an oil based vaccine facilitates active antigen uptake and provides effective tumour control. J Biomed Sci 2018;25:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burdin N, Rousset F, and Banchereau J. B-cell-derived IL-10: production and function. Methods 1997;11:98–111 [DOI] [PubMed] [Google Scholar]
- 20. Camponeschi A, Gerasimcik N, Wang Y, et al. Dissecting integrin expression and function on memory B cells in mice and humans in autoimmunity. Front Immunol 2019;10:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carrasco YR, and Batista FD. B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 2007;27:160–171 [DOI] [PubMed] [Google Scholar]
- 22. Cella M, Sallusto F, and Lanzavecchia A. Origin, maturation and antigen presenting function of dendritic cells. Curr Opin Immunol 1997;9:10–16 [DOI] [PubMed] [Google Scholar]
- 23. Cerf-Bensussan N, and Gaboriau-Routhiau V. The immune system and the gut microbiota: friends or foes? Nat Rev Immunol 2010;10:735–744 [DOI] [PubMed] [Google Scholar]
- 24. Chang S-Y, Ko H-J, and Kweon M-N. Mucosal dendritic cells shape mucosal immunity. Exp Mol Med 2014;46:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charles A, Janeway J, Travers P, Walport M, and Shlomchik MJ. 2001. T cell-mediated cytotoxicity. Immunobiology: The Immune System in Health and Disease. 5th ed. New York, NY: Garland Science [Google Scholar]
- 26. Chen K, and Cerutti A. The function and regulation of immunoglobulin D. Curr Opin Immunol 2011;23:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cinamon G, Matloubian M, Lesneski MJ, et al. Sphingosine 1-phosphate receptor 1 promotes B cell localization in the splenic marginal zone. Nat Immunol 2004;5:713–720 [DOI] [PubMed] [Google Scholar]
- 28. Clark RA. Skin-resident T cells: the ups and downs of on site immunity. J Invest Dermatol 2010;130:362–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Czarnowicki T, Gonzalez J, Bonifacio KM, et al. Diverse activation and differentiation of multiple B-cell subsets in patients with atopic dermatitis but not in patients with psoriasis. J Allergy Clin Immunol 2016;137:118..e5–129.e5. [DOI] [PubMed] [Google Scholar]
- 30. Debes GF, and McGettigan SE. Skin-associated B cells in health and inflammation. J Immunol 2019;202:1659–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. DiLillo DJ, and Ravetch JV. Fc-receptor interactions regulate both cytotoxic and immunomodulatory therapeutic antibody effector functions. Cancer Immunol Res 2015;3:704–713 [DOI] [PubMed] [Google Scholar]
- 32. Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions. Nat Immunol 2009;10:1292–1299 [DOI] [PubMed] [Google Scholar]
- 33. Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007;178:6092–6099 [DOI] [PubMed] [Google Scholar]
- 34. Duddy ME, Alter A, and Bar-Or A. Distinct profiles of human B cell effector cytokines: a role in immune regulation? J Immunol 2004;172:3422–3427 [DOI] [PubMed] [Google Scholar]
- 35. Egbuniwe IU, Karagiannis SN, Nestle FO, et al. Revisiting the role of B cells in skin immune surveillance. Trends Immunol 2015;36:102–111 [DOI] [PubMed] [Google Scholar]
- 36. Elgueta R, Marks E, Nowak E, et al. CCR6-dependent positioning of memory B cells is essential for their ability to mount a recall response to antigen. J Immunol 2015;194:505–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fagarasan S, Kawamoto S, Kanagawa O, et al. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu Rev Immunol 2010;28:243–273 [DOI] [PubMed] [Google Scholar]
- 38. Fan X, and Rudensky AY. Hallmarks of tissue-resident lymphocytes. Cell 2016;164:1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fazilleau N, Eisenbraun MD, Malherbe L, et al. Lymphoid reservoirs of antigen-specific memory T helper cells. Nat Immunol 2007;8:753–761 [DOI] [PubMed] [Google Scholar]
- 40. Fukada K, Sobao Y, Tomiyama H, et al. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J Immunol 2002;168:2225–2232 [DOI] [PubMed] [Google Scholar]
