Abstract
Males and females respond to pathogens differently and exhibit significantly different frequencies of autoimmune disease. For example, vaccinated adult females control influenza virus better than males, but females suffer systemic lupus erythematosus at a 9:1 frequency compared to males. Numerous explanations have been offered for these sex differences, but most have involved indirect mechanisms by which estrogen, a nuclear hormone, modifies cell barriers or immunity. In search of a direct mechanism, we examined the binding of estrogen receptor α (ERα), a class I nuclear hormone receptor, to the immunoglobulin heavy chain locus. Here, we show that in purified murine B cells, ERα and RNA polymerase II (RNA Pol II) exhibit extraordinarily similar DNA binding patterns. We further demonstrate that ERα preferentially binds adenosine–cytidine (AC)-repeats in the immunoglobulin heavy chain locus when supplemental estrogen is added to purified, lipopolysaccharide-activated B cells. Based on these and previous data, we hypothesize that (i) estrogen guides the binding of ERα and its RNA Pol II partner within the locus, which in turn instructs sterile transcription and class switch recombination (CSR), (ii) ERα binding to AC-repeats modifies the DNA architecture and loops associated with CSR, and (iii) by these mechanisms, estrogen instructs antibody expression. By targeting ERα-DNA interactions in the immunoglobulin heavy chain locus, clinicians may ultimately enhance antibody responses in the context of infectious diseases and reduce antibody responses in the context of allergic or autoimmune reactions.
Keywords: antibodies, infectious disease, sex differences
Introduction
Different immune responses in females and males
Females generally express higher levels of serum immunoglobulins compared to males, with a tendency to produce more IgG1 and IgG2 (47,68,88). As a consequence of increased antibody production, females may have an advantage over males in the clearance of certain pathogens. For example, the immune response to influenza virus is better in vaccinated adult females compared to males. Furthermore, males often experience worse disease symptoms after an influenza virus infection compared to females (47,58,90). But enhanced antibody responses in females may come at a cost. Estrogen induces anti-self antibodies and females have extremely high frequencies of the autoimmune disease systemic lupus erythematosus (“lupus”) compared to males (14,16,75,94). During pregnancy, when estrogen may rise to levels of >6,000 pg/mL [compared to levels of <100 pg/mL in males (36,44)], lupus can be life threatening (14,16,18,65,71,75,78).
Differences between females and males in immune responsiveness depend on the environment and target antigens. In vitamin A deficient (VAD) C57BL/6 mice, the female:male differences in IgG2b expression are reversed compared to non-VAD mice (47). Moreover, in the context of a pneumococcus vaccination and infection, C57BL/6 male mice are better protected than females (47).
B cells and class switch recombination
The expression of IgG (including IgG3, IgG1, IgG2b, and IgG2c in C57BL/6 mice), IgE and IgA depends on class switch recombination (CSR), which occurs in B cells following antigen or mitogen stimulation to associate V-D-J gene segments with a constant (C) region gene segment (Cγ, Cɛ, Cα) downstream of Cμ. CSR involves cleavage of DNA in switch (S) regions positioned upstream of C gene segments (e.g., Sμ and Sγ1) and the ligation of two different S regions to delete intervening sequences (86). During CSR, DNA loops are observed that juxtapose promoters, S regions, and enhancers (87).
One of the first steps in CSR is the initiation of sterile germline transcription by RNA Polymerase II (RNA Pol II, spliced transcripts may be integral to the CSR mechanism) (50,66). R loops form (comprising an RNA–DNA hybrid and a single strand of DNA) and stalled RNA Pol II recruits activation induced deaminase (AID) (12,13,50,73). Subsequent steps can include conversion of dC to dU by AID, excision of dU bases by uracil DNA glycosylase (UNG), DNA cleavage by abasic endonucleases, and ligation of cleaved DNA either within or between S regions by nonhomologous end-joining machinery (47).
Multiple enhancers are present in the murine immunoglobulin heavy chain locus, including the Eμ enhancer upstream of Cμ and enhancers within the 3′ regulatory region (3′RR) downstream of Cα (7,56,89). The 3′RR is rich in DNase1 hypersensitive sites (HS) and is required both for CSR and somatic hypermutation (SHM). In mice, a span of 40 kb covers two discrete regions. The upstream segment (∼28 kb) includes four HS sites, HS3A, HS1,2, HS3B, and HS4. The downstream segment (∼12 kb) includes HS5-8, an insulator region (7). In humans, the heavy chain locus is configured differently than in mice. There are two distinct 3′RR regions, one downstream of Cα1, and one downstream of Cα2. As in mice, these 3′RR regions influence CSR and antibody expression. Notably, a human polymorphism that involves an HS1,2 duplication is associated with a significant increase in frequencies of lupus (29,32).
Looping and CSR are influenced by enhanceosomes, the protein complexes associated with enhancers (53). Many proteins are associated with the HS1–4 complex, including Mediator (92), the CCATT enhancer binding protein (CEBP), the octamer binding protein, Pax5/BSAP, and NFκB family members (7). In contrast, CCCTC-binding factor (CTCF) and the subunits of cohesin (SMC1, SMC3) (93) are preferentially bound farther downstream in the insulator region (6). Enhanceosomes containing CEBP, CTCF, and/or cohesin can independently support DNA loop formation. Throughout the genome, signature cooperative protein sets are observed (e.g., STAT5A-CEBPβ-PML or CTCF-RAD21-SMC3 trios [9,95,103,104]).
B cell activation under variable conditions will alter enhanceosome composition, both within and between HS regions (7). Knock-out (KO) mutations have revealed the complex influences of different HS sites on CSR and antibody expression. Deletion of HS3B and HS4 reduced CSR to all isotypes except IgG1 (15) while deletion of the entire upstream (∼28 kb) 3′RR limited CSR to all isotypes and prevented SHM (6,7,82).
