Abstract
Background: Little literature exists on primary care providers' knowledge and preferences toward breast cancer screening for high-risk women.
Materials and Methods: A cross-sectional web-based survey of primary care providers in Minnesota was conducted in 2016. The primary aim was to determine the breast cancer screening practices of primary care providers for women at high risk for breast cancer. A multipart questionnaire focused on breast cancer screening practices for high-risk women and perceived risks/benefits of breast cancer screening was administered. Statistical analyses, included descriptive statistics and tests of differences in screening practices and knowledge across key professional characteristics, were conducted.
Results: Eight hundred five primary care providers completed the survey (7.7% response). Participants were predominantly female (72.2%); 43.9% were physicians, 11.4% physician assistants, and 44.8% advanced practice registered nurses. One-quarter of providers recommended mammography and breast magnetic resonance imaging (MRI) for high-risk women ages 40–49 years. There were no differences in breast MRI recommendations based on years of experience or practice setting. In high-risk women with prior chest radiation and an increased risk of breast cancer, for whom guidelines recommend mammography and MRI, 75.0% of providers recommended mammography, but only 44.3% recommended breast MRI. Recent continuing education on breast cancer screening was associated with providers being more comfortable giving high-risk screening recommendations (p = 0.002).
Conclusions: Most primary care providers believe mammography is helpful in women at high risk for breast cancer. Less than half of practitioners, however, recommend breast MRI to screen women at high risk for breast cancer, despite guidelines promoting the use of breast MRI. Increased provider education is warranted.
Keywords: breast cancer, women's health, preventive care, cancer screening
Introduction
Breast cancer screening recommendations depend on a patient being classified as average risk or high risk. One in eight average-risk women will develop breast cancer by age 80 years, whereas one in three high-risk women will develop breast cancer by age 50 years.1–4 Given these statistics, women considered to be at high risk for breast cancer need more intensive screening that includes initiating breast cancer screening at an earlier age and being screened with both mammography and breast magnetic resonance imaging (MRI).2,5,6 The American Cancer Society (ACS) and the National Cancer Institute define “high risk” as the following (Table 1): any individual with a known germ line BRCA gene mutation or other high-risk genes, a first-degree relative with a known BRCA mutation, a lifetime risk of breast cancer greater than 20%–25% as identified by a variety of clinical and patient characteristics, and cancer survivors who received therapeutic radiation to the chest between the ages of 10–30 years.5,6 While efforts have been made to provide cancer survivors and their providers with a survivorship care plan (SCP) outlining these screening recommendations,7,8 it is not clear if these SCPs are useful in enhancing breast cancer screening and in helping primary care providers follow guideline-specific breast cancer screening recommendations. Intensive breast screening with mammography and breast MRI in all of these patient populations results in an improvement in breast cancer outcomes, including early diagnosis and overall mortality.1 Despite substantial agreement in how and when to initiate breast cancer screening in these women at high risk for breast cancer, it is unclear how these guidelines are followed in practice.
Table 1.
Women at High-Risk for Breast Cancer in Whom Dual Screening with Breast Magnetic Resonance Imaging and Mammography Is Recommended5,14
| 1. Known germ line BRCA gene mutation |
| 2. First-degree relative with a known BRCA mutation |
| 3. A lifetime risk of breast cancer >20%–25% as identified by a variety of clinical and patient characteristics |
| 4. A genetic mutation in genes at high risk for breast cancer (Li-Fraumeni, Cowden, Bannayan-Riley)14 |
Those who received radiation to the chest between the ages of 10–30 years.
Prior studies have identified primary care providers' adherence to recommendations for breast cancer screening in average-risk women.9–12 These studies have focused on physicians' practices and on women at average risk for breast cancer.12,13 Little data exist on the screening practices of the 90,000 advanced practice registered nurses (APRNs) and physician assistants (PAs) who provide primary care in the United States. Furthermore, little data exist on the breast cancer screening recommendations of primary care providers for women at high risk for breast cancer. It is vital that primary care providers understand and follow these guidelines so that the benefits of intensive screening with breast MRI and mammography in women at high risk for breast cancer can be attained.1,14,15 The objective of this study was to determine Minnesota primary care providers' breast cancer screening practices for women at high risk of developing breast cancer, and to examine differences in practices and knowledge of recommendations across provider characteristics such as age, sex, professional background, and years of experience.