- 41. Geherin SA, Fintushel SR, Lee MH, et al. The skin, a novel niche for recirculating B cells. J Immunol 2012;188:6027–6035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geherin SA, Gómez D, Glabman RA, et al. IL-10+ innate-like B cells are part of the skin immune system and require α4β1 integrin to migrate between the peritoneum and inflamed skin. J Immunol 2016;196:2514–2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Golovkina TV, Shlomchik M, Hannum L, et al. Organogenic role of B lymphocytes in mucosal immunity. Science 1999;286:1965–1968 [DOI] [PubMed] [Google Scholar]
- 44. Goodman T, and Lefrançois L. Expression of the γ-δ T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature 1988;333:855–858 [DOI] [PubMed] [Google Scholar]
- 45. Guy-Grand D, Cerf-Bensussan N, Malissen B, et al. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med 1991;173:471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ha S, Tsuji M, Suzuki K, et al. Regulation of B1 cell migration by signals through Toll-like receptors. J Exp Med 2006;203:2541–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hailemichael Y, Dai Z, Jaffarzad N, et al. Persistent antigen at vaccination sites induces tumor-specific CD8+ T cell sequestration, dysfunction and deletion. Nat Med 2013;19:465–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hapfelmeier S, Lawson MAE, Slack E, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science 2010;328:1705–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Harris DP, Haynes L, Sayles PC, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol 2000;1:475–482 [DOI] [PubMed] [Google Scholar]
- 50. Harris RBS. Contribution made by parabiosis to the understanding of energy balance regulation. Biochim Biophys Acta 2013;1832:1449–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Harwood NE, and Batista FD. Early events in B cell activation. Annu Rev Immunol 2010;28:185–210 [DOI] [PubMed] [Google Scholar]
- 52. Hazenberg MD, and Spits H. Human innate lymphoid cells. Blood 2014;124:700–709 [DOI] [PubMed] [Google Scholar]
- 53. Hendricks J, Bos NA, and Kroese FGM. Heterogeneity of memory marginal zone B cells. Crit Rev Immunol 2018;38:145–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Henneken M, Dörner T, Burmester G-R, et al. Differential expression of chemokine receptors on peripheral blood B cells from patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther 2005;7:R1001–R1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu JK, Kagari T, Clingan JM, et al. Expression of chemokine receptor CXCR3 on T cells affects the balance between effector and memory CD8 T-cell generation. Proc Natl Acad Sci U S A 2011;108:E118–E127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ito T, Carson WF, Cavassani KA, et al. CCR6 as a mediator of immunity in the lung and gut. Exp Cell Res 2011;317:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Iwata M, Hirakiyama A, Eshima Y, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity 2004;21:527–538 [DOI] [PubMed] [Google Scholar]
- 58. Iwata Y, Yoshizaki A, Komura K, et al. CD19, a response regulator of B lymphocytes, regulates wound healing through hyaluronan-induced TLR4 signaling. Am J Pathol 2009;175:649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jones DD, Racine R, Wittmer ST, et al. The omentum is a site of protective IgM production during intracellular bacterial infection. Infect Immun 2015;83:2139–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Junt T, Moseman EA, Iannacone M, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 2007;450:110–114 [DOI] [PubMed] [Google Scholar]
- 61. Kamran P, Sereti K-I, Zhao P, et al. Parabiosis in mice: a detailed protocol. J Vis Exp 2013;80:DOI: 10.3791/50556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kantele A, Savilahti E, Tiimonen H, et al. Cutaneous lymphocyte antigen expression on human effector B cells depends on the site and on the nature of antigen encounter. Eur J Immunol 2003;33:3275–3283 [DOI] [PubMed] [Google Scholar]