Estrogen, the estrogen receptor, and the immunoglobulin heavy chain locus
Estrogen functions both within and outside of the nucleus, but is best known for its binding to the class I nuclear hormone receptor estrogen receptor α (ERα), a transcription factor that binds DNA to regulate gene transcription (30,57,99–101). ERα often binds estrogen response elements (EREs, GGTCAnnnTGACC) (28,30,57,63), but ERα–DNA interactions can occur in the absence of an ERE and can be assisted by interactions with other factors including NFκB, AP-1, and SP1 (25,28,60,67,70,76,84). ERα may regulate gene transcription by direct binding to a promoter, although the ERα–DNA interactions responsible for gene regulation are often far more complex. For example, estrogen regulation of the GREB1 gene involves recruitment of ERα and RNA Pol II to three different ERE within 20 kb of upstream flanking sequences, chromatin loop formation, and juxtaposition of EREs with the transcriptional start site (21,91).
Because ERα will influence the functions of virtually every mammalian cell, there are many explanations for female/male differences in influenza virus-specific responses and autoimmune disease. As one example, estrogen may regulate innate cells that in turn regulate B cell functions (37,52). In addition, estrogen can upregulate AID, an enzyme integral to the initiation of CSR (61,69).
We previously hypothesized that ERα might also influence antibody expression by direct binding to the immunoglobulin heavy chain locus, and therefore queried the locus for ERE. In so doing, we discovered hotspots of response elements, both for type I and type II nuclear hormone receptors. These included ERE and retinoic acid response elements [two half-sites, PuG(G/T)TCA, often separated by a short spacer] (42). We then used chromatin immunoprecipitation (ChIP)-seq analyses to confirm that ERα was bound to DNA and found peak binding within enhancers known to influence CSR (46,47). Moreover, when ERE sequences were removed from enhancers in the heavy chain locus using clustered regularly interspaced short palindromic repeats (CRISPR)- CRISPR-associated protein-9 nuclease (Cas9) KO strategies in CH12F3.5B1 cells, the switch in isotype from IgM to IgA expression was inhibited (79).
Here, we examine additional features of ERα binding in the immunoglobulin heavy chain locus to dissect estrogen's influence on CSR and gene expression. We find that ERα and RNA Pol II binding patterns are strikingly similar in Eμ, Sμ, and the 3′RR in purified B cells, supporting our previous finding that supplemental estrogen in purified B cell cultures drives synchronous shifts in ERα and RNA Pol II binding within the locus (47). We also find a propensity for ERα binding to adenosine–cytidine (AC)-rich sequence repeats in the 3′RR of estrogen-supplemented B cell cultures. Results support our hypothesis that estrogen instructs the composition of enhanceosomes and assists DNA loop formation, explaining at least in part why males and females exhibit different antibody expression patterns and are variably susceptible to pathogens, allergies, and autoimmune disease.
Materials and Methods
ChIP-Seq libraries
The ChIP-seq library from lipopolysaccharide (LPS)-stimulated purified B cells has been described previously (46,47). Briefly, B cells were purified from the spleens of C57BL/6J mice by negative selection with anti-CD43 and anti-CD11b microbeads (Miltenyi Biotec) using a MACS LD Column (Miltenyi Biotec). Purified B cells were cultured in RPMI medium (Life Technologies) containing 10% fetal bovine serum, 2 mM l-glutamine, 50 μg/mL gentamicin, and 55 μM 2-mercaptoethanol. LPS (Sigma) was added to a final concentration of 5 μg/mL and cultures were incubated at 37°C in 5% CO2 for 1 day.
Harvested cells were treated with 2 mM disuccinimidyl glutarate (ProteoChem) in Dulbecco's phosphate buffered saline (DPBS) (Lonza) with proteinase inhibitors (PIs) phenylmethylsulfonyl fluoride (PMSF) (Sigma), Pepstatin A (Sigma), and Leupeptin (Sigma) and then washed and fixed in DPBS plus PIs and 1% paraformaldehyde (Sigma, Thermo Scientific) for 5 min with rotation at room temperature. The reaction was quenched with glycine (200 mM final concentration) and rotation for an additional 5 min. The cell pellet was washed with DPBS plus PIs and lysed in Covaris lysing buffer + PIs on ice for 10 min. Nuclei were centrifuged at 1500–1700 g for 5 min and washed 2 × with Covaris wash buffer and then 2 × with shearing buffer with PIs. The pellet was resuspended in Covaris shearing buffer plus PIs at a concentration of 1 mL per initial 2 × 107 cells and sheared in the Covaris E210 or E220 in Covaris MilliTubes with 200 cycles/burst, 20 W for 25–30 min. Sheared chromatin was diluted with Covaris ChIP dilution buffer and immunoprecipitated with anti-ERα antibody (Abcam; Cat#32063, monoclonal E115) or with anti-RNA Polymerase antibody (Active Motif Cat#61081) in combination with anti-mouse IgG bridging antibody (Active Motif Cat#102302) and Protein A/G magnetic beads. DNA was isolated from beads, purified, and quantified using the Quant-iT PicoGreen assay (Life Technologies) Qubit dsDNA HS Assay Kit (ThermoFisher Scientific) or SpectraMax Quant AccuBlue Pico dsDNA assay kit (Molecular Devices).
For the ERα studies with LPS or LPS + E cultured cells, libraries were prepared from DNA using the NEBNext ChIP-Seq Library Prep Reagent Set for Illumina with NEBNext Q5 Hot Start HiFi PCR Master Mix according to the manufacturer's instructions (New England Biolabs, Ipswich, MA) with a modification: a second 1:1 Ampure cleanup was added after adaptor ligation. For RNA Pol II studies with LPS-cultured cells, libraries were prepared from ∼1 to 10 ng of purified DNA using the KAPA HyperPrep Library Preparation Kit (Roche). Fifty cycle single-end or paired-end sequencing was performed on an Illumlina HiSeq 2000 or 2500, NovaSeq 6000, or NextSeq 550 instrument.
For ChIP-Seq data analysis, we followed the guideline of ENCODE for quality control (54). Details and codes have been previously described (19,54,102). The bigwig tracks were normalized to 15M uniquely mapped reads.