Materials and Methods
Study design and participants
A cross-sectional web-based survey of primary care providers licensed to practice in Minnesota was conducted. Licensed health care professionals whose contact information appeared on the State of Minnesota Mailing List Service from the Minnesota Board of Medical Practice or Minnesota Board of Nursing Practice in one of several groups—physicians (family medicine, internal medicine, and obstetrics/gynecology), APRNs (family, adult gerontology, and women's and gender-related health), and PAs—were identified as eligible and included in this study. As PAs are not licensed in a specialty area, all licensed PAs were included. This study was reviewed and deemed exempt from oversight by the University of Minnesota's Institutional Review Board.
Potentially eligible providers were sent an e-mail invitation with an attached letter printed on the University of Minnesota Deborah E. Powell Center of Women's Health letterhead with details about the study. Participants were directed to a unique URL link to the anonymous study survey. Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Minnesota.16 Individuals were able to indicate whether they were not interested in participating in the survey by clicking an “I'm not interested” link. E-mails inviting study participation were sent initially in spring 2016, with up to five reminders through fall 2016.
All participants who completed the survey were eligible for a raffle. After completing the survey, participants were directed to a separate web link to enter their e-mail address to be eligible for the raffle. Entering their e-mail address signified consent to participate in the raffle. A total of twenty $300 Amazon gift cards were distributed to participants. These gift cards were e-mailed to the recipient at his/her participating e-mail address.
Survey instrument
The survey was adapted from measures previously described by Corbelli et al.12 and the National Health Survey of Primary Care Physicians' Recommendations and Practice for Breast, Cervical, Colorectal, and Lung Cancer Screening 2010.12,17 The survey consisted of 37 multipart questions. The questions focused on general breast cancer screening, the risks and benefits of breast cancer screening, and aspects of shared decision-making. To assess providers' screening recommendations by age, providers were asked: “How often do you recommend mammography for high-risk women” and “how often do you recommend breast MRI for high-risk women?” Respondents could choose annually, every 2 years, or “do not recommend screening.” They were also asked: “How comfortable are you in making breast cancer screening recommendations for a particular patient population?” (average-risk women and high-risk women) Respondents could choose “not comfortable at all,” “somewhat comfortable,” or “comfortable.” The survey also included several case vignettes, including one in which respondents were asked which type of breast cancer screening they would recommend for a 40-year-old female with a history of Hodgkin lymphoma at age 20 years treated with mantle radiation (a population known to be at high risk for breast cancer). Available answers included clinical breast examination, mammography, MRI, other, no screening. The ACS, the National Comprehensive Cancer Center, and the Children's Oncology Group guidelines recommend mammogram and breast MRI for all women older than 40 years who are at high risk for developing breast cancer.5,6,14 Thus, participating providers who recommended mammogram and breast MRI in high-risk patients were considered to be adhering to best practices by providing appropriately proactive breast cancer screening suggested by national guidelines. “High risk” was defined in the survey as patients younger than 50 years who have a personal history of breast cancer, have either a family history of breast cancer or other genetic mutation associated with an increased risk of breast cancer, or have received therapeutic chest radiation between the ages of 10 and 30 years.14 While not all women with a personal history of breast cancer require breast MRI and mammography for ongoing breast cancer screening, we chose women younger than 50 with a personal history of breast cancer as being “high risk,” given most of these women have a 20% lifetime risk for breast cancer, a measurement defined by ACS as meeting criteria for breast MRI screening.
The survey closed with several demographic questions. Before survey administration, the survey instrument was pilot tested among several primary care providers.