- 63. Karagiannis P, Gilbert AE, Josephs DH, et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J Clin Invest 2013;123:1457–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kato A, Hulse KE, Tan BK, et al. B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol 2013;131:933–957; quiz 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Khan TN, Mooster JL, Kilgore AM, et al. Local antigen in nonlymphoid tissue promotes resident memory CD8+ T cell formation during viral infection. J Exp Med 2016;213:951–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Klonowska J, Gleń J, Nowicki RJ, et al. New cytokines in the pathogenesis of atopic dermatitis—new therapeutic targets. Int J Mol Sci 2018;19:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kohlmeier JE, Miller SC, Smith J, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity 2008;29:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kool M, Hammad H, and Lambrecht BN. Cellular networks controlling Th2 polarization in allergy and immunity. F1000 Biol Rep 2012;4:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kroese FG, Butcher EC, Stall AM, et al. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol 1989;1:75–84 [DOI] [PubMed] [Google Scholar]
- 70. Krzysiek R, de Goër de Herve M-G, Yang H, et al. Tissue competence imprinting and tissue residency of CD8 T cells. Front Immunol 2013;4:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kubach J, Hubo M, Amendt C, et al. IgG1 anti-epidermal growth factor receptor antibodies induce CD8-dependent antitumor activity. Int J Cancer 2015;136:821–830 [DOI] [PubMed] [Google Scholar]
- 72. Kumar BV, Kratchmarov R, Miron M, et al. Functional heterogeneity of human tissue-resident memory T cells based on dye efflux capacities. JCI Insight 2018;3:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kunisawa J, Kurashima Y, Gohda M, et al. Sphingosine 1-phosphate regulates peritoneal B-cell trafficking for subsequent intestinal IgA production. Blood 2007;109:3749–3756 [DOI] [PubMed] [Google Scholar]
- 74. Kurosaki T, Kometani K, and Ise W. Memory B cells. Nat Rev Immunol 2015;15:149–159 [DOI] [PubMed] [Google Scholar]
- 75. Laichalk LL, Hochberg D, Babcock GJ, et al. The dispersal of mucosal memory B cells: evidence from persistent EBV infection. Immunity 2002;16:745–754 [DOI] [PubMed] [Google Scholar]
- 76. Lambert Emo K, Hyun Y-M, Reilly E, et al. Live imaging of influenza infection of the trachea reveals dynamic regulation of CD8+ T cell motility by antigen. PLoS Pathog 2016;12:e1005881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lau CM, Adams NM, Geary CD, et al. Epigenetic control of innate and adaptive immune memory. Nat Immunol 2018;19:963–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee Y-T, Suarez-Ramirez JE, Wu T, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J Virol 2011;85:4085–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lehmann-Horn K, Sagan SA, Winger RC, et al. CNS accumulation of regulatory B cells is VLA-4-dependent. Neurol Neuroimmunol Neuroinflammation 2016;3:e212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. León B, Ballesteros-Tato A, Browning JL, et al. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol 2012;13:681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Linton P-J, Bautista B, Biederman E, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med 2003;197:875–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liu Y-J, Oldfield S, and Maclennan ICM. Memory B cells in T cell-dependent antibody responses colonize the splenic marginal zones. Eur J Immunol 1988;18:355–362 [DOI] [PubMed] [Google Scholar]
- 83. Lycke N, and Holmgren J. Intestinal mucosal memory and presence of memory cells in lamina propria and Peyer's patches in mice 2 years after oral immunization with cholera toxin. Scand J Immunol 1986;23:611–616 [DOI] [PubMed] [Google Scholar]
- 84. Lycke NY, and Bemark M. The role of Peyer's patches in synchronizing gut IgA responses. Front Immunol 2012;3:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mackay LK, Rahimpour A, Ma JZ, et al. The developmental pathway for CD103(+)CD8+ tissue-resident memory T cells of skin. Nat Immunol 2013;14:1294–1301 [DOI] [PubMed] [Google Scholar]
- 86. Magri G, Comerma L, Pybus M, et al. Human secretory IgM emerges from plasma cells clonally related to gut memory B cells and targets highly diverse commensals. Immunity 2017;47:118..e8–134.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Marrack P, Scott-Browne J, and MacLeod MKL. Terminating the immune response. Immunol Rev 2010;236:5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Martin F, and Kearney JF. Marginal-zone B cells. Nat Rev Immunol 2002;2:323–335 [DOI] [PubMed] [Google Scholar]
