Abstract
Objective: The purpose of this study was to evaluate the effects of a Web-based Multimedia Intervention (WBMI) for breast cancer-related lymphedema (BCRL) patients on symptom burden, function, psychological well-being, costs, and arm volume.
Methods: Women with BCRL were randomized to intervention (n = 80) or control (n = 80) groups. The WBMI offered 12 modules, each of which took about 30 minutes to complete. The Pamphlet took about 2 hours to read. Data on symptom burden, psychological well-being, function, and costs were collected preintervention; and 1, 3, 6, and 12 months postintervention. A subgroup of 45 regional patients had arm extracellular fluid measured by bioimpedance at baseline and at 3, 6, and 12 months postintervention. Intervention perceived value was also captured.
Results: A statistically significant difference (p = 0.011) was observed for rates of intervention completion, WBMI (58%), and Pamphlet (77%). With the exception of the number of biobehavioral symptoms (mood), no statistically significant differences between groups in symptom reduction were apparent between baseline and 1 or 12 months (effect sizes = 0.05–0.28, p > 0.05) based on the Lymphedema Symptom Intensity and Distress Scale–Arm (LSIDS-A). No statistically significant differences between the groups were observed for changes in other variables. The WBMI was perceived as providing better self-care information than the Pamphlet (p = 0.001).
Conclusions: WBMI participants experienced improved biobehavioral symptoms and higher perceived quality of information. The lack of significant differences on other variables may be due to the high percentage of participants who did not complete the WBMI.
Keywords: internet intervention, breast cancer, lymphedema, self-care
Introduction
Upper limb lymphedema is one of the most debilitating complications of breast cancer treatment.1 It is a chronic, progressive condition that presents complex psychological and physical challenges to approximately 20%–30% of breast cancer survivors.2–4 There is currently no surgical or medical cure for breast cancer-related lymphedema (BCRL), and ongoing self-care is needed to reduce the risk of lymphedema progression. Patients with BCRL experience multifaceted symptom burden, which is the collective severity of multiple symptoms that influence physical and emotional well-being.3,5–7 Physical discomforts related to heavy limbs, pain, and other sensations are associated with lymphedema.8,9 From a functional perspective, limb discomfort and limitations in muscle strength and joint mobility can affect activity levels at home and work.8,10–13 Weight-lifting constraints and functional limitations may necessitate lifestyle and occupational role changes, which may increase financial burden.5,14,15 Psychological burdens include the anxiety-provoking uncertainty of potential breast cancer recurrence or lymphedema progression and the stress of high symptom burden and continual self-care demands.7,16 These individuals also face alterations in body image, and body image disturbance has been associated with depression and anxiety in women with breast cancer-related lymphedema.17 Sexual functioning is often affected, as women tend to see themselves as less attractive.18 In addition, the disfiguring aspects of lymphedema may promote self-consciousness, social isolation, and abandonment, which has surfaced as a concern in many qualitative studies.5,19 Stressors related to social impact include perceptions of marginalization by health care providers and general public insensitivity.5,7 Given the multitude of stressors experienced by those with BCRL, psychological distress is well documented in multiple qualitative studies.5,7
Individuals with BCRL face extensive financial burden related to manual lymphatic drainage, pneumatic compression devices, compression garments, and bandages.20–22 The economic burden to patients and society has been documented. Shih et al. found that patients with BCRL were twice as likely to have lymphangitis or cellulitis as breast cancer survivors without lymphedema, and these potentially life-threatening conditions compound medical costs.23
Many individuals find the time commitments involved in self-care overwhelming,24 and as many as 75% of individuals with this condition do not adhere to self-care as directed.15,25 As individuals with lymphedema contend with a progressive condition that taxes their financial, psychological, and social resources, studies of interventions that may improve these outcomes and adherence to self-care merit investigation. There is a need for a low-cost, widely available intervention that will enhance adherence to self-care in BCRL patients. Increasing Internet access and rapid advances in technology are enabling the use of innovative, health promotion strategies, such as a Web-based Multimedia Intervention (WBMI) with breast cancer survivors.26–28 Advantages associated with a WBMI include financial feasibility, optimal accessibility, and maximized convenience for the patient.
The purpose of this study was to evaluate the effects of a WBMI for BCRL patients on symptom burden, function, psychological well-being, costs, and arm volume. Relative to the control condition, it was hypothesized that Stage II lymphedema patients who experience the WBMI would report greater improvements in these outcomes over a 12-month period.