Statistical methods
This analysis was limited to the providers who reported providing primary care to women. Demographic and professional characteristics of providers were summarized using descriptive statistics. Outcomes of interest for this analysis include adherence to breast cancer screening guidelines and comfort making breast cancer screening recommendations for women at high risk for breast cancer. Comparisons of these outcomes across demographic and professional characteristics were conducted using chi-squared and Fisher's exact tests, and multivariate logistic regression analyses when appropriate. Estimates and 95% confidence intervals are provided when appropriate. Data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC). p-Values of <0.05 were considered statistically significant.
Results
A total of 3,800 physicians, 2,132 PAs, 4,000 nurse practitioners/nurse specialists, and 460 nurse midwives were invited to complete the survey, of which 805 invitees participated for a response rate of 7.7%. Of the 805 respondents, 456 (56.7%) reported providing primary care to women. Characteristics of responding providers are outlined in Table 2. Most (72.2%) were women and they were well distributed as follows by professional background: 43.9% were physicians (20.8% internal medicine, 71.7% family medicine, and 6.3% obstetrics/gynecology), 11.4% were PAs, and 44.8% were APRNs (nurse practitioners, nurse midwives, or clinical nurse specialists). The majority (84.8%) were in community practice and had significant years of experience: 38% reported more than 20 years of experience and 27.1% had less than 10 years of experience. On average, participants reported seeing 55.9 ± 22.0 patients per week in their primary care location, and more than half of patients in their primary care practices were older than 40 years.
Table 2.
Survey Professional and Practice Characteristics (N = 456)
| Characteristic | N | % |
|---|---|---|
| Professional background | ||
| Physician | 193 | 43.9 |
| Physician assistant | 50 | 11.4 |
| Advanced practice registered nurse | 197 | 44.8 |
| Physician or physician assistant specialty | ||
| Internal medicine/adult or gerontological health | 50 | 20.8 |
| Family medicine/family practice | 172 | 71.7 |
| Gynecology/women's health/nurse midwifery | 15 | 6.3 |
| Other (geriatrics, nephrology, oncology) | 3 | 1.3 |
| Advanced practice registered nurse specialty | ||
| Adult/gerontological health | 26 | 13.5 |
| Family practice | 98 | 50.8 |
| Nurse midwifery | 25 | 13.0 |
| Women's health | 31 | 16.1 |
| Other | 13 | 6.7 |
| Specialized interest in women's health | ||
| No | 238 | 54.1 |
| Yes | 202 | 45.9 |
| Years of experience | ||
| <5 | 68 | 15.6 |
| 6–10 | 50 | 11.5 |
| 11–15 | 64 | 14.7 |
| 16–20 | 85 | 19.5 |
| >20 | 169 | 38.8 |
| Practice setting | ||
| Academic | 66 | 15.2 |
| Community | 369 | 84.8 |
| Academic affiliation | ||
| No | 308 | 70.0 |
| Yes | 132 | 30.0 |
| Missing | 16 | |
| When was the last time you participated in a continuing education program on breast cancer screening? | ||
| Within the past 3 years | 216 | 49.3 |
| 3–6 years ago | 97 | 22.2 |
| More than 6 years ago | 123 | 28.4 |
| Gender | ||
| Male | 119 | 27.2 |
| Female | 316 | 72.2 |
| Other | 3 | 0.7 |
When asked how effective screening was for reducing cancer mortality in high-risk women, mammography was thought by respondents to be very effective or effective in women ages 40–49 years (95.6% of respondents) and older than 50 years (96.5%). Most (81.6%) respondents thought breast MRI was very effective or effective in reducing cancer mortality in high-risk women.