- 89. Matloubian M, Lo CG, Cinamon G, et al. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004;427:355–360 [DOI] [PubMed] [Google Scholar]
- 90. Matsushita T, Kobayashi T, Mizumaki K, et al. BAFF inhibition attenuates fibrosis in scleroderma by modulating the regulatory and effector B cell balance. Sci Adv 2018;4:eaas9944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. McHeyzer-Williams LJ, Milpied PJ, Okitsu SL, et al. Class-switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol 2015;16:296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mebius RE, and Kraal G. Structure and function of the spleen. Nat Rev Immunol 2005;5:606–616 [DOI] [PubMed] [Google Scholar]
- 93. Mesin L, Ersching J, and Victora GD. Germinal center B cell dynamics. Immunity 2016;45:471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Meza-Perez S, and Randall TD. Immunological functions of the omentum. Trends Immunol 2017;38:526–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Montoya MC, Holtmann K, Snapp KR, et al. Memory B lymphocytes from secondary lymphoid organs interact with E-selectin through a novel glycoprotein ligand. J Clin Invest 1999;103:1317–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Moore BB, Moore TA, and Toews GB. Role of T- and B-lymphocytes in pulmonary host defences. Eur Respir J 2001;18:846–856 [DOI] [PubMed] [Google Scholar]
- 97. Mora JR, Iwata M, Eksteen B, et al. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science 2006;314:1157–1160 [DOI] [PubMed] [Google Scholar]
- 98. Mora JR, and von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Semin Immunol 2009;21:28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Moran I, Nguyen A, Khoo WH, et al. Memory B cells are reactivated in subcapsular proliferative foci of lymph nodes. Nat Commun 2018;9:3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Mueller SN, and Mackay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 2015;16:79–89 [DOI] [PubMed] [Google Scholar]
- 101. Mueller SN, Zaid A, and Carbone FR. Tissue-resident T cells: dynamic players in skin immunity. Front Immunol 2014;5:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Newham P, Craig SE, Seddon GN, et al. Alpha4 integrin binding interfaces on VCAM-1 and MAdCAM-1. Integrin binding footprints identify accessory binding sites that play a role in integrin specificity. J Biol Chem 1997;272:19429–19440 [DOI] [PubMed] [Google Scholar]
- 103. Nihal M, Mikkola D, and Wood GS. Detection of clonally restricted immunoglobulin heavy chain gene rearrangements in normal and lesional skin: analysis of the B cell component of the skin-associated lymphoid tissue and implications for the molecular diagnosis of cutaneous B cell lymphomas. J Mol Diagn 2000;2:5–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Nolz JC, Starbeck-Miller GR, and Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy 2011;3:1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Nutt SL, Hodgkin PD, Tarlinton DM, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol 2015;15:160–171 [DOI] [PubMed] [Google Scholar]
- 106. Ohno H. Intestinal M cells. J Biochem 2016;159:151–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Okabe Y, and Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell 2014;157:832–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Panda SK, and Colonna M. Innate lymphoid cells in mucosal immunity. Front Immunol 2019;10:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pape KA, Taylor JJ, Maul RW, et al. Different B cell populations mediate early and late memory during an endogenous immune response. Science 2011;331:1203–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Park SL, Buzzai A, Rautela J, et al. Tissue-resident memory CD8+ T cells promote melanoma-immune equilibrium in skin. Nature 2019;565:366–371 [DOI] [PubMed] [Google Scholar]