Conceptual Framework
The intervention was based on Lazarus and Folkman's Stress and Coping conceptual model (Fig. 1).29 In this model, primary appraisals include perceived stress related to lymphedema and the demands of its care. Secondary appraisals of resources available to deal with this challenge include efficacy of self-care of lymphedema-related problems and the resource social support. Secondary appraisals of resources influence the selection of coping strategies and adherence behaviors. The model suggests that adaptive coping strategies aimed at problem management, including self-care behaviors, emotional regulation, and meaning-based coping, may enhance patient outcomes. Adaptive changes in appraisals and coping strategies associated with improved self-care patterns should then influence outcomes of symptom burden, psychological well-being, function, cost, and arm volume.
FIG. 1.
Conceptual framework.
Materials and Methods
Design
This was a two-phase study. In Phase 1, focus groups were conducted to solicit patient feedback regarding lymphedema self-care and ideas for content and delivery formats for the WBMI. The details of the focus group content from this phase of the study have been described elsewhere.30,31 In Phase 2, a randomized controlled trial was conducted to assess the effects of the WBMI on lymphedema self-care and associated outcomes compared with those effects using a traditional educational pamphlet.
Procedures
The study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. After obtaining approval from the Vanderbilt University Institutional Review Board and the Vanderbilt Ingram Cancer Center Scientific Review Committee, participants were recruited for the study from a lymphedema patient registry and email advertisements sent to the Vanderbilt Medical Center, Lymphedema Education & Research Network, “Pink Link” Breast Cancer Support Group, Gilda's Club, National Lymphedema Network, and lymphedema therapists.
For Phases 1 and 2, participants were screened by phone by a trained nurse to determine eligibility. Inclusion criteria required patient participants to: (1) have a history of breast cancer, (2) a diagnosis of Stage II lymphedema based on the International Society of Lymphedema, (3) be age 18 or older, and (4) be able to see and read printed documents in English. In addition to the inclusion criteria cited earlier, participants had to have access to the Internet or a smart phone. Exclusion criteria required that participants not be: (1) undergoing chemotherapy or radiation, or (2) receiving hospice care. Informed consent was obtained via hard copy for Phase one and via REDCap eConsent for Phase 2 before study enrollment.
Intervention development
Using the conceptual framework as a guide, authors S.H.R. and V.S. conducted an extensive review of the literature to identify key areas of potential focus for a WBMI focused on lymphedema self-care. Multiple research studies were included in this review, along with position papers from nonprofits with a lymphedema focus, and international clinical guidelines. The resultant topics identified included: managing self-care tasks, goal setting, using self-reward, dealing with negative feelings, building social support, and reappraisal of meaning.
Focus groups were then held with BCRLs to discuss these topics and solicit feedback from the affected population.30,31 On completion of the focus groups, participants were asked whether they would be interested in being in the videos and whether they have any caregivers who might wish to be in the videos. After focus group data were analyzed, scripts were written by authors S.H.R. and V.S.; and, stakeholders (e.g., patients, caregivers, and therapists) reviewed and modified the scripts. Revisions were made and videos were filmed by Vanderbilt University School of Nursing videographers by using indoor and outdoor locations across Nashville, Tennessee. Focus group volunteers along with their family members and volunteer health care professionals performed in the videos. A professional narrator was employed to introduce, narrate, and assist with transitions to vignettes.
In Phase 2, after completion of the eConsent process, consented participants were immediately directed to a site within REDCap for completion of the baseline survey instruments.32 On completion of the baseline documentation, participants were randomly assigned to one of two study conditions via the use of a computer-generated, permuted block program developed and executed by the study biostatistician.
Participants randomized to the control group received a hard copy of an educational pamphlet titled, “Guide to Understanding Lymphedema”33 (Pamphlet). Pamphlet topics included lymphedema risk reduction, early warning signs, advice regarding lymphedema treatment, emotions and lymphedema, and paying for treatment. Participants randomized to the WBMI group were offered 12 sessions of videos, each 20–45 minutes long, that featured narration, reflective questions, and interviews with patients sharing stories about living with lymphedema. The 12 sessions covered basic physiology of lymphedema and self-care, goal setting and self-reward, diet and exercise strategies, methods of dealing with negative emotions and stress, body image changes, uncertainty, and enhancing emotional and instrumental social support. The last session discussed looking at life from a different perspective and finding a new identity.
All participants (regardless of study group) were asked to complete their intervention (either the WBMI or the Pamphlet), within 1 month. After completing the intervention, participants completed an evaluation form containing questions about their level of satisfaction with their respective program (WBMI or Pamphlet) as well as the amount of time spent on the activities. In addition to completing the evaluation form, participants in both groups also completed several measures at the end of month 1 and then again at 3, 6, and 12 months postintervention. All measures could be completed by participants online, via REDCap. All study participants were provided a toll-free number to call should they have any questions or difficulties. Based on participant preferences, they were sent email or postal mail reminders 2 weeks before each follow-up assessment was due. Reminder phone calls (no more than three over a 1-month period) were made if the follow-up surveys were not completed.