When asked what method of breast cancer screening they recommended to high-risk women ages 40–44 years (Table 3), approximately one-quarter of providers recommended both mammography and breast MRI for high-risk women, in line with clinical guidelines.5,14 In the univariate analyses, no differences were observed in reported mammography and breast MRI screening recommendations by professional background (p = 0.51), years of experience (p = 0.12), or practice setting (p = 0.88). Female practitioners, compared with male practitioners (p = 0.003), those working in gynecology, compared with other specialties (p = 0.04), and those specializing in women's health (p = 0.0006) more commonly recommended mammography and breast MRI for high-risk women. In multivariable analyses, being a physician (p = 0.04), having an interest in women's health (p = 0.02), and being a female provider (p = 0.02) were statistically significantly associated with mammography and breast MRI screening recommendations. The relationships were similar for responses regarding high-risk women 45–49 years old, with being a physician (p = 0.01), having an interest in women's health (p = 0.02), and being a female provider (p = 0.009) were statistically significantly associated with mammography and breast MRI screening recommendations in the multivariable model.
Table 3.
Characteristics of Providers' Guideline Adherence to Recommending Both Mammogram and Breast Magnetic Resonance Imaging Screening in Young Women at High Risk for Breast Cancer
| Characteristic | 40–44 years |
45–49 years |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No |
Yes |
|
No |
Yes |
|
|||||
| N | % | N | % | p | N | % | N | % | p | |
| Professional background | 0.47 | 0.16 | ||||||||
| Physician | 146 | 75.7 | 47 | 24.4 | 144 | 74.6 | 49 | 25.4 | ||
| Physician assistant | 41 | 82.0 | 9 | 18.0 | 43 | 86.0 | 7 | 14.0 | ||
| Advanced practice registered nurse | 145 | 73.6 | 52 | 26.4 | 144 | 73.1 | 53 | 26.9 | ||
| Specialty (among physicians) | 0.04 | 0.04 | ||||||||
| Internal medicine/adult or gerontological health | 35 | 72.9 | 13 | 27.1 | 34 | 70.9 | 14 | 29.2 | ||
| Family medicine/family practice | 100 | 80.0 | 25 | 20.0 | 99 | 79.2 | 26 | 20.8 | ||
| Gynecology/women's health/nurse midwifery | 7 | 46.7 | 8 | 53.3 | 7 | 46.7 | 8 | 53.3 | ||
| Other (geriatrics, nephrology, oncology) | 2 | 100.0 | 0 | 0.0 | 2 | 100.0 | 0 | 0.0 | ||
| Specialized interest in women's health | 0.0004 | 0.0002 | ||||||||
| No | 195 | 81.9 | 43 | 18.1 | 196 | 82.4 | 42 | 17.7 | ||
| Yes | 136 | 67.3 | 66 | 32.7 | 135 | 66.8 | 67 | 33.2 | ||
| Years of experience | 0.14 | 0.17 | ||||||||
| <5 | 51 | 75.0 | 17 | 25.0 | 52 | 76.5 | 16 | 23.5 | ||
| 6–10 | 35 | 70.0 | 15 | 30.0 | 36 | 72.0 | 14 | 28.0 | ||
| 11–15 | 44 | 68.8 | 20 | 31.3 | 44 | 68.8 | 20 | 31.3 | ||
| 16–20 | 60 | 70.6 | 25 | 29.4 | 59 | 69.4 | 26 | 30.6 | ||
| >20 | 138 | 81.7 | 31 | 18.3 | 137 | 81.1 | 32 | 18.9 | ||
| Practice setting | 0.89 | 0.89 | ||||||||
| Academic | 49 | 74.2 | 17 | 25.8 | 49 | 15.0 | 17 | 15.6 | ||
| Community | 277 | 75.1 | 92 | 24.9 | 277 | 85.0 | 92 | 84.4 | ||
| Gender | 0.003 | 0.003 | ||||||||
| Male | 102 | 85.7 | 17 | 14.3 | 102 | 85.7 | 17 | 14.3 | ||
| Female | 226 | 71.5 | 90 | 28.5 | 226 | 71.5 | 90 | 28.5 | ||
| Other | 3 | 100.0 | 0 | 0.0 | 3 | 100.0 | 0 | 0.0 | ||
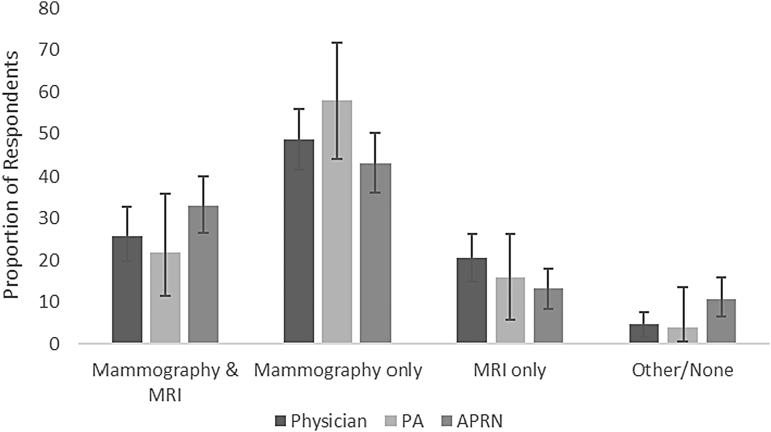
