Abstract
Adaptive antibody responses provide a crucial means of host defense against viral infections by mediating the neutralization and killing infectious pathogens. At the forefront of humoral defense against viruses lie a subset of innate-like serum antibodies known as natural antibodies (NAbs). NAbs serve multifaceted functions in host defense and play an essential role in early immune responses against viruses. However, there remain many unanswered questions with regard to both the breadth of viral antigens recognized by NAbs, and how B cell ontology and individual antigenic histories intersect to control the development and function of antiviral human NAbs. In the following article we briefly review the current understanding of the functions and source of NAbs in the immune repertoire, their role during antiviral immune responses, the factors influencing the maturation of the NAb repertoire, and finally, the gaps and future research needed to advance our understanding of innate-like B cell biology for the purpose of harnessing NAbs for host defense against viral infections.
Keywords: natural antibodies, innate-like B cells, antiviral immunity, glycan-reactive antibodies
What Are Natural Antibodies?
The term “natural antibody” broadly encompasses antibody reactivity present in sera, in the absence of infection or deliberate immunization. The evolutionary conservation of natural antibodies (NAbs) in virtually all vertebrate species strongly suggests they play a significant role in the host immune system. Immunoglobulin M (IgM) antibodies arise first during B cell ontogeny and constitute a significant portion of the natural immunoglobulin repertoire. In adults, NAbs are maintained at relatively stable concentrations, and broaden to encompass antibodies of multiple immunoglobulin isotypes, including IgG and IgA antibodies (67). Natural human serum Ig contains a diverse repertoire of antibodies that bind to distinct carbohydrate and phospholipid epitopes, which occur as constituents of diverse classes of macromolecules including antigens of viral, bacterial, and fungal pathogen origin and self-antigens (42,68).
NAbs serve multiple primary functions within the host immune system; first, they provide an important first-line defense against virulent infectious microorganisms, including viral, bacterial, and fungal pathogens (11,21,26,61,103,110,124). The role of NAbs in host antiviral immunity will be discussed in greater detail later in this article. Second, NAbs serve “housekeeping” functions through the recognition of neo-self-epitopes generated during various cellular processes. NAbs promote the clearance of self-antigens generated during natural cellular senescence, which otherwise potentiate autoimmune disease, and modulate the activation of phagocytes after engulfment of IgM-opsonized apoptotic cells (59,130). NAbs have similarly been shown to modulate immune responsiveness to products of host molecule oxidation (19,36) and allergenic particles (77,107,108), and additionally exhibit tumor surveillance functions through the recognition of altered self-oncoantigens (25,85,133). Finally, NAbs play roles in the development and selection of the B cell repertoire, and the regulation of antigen-specific B cell responses (20,46,99,100).
Ontogeny of NAb-Producing B Cells in Mice
B cell subsets in mice are anatomically compartmentalized and exhibit distinct functions. The production of NAbs is associated with innate-like subsets of B cells, including the B-1 and, to a lesser extent, marginal zone (MZ) B cells (15,35,74,91). B-1 B and MZ B cells express Ig receptors that react with highly conserved antigens and mediate innate-like B cell effector functions, in contrast to B-2 B cells (also known as follicular B cells), which participate in germinal center-dependent adaptive immune responses (15). Mouse B-1 B cells express a CD19+B220LowCD5+/−CD43+CD11b+/− phenotype and can be further differentiated into CD5+ B-1a B cells and CD5− B-1b B cells (72). B-1 B cells are enriched in the peritoneal and pleural cavities, and the omentum, a site of fetal B cell lymphopoiesis and a CXCL13-dependent point of entry for B-1 B cells to the peritoneal cavity (PerC) (5,119,121). The low frequencies of B-1 B cells in spleen and lymph nodes, suggest that they also circulate through secondary lymphoid organs (72). In addition to producing NAbs, B-1 B cells have been shown to contribute to the generation of adaptive antibody responses to T-independent antigens during infection with a variety of pathogens, including influenza virus (11,34) and multiple bacterial species (3,4,26,53,61,141,142). They also contribute to secretory IgA in response to the commensal microbiota (28,79,84). MZ B cells, that express a CD19+B220+CD21HiCD23LowCD9+CD36+ phenotype, populate the MZ at the border between lymphoid follicles and red pulp of the spleen, and provide surveillance and rapid defense against blood-borne particulate antigens (90,92,136,137).
In mice innate-like B cell subsets are the product of specialized developmental programs that enrich for autoreactive B cell receptor clonotypes (72). B-1 B cell precursors arise predominantly during fetal and neonatal life from distinct progenitors (52,73,96). Their development is dependent on the induction of the transcription factor Arid3a (146), which is reciprocally regulated by the Lin28b/Let-7 axis that governs fetal versus adult lymphopoietic programs (145). MZ B cells are derived from adult bone marrow B cell progenitors and depend on signaling through Notch2 after engagement of the ligand delta-like 1, expressed by stromal cells of the splenic MZ (115,128). The selection of innate-like B cell precursors into both the mature B-1 and MZ B cell compartments requires B cell receptor signaling-dependent selection (15,64). Innate-like mouse B cells formed during fetal and perinatal development exhibit preferential utilization of IGHD-proximal IGHV variable-region regions (87,109,139,140), and minimal junctional diversity because of reduced terminal deoxynucleotidyl transferase (TdT) activity during these periods (16). These developmental constraints are paramount to the establishment of stereotyped B cell clonotypes that comprise a large proportion of the NAb-producing B cell repertoire, as ectopic expression of TdT transgenes was shown to disrupt the programmed development of highly conserved TEPC15+ B cell clonotypes, which normally protect mice from infection with Streptococcus pneumoniae through their recognition of phosphorylcholine (PC)-containing cell wall antigens (17,89). The unique developmental pathway of innate-like B cells is therefore central to their role in the immune system.
Innate-Like B Cells in Host Defense
NAbs preexisting in serum represent an important first line of defense that can neutralize and, in some cases, directly kill invading pathogens. Although the nature of NAb-producing cells is subject to debate, many studies have demonstrated that B-1 B cells are a prominent source of NAb in mice (11,80). Within the B-1 B cell compartment, both B-1a and B-1b B cells have been shown to contribute to the secretion of NAb (34,35,53,61,66,105). MZ B cells (69,91) and non-B-1 fetal precursors (111) have additionally been shown to contribute to natural IgM production. PerC-localized B-1 B cells, which do not spontaneously secrete antibody in situ, are precursors to NAb-producing cells that populate the spleen and bone marrow of mice, suggesting that some degree of differentiation is necessary to initiate NAb secretion by innate-like B cells (74,106). In fact, two pathways through which innate-like B cells produce NAb have been described. The first parallels the classical differentiation of B cells into plasma cells, and relies on Blimp1 to promote the acquisition of plasma cell phenotypes, characterized by reduced expression of CD19 and expression of CD138 (111,116) (Fig. 1A). Of interest, NAb-producing plasma cells in bone marrow have been found to exhibit a dependency on interleukin-5 (IL-5), like B-1 B cell-derived IgA+ plasma cells that populate the intestinal mucosa (9,97), distinguishing them from B-2 B cell-derived IgG-producing plasma cells that are dependent on IL-6 and other niche-derived factors (111). B-1 B cells have additionally been shown to contribute to NAb production as nonterminally differentiated antibody-secreting cells (ASCs), through a Blimp1-independent pathway that does not result in expression of CD138 or the downregulation of surface immunoglobulin (35,116) (Fig. 1A). Although the molecular signature of B-1 B cells remains to be fully characterized, the enrichment of natural IgG3 ASCs in this compartment suggests it will be interesting to learn how these pathways contribute differentially to host immunity (116).