Patients who lived within 35 miles of Nashville were asked whether they would be willing to have their extracellular arm volume measured by bioimpedance at baseline and 3, 6, and 12 months postintervention. Month 1 was left out to mitigate patient burden of travel to complete testing. If they agreed to do so, those individuals were measured by trained staff either in their own homes or at Vanderbilt University School of Nursing.
Sample
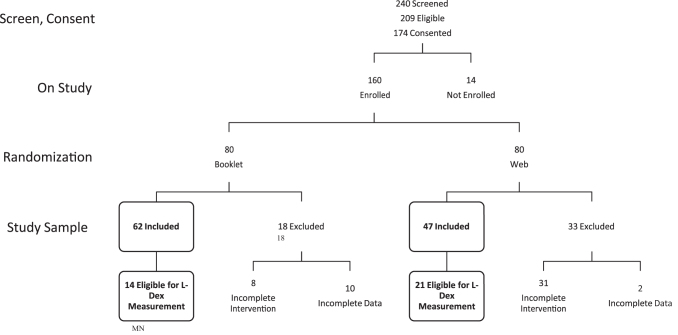
Of the 240 people who inquired about the study and were screened, 209 were found to be eligible (Fig. 2). Of those eligible participants, 174 completed the study eConsent process. Subsequently, 160 of those consented individuals completed all baseline activities and were randomized (Pamphlet: n = 80, WBMI: n = 80).
FIG. 2.
CONSORT diagram.
Measures
On study enrollment, participants provided one-time-only demographic information, including date of birth, years of education completed, race, ethnicity, marital status, income, employment status, concurrent medical conditions, current medication use, area of residence, and insurance status. Breast cancer history and treatment information included date of diagnosis of breast cancer, location, and types and dates of treatment. Lymphedema history and treatment information included date of diagnosis of lymphedema, location of lymphedema, and types and dates of initial and current treatment.
The following instruments were completed at each follow-up time point:
Symptom burden was measured with the Lymphedema Symptom Intensity and Distress Scale–Arm (LSIDS-A).34 This 30-item, validated instrument evaluates 7 domains of physical and psychological symptoms observed in patients with lymphedema. At the time of this study, only the total number of symptoms in each of the domains was used for longitudinal work. Since then, weighted scores for each domain incorporate intensity and distress into the scores. The Cronbach's alphas for those domain scores across the study period ranged from 0.64 to 0.96, whereas the overall symptom score alphas ranged from 0.92 to 0.95.
The Profile of Mood States-Short Form (POMS-SF), a validated tool, was utilized to assess psychological well-being.35 This 37-item adjective checklist asks respondents to rate their feelings in the last week on a 5-point Likert scale. The measure provides an overall Total Mood Disturbance score along with scores for each of six subscales. The Cronbach's alpha for total scores throughout this study ranged from 0.88 to 0.92, and subscales ranged from 0.74 to 0.94.
The validated 11-item Quick-Disabilities of Arm, Shoulder, and Hand (QuickDASH) tool was used to measure function.36 The QuickDASH asks respondents to rate their ability to perform specific activities (e.g., heavy household chores or carrying a shopping bag); degree of interference with normal social activities, work activities, sleep impairment, ratings of pain, and altered sensations in the upper extremity. Cronbach's alpha across the study period was 0.90–0.92.
The 28-item Brief COPE is a validated, multidimensional coping inventory that assesses concepts such as adaptive, maladaptive, and measured coping.37 It produces 14 subscale scores, only 3 of which had sufficient reliability for use in the study analysis: positive coping (Cronbach's alphas of 0.72–0.81), active coping (Cronbach's alphas of 0.77–0.84), and supportive coping (Cronbach's alphas of 0.78–0.86).
The 4-item Perceived Stress Scale-4 was used to measure stress in the last 30 days, with Cronbach's alpha scores ranging from 0.75 to 0.82 over the study period.38
The validated Perceived Medical Condition Self-Management Scale was used to assess perceived ability to self-manage specific, chronic medical conditions, in this case lymphedema.39,40 This 8-item scale uses a 5-point Likert scale to provide an overall score of health competence. Cronbach's alpha over the course of this study ranged from 0.90 to 0.93.