ENCODE ChIP-seq libraries were produced with purified C57BL/6J splenic B cells, negatively selected for CD43+ and CD11b+ cells. Bigwig tracks were downloaded from the ENCODE portal with the following identifiers: SERIES ENCSR902FHL, ENCSR000CBC, ENCSR000CBD, ENCSR000CBE, ENCSR000CBY, ENCSR000CBZ, ENCSR000CDJ, ENCSR000CFT, and ENCSR000CGA (17,19). Libraries were evaluated using Integrative Genomics Viewer (IGV) software.
Results
ERα and RNA Pol II co-bind the immunoglobulin heavy chain locus
We previously described ERα binding patterns in the immunoglobulin heavy chain locus of purified, LPS-activated C57BL/6 splenic B cells (46,47,79). Because RNA Pol II recruitment has been previously described as an integral step in estrogen-induced gene regulation (21,101), we queried the relationships between ERα and RNA Pol II binding within the immunoglobulin heavy chain locus. To this end, we aligned our ERα ChIP-seq data with RNA Pol II ChIP-seq data from ENCODE (Library ENCSR000CBZ, Target POLR2A). Both libraries originated from purified (negatively selected for CD43+ and CD11b+ cells), C57BL/6 splenic B cells, in one case collected after LPS stimulation. As shown in Figure 1, the alignment revealed strikingly similar binding patterns between ERα and RNA Pol II; each protein bound Sμ and 3′RR HS sites within the immunoglobulin heavy chain locus.
FIG. 1.
ERα binding and RNA Pol II binding patterns match. ChIP-seq libraries are aligned using IGV software (mm9, chromosome 12). The locations of switch regions and 3′RR enhancers are indicated. The ChIP-seq library with LPS-stimulated, purified splenic B cells from C57BL/6 mice was described previously (46,47,79) (range 0–138). Data were normalized against 15M uniquely mapped reads (102). Additional ChIP-seq libraries were from ENCODE. These used unstimulated, purified splenic B cells from C57BL/6 mice. ENCODE ChIP-seq libraries examined RNA Pol II (range 0–2.74), H3K27ac (range 0–37), H3K4me1 (range 0–4.41), H3K36me3 (range 0–3.38), H3K4me3 (range 0–31), and CTCF (range 0–18). For input, the range was 0–2.23. Patterns were most similar between ERα and RNA Pol II binding. ER, estrogen receptor; RNA Pol II, RNA polymerase II; CTCF, CCCTC binding factor; HS, (DNase I) hypersensitive site; ChIP, chromatin immunoprecipitation; LPS, lipopolysaccharide; RR, regulatory region; IGV, Integrative Genomics Viewer.
We aligned additional ENCODE ChIP-seq data (ENCSR000CBC, ENCSR000CBD, ENCSR000CBE, ENCSR000CBY, ENCSR000CDJ, ENCSR000CFT, and ENCSR000CGA) to examine positions of histone modifications and CTCF binding in the immunoglobulin heavy chain locus of purified B cells. We found that H3K27ac and H3K4me1 binding patterns were similar to ERα and RNA Pol II (Fig. 1). In contrast, H3K36me3 and H3K4me3 bound predominantly to upstream sequences and poorly to the 3′RR region. CTCF bound predominantly in the insulator region as previously described (7). Overall, the ERα and RNA Pol II binding patterns were best matched, illustrating a partnership of the two proteins within the immunoglobulin heavy chain locus.
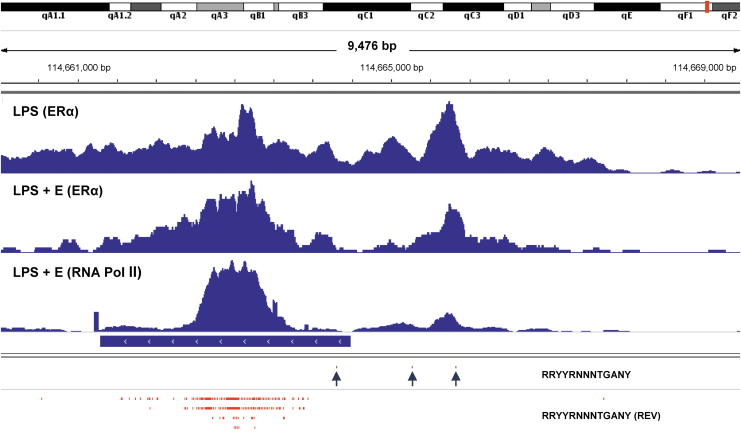
As shown in Figure 2, when we added supplemental estrogen to purified, LPS-stimulated B cell cultures, ERα binding exhibited improved focus on the ERE hotspot in Sμ. RNA Pol II was similarly targeted to this site. Results supplement our previous discovery of synchronous shifts in ERα and RNA Pol II DNA binding patterns when supplemental estrogen was added to LPS-stimulated B cell cultures (47).
FIG. 2.
Focused binding of ERα and RNA Pol II on an ERE hotspot in Sμ in estrogen-supplemented B cell cultures. ChIP-seq libraries were prepared from purified B cells stimulated with LPS or LPS plus estrogen (LPS + E). In the latter case, both ERα and RNA Pol II were tested. Potential ERE (RRYYRNNNTGANY) were mapped using the IGV “Find Motif” function. ERE were identified in forward (blue ticks and arrows) and reverse (REV, red ticks) directions. The position of Sμ is indicated by a horizontal blue bar. Data ranges were 0–150 for the LPS library with ERα, 0–106 for the LPS + E library with ERα, and 0–233 for the LPS + E library with RNA Pol II. ERE, estrogen response element.
Focused binding of ERα to AC-repeats in the presence of supplemental estrogen
ERE hotspots (42) and individual ERE clearly mark some, but not all sites of ERα binding in the immunoglobulin heavy chain locus (46,47,79). We and others have previously identified AC-repeat sequences and other repetitive elements in and near the 3′RR among loci of rodents and primates (7,79,81,82). We therefore asked whether ERα binding associated with these sites (7,47) in B cells activated with LPS or LPS plus supplemental estrogen (LPS + E).