- 111. Pascual V, Liu YJ, and Banchereau J. Normal human B cell sub-populations and their malignant counterparts. Baillieres Clin Haematol 1997;10:525–538 [DOI] [PubMed] [Google Scholar]
- 112. Pasparakis M, Haase I, and Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol 2014;14:289–301 [DOI] [PubMed] [Google Scholar]
- 113. Pérez ME, Billordo LA, Baz P, et al. Human memory B cells isolated from blood and tonsils are functionally distinctive. Immunol Cell Biol 2014;92:882–887 [DOI] [PubMed] [Google Scholar]
- 114. Pérez-Sánchez A, Barrajón-Catalán E, Herranz-López M, et al. Nutraceuticals for skin care: a comprehensive review of human clinical studies. Nutrients 2018;10:1–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Phan TG, Grigorova I, Okada T, et al. Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol 2007;8:992–1000 [DOI] [PubMed] [Google Scholar]
- 116. Platts-Mills TAE, Schuyler AJ, Erwin EA, et al. IgE in the diagnosis and treatment of allergic disease. J Allergy Clin Immunol 2016;137:1662–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rahimi RA, and Luster AD. Chemokines: critical regulators of memory T cell development, maintenance, and function. Adv Immunol 2018;138:71–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, et al. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 2011;12:639–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, et al. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity 2009;30:731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ratajczak W, Niedźwiedzka-Rystwej P, Tokarz-Deptuła B, et al. Immunological memory cells. Cent Eur J Immunol 2018;43:194–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Rauch PJ, Chudnovskiy A, Robbins CS, et al. Innate response activator B cells protect against microbial sepsis. Science 2012;335:597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Ray SJ, Franki SN, Pierce RH, et al. The collagen binding alpha1beta1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity 2004;20:167–179 [DOI] [PubMed] [Google Scholar]
- 123. Reboldi A, and Cyster JG. Peyer's patches: organizing B-cell responses at the intestinal frontier. Immunol Rev 2016;271:230–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Richter M, Ray SJ, Chapman TJ, et al. Collagen distribution and expression of collagen-binding α1β1 (VLA-1) and α2β1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J Immunol 2007;178:4506–4516 [DOI] [PubMed] [Google Scholar]
- 125. Ridderstad A, and Tarlinton DM. Kinetics of establishing the memory B cell population as revealed by CD38 expression. J Immunol 1998;160:4688–4695 [PubMed] [Google Scholar]
- 126. Riedhammer C, and Weissert R. Antigen presentation, autoantigens, and immune regulation in multiple sclerosis and other autoimmune diseases. Front Immunol 2015;6:1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Roguedas A-M, Pers J-O, Lemasson G, et al. Memory B-cell aggregates in skin biopsy are diagnostic for primary Sjögren's syndrome. J Autoimmun 2010;35:241–247 [DOI] [PubMed] [Google Scholar]
- 128. Rosé JR, Williams MB, Rott LS, et al. Expression of the mucosal homing receptor alpha4beta7 correlates with the ability of CD8+ memory T cells to clear rotavirus infection. J Virol 1998;72:726–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rott LS, Rosé JR, Bass D, et al. Expression of mucosal homing receptor alpha4beta7 by circulating CD4+ cells with memory for intestinal rotavirus. J Clin Invest 1997;100:1204–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Roy B, Brennecke A-M, Agarwal S, et al. An intrinsic propensity of murine peritoneal B1b cells to switch to IgA in presence of TGF-β and retinoic acid. PLoS One 2013;8:e82121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sallusto F, Geginat J, and Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 2004;22:745–763 [DOI] [PubMed] [Google Scholar]