In the clinical vignette for a 40-year-old Hodgkin lymphoma survivor treated 20 years prior with mantle radiation, 75.0% of respondents recommended mammography and 44.3% recommended breast MRI (Fig. 1). The recommendations significantly differed by practitioner background (p = 0.04), with 25.9%, 22.0%, and 33.0% of physicians, PAs, and APRNs, respectively, reporting that they recommend both breast MRI and mammography (the guideline-adherent response) in this clinical scenario. A total of 64.4% reported never having received an SCP outlining breast imaging recommendations for a patient with such a cancer history. Of those who had received an SCP, 94.7% reported it was helpful.
FIG. 1.
Providers' recommendations for breast cancer screening of a 40-year old treated with mantle radiation for Hodgkin lymphoma at 20 years. National guidelines recommend breast MRI and mammogram. MRI, magnetic resonance imaging.
Less than half of the primary care providers reported being comfortable making breast cancer screening recommendations for high-risk women (Table 4). The proportion who reported being comfortable did not vary by professional background, special interest in women's health, years of experience (p = 0.15), gender of provider, practice setting, or academic affiliation. Those who had participated in a continuing education program (CME) on breast cancer screening within the past 3 years were more likely to be comfortable providing breast cancer screening recommendations for high-risk women (p = 0.002).
Table 4.
Comfort Making Breast Cancer Screening Recommendations for High-Risk Women
| Not/Somewhat comfortable |
Comfortable |
p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Professional background | 0.76 | ||||
| Physician | 109 | 56.8 | 83 | 43.2 | |
| Physician assistant | 27 | 55.1 | 22 | 44.9 | |
| Advanced practice registered nurse | 116 | 59.8 | 78 | 40.2 | |
| Specialized interest in women's health | 0.15 | ||||
| No | 145 | 61.2 | 92 | 38.8 | |
| Yes | 108 | 54.3 | 91 | 45.7 | |
| Years of experience | 0.15 | ||||
| <5 | 42 | 61.8 | 26 | 14.2 | |
| 6–10 | 32 | 65.3 | 17 | 9.3 | |
| 11–15 | 43 | 67.2 | 21 | 11.5 | |
| 16–20 | 45 | 53.6 | 39 | 21.3 | |
| >20 | 87 | 52.1 | 80 | 43.7 | |
| Practice setting | 0.91 | ||||
| Academic | 37 | 56.9 | 28 | 43.1 | |
| Community | 211 | 57.7 | 155 | 42.4 | |
| Academic affiliation | 0.12 | ||||
| No | 186 | 60.8 | 120 | 39.2 | |
| Yes | 68 | 52.7 | 61 | 47.3 | |
| When was the last time you participated in a continuing education program on breast cancer screening? | 0.002 | ||||
| Within the past 3 years | 106 | 49.8 | 107 | 50.2 | |
| 3–6 years ago | 64 | 66.7 | 32 | 33.3 | |
| More than 6 years ago | 82 | 66.1 | 42 | 33.9 | |
| Gender | 0.95 | ||||
| Male | 69 | 58.5 | 49 | 41.5 | |
| Female | 181 | 58.0 | 131 | 42.0 | |
| Other | 2 | 66.7 | 1 | 33.3 | |
Discussion
In this survey, few primary care providers reported following appropriate recommendations to screen women at high risk for breast cancer with mammography and breast MRI. In addition, only one-third were able to identify intensive guideline-adherent screening recommendations for Hodgkin lymphoma survivors. This is the first study to describe screening practices for women at high risk for breast cancer distinguishing by provider type. Although most respondents believe both mammogram and breast MRI are effective in reducing cancer mortality, only one-quarter said they actually recommend both mammogram and breast MRI for women at high risk for breast cancer in their fourth decade. Female physician providers, those interested in gynecology, and those who recently completed continuing medical education were more likely to make recommendations for screening women at high risk for breast cancer that were consistent with guidelines provided by the National Comprehensive Cancer Network and the ACS national guidelines.6,14