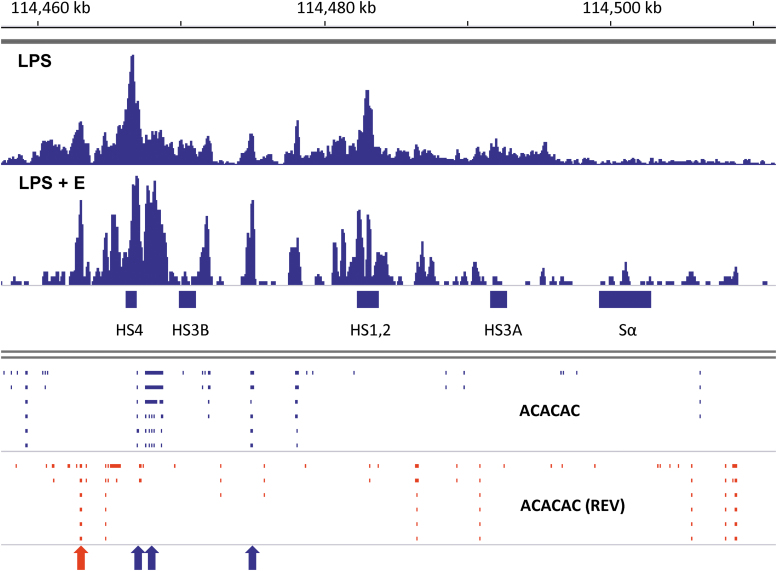
As shown in Figure 3, the ERα binding peaks were indeed aligned with AC-repeat sequences (mapped as “ACACAC” using the “Find Motif” function of IGV), particularly when B cells were stimulated in the presence of supplemental estrogen (LPS + E). AC-repeats are shown within the 3′RR in Figure 3 with blue and red ticks (indicating forward [left to right] and reverse (REV) sequence orientations, respectively). The four highest “LPS + E” peaks are indicated with arrows. Each peak aligned with at least one AC-repeat (at least 44 bases in length), either in a forward or reverse orientation. For example, the highest peak of ERα binding in Figure 3 (marked with a blue arrow) mapped to a sequence containing a 54 base AC-repeat straddled on both sides by one or two TGACC ERE half-sites; half sites were each located within 70 bases of the AC-repeat. We also observed focused ERα binding to AC-repeats and poly A sequences in and near Sμ, Cμ, and Cδ gene segments when supplemental estrogen was added to B cell cultures (45).
FIG. 3.
Shifts in ERα toward improved binding of AC-repeat sequences in the presence of supplemental estrogen. ChIP-seq libraries were from purified B cell cultures with LPS or LPS plus estrogen (LPS + E), shown using IGV software (mm9, chromosome 12). Data were normalized against 15M uniquely mapped reads (102). Data ranges were 0–134 for the LPS library and 0–87 for the LPS + E library. AC-repeat sequences (ACACAC) were mapped using the IGV “Find Motif” function. Ticks mark each sequence with a 5′–3′ orientation from left to right (blue) or right to left (red, termed “REV”). The four highest peaks of ERα binding in the LPS + E culture are marked with arrows, color-coded to match corresponding AC-repeat sequence orientations. AC, adenosine–cytidine.
Discussion
Synchronous binding of ERα and RNA Pol II
Data in this report reveal a striking similarity between patterns of ERα and RNA Pol II binding within the immunoglobulin heavy chain locus. Results supplement our previous finding that when estrogen was added to purified LPS-stimulated splenic B cells, there were synchronous shifts in binding patterns for ERα and RNA Pol II (47). Apparently, when estrogen ligands alter the conformation of ERα, both ERα and RNA pol II can be repositioned within the immunoglobulin heavy chain locus. These two transcription factors are clearly integral members of switchosomes (47,79) and enhanceosomes that regulate CSR and immunoglobulin expression patterns (99–101).
In purified murine B cells, H3K27ac binding patterns were closely matched to those of ERα and RNA Pol II and H3K4me1 exhibited the next-best match. H3K36me3 and H3K4me3 associated predominantly with Sμ and surrounding regions, perhaps indicative of their roles in transcription initiation and elongation (33). The various histone marks will recruit different readers and thereby impact functions of RNA Pol II (2,33,35,59). CTCF, another factor known to regulate histone modifications and influence DNA architecture of the immunoglobulin heavy chain locus throughout B cell development, was associated with the HS5-8 insulator region as previously described (6,20,32). These proteins define only a fraction of enhanceosome composition, illustrating the enormous complexity of factors that influence enhanceosome function (7).
ER binds AC-repeat sequences, particularly when supplemental estrogen is added
We found that in the context of estrogen-supplementation, ERα binding to DNA was well focused on AC-repeats (Fig. 3). Sequence repeats were previously identified throughout the immunoglobulin heavy chain locus in both primates and rodents (79,82) and AC-repeats have been previously described as regulatory elements (41). These repeats are somewhat reminiscent of the heptamer-nonamer sequences instrumental in V-D-J joining during B cell development (e.g., a typical heptamer has the sequence CACAGTG). Possibly, for both V-D-J joining and CSR, ERα binding to AC-repeats assists DNA looping, alignment of DNA strands, and juxtaposition of regulatory elements, as is necessary for the initiation of DNA rearrangement events (26,30,98).
Cross-regulation of transcription factors
Transcription factors are cross-regulatory whereby changes in one hormone or transcription factor will alter the functions of others. As an example, as stated above, DNA loop formation is signaled by cooperative protein sets such as STAT5A, CEBPβ and PML or CTCF, RAD21, and SMC3 (9,95,103,104). Interactions have been described between ERα and NFκB (8,48,64), ERα and PPAR (49), ERα and STAT-5A (43,51,97), ERα and retinoic acid receptors (RAR) (55), and RAR and CTCF (43).