Four types of social support (emotional/informational, tangible, affectionate, and positive social interaction) were assessed via the 19-item Medical Outcomes Study Social Support Survey (MOS Social Support Survey).41 Scoring of the MOS also produces an overall functional social support index. Cronbach's alphas in this study were 0.97 for the overall index, and they ranged from 0.95 to 0.999 for the subscales.
Patient-reported information specific to lymphedema treatment expenses (e.g., bandages, antibiotics) over the past 2 weeks was collected via the Resource Utilization and Economic Burden Questionnaire (RUEBQ).23 A self-care checklist was used to assess self-care activities in the last 7 days.
Finally, participants were also asked to evaluate their respective interventions—either Pamphlet or WBMI. A seven-item form based on the program evaluation format used by Cook et al. was used.42 The five-point scale (1 = strongly disagree to 5 = strongly agree) enabled participants to evaluate informativeness/usefulness (items 1–5) and appeal/ease of use (items 6–7) of either the printed Pamphlet or the WBMI. The length of time spent on each intervention was also collected on this form.
For the subset of patients whose arms were measured for extracellular fluid, bioelectrical impedance was used to accurately measure extracellular fluid; the XCA single-frequency bioimpedance device was used.43,44
Data analysis
Data analysis was completed by using IBM SPSS Statistics (Version 25).45 Nominal and ordinal variables were summarized by using frequency distributions. If normally distributed, continuous variables were summarized by using mean and standard deviation (SD); if skewed, median and interquartile range were used. Differences between the groups in demographic and clinical characteristics were conducted by using Chi-Square Tests of Independence (nominal, ordinal) or Mann–Whitney (continuous) tests. Generalized linear models that controlled for the baseline values were used to assess differences between groups in the amount of change between baseline and end of study in the study outcome variables. Cohen's d effect size indices were used to quantify the effects of the WBMI on those outcomes. Effect sizes were of primary importance in this feasibility work, whereas an alpha of 0.05 was used for determining statistical significance.
Results
Participants who completed their randomized assignment (Pamphlet or WBMI) were included in the study analyses. Of those randomized to the Pamphlet group, 62 (77.5%) completed that program, and 47 (58.8%) of the WBMI group completed that program (p = 0.011). There were no statistically significant differences in demographic characteristics between those who completed the assigned tasks and were included in the study sample and those who did not complete the assigned tasks and were excluded from the analyses.
Demographic and clinical characteristics of the participants who did complete their respective interventions are summarized in Table 1. Participants were all female, with a mean age of 57.6 years (SD = 9.1), primarily white (86.2%), married or living with a partner (67.6%), and reported living in a city (64.2%). Approximately half were employed (51.9%) and had private insurance (54.1%). Approximately two-thirds of the sample (64.8%) had undergone a combined treatment of surgery, radiation, and chemotherapy; only 9% reported that they were not currently using any type of lymphedema treatment measures (such as use of complete decongestive therapy, pump, compression sleeve, arm elevation, and night bandaging). The only statistically significant difference between the groups was that those completing the WBMI were slightly older than those who completed the Pamphlet intervention (mean = 59.8 vs. 56.0 years, respectively, p = 0.035, see Table 1).
Table 1.
Demographic and Clinical Characteristics by Group (N = 109)
| Overall (N = 109) | Booklet (N = 62) | Web (N = 47) | p | |
|---|---|---|---|---|
| Mean (SD) | ||||
| Demographic characteristicsa | ||||
| Ageb | 57.6 (9.1) | 56.0 (9.2) | 59.8 (8.6) | 0.035 |
| Years of education | 15.5 (2.4) | 15.4 (2.4) | 15.6 (2.5) | 0.753 |
| N (%) | ||||
| Race | 0.864 | |||
| Black or African American | 11 (10.1) | 7 (11.3) | 4 (8.5) | |