Recent studies have demonstrated that breast MRI can detect more breast cancers and improve breast cancer mortality, compared with mammography alone in women at high risk for developing breast cancer.2,18,19 In 1,000 breast examinations, 21.8 cancers will be detected with MRI compared with 7.2 cancers by mammogram.19 The sensitivity and specificity of MRI are 96% and 78%, respectively, compared with 31% and 89% (p < 0.0001) for mammogram. The MRI recall images, however, are slightly increased compared with mammogram (9.3% vs. 6.5%).18,20 Breast MRI has been shown to increase detection and identify breast cancer at an earlier stage with no lymph node involvement.21 Recent studies also suggest that breast MRI improves breast cancer mortality.1
There are over 10 organizations with established clinical guidelines or recommendations for breast cancer screening, including the U.S. Preventive Services Task Force (USPSTF), the American College of Obstetrics and Gynecology, and the ACS.14,22,23 For women at average risk for breast cancer, these different organizations have different recommendations regarding when to start screening mammography, when to stop screening, and at what interval to screen. There is significantly more consensus regarding screening recommendations for women at high risk for breast cancer. Nevertheless, there are discrepancies across organizations; for example, the USPSTF does not have recommendations for high-risk individuals.23 In contrast, the ACS and the National Comprehensive Cancer Network and other organizations recommend both mammography and breast MRI as screening tools.6,14,23 Studies suggest there are negative consequences on clinical care when multiple guidelines exist.12 The differences in these guidelines may explain why, in our survey of primary care providers, many providers did not recommend mammography and MRI breast cancer screening for high-risk women.
Cancer survivors, particularly Hodgkin lymphoma survivors who received mantle radiation, are at extremely high risk for developing breast cancer with the risk of breast cancer in a patient treated for Hodgkin lymphoma with mantle radiation at the age of 20 years to be ∼35%.2 This increased risk has been well known for over a decade, with the Institute of Medicine reporting this in 2005.7 The Children's Oncology Group, the ACS, and the National Comprehensive Cancer Network have all recommended breast MRI and mammography on an annual basis in this patient population.5,22 Despite efforts to promote awareness of this topic in cancer survivors, it appears there is still a deficit in knowledge regarding screening recommendations in cancer survivors.10,11 Receipt of an SCP or a visit in a survivorship clinic may increase these screening recommendations,8,24 yet only half of providers in our sample report ever having received an SCP. With the recent Commission on Cancer mandates that individuals completing cancer therapy receive an SCP, it is possible that the delivery of an SCP will improve both awareness of these guidelines and the appropriate screening recommendations in this high-risk patient population.25
Other studies have suggested that breast MRI is underutilized in women at high risk for breast cancer.26 In our study, however, in general there were few differences in breast cancer screening recommendations by provider type. Years of practice and type of practice did not influence screening practices. Recent breast cancer CME appeared to increase the comfort of providers in recommending breast cancer screening to high-risk women. As a result, it appears that, across all provider types, provider education remains critical.