Nuclear hormone receptors compete both for DNA binding sites and ligands (39). This explains why patterns of antibody isotype expression are difficult to predict in vivo (3,24,39,48,51) and helps account for our previous finding that IgG2b is generally higher in C57BL/6 females compared to males, but that ratios can be reversed in the context of VAD (47). Perhaps estrogen supports IgG2b production, but ERα and RAR have competitive influences on CSR [vitamin A often drives the switch to IgA (55,77)]. If this is the case, estrogen's capacity to upregulate IgG2b in male mice may be more evident when vitamin A is absent.
From flu to lupus
As stated above, females and males respond differently to influenza virus (and other) infections and exhibit different frequencies of autoimmune disease. These differences are due, at least in part to variant estrogen levels, influenced by factors including sex, age, pregnancy, and hormone replacement therapies (34). We suggest that changes in estrogen and ERα binding to DNA may have profound influences on gene rearrangement and antibody output [as is the case for other factors such as Ikaros, Mediator, and the histone-reader bromodomain family member BRWD1 (62,80,92)]. Changes in antibody output may, in turn, translate to serious disease consequences (16,29).
Intentional and targeted manipulations of ERα binding within enhanceosomes and switchosomes of the immunoglobulin heavy chain locus (e.g., by using CRISPR-Cas9 technologies) (4,5,10,22–23,27,31,38,40,72,74,83,85,96), may eventually allow clinicians to improve control of pathogens and to reduce threats of autoimmune disease.
Conclusion
We previously identified hotspots for ERE in the immunoglobulin heavy chain locus, identified ERα binding to the locus, showed that estrogen induced synchronous shifts in DNA binding for ERα and RNA Pol II, and showed that deletion of ERE in HS1,2 or Eμ reduced CSR in a B cell line (7,46,47,79). Here, we show that ERα and RNA Pol II binding patterns within the immunoglobulin heavy chain locus have an extraordinary similarity and we show that ERα has a preference for binding to AC-repeat sequences in the 3′RR in the presence of supplemental estrogen. Data are presented to encourage further research to define functions of ERα and related nuclear hormones in the immunoglobulin heavy chain locus.
We emphasize that the binding of nuclear hormones to regulatory elements defines just one of many mechanisms by which nuclear hormones influence pathogens and pathogen control in mammals. Important ERα binding sites are also situated in T cell receptor loci and among V, D, and J gene fragments (47). Next steps will be to employ new molecular technologies to modify ERE and ERα-DNA binding patterns in vivo (1,4,5,10,11,23,27,31,38,40,72,74,83,85,96). Better understanding and control of ERα-DNA binding in the immunoglobulin heavy chain locus may ultimately allow clinicians to improve immune responses in cases of immunodeficiency and reduce immune responses in cases of allergic reactions or autoimmunity.
Acknowledgments
We thank Scott Olsen for assistance with library production. We thank the ENCODE consortium, the ENCODE production laboratories, John Stamatoyannopoulos (UW) and Bing Ren (UCSD) for generating and sharing ChIP-seq data sets (ENCODE ENCSR000CBC, ENCSR000CBD, ENCSR000CBE, ENCSR000CBY, ENCSR000CBZ, ENCSR000CDJ, ENCSR000CFT, ENCSR000CGA).
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by NCI P30 CA21765, the intramural research program of the National Institutes of Health, National Institute of Aging, and ALSAC.
References
- 1. Aguiar S, Dias J, Manuel AM, et al. Chimeric small antibody fragments as strategy to deliver therapeutic payloads. Adv Protein Chem Struct Biol 2018;112:143–182 [DOI] [PubMed] [Google Scholar]
- 2. Allfrey VG, Faulkner R, and Mirsky AE. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc Natl Acad Sci U S A 1964;51:786–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barish GD, Yu RT, Karunasiri M, et al. Bcl-6 and NF-kappaB cistromes mediate opposing regulation of the innate immune response. Genes Dev 2010;24:2760–2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barolo S. How to tune an enhancer. Proc Natl Acad Sci U S A 2016;113:6330–6331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bennett CF, Baker BF, Pham N, et al. Pharmacology of antisense drugs. Annu Rev Pharmacol Toxicol 2017;57:81–105 [DOI] [PubMed] [Google Scholar]
- 6. Birshtein BK. The role of CTCF binding sites in the 3′ immunoglobulin heavy chain regulatory region. Front Genet 2012;3:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Birshtein BK. Epigenetic regulation of individual modules of the immunoglobulin heavy chain locus 3′ regulatory region. Front Immunol 2014;5:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Biswas DK, Singh S, Shi Q, et al. Crossroads of estrogen receptor and NF-kappaB signaling. Sci STKE 2005;2005:pe27. [DOI] [PubMed] [Google Scholar]
- 9. Busslinger GA, Stocsits RR, van der Lelij P, et al. Cohesin is positioned in mammalian genomes by transcription, CTCF and Wapl. Nature 2017;544:503–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cabrini G, Bezzerri V, Mancini I, et al. Targeting transcription factor activity as a strategy to inhibit pro-inflammatory genes involved in cystic fibrosis: decoy oligonucleotides and low-molecular weight compounds. Curr Med Chem 2010;17:4392–4404 [DOI] [PubMed] [Google Scholar]
- 11. Callaway E. CRISPR cuts turn gels into biological watchdogs. Nature 2019;572:574. [DOI] [PubMed] [Google Scholar]
- 12. Chaudhuri J, and Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol 2004;4:541–552 [DOI] [PubMed] [Google Scholar]
- 13. Chaudhuri J, Tian M, Khuong C, et al. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature 2003;422:726–730 [DOI] [PubMed] [Google Scholar]
- 14. Clowse ME. Lupus activity in pregnancy. Rheum Dis Clin North Am 2007;33:237–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cogne M, Lansford R, Bottaro A, et al. A class switch control region at the 3′ end of the immunoglobulin heavy chain locus. Cell 1994;77:737–747 [DOI] [PubMed] [Google Scholar]