- 132. Sandoval F, Terme M, Nizard M, et al. Mucosal imprinting of vaccine-induced CD8+ T cells is crucial to inhibit the growth of mucosal tumors. Sci Transl Med 2013;5:172ra20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Saul L, Ilieva KM, Bax HJ, et al. IgG subclass switching and clonal expansion in cutaneous melanoma and normal skin. Sci Rep 2016;6:29736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Schenkel JM, and Masopust D. Tissue-resident memory T cells. Immunity 2014;41:886–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schietinger A, Philip M, Krisnawan VE, et al. Tumor-specific T cell dysfunction is a dynamic antigen-driven differentiation program initiated early during tumorigenesis. Immunity 2016;45:389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Shen P, Roch T, Lampropoulou V, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014;507:366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sheridan BS, and Lefrançois L. Regional and mucosal memory T cells. Nat Immunol 2011;12:485–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Shimoda M, Nakamura T, Takahashi Y, et al. Isotype-specific selection of high affinity memory B cells in nasal-associated lymphoid tissue. J Exp Med 2001;194:1597–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Shirakawa A-K, Nagakubo D, Hieshima K, et al. 1,25-Dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B cells. J Immunol 2008;180:2786–2795 [DOI] [PubMed] [Google Scholar]
- 140. Sîrbulescu RF, Boehm CK, Soon E, et al. Mature B cells accelerate wound healing after acute and chronic diabetic skin lesions. Wound Repair Regen 2017;25:774–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Sitaru C, Mihai S, and Zillikens D. The relevance of the IgG subclass of autoantibodies for blister induction in autoimmune bullous skin diseases. Arch Dermatol Res 2007;299:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Skon CN, Lee J-Y, Anderson KG, et al. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 2013;14:1285–1293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Smith-Garvin JE, Koretzky GA, and Jordan MS. T cell activation. Annu Rev Immunol 2009;27:591–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Snyder ME, Finlayson MO, Connors TJ, et al. Generation and persistence of human tissue-resident memory T cells in lung transplantation. Sci Immunol 2019;4:eaav5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Spencer J, and Sollid LM. The human intestinal B-cell response. Mucosal Immunol 2016;9:1113–1124 [DOI] [PubMed] [Google Scholar]
- 146. Steegmaier M, Levinovitz A, Isenmann S, et al. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature 1995;373:615–620 [DOI] [PubMed] [Google Scholar]
- 147. Steiniger B, Timphus EM, and Barth PJ. The splenic marginal zone in humans and rodents: an enigmatic compartment and its inhabitants. Histochem Cell Biol 2006;126:641–648 [DOI] [PubMed] [Google Scholar]
- 148. Suan D, Kräutler NJ, Maag JLV, et al. CCR6 defines memory B cell precursors in mouse and human germinal centers, revealing light-zone location and predominant low antigen affinity. Immunity 2017;47:1142..e4–1153.e4. [DOI] [PubMed] [Google Scholar]
- 149. Swanson JA, and Hoppe AD. The coordination of signaling during Fc receptor-mediated phagocytosis. J Leukoc Biol 2004;76:1093–1103 [DOI] [PubMed] [Google Scholar]
- 150. Takamura S. Niches for the long-term maintenance of tissue-resident memory T cells. Front Immunol 2018;9:1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Takamura S, Yagi H, Hakata Y, et al. Specific niches for lung-resident memory CD8+ T cells at the site of tissue regeneration enable CD69-independent maintenance. J Exp Med 2016;213:3057–3073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Talayero P, Mancebo E, Calvo-Pulido J, et al. Innate lymphoid cells groups 1 and 3 in the epithelial compartment of functional human intestinal allografts. Am J Transplant 2016;16:72–82 [DOI] [PubMed] [Google Scholar]
- 153. Tamura S, and Kurata T. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn J Infect Dis 2004;57:236–247 [PubMed] [Google Scholar]
- 154. Tan BK, Peters AT, Schleimer RP, et al. Pathogenic and protective roles of B cells and antibodies in patients with chronic rhinosinusitis. J Allergy Clin Immunol 2018;141:1553–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Tarlinton D. Antigen presentation by memory B cells: the sting is in the tail. Science 1997;276:374–375 [DOI] [PubMed] [Google Scholar]