This study has several limitations, including the response rate and potential biases associated with self-reported data. Although the response rate was low, it is similar to other studies of physicians.27–30 The response rate may have been low due to our sampling strategy. All licensed providers were targeted; this may have affected response rates. In some studies of providers, monetary incentives increased response rates. This was attempted in our study although the response rate remained low. At least one review suggests there is less concern of nonresponse bias in physician surveys.27 Another potential limitation of this study is it was conducted in the state of Minnesota. Given that Minnesota is known to have high access rates to health care and health care quality compared with most other states, these results may not be generalizable to all primary care providers in the United States where patients may have difficulty with insurance coverage or accessing care.31 It is also possible that the providers who answered the survey had an interest in breast health; thus, these results may actually be an overestimation of adherence to breast cancer screening practices in high-risk women. This study did not explore barriers to providing guideline-adherent recommendations. For example, it is possible that in rural areas, breast MRI may not be readily available. It is also possible that there are concerns about cost, and thus, breast MRI subsequently is not routinely ordered. Despite these limitations, this study is one of the few to query different primary care providers—physicians, APRNs, and PAs—about breast cancer screening practices, and the first to ask about screening practices for high-risk women.
Further work is needed to understand the barriers around providing breast cancer screening guidelines for women at high risk. It is not clear from our study if adherence to performing guideline-specific care is a function of provider knowledge, the variation in guidelines, or something else. Identifying these barriers would help provide the next steps for intervention. For example, best practice alerts in the electronic medical record, patient education materials, or further shared decision-making models could be developed, depending on the barriers identified.
Conclusions
While most providers believe breast MRI and mammography are effective in reducing breast cancer mortality for high-risk women, only about one-third suggest ordering these screenings for a hypothetical cancer survivor or other women at high risk for breast cancer. Education of health care providers on the benefits of high-risk breast cancer screening, and possible receipt of an SCP, may be beneficial in improving adherence. Identifying barriers to providing this specific care in women at high risk for breast cancer is an important next step.
Acknowledgment
This study was conducted with the support of K12-HD055887.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Hodgson DC, Cotton C, Crystal P, Nathan PC. Impact of early breast cancer screening on mortality among young survivors of childhood Hodgkin's lymphoma. J Natl Cancer Inst 2016;108. [DOI] [PubMed] [Google Scholar]
- 2. Moskowitz CS, Chou JF, Wolden SL, et al. . Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol 2014;32:2217–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Cancer Society. American Cancer Society Facts and Figures 2008: Atlanta: American Cancer Society 2008
- 4. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf Accessed March1, 2019
- 5. Hoppe RT, Advani RH, Ai WZ, et al. . Hodgkin Lymphoma Version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15:608–638 [DOI] [PubMed] [Google Scholar]
- 6. Saslow D, Boetes C, Burke W, et al. . American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 2007;57:75–89 [DOI] [PubMed] [Google Scholar]
- 7. Hewitt M, Rowland JH, Yancik R. Cancer survivors in the United States: Age, health, and disability. J Gerontol A Biol Sci Med Sci 2003;58:82–91 [DOI] [PubMed] [Google Scholar]
- 8. Baxstrom K, Peterson BA, Vogel RI, Blaes AH. Impact of consultation in a long term follow-up clinic on breast cancer and cardiovascular screening in Hodgkin lymphoma survivors. J Clin Oncol 2015;33:abstract e20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oeffinger KC, Ford JS, Moskowitz CS, et al. . Breast cancer surveillance practices among women previously treated with chest radiation for a childhood cancer. JAMA 2009;301:404–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nathan PC, Daugherty CK, Wroblewski KE, et al. . Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv 2013;7:275–282 [DOI] [PubMed] [Google Scholar]