| White | 94 (86.2) | 53 (85.5) | 41 (87.2) | |
| Multiple and Other | 4 (3.7) | 2 (3.2) | ||
| Ethnicity | 0.190 | |||
| Hispanic or Latina | 4 (3.7) | 1 (1.6) | 3 (6.4) | |
| Not Hispanic or Latina | 105 (96.3) | 661 (98.4) | 44 (93.6) | |
| Partnered (married/living with partner) | N = 108 | N = 61 | 0.924 | |
| No | 35 (32.4) | 20 (32.8) | 15 (31.9) | |
| Yes | 73 (67.6) | 41 (67.2) | 32 (68.1) | |
| Residence | 0.148 | |||
| City | 70 (64.2) | 35 (56.5) | 35 (74.5) | |
| Country | 25 (22.9) | 17 (27.4) | 8 (17.0) | |
| Other | 14 (12.8) | 10 (16.1) | 4 (8.5) | |
| Employment | N = 108 | N = 61 | 0.844 | |
| Employed | 56 (51.9) | 33 (54.1) | 23 (48.9) | |
| Not employed | 44 (40.7) | 24 (39.3) | 20 (42.6) | |
| Other | 8 (7.4) | 4 (6.6) | 4 (8.5) | |
| Insurance | 0.347 | |||
| Government | 22 (20.2) | 10 (16.1) | 12 (25.5) | |
| Private | 59 (54.1) | 37 (59.7) | 22 (46.8) | |
| Multiple and other | 28 (25.7) | 15 (24.2) | 13 (27.7) | |
| Annual income | N = 108 | N = 61 | 0.983 | |
| Up to $30,000 | 19 (17.6) | 11 (18.0) | 8 (17.0) | |
| More than $30,000 | 69 (63.9) | 39 (63.9) | 30 (63.8) | |
| Do not care to respond | 20 (18.5) | 11 (18.0) | 9 (19.1) | |
| Clinical characteristics | ||||
| Chemotherapy | N = 106 | N = 61 | N = 45 | 0.664 |
| None | 16 (15.1) | 10 (16.4) | 6 (13.3) | |
| Some | 90 (84.9) | 51 (83.6) | 39 (86.7) | |
| Taxane Chemo | N = 81 | N = 43 | N = 38 | 0.249 |
| No | 13 (16.0) | 5 (11.6) | 8 (21.1) | |
| Yes | 68 (84.0) | 38 (88.4) | 30 (78.9) | |
| Radiation therapy | 0.735 | |||
| None | 32 (29.4) | 19 (30.6) | 13 (27.7) | |
| Some | 77 (70.6) | 43 (69.4) | 34 (72.3) | |
| Complete treatment received | N = 108 | N = 61 | 0.580 | |
| Surgery alone | 7 (6.5) | 3 (4.9) | 4 (8.5) | |
| Surgery and radiation | 8 (7.4) | 6 (9.8) | 2 (4.3) | |
| Surgery and chemotherapy | 23 (21.3) | 14 (23.0) | 9 (19.1) | |
| Surgery, radiation, and chemotherapy | 70 (64.8) | 38 (62.3) | 32 (68.1) | |
| Surgery type | 0.350 | |||
| Breast conserving | 30 (27.5) | 18 (29.0) | 12 (25.5) | |
| Breast sacrificing | 57 (52.3) | 29 (46.8) | 28 (59.6) | |
| Multiple and other | 22 (20.2) | 15 (24.2) | 7 (14.9) | |
| Reconstruction | 0.952 | |||
| None | 56 (51.4) | 32 (51.6) | 24 (51.1) | |
| Immediate | 22 (20.2) | 13 (21.0) | 9 (19.1) | |
| Delayed | 31 (28.4) | 17 (27.4) | 14 (29.8) | |
| Hormone inhibitor use | N = 100 | N = 55 | N = 45 | 0.809 |
| No | 52 (52.0) | 28 (50.9) | 24 (53.3) | |
| Yes | 48 (48.0) | 27 (49.1) | 21 (46.7) | |
| Regular medications | N = 108 | N = 61 | 0.957 | |
| No | 14 (13.0) | 8 (13.1) | 6 (12.8) | |
| Yes | 94 (87.0) | 53 (86.9) | 41 (87.2) | |
| Menstrual status | N = 108 | N = 61 | 0.115 | |
| Premenopausal | 10 (9.3) | 8 (13.1) | 2 (4.3) | |
| Postmenopausal | 98 (90.7) | 53 (86.9) | 45 (95.7) | |
| Current treatment for lymphedema | N = 107 | N = 61 | N = 46 | |
| None | 10 (9.3) | 7 (11.5) | 3 (6.5) | 0.383 |
| CDT | 31 (29.0) | 17 (27.9) | 14 (30.4) | 0.772 |
| Pump | 1 (0.9) | 1 (1.6) | 0 (0.0) | 0.383 |
| Compression sleeve | 41 (38.3) | 20 (32.8) | 21 (45.7) | 0.175 |
| Arm elevation | 14 (13.1) | 5 (8.2) | 9 (19.6) | 0.084 |
| Medication only | 1 (0.9) | 0 (0.0) | 1 (2.2) | 0.247 |
| CDT and pump | 5 (4.7) | 4 (6.6) | 1 (2.2) | 0.288 |
| Pump and compression sleeve | 19 (17.8) | 12 (19.7) | 7 (15.2) | 0.551 |
| Arm elevation and medication | 4 (3.7) | 3 (4.9) | 1 (2.2) | 0.459 |
| Night bandaging | 26 (24.3) | 13 (21.3) | 13 (28.3) | 0.407 |
| Arm exercises, skin care, compression sleeve, and bandaging | 52 (48.6) | 26 (42.6) | 26 (56.5) | 0.154 |
| Other | 25 (23.4) | 13 (21.3) | 12 (26.1) | 0.563 |
| Median [IQR], (minimum, maximum) N | ||||
| Months since first surgery | 79.1 [34–139], (0, 535) | 84.0 [35–172], (0, 500) | 77.2 [33–113], (1, 535) | 0.485 |
| Months since last surgery | 75.0 [24–129], (0, 535) | 80.4 [24–146], (0, 385) | 73.9 [22–112], (0, 535) | 0.664 |
| Months since last treatment | 68.2 [19–122], (0, 529) | 60.7 [14–122], (0, 499) | 68.7 [23–118], (0, 529) | 0.997 |
| No. of skin conditions at baseline, affected arm | 2.0 [1–4], (0, 9) | 2.0 [1–5], (0, 8) | 2.0 [1–3], (0, 9) | 0.098 |
| No. of skin conditions at baseline, unaffected arm | 1.0 [0–1], (0, 9) | 1.0 [0–2], (0, 8) | 1.0 [0–1], (0, 9) | 0.373 |
All participants were female.