FIG. 1.
Immune functions of innate-like B cells. Innate-like B cells play a pivotal, multifaceted role in host defense against infection by bacterial and viral pathogens. (A) Homeostatic production of natural IgM antibody by innate-like B-1 B cells in spleen and bone marrow generates a first line of defense against blood-borne pathogens and occurs through two known pathways. Left: B-1 B cells may differentiate into NAb-producing plasma cells through a Blimp1-dependent pathway that yields CD19-CD138+Blimp1Hi plasma cells, which lack sIg expression. B-1 B cell-derived plasma cells are maintained by IL-5, which may be derived from stromal cells and hematopoietic sources. B-1 B cell-derived plasma cells have also been implicated in the production of secretory IgA at mucosal barriers. Right: B-1 B cells can also secrete NAb through a Blimp1-independent pathway, as nonterminally differentiated ASCs that lack CD138 expression, and maintain expression of CD19 and sIg, which produce NAbs of IgM and IgG3 isotypes. (B) Innate-like B cells are also poised to generate rapid antibody response upon exposure to pathogens. Intravenous challenge of mice with bacterial polysaccharides and glycolipids (92) engages MZ B cells through their cognate antigen receptors. This activating event drives MZ B cells into the lymphoid follicle, which subsequently migrate into the red pulp and rapidly differentiate into antibody-secreting plasma cells, yielding large increases in serum IgM and IgG3 antibodies. (C) B-1 B lymphocytes may also be recruited to the site of pathogen encounter through chemotactic gradients and homing receptor expression. B-1 B cells have been found to migrate to lung and gut tissues, and draining lymph nodes, to produce antibody at local sites. ASCs, antibody-secreting cells; IgM, immunoglobulin M; IL, interleukin; 5MZ, marginal zone; NAb, natural antibody; sIg, surface immunoglobulin.
Both B-1 B cells and MZ B cells are additionally poised for rapid mobilization and secretion of antigen-specific antibodies, which provides an additional fast-acting defense against pathogens (90,92). B-1 B cells rapidly differentiate into ASCs after stimulation by molecules such as lipopolysaccharide and peptidoglycan, which is accompanied by limited clonal proliferation (143). The Toll-like receptor 7 agonist Resiquimod (R848), which bears structural similarities to viral nucleic acids, has also been shown induce proliferation and Ig secretion by mouse B-1 B cells, suggesting B-1 cells are additionally poised to mount rapid humoral responses after the encounter of viral antigens (51,125). Both B-1a and B-1b B cells have been shown to contribute to antigen-specific immunity in mice (4,61,141). Similarly, challenge with bacterial glycolipid antigens drives rapid migration of antigen-specific MZ B cells to splenic follicles, which subsequently translocate to the red pulp as plasma cells producing antigen-specific IgM and IgG3 antibodies (92) (Fig. 1B). Although NAbs and extrafollicular immune response-derived antibodies exhibit lower affinity for their antigens than GC-derived antibody and in some cases are polyreactive, they provide an essential rapid protection during the time required to form later appearing GC-dependent adaptive immune responses (98).
B-1 B cells in mice further contribute to host defense by localizing to sites of infection and producing antibody locally (Fig. 1C). In mouse models of viral pneumonia associated with influenza, infection-induced type 1 interferon mobilizes B-1 B cell migration to lung-draining mediastinal lymph nodes through the activation of CD11b integrin, which is expressed by a subset of mouse B-1 B cells (134). B-1 B cells secreting NAb have also been reported in the lung parenchyma at steady state (34). Innate-like B cell subsets in mice therefore serve to limit the dissemination of infectious pathogens through their expression of templated B cell receptors reactive with conserved antigens, the steady-state secretion of NAb, and their capacity to mobilize during antigen challenge to produce rapid increases in antigen-specific serum Ab.
NAbs in the Human Immune System?
The existence of a human equivalent of mouse B-1 B cells is somewhat controversial. In contrast to CD5 and CD43 expression by mouse B cells, which effectively differentiates B-1 B cells from other B cell subsets, these markers are modulated by cellular activation in human B cells (131). Furthermore, whereas the unique anatomical distribution of mouse B-1 B cells has aided in their characterization, the analysis of resident cells of the peritoneal and pleural cavities is not easily accomplished in humans. However, like mouse B-1 B cells, human fetal B cells are enriched for clonotypes exhibiting polyreactivity for autoantigens (33,83,95), and fetal human B cells additionally exhibit lower levels of TdT-dependent junctional diversity and a preferential utilization of specific VH genes (95,113). The human omentum is also a site of fetal B cell lymphopoiesis (119,120); thus, there are several conserved features of fetal B cell lymphopoiesis between humans and mice. CD20+CD27+CD43+CD70− B cells in umbilical cord blood and adult peripheral blood bear similarities to mouse B-1 B cells, including the spontaneous secretion of antibody and the expression of largely unmutated immunoglobulin genes enriched for reactivity to common NAb targets, such as PC (56,58,114). However, the classification of these cells as human B-1 B cells has been contested (37,39,57). Although humans possess a MZ-like B cell population, characterized by CD27-expressing IgM+ memory B cell phenotype, human MZ B cells are more widely distributed than those of mice, and it is not clear to what extent they contribute to homeostatic NAb production (32,135). Thus, the precise nature of NAb-producing B cells in humans remains unclear, and to date it is unknown whether NAbs in humans share a similar ontogenetic program as those in mice.
It is clear, however, that humans possess serum antibodies functionally analogous to mouse NAbs. Although rare in humans, selective IgM deficiency commonly presents in the clinic with repeated chronic infections by bacterial, viral, fungal, and protozoan pathogens (60,65,71), mirroring the essential role of NAbs and induced IgM in host defense of mice. IgM-deficient patients additionally exhibit high frequencies of allergic disease (81), and there are reported cases of IgM deficiency co-associating with autoimmune disease including rheumatoid arthritis (8), systemic lupus erythematosus (126), glomerulonephritis (6), and myriad of other human autoimmune diseases (81). These findings strongly suggest that certain human antibodies perform “housekeeping” functions, similar to those described for mouse NAbs. Moreover, serum concentrations of specific antibodies have been shown to be predictive of disease progression in several settings, including: (i) oxidized low-density lipoprotein and advanced glycation end product modification-reactive Abs in cardiovascular disease (43,47), (ii) laminaribiose (Glcβ1–3Glcβ)- and chitobiose (GlcNAcβ1–4GlcNAcβ)-reactive Abs in Crohn's disease (40) and (iii) Glcα1–4Glcα-specific antibodies in relapsing–remitting multiple sclerosis (117).
Antibodies reactive with a multitude of phospholipid and carbohydrate epitopes are abundant in the human serum Ig repertoire (14,42,68). Encompassed in the human serum NAb repertoire are antibodies reactive with common antigen targets of mouse NAbs, such as PC and N-acetyl-d-gluosamine (GlcNAc). Humans also exhibit high levels of xenoantigen-reactive NAbs, including α1–3Galactose (αGal)-reactive NAbs, which are universally present in humans and in some situations may comprise as much as 1% of total serum Ig (48). It is also widely accepted that humans produce high titers of NAbs against blood group A/B alloantigens, when those antigens are absent from host red blood cells (24). Epstein–Barr virus (EBV) immortalization of peripheral blood B cells has substantiated the notion that B cells expressing Ig reactive with these NAb antigen targets are represented at high frequencies within the mature, adult human B cell repertoire (45,48,49). Of importance, recent microarray-based analyses of serum antibody specificities in umbilical cord blood revealed that NAbs were of lower abundance than in adult blood, suggesting maturation and development of the NAb repertoire occurred after birth (138).