- 11. Suh E, Daugherty CK, Wroblewski K, et al. . General internists' preferences and knowledge about the care of adult survivors of childhood cancer: A cross-sectional survey. Ann Internl Med 2014;160:11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Corbelli J, Borrero S, Bonnema R, et al. . Physician adherence to U.S. Preventive Services Task Force mammography guidelines. Womens Health Issues 2014;24:e313–e319 [DOI] [PubMed] [Google Scholar]
- 13. Yasmeen S, Romano PS, Tancredi DJ, Saito NH, Rainwater J, Kravitz RL. Screening mammography beliefs and recommendations: A web-based survey of primary care physicians. BMC Health Serv Res 2012;12:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gradishar WJ, Anderson BO, Balassanian R, et al. . NCCN Guidelines Insights: Breast Cancer, Version 1.2017. J Natl Compr Canc Netw 2017;15:433–451 [DOI] [PubMed] [Google Scholar]
- 15. Narayan AK, Visvanathan K, Harvey SC. Comparative effectiveness of breast MRI and mammography in screening young women with elevated risk of developing breast cancer: A retrospective cohort study. Breast Cancer Res Treat 2016;158:583–589 [DOI] [PubMed] [Google Scholar]
- 16. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zapka JM, Klabunde CN, Arora NK, Yuan G, Smith JL, Kobrin SC. Physicians' colorectal cancer screening discussion and recommendation patterns. Cancer Epidemiol Biomarkers Prev 2011;20:509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lo G, Scaranelo AM, Aboras H, et al. . Evaluation of the utility of screening mammography for high-risk women undergoing screening breast MR imaging. Radiology 2017;285:36–43 [DOI] [PubMed] [Google Scholar]
- 19. Leach MO, Boggis CR, Dixon AK, et al. . Screening with magnetic resonance imaging and mammography of a UK population at high familial risk of breast cancer: A prospective multicentre cohort study (MARIBS). Lancet 2005;365:1769–1778 [DOI] [PubMed] [Google Scholar]
- 20. Berg WA, Zhang Z, Lehrer D, et al. . Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 2012;307:1394–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Passaperuma K, Warner E, Causer PA, et al. . Long-term results of screening with magnetic resonance imaging in women with BRCA mutations. Br J Cancer 2012;107:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oeffinger KC, Fontham ET, Etzioni R, et al. . Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA 2015;314:1599–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moyer VA, United States Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:271–281 [DOI] [PubMed] [Google Scholar]
- 24. Hill-Kayser CE, Vachani CC, Hampshire MK, Di Lullo G, Jacobs LA, Metz JM. Impact of internet-based cancer survivorship care plans on health care and lifestyle behaviors. Cancer 2013;119:3854–3860 [DOI] [PubMed] [Google Scholar]
- 25. Stricker CT, O'Brien M. Implementing the commission on cancer standards for survivorship care plans. Clin J Oncol Nurs 2014;18 Suppl:15–22 [DOI] [PubMed] [Google Scholar]
- 26. Miles R, Wan F, Onega TL, et al. . Underutilization of supplemental magnetic resonance imaging screening among patients at high breast cancer risk. J Womens Health (Larchmt) 2018;27:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kellerman SE, Herold J. Physician response to surveys. A review of the literature. Am J Prev Med 2001;20:61–67 [DOI] [PubMed] [Google Scholar]
- 28. Martins Y, Lederman RI, Lowenstein CL, et al. . Increasing response rates from physicians in oncology research: A structured literature review and data from a recent physician survey. Br J Cancer 2012;106:1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cunningham CT, Quan H, Hemmelgarn B, et al. . Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cook DA, Wittich CM, Daniels WL, West CP, Harris AM, Beebe TJ. Incentive and reminder strategies to improve response rate for internet-based physician surveys: A randomized experiment. J Med Internet Res 2016;18:e244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. www.health.state.mn.us/divs/healthimprovement/content/documents/CancerFandF.pdf Accessed March1, 2019