N = 105, Booklet N = 60, Web N = 45.
CDT, complex decongestive therapy; IQR, interquartile range; SD, standard deviation.
A median 12 symptoms of a total 30 possible were reported at baseline. Domains with the highest numbers of symptoms were swelling (median 3 out of possible 4) and mood (median 4 out of possible 9). With the exception of the number of biobehavioral symptoms, no statistically significant differences between the groups in the reduction of symptoms were apparent between baseline and 1 or 12 months (effect sizes = 0.05–0.28, p > 0.05). There was a slightly greater reduction in the number of biobehavioral symptoms in the WBMI group than in the Pamphlet group. The median score at baseline was four symptoms in the WBMI group and three symptoms in the Pamphlet group. Of the participants in the WBMI group, 25% had two or fewer mood symptoms at the 1-month assessment whereas most had not reduced those symptoms at all in the Pamphlet group (p < 0.05, effect size = 0.53). Although 25% of the Pamphlet participants had a reduction of at least one biobehavioral symptom at the 12-month assessment, the reduction of at least two symptoms in 25% of the WBMI participants remained from 1 month onward (p < 0.05, effect size = 0.47). Summaries of the symptom domain scores (that incorporate the intensity and distress from symptoms) are shown in Table 2. There were very low ratings of intensity and distress from the symptoms reported; therefore, the symptom domain scores observed at baseline were low (median values 0.0–2.6 on a scale from 0 to 10). Thus, there was little opportunity for a statistically significant decrease in those scores over the study period. Cohen's d values for the effects of the WBMI protocol ranged from 0.03 to 0.34.
Table 2.
Summaries of Lymphedema Symptom Intensity and Distress Scale–Arm Baseline Scores and Change Values
| Baseline, median (IQR) | 1 Month, median (IQR) | Cohen's d | 12 Months, median (IQR) | Cohen's d | |
|---|---|---|---|---|---|
| Soft tissue sensation | |||||
| Booklet | 2.6 (1 to 5) | 0.0 (−1.3 to 0.6) | 0.15 | 0.0 (−1.3 to 0.5) | 0.06 |
| Web | 2.5 (1 to 4) | −0.5 (−1.0 to 0.3) | 0.0 (−2.0 to 1.0) | ||
| Neurological sensation | |||||
| Booklet | 0.9 (0 to 3) | 0.0 (−0.6 to 0.4) | 0.13 | 0.0 (−0.6 to 0.6) | 0.16 |
| Web | 0.9 (0 to 3) | 0.0 (−0.6 to 0.3) | 0.0 (−0.8 to 0.9) | ||
| Biobehavioral | |||||
| Booklet | 2.2 (0 to 4) | −0.1 (−0.7 to 0.5) | 0.24 | −0.2 (−1.3 to 0.4) | 0.03 |
| Web | 1.7 (1 to 4) | −0.2 (−1.0 to 0.3) | −0.4 (−1.4 to 0.3) | ||
| Sexuality | |||||
| Booklet | 2.0 (0 to 7) | 0.0 (−0.5 to 0.2) | 0.34 | 0.0 (−1.4 to 0.0) | 0.18 |
| Web | 2.0 (0 to 6) | 0.0 (−1.9 to 0.0) | 0.0 (−0.7 to 0.0) | ||
| Activity | |||||
| Booklet | 0.0 (0 to 4) | 0.0 (−1.4 to 0.2) | 0.13 | 0.0 (−1.2 to 0.4) | 0.14 |
| Web | 1.3 (0 to 50 | 0.0 (−1.7 to 0.7) | 0.0 (−2.4 to 0.0) | ||
| Overall | |||||
| Booklet | 1.8 (0 to 3) | −0.2 (−0.7 to 0.4) | 0.28 | −0.2 (−0.8 to 0.4) | 0.03 |
| Web | 1.6 (1 to 3) | −0.2 (−0.6 to 0.2) | −0.2 (−1.0 to 0.3) | ||
N unless explicitly noted: Booklet = 61–62, Web = 47. Two clusters (Function and Resources) are not summarized in this table due to very low incidence of those symptoms at baseline. Function: 92.7% had ≤1 symptom at baseline (73.4% had none); Resources: 86.2% had ≤1 symptom at baseline (67.9% had none).