NAbs in Antiviral Immunity
The impact of experimental conventions used during the study of host immune responses on our ability to observe the contributions of NAbs in antiviral immunity was succinctly described by Ochsenbein and Zinkernagel:
“In many virological and immunological assays it is a convenient practice to predilute sera to variable extents, from 1:10 up to 1:80, to avoid so called ‘nonspecific background’ signals; the consequence of this practice is that naturally occurring antibodies are not normally detected or are not registered.” (103)
Thus, serum is often diluted in immunological assays beyond the functional range of NAbs and their reactivity for viral antigens, or neutralizing activity against infectious viruses are therefore imperceptible and have remained largely unstudied.
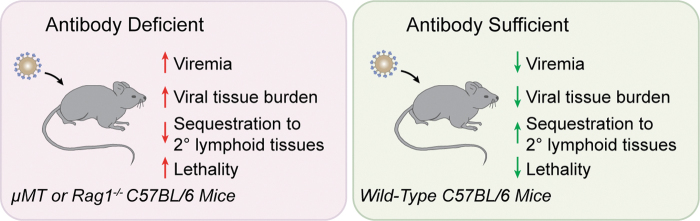
However, studies specifically examining NAbs have found significant evidence for their early and critical role in preventing the tissue dissemination of viruses. In fact, mice deficient in Ig production, as a result of genetic ablation of recombination activation genes (RAG) or the IgM heavy chain (μMT) exhibit viral titers 10–100 times greater in peripheral tissues than wild-type mice and show increased rates of morbidity after viral challenge (103) (Fig. 2). Similarly, mice deficient exclusively in secretory IgM exhibit impaired clearance of influenza virus, indicating that B-1 B cell-derived natural IgM serves an important role in host defense against viruses (12). Low titers of IgM NAbs that recognize vesicular stomatitis virus (VSV) protect against VSV-induced encephalitis and have long been appreciated as components of the NAb repertoire in mice (54,103), and NAbs have also been shown to be an important defense against infectious influenza virus (11,34,70). The transfer of natural serum antibodies to antibody-deficient mice is sufficient to delay mortality after challenge with infectious VSV or influenza virus, indicating the significance of their early role during antiviral immunity (10,103). Like mice, humans with selective IgM deficiency are at an increased risk of viral infection, and often present with infections caused by varicella zoster virus, cytomegalovirus, and molluscum contagiosum virus (81). Similarly, humans have been shown to possess low titers of natural IgM antibodies that recognize influenza virus (13). Thus, as is the case in mice, IgM represents an important front-line of defense against infectious viruses in humans.
FIG. 2.
Phenotype of NAb-deficient mice after viral challenge. NAbs play an essential role in the early defense against viral infection in mammalian hosts. When challenged with a low virus dose, mice lacking B cells and deficient in normal serum antibodies exhibit increased mortality (103). Viral burden in blood and tissue is high in these animals, relative to their antibody-sufficient counterparts, and the sequestration of viral particles to secondary lymphoid tissues is reduced.
In vitro neutralization of viral particles mediated by mouse natural IgM has been reported for lymphocytic choriomeningitis virus (LCMV) (103), vaccinia virus (54), and influenza (70), and the mechanisms underlying NAb-mediated virus neutralization appear heterogenous. Passive NAb transfer to antibody-deficient mice does not require intact complement activity to neutralize VSV, as depletion of complement components by treatment with cobra venom factor did not significantly impact the efficacy of transferred protection (103). It is possible that NAbs neutralize VSV directly by interfering with its invasion of host cells (Fig. 3A). Conversely, in the case of influenza infection, NAb-dependent activation of complement pathways was required for effective neutralization of virus (70). Consistent with the notion that viruses are considered to be resistant to complement-mediated lysis, the neutralization of influenza virus was instead dependent on robust deposition of C3b and occlusion of hemagglutinin epitopes, necessary for recognition and fusion with host cells (Fig. 3A) (70). Human influenza-reactive NAbs were similarly shown to neutralize virus in vitro through complement-dependent means (13). Similarly, antibody-mediated deposition of C4 was recently shown to neutralize human adenovirus 5 (Ad5), a model organism for nonenveloped viruses, by blocking entry to the cell cytosol through inhibition of the capsid disassembly process in a C3 independent pathway (23). Thus, NAbs reactive with viral antigens serve multifaceted roles with respect to co-opting the classical complement pathway to neutralize infectious viruses.
FIG. 3.
Modes of NAb function against viral infection. NAbs function in several modes contributing to host defense against viruses. (A) Spontaneous production of NAbs by innate-like B cell-derived plasma cells may neutralize viruses directly, by direct killing of viruses or by blocking binding and entry of viral particles to host cells. NAbs are also efficient at initiating the classical complement cascade, which generates virus bound C4 and C3 that have been shown to block viral entry of host cells. (B) Antiviral immunity is also augmented by NAb through Fc receptor-dependent mechanisms. Ig opsonization of viruses can promote antiviral immunity after viral infection of host cells through the engagement of the cytosolic Fc receptor Trim21, which targets viruses to the proteasome for degradation and has also been shown to bind IgM (86). Expression of the IgM-specific Fcμ receptor by mouse B cells has been shown to contribute to the clonal expansion of hemagglutinin-reactive B cells during influenza infection and the development of IgG BMem and PCs (101). (C) Complement activation by NAbs further results in the efficient localization of opsonized antigens to lymphoid follicles for the induction of subsequent adaptive immune responses. Complement opsonized antigens localize to fDC networks that promote the induction of adaptive immune response to viral antigens, yielding the generation of high-affinity BMem, PCs and CTLs. BMem, memory B cells; CTLs, cytotoxic T lymphocytes; fDC, follicular dendritic cell; PCs, plasma cells.
NAbs may further promote protection from viral infections through interactions with receptors for the fragment crystallizable (Fc) region of immunoglobulin molecules. With respect to natural IgM, two such Fc receptors have been directly implicated in antiviral immune responses. Trim21, an intracellular receptor for Ig Fc regions with high affinity for IgG molecules has also been shown to bind to Fc region of IgM molecules and neutralize infection by the adenovirus (86) (Fig. 3B). Trim21 was also shown to work synergistically with antibody-dependent C4 deposition to mediate robust neutralization of adenovirus (23). The IgM-specific Fcμ receptor has also been shown to play vital B cell intrinsic roles in the early response to viral infection. Nguyen et al. demonstrated that conditional deletion of Fcμ receptor in mouse B cells results in impaired clonal expansion of hemagglutinin-reactive B cells during the early phases of infection, and reduced numbers hemagglutinin-reactive IgG plasma cells and memory B cells (101) (Fig. 3B). Thus, in addition to mediating complement-dependent neutralization of infectious viruses, natural IgM seems to play important roles in Fc receptor interactions that promote virus neutralization and the generation of high-affinity antibody memory B cells.
Perhaps the most significant outcome for NAb-mediated opsonization of viruses is to sequester them to secondary lymphoid tissues (93,103,104). This occurs, in part, through the localization of antigen–antibody complexes to follicular dendritic cell networks in lymphoid follicles through interactions with CD35 (complement receptor 1) and CD21 (complement receptor 2), which promotes induction of germinal center-dependent adaptive immune responses to viral antigens (112) (Fig. 3C). This effect has been observed in mouse models of infection by influenza and VSV, wherein NAbs lower infectious influenza virus titers, and impede tissue dissemination (10,103). These features of NAb activity seem to be essential for the development of class-switched antiviral antibodies, effective T lymphocyte activation, and the generation of long-term immunological memory to influenza virus (12,44,78). Unfortunately, in most cases, including the examples in relation to influenza, VSV, and LCMV cited previously, the antigenic determinants recognized by NAb have not been characterized, and the origins of the B cell clonotypes producing these NAbs remain unknown.