- 16. Cohen-Solal JF, Jeganathan V, Grimaldi CM, et al. Sex hormones and SLE: influencing the fate of autoreactive B cells. Curr Top Microbiol Immunol 2006;305:67–88 [DOI] [PubMed] [Google Scholar]
- 17. Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature 2012;489:57–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cutolo M, Capellino S, Sulli A, et al. Estrogens and autoimmune diseases. Ann N Y Acad Sci 2006;1089:538–547 [DOI] [PubMed] [Google Scholar]
- 19. Davis CA, Hitz BC, Sloan CA, et al. The encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res 2018;46:D794–D801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Degner SC, Verma-Gaur J, Wong TP, et al. CCCTC-binding factor (CTCF) and cohesin influence the genomic architecture of the Igh locus and antisense transcription in pro-B cells. Proc Natl Acad Sci U S A 2011;108:9566–9571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deschenes J, Bourdeau V, White JH, et al. Regulation of GREB1 transcription by estrogen receptor alpha through a multipartite enhancer spread over 20 kb of upstream flanking sequences. J Biol Chem 2007;282:17335–17339 [DOI] [PubMed] [Google Scholar]
- 22. Dias N, and Stein CA. Potential roles of antisense oligonucleotides in cancer therapy. The example of Bcl-2 antisense oligonucleotides. Eur J Pharm Biopharm 2002;54:263–269 [DOI] [PubMed] [Google Scholar]
- 23. Dias N, and Stein CA. Antisense oligonucleotides: basic concepts and mechanisms. Mol Cancer Ther 2002;1:347–355 [PubMed] [Google Scholar]
- 24. Ding N, Yu RT, Subramaniam N, et al. A vitamin D receptor/SMAD genomic circuit gates hepatic fibrotic response. Cell 2013;153:601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Driscoll MD, Sathya G, Muyan M, et al. Sequence requirements for estrogen receptor binding to estrogen response elements. J Biol Chem 1998;273:29321–29330 [DOI] [PubMed] [Google Scholar]
- 26. Dumbovic G, Forcales SV, and Perucho M. Emerging roles of macrosatellite repeats in genome organization and disease development. Epigenetics 2017;12:515–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eimer H, Sureshkumar S, Singh Yadav A, et al. RNA-dependent epigenetic silencing directs transcriptional downregulation caused by intronic repeat expansions. Cell 2018;174:1095–1105.e11. [DOI] [PubMed] [Google Scholar]
- 28. Evans RM, and Mangelsdorf DJ. Nuclear receptors, RXR, and the big bang. Cell 2014;157:255–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frezza D, Tolusso B, Giambra V, et al. Polymorphisms of the IgH enhancer HS1.2 and risk of systemic lupus erythematosus. Ann Rheum Dis 2012;71:1309–1315 [DOI] [PubMed] [Google Scholar]
- 30. Fullwood MJ, Liu MH, Pan YF, et al. An oestrogen-receptor-alpha-bound human chromatin interactome. Nature 2009;462:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gambari R. Recent patents on therapeutic applications of the transcription factor decoy approach. Expert Opin Ther Pat 2011;21:1755–1771 [DOI] [PubMed] [Google Scholar]
- 32. Garrett FE, Emelyanov AV, Sepulveda MA, et al. Chromatin architecture near a potential 3′ end of the igh locus involves modular regulation of histone modifications during B-Cell development and in vivo occupancy at CTCF sites. Mol Cell Biol 2005;25:1511–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gates LA, Foulds CE, and O'Malley BW. Histone marks in the ‘driver's seat’: functional roles in steering the transcription cycle. Trends Biochem Sci 2017;42:977–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ghosh M, Rodriguez-Garcia M, and Wira CR. The immune system in menopause: pros and cons of hormone therapy. J Steroid Biochem Mol Biol 2014;142:171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Giambra V, Volpi S, Emelyanov AV, et al. Pax5 and linker histone H1 coordinate DNA methylation and histone modifications in the 3′ regulatory region of the immunoglobulin heavy chain locus. Mol Cell Biol 2008;28:6123–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Handelsman DJ, Newman JD, Jimenez M, et al. Performance of direct estradiol immunoassays with human male serum samples. Clin Chem 2014;60:510–517 [DOI] [PubMed] [Google Scholar]
- 37. Heath WR, Kato Y, Steiner TM, et al. Antigen presentation by dendritic cells for B cell activation. Curr Opin Immunol 2019;58:44–52 [DOI] [PubMed] [Google Scholar]
- 38. Hecker M, and Wagner AH. Transcription factor decoy technology: a therapeutic update. Biochem Pharmacol 2017;144:29–34 [DOI] [PubMed] [Google Scholar]
- 39. Hua S, Kittler R, and White KP. Genomic antagonism between retinoic acid and estrogen signaling in breast cancer. Cell 2009;137:1259–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hua Y, Vickers TA, Okunola HL, et al. Antisense masking of an hnRNP A1/A2 intronic splicing silencer corrects SMN2 splicing in transgenic mice. Am J Hum Genet 2008;82:834–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hui J, Hung LH, Heiner M, et al. Intronic CA-repeat and CA-rich elements: a new class of regulators of mammalian alternative splicing. EMBO J 2005;24:1988–1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hurwitz JL, Penkert RR, Xu B, et al. Hotspots for vitamin-steroid-thyroid hormone response elements within switch regions of immunoglobulin heavy chain loci predict a direct influence of vitamins and hormones on B cell class switch recombination. Viral Immunol 2016;29:132–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ishihara K, Nakamoto M, and Nakao M. DNA methylation-independent removable insulator controls chromatin remodeling at the HOXA locus via retinoic acid signaling. Hum Mol Genet 2016;25:5383–5394 [DOI] [PubMed] [Google Scholar]
- 44. James D, Steer P, Weiner C, et al. Pregnancy and laboratory studies: a reference table for clinicians. Obstet Gynecol 2010;115:868.; author reply 868–869. [DOI] [PubMed] [Google Scholar]