Summaries and analyses of changes in function (QuickDASH) and well-being (POMS) also revealed no statistically significant differences between the groups during the study period (p > 0.05). Effect sizes for the differences between the groups ranged from −0.19 (indicating a greater increase function/well-being in the Pamphlet group than the WBMI group) to +0.30 (WBMI > Pamphlet, see Table 3).
Table 3.
Summaries of Quick Disabilities of Arm, Shoulder, and Hand and Profile of Mood States-Short Form Baseline and Change Values
| Measure | Baseline, median (IQR) | Change from baseline |
|||
|---|---|---|---|---|---|
| 1 Month, median (IQR) | Cohen's d | 12 Months, median (IQR) | Cohen's d | ||
| QuickDASH (0–100) | |||||
| Booklet | 25.0 (9 to 37) | −2.3 (−9.0 to 2.3) | 0.03 | −2.3 (−9.0 to 4.6) | −0.04 |
| Web | 27.3 (13 to 41) | −2.3 (−11.3 to 4.6) | −2.3 (−9.0 to 2.3) | ||
| POMS | |||||
| Tension (0–24) | |||||
| Booklet | 5.0 (2 to 7) | 0.0 (−2.0 to 2.0) | −0.19 | −1.0 (−2.0 to 0.0) | 0.22 |
| Web | 4.0 (2 to 6) | 0.0 (−2.0 to 1.0) | −1.0 (−2.0 to 1.0) | ||
| Depression (0–32) | |||||
| Booklet | 3.0 (0 to 5) | 0.0 (−2.0 to 1.0) | 0.19 | 0.0 (−3.0 to 1.0) | 0.23 |
| Web | 3.0 (1 to 6) | 0.0 (−2.0 to 3.0) | 0.0 (−1.3 to 1.3) | ||
| Anger (0–28) | |||||
| Booklet | 3.0 (1 to 5) | 0.0 (−2.8 to 1.0) | −0.08 | −1.0 (−3.0 to 1.0) | −0.11 |
| Web | 3.0 (0 to 5) | 0.0 (−2.0 to 1.0) | 0.0 (−3.0 to 1.0) | ||
| Vigor (0–24) | |||||
| Booklet | 9.5 (5 to 14) | 1.0 (−2.0 to 4.0) | −0.19 | 0.0 (−2.0 to 4.0) | −0.16 |
| Web | 10.0 (6 to 13) | 0.5 (−1.3 to 3.0) | 0.0 (−2.0 to 3.0) | ||
| Fatigue (0–20) | |||||
| Booklet | 6.5 (3 to 12) | −1.0 (−3.0 to 2.0) | 0.02 | −2.0 (−5.0 to 0.0) | 0.30 |
| Web | 5.0 (3 to 8) | 0.0 (−3.0 to 1.0) | 0.0 (−4.0 to 2.0) | ||
| Confusion (0–20) | |||||
| Booklet | 3.0 (1 to 7) | 0.0 (−2.0 to 1.0) | <0.01 | 0.0 (−2.3 to 0.0) | 0.26 |
| Web | 3.0 (1 to 5) | 0.0 (−2.0 to 1.0) | 0.0 (−2.0 to 1.0) | ||
| Total (0–168) | |||||
| Booklet | 39.0 (32 to 49) | 0.0 (−6.0 to 5.0) | −0.09 | −3.0 (−10.8 to 1.8) | 0.07 |
| Web | 36.0 (29 to 46) | −1.0 (−5.5 to 5.5) | −3.0 (−7.8 to 3.0) | ||
N: Booklet = 52–62, Web = 44–47.
POMS, Profile of Mood States; QuickDASH, Quick-Disabilities of Arm, Shoulder, and Hand.