NAb Responses to Viral Envelope Glycoproteins
Viruses often utilize host glycosylation machinery during viral replication and assembly in host cells. NAb reactivity and neutralization potential against viral pathogens may therefore be closely associated with the host species from which a virus originates. Because of this feature of viral replication, certain self-reactive NAbs can provide protection against chronic viral infections. This effect is exemplified by the family of human broadly neutralizing HIV antibodies that react with high-mannose modifications of gp120 viral envelope glycoprotein, such as the monoclonal antibody 2G12. 2G12 potently binds and neutralizes primary isolates of HIV and SIV through recognition of a conserved Manα1–2Man motif on the oligomannose glycan (29,82). High-mannose-binding antibodies that recognize the HIV glycoproteins exhibit features of autoreactive antibodies, perhaps because of the derivation of viral antigenic epitopes from host carbohydrates. Of interest, 2G12 also binds mannose-containing cell walls of some Candida species, suggesting the HIV-neutralizing potential of the human NAb repertoire may be influenced by exposure to pathogens bearing conserved mannose structures (41). Self-reactivity has been reported in other broadly neutralizing HIV human monoclonal antibodies, such as MAbs 2F5 and 4E10, which show reactivity to the widely expressed lipid cardiolipin (1), and broadly neutralizing antibodies against stem epitopes of influenza hemagglutinin have also been reported to exhibit autoreactivity (7). The autoreactivity of these antibodies have given rise to speculation that the NAb-producing B cell repertoire could be leveraged through vaccine design to elicit broadly neutralizing antibodies.
NAb-mediated viral neutralization through recognition of viral envelope glycoproteins may be especially important in protection against zoonotic viral infections. For example, humans and old-world primates, which do not generate endogenous αGal1–3αGal glycan modifications because of the evolutionary loss of the α-galactosyl transferase (αGalT) enzyme, generate high titers of αGal-reactive serum antibodies. Natural anti-αGal antibodies effectively opsonize virus containing αGal-modified capsid antigens through antibody-mediated classical complement cascade activation (127). αGal-modified capsid antigens are common to animal viruses, including rhabdoviruses, rabies virus, some nonprimate lentiviruses, and foamy viruses, indicating the breadth of protection potentially provided by this NAb defense (127). Similarly, when considering infection by arthropod-borne arboviruses such as dengue flavivirus, which originate from insect cells and express insect host cell-derived glycans, insect glycan-reactive NAbs may represent an important front-line defense, before the virus's acquisition of host glycosylation during replication within mammalian host. Natural anti-αGal antibodies provide immediate protection in mouse models of insect-borne malaria transmission, and can be boosted by vaccination with αGal conjugates to provide sterilizing immunity in αGalT-deficient mice (144). Of interest, similar effects have been reported during transmission of hepatitis C virus (HCV), wherein viral capsid glycoproteins are modulated by host ABO blood group oligosaccharide alloantigens and some degree of immunity to HCV can be demonstrated in allotype mismatched infections (132). Although the presence of blood group alloantigen-reactive NAbs in the human immune repertoire remains enigmatic, perhaps they represent a barrier to infection against viruses bearing alloantigen-derived carbohydrate epitopes.
NAb recognition of viral envelope glycoprotein modifications hold implications beyond host defense and can significantly impact the efficacy of therapeutic viral vectors. Specifically, the galactosylation of VSV G glycoprotein, after production of VSV in αGal-positive cell lines, resulted in failed gene delivery to human cells because of anti-αGal antibody-dependent, complement-mediated inactivation and blockade of viral particle replication (50). Conversely, during treatment with PROSTVAC-VF, a poxvirus-based therapeutic cancer vaccine now in phase III clinical trials for the treatment of advanced prostate cancer, preexisting serum NAbs to blood group A trisaccharide (BG-ATri), and antibodies reactive with the Forssman disaccharide (GalNAcα1–3GalNAcβ) induced during treatment correlated with enhanced antitumor immune responses in recipients (30,31). αGal modification of tumor vaccine antigens has also been used to improve antitumor responses in mouse models of pancreatic cancer (38), and has been shown to enhance phagocytosis and cross-presentation of tumor antigens by human antigen-presenting cells in the presence of purified anti-αGal human antibodies (88). Thus, NAb responses to viral glycoproteins can significantly impact the successful delivery of viral vector-based gene therapies and tumor antigen vaccines.
Origins of the NAb Repertoire
As discussed previously, the recruitment of B cell progenitors into NAb-producing B cell subsets occurs by positive selection through B cell receptor signaling. During fetal B cell lymphopoiesis, this is thought to occur predominantly in the context of self-antigens. Unlike class-switched antibody isotypes, serum concentration of IgM antibody is not affected in germ-free mice, and some classes of NAbs are found at comparable levels in germ-free and conventionally raised mice (36,63,103). However, microarray analysis of global serum antibody reactivity in germ-free mice revealed significant deficiencies in carbohydrate-reactive antibodies commonly observed in conventionally raised mice (22). Germ-free (GF) mice also exhibit blunted antibody T-independent antibody responses to α1–3 glucan carbohydrate antigens after immunization with Enterobacter cloacae, indicating that microbial colonization can alter the clonal response to polysaccharide vaccine antigens (75). Moreover, it is clear that neonatal exposure to bacterial antigens can result in striking and long-lasting changes to the clonal composition of antigen-specific NAbs. Neonatal immunization of mice with S. pneumoniae and E. cloacae results in the expansion of B cells expressing immunoglobulin idiotype determinants distinct from those that dominate the resting repertoire of unimmunized adult mice (76). Thus, the clonal repertoire of NAb-producing innate-like B cells may be shaped significantly by the early-life antigenic history of an individual that, by extension, may impact their susceptibility to infectious, autoimmune, or allergic disease.
Human antibodies with specificities similar to mouse NAbs also bear signatures of environmental antigen modulation. Although found at low levels in umbilical cord blood, antibodies reactive with carbohydrate and phospholipid epitopes increase significantly with age (62,76,138), and serum concentrations of NAbs directed toward PC and GlcNAc antigens are clearly modulated by infection with S. pneumoniae and Streptococcus pyogenes (Group A Streptococcus), respectively (55,118). Unlike the NAb-producing B cell clonotypes of mice, which express predominantly germline Ig gene rearrangements, PC-reactive human B cells exhibit significant somatic mutation of their Ig gene variable regions (45). Although the relationship of these human B cells to NAb production is unclear, these findings may support a role for progressive antigen encounter at pulmonary and gut mucosal tissues in the selection of B cells that contribute to NAb production in humans. The effects of IG gene mutations on antigen specificity, affinity, pathogen-neutralizing potential, and immune modulating activity of human NAbs are poorly characterized, but their presence suggests that early life encounters with pathogen-, commensal microorganism-, and vaccine-derived antigens may modulate the antiviral activity of NAbs discussed previously.