- 45. Jones BG, Penkert RR, Surman SL, et al. Matters of life and death: how estrogen and estrogen receptor binding to the immunoglobulin heavy chain locus may influence outcomes of infection, allergy, and autoimmune disease. Cell Immunol 2019;346:103996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jones BG, Penkert RR, Xu B, et al. Binding of estrogen receptors to switch sites and regulatory elements in the immunoglobulin heavy chain locus of activated B cells suggests a direct influence of estrogen on antibody expression. Mol Immunol 2016;77:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jones BG, Sealy RE, Penkert RR, et al. Complex sex-biased antibody responses: estrogen receptors bind estrogen response elements centered within immunoglobulin heavy chain gene enhancers. Int Immunol 2019;31:141–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kalaitzidis D, and Gilmore TD. Transcription factor cross-talk: the estrogen receptor and NF-kappaB. Trends Endocrinol Metab 2005;16:46–52 [DOI] [PubMed] [Google Scholar]
- 49. Keller H, Givel F, Perroud M, et al. Signaling cross-talk between peroxisome proliferator-activated receptor/retinoid X receptor and estrogen receptor through estrogen response elements. Mol Endocrinol 1995;9:794–804 [DOI] [PubMed] [Google Scholar]
- 50. Kenter AL. AID targeting is dependent on RNA polymerase II pausing. Semin Immunol 2012;24:281–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Khorasanizadeh S, and Rastinejad F. Visualizing the architectures and interactions of nuclear receptors. Endocrinology 2016;157:4212–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Laffont S, Seillet C, and Guery JC. Estrogen receptor-dependent regulation of dendritic cell development and function. Front Immunol 2017;8:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Laiosa CV, Stadtfeld M, and Graf T. Determinants of lymphoid-myeloid lineage diversification. Annu Rev Immunol 2006;24:705–738 [DOI] [PubMed] [Google Scholar]
- 54. Landt SG, Marinov GK, Kundaje A, et al. ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res 2012;22:1813–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lee SK, Choi HS, Song MR, et al. Estrogen receptor, a common interaction partner for a subset of nuclear receptors. Mol Endocrinol 1998;12:1184–1192 [DOI] [PubMed] [Google Scholar]
- 56. Lennon GG, and Perry RP. C mu-containing transcripts initiate heterogeneously within the IgH enhancer region and contain a novel 5′-nontranslatable exon. Nature 1985;318:475–478 [DOI] [PubMed] [Google Scholar]
- 57. Lin CY, Vega VB, Thomsen JS, et al. Whole-genome cartography of estrogen receptor alpha binding sites. PLoS Genet 2007;3:e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lorenzo ME, Hodgson A, Robinson DP, et al. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine 2011;29:9246–9255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Luger K, Mader AW, Richmond RK, et al. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997;389:251–260 [DOI] [PubMed] [Google Scholar]
- 60. Mader S, Leroy P, Chen JY, et al. Multiple parameters control the selectivity of nuclear receptors for their response elements. Selectivity and promiscuity in response element recognition by retinoic acid receptors and retinoid X receptors. J Biol Chem 1993;268:591–600 [PubMed] [Google Scholar]
- 61. Mai T, Zan H, Zhang J, et al. Estrogen receptors bind to and activate the HOXC4/HoxC4 promoter to potentiate HoxC4-mediated activation-induced cytosine deaminase induction, immunoglobulin class switch DNA recombination, and somatic hypermutation. J Biol Chem 2010;285:37797–37810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mandal M, Hamel KM, Maienschein-Cline M, et al. Histone reader BRWD1 targets and restricts recombination to the Igk locus. Nat Immunol 2015;16:1094–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mason CE, Shu FJ, Wang C, et al. Location analysis for the estrogen receptor-alpha reveals binding to diverse ERE sequences and widespread binding within repetitive DNA elements. Nucleic Acids Res 2010;38:2355–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. McKay LI, and Cidlowski JA. Cross-talk between nuclear factor-kappa B and the steroid hormone receptors: mechanisms of mutual antagonism. Mol Endocrinol 1998;12:45–56 [DOI] [PubMed] [Google Scholar]
- 65. Murphy VE. Managing asthma in pregnancy. Breathe (Sheff) 2015;11:258–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Neale GA, and Kitchingman GR. mRNA transcripts initiating within the human immunoglobulin mu heavy chain enhancer region contain a non-translatable exon and are extremely heterogeneous at the 5′ end. Nucleic Acids Res 1991;19:2427–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. O'Lone R, Frith MC, Karlsson EK, et al. Genomic targets of nuclear estrogen receptors. Mol Endocrinol 2004;18:1859–1875 [DOI] [PubMed] [Google Scholar]
- 68. Pasternak G, Lewandowicz-Uszynska A, and Pentos K. Analysis of differences between total IgG and sum of the IgG subclasses in children with suspected immunodeficiency-indication of determinants. BMC Immunol 2018;19:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Pauklin S, Sernandez IV, Bachmann G, et al. Estrogen directly activates AID transcription and function. J Exp Med 2009;206:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Penvose A, Keenan JL, Bray D, et al. Comprehensive study of nuclear receptor DNA binding provides a revised framework for understanding receptor specificity. Nat Commun 2019;10:2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Platts-Mills TA. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med 2001;164:S1–S5 [DOI] [PubMed] [Google Scholar]
- 72. Rad SM, Langroudi L, Kouhkan F, et al. Transcription factor decoy: a pre-transcriptional approach for gene downregulation purpose in cancer. Tumour Biol 2015;36:4871–4881 [DOI] [PubMed] [Google Scholar]
- 73. Rajagopal D, Maul RW, Ghosh A, et al. Immunoglobulin switch mu sequence causes RNA polymerase II accumulation and reduces dA hypermutation. J Exp Med 2009;206:1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rao VK, Kapp D, and Schroth M. Gene therapy for spinal muscular atrophy: an emerging treatment option for a devastating disease. J Manag Care Spec Pharm 2018;24:S3–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ritchie J, Smyth A, Tower C, et al. Maternal deaths in women with lupus nephritis: a review of published evidence. Lupus 2012;21:534–541 [DOI] [PubMed] [Google Scholar]