- 156. Taylor JJ, Pape KA, and Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med 2012;209:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Tietz W, Allemand Y, Borges E, et al. CD4+ T cells migrate into inflamed skin only if they express ligands for E- and P-selectin. J Immunol 1998;161:963–970 [PubMed] [Google Scholar]
- 158. Tokunaga R, Zhang W, Naseem M, et al. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—a target for novel cancer therapy. Cancer Treat Rev 2018;63:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Tomayko MM, Steinel NC, Anderson SM, et al. Cutting edge: hierarchy of maturity of murine memory B cell subsets. J Immunol 2010;185:7146–7150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Turchinovich G, Vu TT, Frommer F, et al. Programming of marginal zone B-cell fate by basic Krüppel-like factor (BKLF/KLF3). Blood 2011;117:3780–3792 [DOI] [PubMed] [Google Scholar]
- 161. Turner DL, Bickham KL, Thome JJ, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol 2014;7:501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Vazquez MI, Catalan-Dibene J, and Zlotnik A. B cells responses and cytokine production are regulated by their immune microenvironment. Cytokine 2015;74:318–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Venkataraman G, Aguhar C, Kreitman RJ, et al. Characteristic CD103 and CD123 expression pattern defines hairy cell leukemia: usefulness of CD123 and CD103 in the diagnosis of mature B-cell lymphoproliferative disorders. Am J Clin Pathol 2011;136:625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Walker WS. Mediation of macrophage cytolytic and phagocytic activities by antibodies of different classes and class-specific Fc-receptors. J Immunol 1977;119:367–373 [PubMed] [Google Scholar]
- 165. Watanabe R, Fujimoto M, Ishiura N, et al. CD19 expression in B cells is important for suppression of contact hypersensitivity. Am J Pathol 2007;171:560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Weber GF, Chousterman BG, Hilgendorf I, et al. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J Exp Med 2014;211:1243–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Weisel F, and Shlomchik M. Memory B cells of mice and humans. Annu Rev Immunol 2017;35:255–284 [DOI] [PubMed] [Google Scholar]
- 168. Weisel FJ, Zuccarino-Catania GV, Chikina M, et al. A temporal switch in the germinal center determines differential output of memory B and plasma cells. Immunity 2016;44:116–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Weller S, Braun MC, Tan BK, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 2004;104:3647–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Wojciechowski W, Harris DP, Sprague F, et al. Regulation of type 2 immunity to H. polygyrus by effector B cells. Immunity 2009;30:421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Yang X, Yang J, Chu Y, et al. T follicular helper cells and regulatory B cells dynamics in systemic lupus erythematosus. PLoS One 2014;9:e88441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Yoshikawa M, Nakayamada S, Kubo S, et al. Type I and II interferons commit to abnormal expression of chemokine receptor on B cells in patients with systemic lupus erythematosus. Clin Immunol 2019;200:1–9 [DOI] [PubMed] [Google Scholar]
- 173. Yuan D, Dang T, and Bibi R. Inappropriate expression of IgD from a transgene inhibits the function of antigen-specific memory B cells. Cell Immunol 2001;211:61–70 [DOI] [PubMed] [Google Scholar]
- 174. Yuan H, Zhou S, Liu Z, et al. Pivotal role of lesional and perilesional T/B lymphocytes in pemphigus pathogenesis. J Invest Dermatol 2017;137:2362–2370 [DOI] [PubMed] [Google Scholar]
- 175. Zaid A, Hor JL, Christo SN, et al. Chemokine receptor-dependent control of skin tissue-resident memory T cell formation. J Immunol 2017;199:2451–2459 [DOI] [PubMed] [Google Scholar]
- 176. Zan H, and Casali P. Epigenetics of peripheral B-cell differentiation and the antibody response. Front Immunol 2015;6:631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Zuccarino-Catania GV, Sadanand S, Weisel FJ, et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol 2014;15:631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]