Heterogeneity of Fine Specificity Within the Human NAb Repertoire
The global glycan and phospholipid reactivity observed in human sera results from myriad individual antibodies with distinct, and in some cases, overlapping fine specificities. However, the NAb repertoire is often described in aggregate as a single entity, and few studies have extensively analyzed individual NAb specificities within the global repertoire. Although human sera clearly exhibit substantial reactivity toward host glycan and glycolipid structures, the fine antibody specificity of NAbs may differ broadly among individuals, and across Ig subclasses within individuals (14,42,68). Despite the well-conserved, oligoclonal nature of human antibodies directed toward PC, GlcNAc, αGal and A/B blood group antigens, individual clones comprising these antibody pools exhibit variable fine specificity: PC-reactive monoclonal antibodies derived from EBV immortalized human B cells were shown to exhibit considerable heterogeneity in terms of affinity to different synthetic derivatives of PC (123), and several studies have described differences in the fine specificity of αGal-reactive antibodies in human sera (18,102), which is clearly modulated in cases of blood group B alloantigen-positive individuals (49). Moreover, although the human NAb repertoire contains abundant mannose-reactive antibody that can bind to HIV envelope glycoproteins, the majority of these polyclonal antibodies exhibit no neutralizing activity, indicating that distinct mannose-reactive B cell clonotypes are required for effective neutralizing NAb responses (129). Thus, subtle differences in the sequence and the degree of mutations of immunoglobulin genes may produce significant differences in the capacity of antibodies to recognize defined T-independent epitopes as arrayed in different contexts, which may subsequently affect their function during the opsonization, neutralization, and lysis of infectious pathogens.
Remaining Questions
Despite the fact that NAbs have been recognized for decades as an important component of the host immune system, very little is known regarding the origin and differentiation of NAb-producing plasma cells or the regulation of NAb production in serum and mucosal secretions in humans. These shortcomings have limited the characterization of the Ig gene repertoire of human NAb-producing B cells and the exploration of the mechanisms underlying the conserved NAb reactivity to glycan glycolipids, including viral antigens. We must address these gaps in knowledge, as glycans exist at the forefront of most cellular interactions; in the glycocalyxes that decorate each cell in the body, on mucin proteins that constitute important barriers in mucosal tissues, and as surface antigens expressed by myriad pathogens where they play essential roles during infection.
The advent of high-throughput sequencing approaches to characterize immunoglobulin heavy chain gene rearrangements has provided unprecedented glimpses into the clonal diversity of the human B cell repertoire, but has also revealed convergent and conserved elements (27,122). Although past sequencing-based analyses of the human B cell repertoire have been heavily skewed toward the analysis of blood or tonsil-derived B cells, recent efforts to establish a VH gene-rearrangement-based atlas of B cell clonal distribution over multiple tissues of individual human organ donors revealed distinct networks of B cell clonal lineages that distribute across and inhabit tissues in the human body (94). The recent demonstration that lung tissue-resident B cells play an important role during secondary immune responses to influenza infection in mice (2) strongly suggest that coupling high-throughput antigen receptor sequencing efforts, such as those pioneered in the formation of the Human B cell Atlas, with the targeted analysis of antigen-specific B cells will provide unprecedented clarity of the relatedness of systemic and mucosal B cell responses to viral antigens. Extending such strategies to the analysis of B cells bearing reactivity toward known NAb antigens may provide insight into the origins of NAb-producing B cells in humans, and the distribution of these cells throughout the body. Given the role NAbs play in the context of antiviral immunity, these approaches may illuminate many of the outstanding questions outlined above and provide avenues to harness or manipulate humoral NAb immunity to improve the efficacy of virus-based vaccines and immunotherapies.
Acknowledgments
The authors thank Dr. Denise Kaminski for her support in editing and revising this review.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This study was supported by NIH grants R01 AI14782, U01 AI100005 and U19 AI142637 (Project 1) awarded to J.F.K.
References
- 1. Alam SM, McAdams M, Boren D, et al. The role of antibody polyspecificity and lipid reactivity in binding of broadly neutralizing anti-HIV-1 envelope human monoclonal antibodies 2F5 and 4E10 to glycoprotein 41 membrane proximal envelope epitopes. J Immunol 2007;178:4424–4435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Allie SR, Bradley JE, Mudunuru U, et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat Immunol 2019;20:97–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alugupalli KR, Gerstein RM, Chen J, et al. The resolution of relapsing fever borreliosis requires IgM and is concurrent with expansion of B1b lymphocytes. J Immunol 2003;170:3819–3827 [DOI] [PubMed] [Google Scholar]
- 4. Alugupalli KR, Leong JM, Woodland RT, et al. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity 2004;21:379–390 [DOI] [PubMed] [Google Scholar]
- 5. Ansel KM, Harris RB, and Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity 2002;16:67–76 [DOI] [PubMed] [Google Scholar]
- 6. Antar M, Lamarche J, Peguero A, et al. A case of selective immunoglobulin M deficiency and autoimmune glomerulonephritis. Clin Exp Nephrol 2008;12:300–304 [DOI] [PubMed] [Google Scholar]
- 7. Bajic G, van der Poel CE, Kuraoka M, et al. Autoreactivity profiles of influenza hemagglutinin broadly neutralizing antibodies. Sci Rep 2019;9:3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bandilla KK, Pitts NC, and McDuffie FC. Immunoglobulin M deficiency in the immune response of patients with rheumatoid arthritis. Arthritis Rheum 1970;13:214–221 [DOI] [PubMed] [Google Scholar]
- 9. Bao S, Beagley KW, Murray AM, et al. Intestinal IgA plasma cells of the B1 lineage are IL-5 dependent. Immunology 1998;94:181–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baumgarth N. B-1 cell heterogeneity and the regulation of natural and antigen-induced IgM production. Front Immunol 2016;7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baumgarth N, Herman OC, Jager GC, et al. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A 1999;96:2250–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baumgarth N, Herman OC, Jager GC, et al. B-1 and B-2 cell-derived immunoglobulin M antibodies are nonredundant components of the protective response to influenza virus infection. J Exp Med 2000;192:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beebe DP, Schreiber RD, and Cooper NR. Neutralization of influenza virus by normal human sera: mechanisms involving antibody and complement. J Immunol 1983;130:1317–1322 [PubMed] [Google Scholar]
- 14. Bello-Gil D, Khasbiullina N, Shilova N, et al. Repertoire of BALB/c mice natural anti-carbohydrate antibodies: mice vs. humans difference, and otherness of individual animals. Front Immunol 2017;8:1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bendelac A, Bonneville M, and Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol 2001;1:177–186 [DOI] [PubMed] [Google Scholar]
- 16. Benedict CL, Gilfillan S, Thai TH, et al. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev 2000;175:150–157 [PubMed] [Google Scholar]
- 17. Benedict CL, and Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity 1999;10:607–617 [DOI] [PubMed] [Google Scholar]
- 18. Bernth-Jensen JM, Moller BK, Jensenius JC, et al. Biological variation of anti-alphaGal-antibodies studied by a novel time-resolved immunofluorometric assay. J Immunol Methods 2011;373:26–35 [DOI] [PubMed] [Google Scholar]