- 76. Rousseau C, Pettersson F, Couture MC, et al. The N-terminal of the estrogen receptor (ERalpha) mediates transcriptional cross-talk with the retinoic acid receptor in human breast cancer cells. J Steroid Biochem Mol Biol 2003;86:1–14 [DOI] [PubMed] [Google Scholar]
- 77. Rudraraju R, Jones BG, Surman SL, et al. Respiratory tract epithelial cells express retinaldehyde dehydrogenase ALDH1A and enhance IgA production by stimulated B cells in the presence of vitamin A. PLoS One 2014;9:e86554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Schreiber K, Stach K, and Sciascia S. Lupus nephritis and pregnancy outcome. Autoimmun Rev 2017;16:433–434 [DOI] [PubMed] [Google Scholar]
- 79. Sealy RE, Jones BG, Surman SL, et al. Will attention by vaccine developers to the host's nuclear hormone levels and immunocompetence improve vaccine success? Vaccines (Basel) 2019;7:pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sellars M, Reina-San-Martin B, Kastner P, et al. Ikaros controls isotype selection during immunoglobulin class switch recombination. J Exp Med 2009;206:1073–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sepulveda MA, Emelyanov AV, and Birshtein BK. NF-kappa B and Oct-2 synergize to activate the human 3′ Igh hs4 enhancer in B cells. J Immunol 2004;172:1054–1064 [DOI] [PubMed] [Google Scholar]
- 82. Sepulveda MA, Garrett FE, Price-Whelan A, et al. Comparative analysis of human and mouse 3′ Igh regulatory regions identifies distinctive structural features. Mol Immunol 2005;42:605–615 [DOI] [PubMed] [Google Scholar]
- 83. Shin HY. Targeting super-enhancers for disease treatment and diagnosis. Mol Cells 2018;41:506–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shu FJ, Sidell N, Yang D, et al. The tri-nucleotide spacer sequence between estrogen response element half-sites is conserved and modulates ERalpha-mediated transcriptional responses. J Steroid Biochem Mol Biol 2010;120:172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Singh NK, Singh NN, Androphy EJ, et al. Splicing of a critical exon of human survival motor neuron is regulated by a unique silencer element located in the last intron. Mol Cell Biol 2006;26:1333–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Stavnezer J, Bjorkman A, Du L, et al. Mapping of switch recombination junctions, a tool for studying DNA repair pathways during immunoglobulin class switching. Adv Immunol 2010;108:45–109 [DOI] [PubMed] [Google Scholar]
- 87. Stavnezer J, and Schrader CE. IgH chain class switch recombination: mechanism and regulation. Journal of immunology 2014;193:5370–5378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Straub RH. The complex role of estrogens in inflammation. Endocr Rev 2007;28:521–574 [DOI] [PubMed] [Google Scholar]
- 89. Su LK, and Kadesch T. The immunoglobulin heavy-chain enhancer functions as the promoter for I mu sterile transcription. Mol Cell Biol 1990;10:2619–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sue K. The science behind “man flu”. BMJ 2017;359:j5560. [DOI] [PubMed] [Google Scholar]
- 91. Sun J, Nawaz Z, and Slingerland JM. Long-range activation of GREB1 by estrogen receptor via three distal consensus estrogen-responsive elements in breast cancer cells. Mol Endocrinol 2007;21:2651–2662 [DOI] [PubMed] [Google Scholar]
- 92. Thomas-Claudepierre AS, Robert I, Rocha PP, et al. Mediator facilitates transcriptional activation and dynamic long-range contacts at the IgH locus during class switch recombination. J Exp Med 2016;213:303–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Thomas-Claudepierre AS, Schiavo E, Heyer V, et al. The cohesin complex regulates immunoglobulin class switch recombination. J Exp Med 2013;210:2495–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Verthelyi D, and Ansar Ahmed S. Characterization of estrogen-induced autoantibodies to cardiolipin in non-autoimmune mice. J Autoimmun 1997;10:115–125 [DOI] [PubMed] [Google Scholar]
- 95. Vierstra J, Rynes E, Sandstrom R, et al. Mouse regulatory DNA landscapes reveal global principles of cis-regulatory evolution. Science 2014;346:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Wang LH, Yang XY, Zhang X, et al. The cis decoy against the estrogen response element suppresses breast cancer cells via target disrupting c-fos not mitogen-activated protein kinase activity. Cancer Res 2003;63:2046–2051 [PubMed] [Google Scholar]
- 97. Wang Y, and Cheng CH. ERalpha and STAT5a cross-talk: interaction through C-terminal portions of the proteins decreases STAT5a phosphorylation, nuclear translocation and DNA-binding. FEBS Lett 2004;572:238–244 [DOI] [PubMed] [Google Scholar]
- 98. Weber J, Bao H, Hartlmuller C, et al. Structural basis of nucleic-acid recognition and double-strand unwinding by the essential neuronal protein Pur-alpha. Elife 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Welboren WJ, Stunnenberg HG, Sweep FC, et al. Identifying estrogen receptor target genes. Mol Oncol 2007;1:138–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Welboren WJ, Sweep FC, Span PN, et al. Genomic actions of estrogen receptor alpha: what are the targets and how are they regulated? Endocr Relat Cancer 2009;16:1073–1089 [DOI] [PubMed] [Google Scholar]
- 101. Welboren WJ, van Driel MA, Janssen-Megens EM, et al. ChIP-Seq of ERalpha and RNA polymerase II defines genes differentially responding to ligands. EMBO J 2009;28:1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Yang X, Xu B, Mulvey B, et al. Differentiation of human pluripotent stem cells into neurons or cortical organoids requires transcriptional co-regulation by UTX and 53BP1. Nat Neurosci 2019;22:362–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang K, Li N, Ainsworth RI, et al. Systematic identification of protein combinations mediating chromatin looping. Nat Commun 2016;7:12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Zuin J, Dixon JR, van der Reijden MI, et al. Cohesin and CTCF differentially affect chromatin architecture and gene expression in human cells. Proc Natl Acad Sci U S A 2014;111:996–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]