- 19. Binder CJ, Horkko S, Dewan A, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med 2003;9:736–743 [DOI] [PubMed] [Google Scholar]
- 20. Boes M, Esau C, Fischer MB, et al. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol 1998;160:4776–4787 [PubMed] [Google Scholar]
- 21. Boes M, Prodeus AP, Schmidt T, et al. A critical role of natural immunoglobulin M in immediate defense against systemic bacterial infection. J Exp Med 1998;188:2381–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bos NA, Kimura H, Meeuwsen CG, et al. Serum immunoglobulin levels and naturally occurring antibodies against carbohydrate antigens in germ-free BALB/c mice fed chemically defined ultrafiltered diet. Eur J Immunol 1989;19:2335–2339 [DOI] [PubMed] [Google Scholar]
- 23. Bottermann M, Foss S, Caddy SL, et al. Complement C4 prevents viral infection through capsid inactivation. Cell Host Microbe 2019;25:617–629 e617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Boyden SV. Natural antibodies and the immune response. Adv Immunol 1966;5:1–28 [DOI] [PubMed] [Google Scholar]
- 25. Brandlein S, Pohle T, Ruoff N, et al. Natural IgM antibodies and immunosurveillance mechanisms against epithelial cancer cells in humans. Cancer Res 2003;63:7995–8005 [PubMed] [Google Scholar]
- 26. Briles DE, Nahm M, Schroer K, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med 1981;153:694–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Briney B, Inderbitzin A, Joyce C, et al. Commonality despite exceptional diversity in the baseline human antibody repertoire. Nature 2019;566:393–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bunker JJ, Flynn TM, Koval JC, et al. Innate and adaptive humoral responses coat distinct commensal bacteria with immunoglobulin A. Immunity 2015;43:541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calarese DA, Lee HK, Huang CY, et al. Dissection of the carbohydrate specificity of the broadly neutralizing anti-HIV-1 antibody 2G12. Proc Natl Acad Sci U S A 2005;102:13372–13377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Campbell CT, Gulley JL, Oyelaran O, et al. Serum antibodies to blood group A predict survival on PROSTVAC-VF. Clin Cancer Res 2013;19:1290–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Campbell CT, Gulley JL, Oyelaran O, et al. Humoral response to a viral glycan correlates with survival on PROSTVAC-VF. Proc Natl Acad Sci U S A 2014;111:E1749–E1758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cerutti A, Cols M, and Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol 2013;13:118–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen ZJ, Wheeler CJ, Shi W, et al. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur J Immunol 1998;28:989–994 [DOI] [PubMed] [Google Scholar]
- 34. Choi YS, and Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J Exp Med 2008;205:3053–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Choi YS, Dieter JA, Rothaeusler K, et al. B-1 cells in the bone marrow are a significant source of natural IgM. Eur J Immunol 2012;42:120–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chou MY, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest 2009;119:1335–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Covens K, Verbinnen B, Geukens N, et al. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood 2013;121:5176–5183 [DOI] [PubMed] [Google Scholar]
- 38. Deguchi T, Tanemura M, Miyoshi E, et al. Increased immunogenicity of tumor-associated antigen, mucin 1, engineered to express alpha-gal epitopes: a novel approach to immunotherapy in pancreatic cancer. Cancer Res 2010;70:5259–5269 [DOI] [PubMed] [Google Scholar]
- 39. Descatoire M, Weill JC, Reynaud CA, et al. A human equivalent of mouse B-1 cells? J Exp Med 2011;208:2563–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dotan I, Fishman S, Dgani Y, et al. Antibodies against laminaribioside and chitobioside are novel serologic markers in Crohn's disease. Gastroenterology 2006;131:366–378 [DOI] [PubMed] [Google Scholar]
- 41. Dunlop DC, Ulrich A, Appelmelk BJ, et al. Antigenic mimicry of the HIV envelope by AIDS-associated pathogens. AIDS 2008;22:2214–2217 [DOI] [PubMed] [Google Scholar]
- 42. Durbin SV, Wright WS, and Gildersleeve JC. Development of a multiplex glycan microarray assay and comparative analysis of human serum anti-glycan IgA, IgG, and IgM repertoires. ACS Omega 2018;3:16882–16891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Engelbertsen D, Vallejo J, Quach TD, et al. Low levels of IgM antibodies against an advanced glycation endproduct-modified apolipoprotein B100 peptide predict cardiovascular events in nondiabetic subjects. J Immunol 2015;195:3020–3025 [DOI] [PubMed] [Google Scholar]
- 44. Fernandez Gonzalez S, Jayasekera JP, and Carroll MC. Complement and natural antibody are required in the long-term memory response to influenza virus. Vaccine 2008;26 Suppl 8:I86–I93 [DOI] [PubMed] [Google Scholar]
- 45. Fiskesund R, Steen J, Amara K, et al. Naturally occurring human phosphorylcholine antibodies are predominantly products of affinity-matured B cells in the adult. J Immunol 2014;192:4551–4559 [DOI] [PubMed] [Google Scholar]
- 46. Freitas AA, Viale AC, Sundblad A, et al. Normal serum immunoglobulins participate in the selection of peripheral B-cell repertoires. Proc Natl Acad Sci U S A 1991;88:5640–5644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frostegard J, Tao W, Georgiades A, et al. Atheroprotective natural anti-phosphorylcholine antibodies of IgM subclass are decreased in Swedish controls as compared to non-westernized individuals from New Guinea. Nutr Metab (Lond) 2007;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galili U, Anaraki F, Thall A, et al. One percent of human circulating B lymphocytes are capable of producing the natural anti-Gal antibody. Blood 1993;82:2485–2493 [PubMed] [Google Scholar]
- 49. Galili U, Buehler J, Shohet SB, et al. The human natural anti-Gal IgG. III. The subtlety of immune tolerance in man as demonstrated by crossreactivity between natural anti-Gal and anti-B antibodies. J Exp Med 1987;165:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Galili U, and Swanson K. Gene sequences suggest inactivation of alpha-1,3-galactosyltransferase in catarrhines after the divergence of apes from monkeys. Proc Natl Acad Sci U S A 1991;88:7401–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Genestier L, Taillardet M, Mondiere P, et al. TLR agonists selectively promote terminal plasma cell differentiation of B cell subsets specialized in thymus-independent responses. J Immunol 2007;178:7779–7786 [DOI] [PubMed] [Google Scholar]
- 52. Ghosn EE, Sadate-Ngatchou P, Yang Y, et al. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci U S A 2011;108:2879–2884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gil-Cruz C, Bobat S, Marshall JL, et al. The porin OmpD from nontyphoidal Salmonella is a key target for a protective B1b cell antibody response. Proc Natl Acad Sci U S A 2009;106:9803–9808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gobet R, Cerny A, Ruedi E, et al. The role of antibodies in natural and acquired resistance of mice to vesicular stomatitis virus. Exp Cell Biol 1988;56:175–180 [DOI] [PubMed] [Google Scholar]
- 55. Gray BM, Dillon HC Jr., and Briles DE. Epidemiological studies of Streptococcus pneumoniae in infants: development of antibody to phosphocholine. J Clin Microbiol 1983;18:1102–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Griffin DO, Holodick NE, and Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med 2011;208:67–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Griffin DO, Quach T, Batliwalla F, et al. Human CD11b+ B1 cells are not monocytes: a reply to “Gene profiling of CD11b+ and CD11b- B1 cell subsets reveals potential cell sorting artifacts”. J Exp Med 2012;209:434–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Griffin DO, and Rothstein TL. Human b1 cell frequency: isolation and analysis of human b1 cells. Front Immunol 2012;3:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gronwall C, Chen Y, Vas J, et al. MAPK phosphatase-1 is required for regulatory natural autoantibody-mediated inhibition of TLR responses. Proc Natl Acad Sci U S A 2012;109:19745–19750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gupta S, and Gupta A. Selective IgM deficiency—an underestimated primary immunodeficiency. Front Immunol 2017;8:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haas KM, Poe JC, Steeber DA, et al. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 2005;23:7–18 [DOI] [PubMed] [Google Scholar]
- 62. Hamanova M, Chmelikova M, Nentwich I, et al. Anti-Gal IgM, IgA and IgG natural antibodies in childhood. Immunol Lett 2015;164:40–43 [DOI] [PubMed] [Google Scholar]
- 63. Haury M, Sundblad A, Grandien A, et al. The repertoire of serum IgM in normal mice is largely independent of external antigenic contact. Eur J Immunol 1997;27:1557–1563 [DOI] [PubMed] [Google Scholar]
- 64. Hayakawa K, Asano M, Shinton SA, et al. Positive selection of natural autoreactive B cells. Science 1999;285:113–116 [DOI] [PubMed] [Google Scholar]
- 65. Hobbs JR, Milner RD, and Watt PJ. Gamma-M deficiency predisposing to meningococcal septicaemia. Br Med J 1967;4:583–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Holodick NE, Repetny K, Zhong X, et al. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol 2009;39:2383–2394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Holodick NE, Rodriguez-Zhurbenko N, and Hernandez AM. Defining natural antibodies. Front Immunol 2017;8:872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Huflejt ME, Vuskovic M, Vasiliu D, et al. Anti-carbohydrate antibodies of normal sera: findings, surprises and challenges. Mol Immunol 2009;46:3037–3049 [DOI] [PubMed] [Google Scholar]
- 69. Ichikawa D, Asano M, Shinton SA, et al. Natural anti-intestinal goblet cell autoantibody production from marginal zone B cells. J Immunol 2015;194:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jayasekera JP, Moseman EA, and Carroll MC. Natural antibody and complement mediate neutralization of influenza virus in the absence of prior immunity. J Virol 2007;81:3487–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Jones DM, Tobin BM, and Butterworth A. Three cases of meningococcal infection in a family, associated with a deficient immune response. Arch Dis Child 1973;48:742–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kantor AB, and Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol 1993;11:501–538 [DOI] [PubMed] [Google Scholar]
- 73. Kantor AB, Stall AM, Adams S, et al. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A 1992;89:3320–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kawahara T, Ohdan H, Zhao G, et al. Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J Immunol 2003;171:5406–5414 [DOI] [PubMed] [Google Scholar]
- 75. Kearney JF, McCarthy MT, Stohrer R, et al. Induction of germ-line anti-alpha 1–3 dextran antibody responses in mice by members of the Enterobacteriaceae family. J Immunol 1985;135:3468–3472 [PubMed] [Google Scholar]
- 76. Kearney JF, Patel P, Stefanov EK, et al. Natural antibody repertoires: development and functional role in inhibiting allergic airway disease. Annu Rev Immunol 2015;33:475–504 [DOI] [PubMed] [Google Scholar]
- 77. Kin NW, Stefanov EK, Dizon BL, et al. Antibodies generated against conserved antigens expressed by bacteria and allergen-bearing fungi suppress airway disease. J Immunol 2012;189:2246–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kopf M, Abel B, Gallimore A, et al. Complement component C3 promotes T-cell priming and lung migration to control acute influenza virus infection. Nat Med 2002;8:373–378 [DOI] [PubMed] [Google Scholar]
- 79. Kroese FG, Butcher EC, Stall AM, et al. Many of the IgA producing plasma cells in murine gut are derived from self-replenishing precursors in the peritoneal cavity. Int Immunol 1989;1:75–84 [DOI] [PubMed] [Google Scholar]
- 80. Lalor PA, Herzenberg LA, Adams S, et al. Feedback regulation of murine Ly-1 B cell development. Eur J Immunol 1989;19:507–513 [DOI] [PubMed] [Google Scholar]
- 81. Louis AG, and Gupta S. Primary selective IgM deficiency: an ignored immunodeficiency. Clin Rev Allergy Immunol 2014;46:104–111 [DOI] [PubMed] [Google Scholar]
- 82. Luallen RJ, Agrawal-Gamse C, Fu H, et al. Antibodies against Manalpha1,2-Manalpha1,2-Man oligosaccharide structures recognize envelope glycoproteins from HIV-1 and SIV strains. Glycobiology 2010;20:280–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lydyard PM, Quartey-Papafio R, Broker B, et al. The antibody repertoire of early human B cells. I. High frequency of autoreactivity and polyreactivity. Scand J Immunol 1990;31:33–43 [DOI] [PubMed] [Google Scholar]
- 84. Macpherson AJ, Gatto D, Sainsbury E, et al. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 2000;288:2222–2226 [DOI] [PubMed] [Google Scholar]
- 85. Madi A, Bransburg-Zabary S, Maayan-Metzger A, et al. Tumor-associated and disease-associated autoantibody repertoires in healthy colostrum and maternal and newborn cord sera. J Immunol 2015;194:5272–5281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mallery DL, McEwan WA, Bidgood SR, et al. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A 2010;107:19985–19990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Malynn BA, Yancopoulos GD, Barth JE, et al. Biased expression of JH-proximal VH genes occurs in the newly generated repertoire of neonatal and adult mice. J Exp Med 1990;171:843–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Manches O, Plumas J, Lui G, et al. Anti-Gal-mediated targeting of human B lymphoma cells to antigen-presenting cells: a potential method for immunotherapy using autologous tumor cells. Haematologica 2005;90:625–634 [PubMed] [Google Scholar]
- 89. Marshall AJ, Doyen N, Bentolila LA, et al. Terminal deoxynucleotidyl transferase expression during neonatal life alters D(H) reading frame usage and Ig-receptor-dependent selection of V regions. J Immunol 1998;161:6657–6663 [PubMed] [Google Scholar]
- 90. Martin F, and Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev 2000;175:70–79 [PubMed] [Google Scholar]
- 91. Martin F, and Kearney JF. Marginal-zone B cells. Nat Rev Immunol 2002;2:323–335 [DOI] [PubMed] [Google Scholar]
- 92. Martin F, Oliver AM, and Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 2001;14:617–629 [DOI] [PubMed] [Google Scholar]
- 93. Matter MS, and Ochsenbein AF. Natural antibodies target virus-antibody complexes to organized lymphoid tissue. Autoimmun Rev 2008;7:480–486 [DOI] [PubMed] [Google Scholar]
- 94. Meng W, Zhang B, Schwartz GW, et al. An atlas of B-cell clonal distribution in the human body. Nat Biotechnol 2017;35:879–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Merbl Y, Zucker-Toledano M, Quintana FJ, et al. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest 2007;117:712–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Montecino-Rodriguez E, Leathers H, and Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol 2006;7:293–301 [DOI] [PubMed] [Google Scholar]
- 97. Moon BG, Takaki S, Miyake K, et al. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. J Immunol 2004;172:6020–6029 [DOI] [PubMed] [Google Scholar]
- 98. Mouquet H, and Nussenzweig MC. Polyreactive antibodies in adaptive immune responses to viruses. Cell Mol Life Sci 2012;69:1435–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nguyen TT, Elsner RA, and Baumgarth N. Natural IgM prevents autoimmunity by enforcing B cell central tolerance induction. J Immunol 2015;194:1489–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Nguyen TT, Klasener K, Zurn C, et al. The IgM receptor FcmuR limits tonic BCR signaling by regulating expression of the IgM BCR. Nat Immunol 2017;18:321–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nguyen TTT, Graf BA, Randall TD, et al. sIgM-FcmuR interactions regulate early B cell activation and plasma cell development after influenza virus infection. J Immunol 2017;199:1635–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Obukhova P, Rieben R, and Bovin N. Normal human serum contains high levels of anti-Gal alpha 1–4GlcNAc antibodies. Xenotransplantation 2007;14:627–635 [DOI] [PubMed] [Google Scholar]
- 103. Ochsenbein AF, Fehr T, Lutz C, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science 1999;286:2156–2159 [DOI] [PubMed] [Google Scholar]
- 104. Ochsenbein AF, and Zinkernagel RM. Natural antibodies and complement link innate and acquired immunity. Immunol Today 2000;21:624–630 [DOI] [PubMed] [Google Scholar]
- 105. Ohdan H, Swenson KG, Kruger Gray HS, et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Gal alpha 1,3Gal epitopes in alpha 1,3-galactosyltransferase-deficient mice. J Immunol 2000;165:5518–5529 [DOI] [PubMed] [Google Scholar]
- 106. Pages J, and Bussard AE. Precommitment of normal mouse peritoneal cells by erythrocyte antigens in relation to auto-antibody production. Nature 1975;257:316–317 [DOI] [PubMed] [Google Scholar]
- 107. Patel PS, and Kearney JF. Neonatal exposure to pneumococcal phosphorylcholine modulates the development of house dust mite allergy during adult life. J Immunol 2015;194:5838–5850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Patel PS, King RG, and Kearney JF. Pulmonary alpha-1,3-glucan-specific IgA-secreting B cells suppress the development of cockroach allergy. J Immunol 2016;197:3175–3187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Perlmutter RM, Kearney JF, Chang SP, et al. Developmentally controlled expression of immunoglobulin VH genes. Science 1985;227:1597–1601 [DOI] [PubMed] [Google Scholar]
- 110. Rapaka RR, Ricks DM, Alcorn JF, et al. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med 2010;207:2907–2919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Reynolds AE, Kuraoka M, and Kelsoe G. Natural IgM is produced by CD5- plasma cells that occupy a distinct survival niche in bone marrow. J Immunol 2015;194:231–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Roozendaal R, and Carroll MC. Complement receptors CD21 and CD35 in humoral immunity. Immunol Rev 2007;219:157–166 [DOI] [PubMed] [Google Scholar]
- 113. Rother MB, Jensen K, van der Burg M, et al. Decreased IL7Ralpha and TdT expression underlie the skewed immunoglobulin repertoire of human B-cell precursors from fetal origin. Sci Rep 2016;6:33924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rothstein TL, Griffin DO, Holodick NE, et al. Human B-1 cells take the stage. Ann N Y Acad Sci 2013;1285:97–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Saito T, Chiba S, Ichikawa M, et al. Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity 2003;18:675–685 [DOI] [PubMed] [Google Scholar]
- 116. Savage HP, Yenson VM, Sawhney SS, et al. Blimp-1-dependent and -independent natural antibody production by B-1 and B-1-derived plasma cells. J Exp Med 2017;214:2777–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Schwarz M, Spector L, Gortler M, et al. Serum anti-Glc(alpha1,4)Glc(alpha) antibodies as a biomarker for relapsing-remitting multiple sclerosis. J Neurol Sci 2006;244:59–68 [DOI] [PubMed] [Google Scholar]
- 118. Shackelford PG, Nelson SJ, Palma AT, et al. Human antibodies to group A streptococcal carbohydrate. Ontogeny, subclass restriction, and clonal diversity. J Immunol 1988;140:3200–3205 [PubMed] [Google Scholar]
- 119. Solvason N, Chen X, Shu F, et al. The fetal omentum in mice and humans. A site enriched for precursors of CD5 B cells early in development. Ann N Y Acad Sci 1992;651:10–20 [DOI] [PubMed] [Google Scholar]
- 120. Solvason N, and Kearney JF. The human fetal omentum: a site of B cell generation. J Exp Med 1992;175:397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Solvason N, Lehuen A, and Kearney JF. An embryonic source of Ly1 but not conventional B cells. Int Immunol 1991;3:543–550 [DOI] [PubMed] [Google Scholar]
- 122. Soto C, Bombardi RG, Branchizio A, et al. High frequency of shared clonotypes in human B cell receptor repertoires. Nature 2019;566:398–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stein LD, and Sigal NH. Heterogeneity of the human phosphocholine-specific B cell repertoire. J Immunol 1984;132:1329–1335 [PubMed] [Google Scholar]
- 124. Subramaniam KS, Datta K, Quintero E, et al. The absence of serum IgM enhances the susceptibility of mice to pulmonary challenge with Cryptococcus neoformans. J Immunol 2010;184:5755–5767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Takeda K, and Akira S. Toll-like receptors. Curr Protoc Immunol 2007;Chapter 14:Unit 14.12. [DOI] [PubMed] [Google Scholar]
- 126. Takeuchi T, Nakagawa T, Maeda Y, et al. Functional defect of B lymphocytes in a patient with selective IgM deficiency associated with systemic lupus erythematosus. Autoimmunity 2001;34:115–122 [DOI] [PubMed] [Google Scholar]
- 127. Takeuchi Y, Liong SH, Bieniasz PD, et al. Sensitization of rhabdo-, lenti-, and spumaviruses to human serum by galactosyl(alpha1-3)galactosylation. J Virol 1997;71:6174–6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Tanigaki K, Han H, Yamamoto N, et al. Notch-RBP-J signaling is involved in cell fate determination of marginal zone B cells. Nat Immunol 2002;3:443–450 [DOI] [PubMed] [Google Scholar]
- 129. Tomiyama T, Lake D, Masuho Y, et al. Recognition of human immunodeficiency virus glycoproteins by natural anti-carbohydrate antibodies in human serum. Biochem Biophys Res Commun 1991;177:279–285 [DOI] [PubMed] [Google Scholar]
- 130. Vas J, Gronwall C, Marshak-Rothstein A, et al. Natural antibody to apoptotic cell membranes inhibits the proinflammatory properties of lupus autoantibody immune complexes. Arthritis Rheum 2012;64:3388–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Vernino LA, Pisetsky DS, and Lipsky PE. Analysis of the expression of CD5 by human B cells and correlation with functional activity. Cell Immunol 1992;139:185–197 [DOI] [PubMed] [Google Scholar]
- 132. Vieyres G, Thomas X, Descamps V, et al. Characterization of the envelope glycoproteins associated with infectious hepatitis C virus. J Virol 2010;84:10159–10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Vollmers HP, and Brandlein S. Natural antibodies and cancer. J Autoimmun 2007;29:295–302 [DOI] [PubMed] [Google Scholar]
- 134. Waffarn EE, Hastey CJ, Dixit N, et al. Infection-induced type I interferons activate CD11b on B-1 cells for subsequent lymph node accumulation. Nat Commun 2015;6:8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Weller S, Braun MC, Tan BK, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 2004;104:3647–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Won WJ, Bachmann MF, and Kearney JF. CD36 is differentially expressed on B cell subsets during development and in responses to antigen. J Immunol 2008;180:230–237 [DOI] [PubMed] [Google Scholar]
- 137. Won WJ, and Kearney JF. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J Immunol 2002;168:5605–5611 [DOI] [PubMed] [Google Scholar]
- 138. Xia L, and Gildersleeve JC. Anti-glycan IgM repertoires in newborn human cord blood. PLoS One 2019;14:e0218575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Yancopoulos GD, Desiderio SV, Paskind M, et al. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature 1984;311:727–733 [DOI] [PubMed] [Google Scholar]
- 140. Yancopoulos GD, Malynn BA, and Alt FW. Developmentally regulated and strain-specific expression of murine VH gene families. J Exp Med 1988;168:417–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Yang Y, Ghosn EE, Cole LE, et al. Antigen-specific antibody responses in B-1a and their relationship to natural immunity. Proc Natl Acad Sci U S A 2012;109:5382–5387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Yang Y, Ghosn EE, Cole LE, et al. Antigen-specific memory in B-1a and its relationship to natural immunity. Proc Natl Acad Sci U S A 2012;109:5388–5393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Yang Y, Tung JW, Ghosn EE, et al. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci U S A 2007;104:4542–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Yilmaz B, Portugal S, Tran TM, et al. Gut microbiota elicits a protective immune response against malaria transmission. Cell 2014;159:1277–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Yuan J, Nguyen CK, Liu X, et al. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 2012;335:1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhou Y, Li YS, Bandi SR, et al. Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J Exp Med 2015;212:569–580 [DOI] [PMC free article] [PubMed] [Google Scholar]