Consistent with the other measures in this study, most of the participants reported relatively high levels of coping at baseline for all of the domains assessed by the Brief COPE measure. Nevertheless, at the 1-month postintervention completion assessment point, the domain for supportive coping did show increased improvement over time for the WBMI group relative to the Pamphlet (Cohen's d = 0.54, p = 0.002); however, this effect was no longer apparent at the 12-month postintervention assessment (Cohen's d = 0.22, p > 0.05). Relative to the Pamphlet participants, the participants in the WBMI group demonstrated a reduction in the use of humor as a coping strategy at the 1-month assessment (Cohen's d = −0.37, p = 0.038) that also dissipated by 12 months (Cohen's d = −0.22, p > 0.05). Findings at 12 months postintervention completion suggested that the Pamphlet group demonstrated a greater reduction in the use of self-distraction as a coping strategy relative to any change in the WBMI group (Cohen's d = 0.35, p = 0.034, see Table 4).
Table 4.
Summaries of COPE and Self-Care Baseline and Change Values
| Measure | Baseline, median, IQR | Change from baseline |
|||
|---|---|---|---|---|---|
| 1 Month, median, IQR | Cohen's d | 12 Months, median, IQR | Cohen's d | ||
| COPE | |||||
| Positive coping (4–16) | |||||
| Booklet | 11.0 (7 to 14) | 0.0 (−1.0 to 2.0) | 0.04 | 0.0 (−1.8 to 1.0) | <0.01 |
| Web | 12.0 (9 to 14) | 0.0 (−2.0 to 2.0) | −0.5 (−2.3 to 1.0) | ||
| Active coping (4–16) | |||||
| Booklet | 11.0 (9 to 13) | 0.0 (−1.0 to 2.0) | 0.16 | 0.0 (−3.0 to 2.0) | 0.08 |
| Web | 12.0 (10 to 15) | 0.0 (−2.0 to 1.0) | 0.0 (−4.0 to 2.0) | ||
| Supportive coping (4–16) | |||||
| Booklet | 11.0 (8 to 13) | −1.0 (−3.0 to 0.0) | 0.54a | −1.0 (−3.0 to 1.0) | 0.22 |
| Web | 10.0 (8 to 12) | 0.0 (−1.0 to 2.0) | 0.0 (−2.0 to 1.0) | ||
| Acceptance (2–8) | |||||
| Booklet | 7.0 (6 to 8) | 0.0 (−0.8 to 1.0) | −0.02 | 0.0 (−1.0 to 1.0) | −0.14 |
| Web | 7.0 (6 to 8) | 0.0 (−1.0 to 0.0) | 0.0 (−1.0 to 1.0) | ||
| Humor (2–8) | |||||
| Booklet | 4.0 (2 to 5) | 0.0 (0.0 to 1.0) | −0.37a | 0.0 (−1.0 to 1.0) | −0.22 |
| Web | 4.0 (2 to 6) | 0.0 (−2.0 to 0.0) | −1.0 (−2.0 to 0.0) | ||
| Venting (2–8) | |||||
| Booklet | 3.0 (2 to 5) | 0.0 (0.0 to 1.0) | 0.10 | 0.0 (−1.0 to 1.0) | 0.02 |
| Web | 4.0 (3 to 5) | 0.0 (−1.0 to 1.0) | 0.0 (−1.0 to 1.0) | ||
| Self-blame (2–8) | |||||
| Booklet | 3.0 (2 to 4) | 0.0 (−1.0 to 1.0) | 0.06 | 0.0 (−1.0 to 0.0) | 0.18 |
| Web | 3.0 (2 to 4) | 0.0 (0.0 to 1.0) | 0.0 (−1.0 to 1.0) | ||
| Self-distraction (2–8) | |||||
| Booklet | 5.0 (4 to 7) | 0.0 (−1.0 to 1.0) | 0.24 | 0.0 (−2.0 to 0.8) | 0.35a |
| Web | 6.0 (5 to 6) | 0.0 (−1.0 to 1.0) | 0.0 (−1.0 to 1.0) | ||
N: Booklet = 58–62, Web = 46–47.
p < 0.05.
Participants were conducting self-care activities a median of 3 days a week at baseline. There were no statistically significant differences between the study groups in the amount of change in self-care activities from baseline to either 1 or 12 months postcompletion of the intervention (Cohen's d = 0.20 and, −0.04 respectively). Further, participants reported low levels of stress, as well as high levels of social support and health competency at baseline, with no statistically significant differences between the groups in change over the study period. Most participants had no or minimal lymphedema health care expenses at baseline. There was insufficient variability across the duration of the study in those expenses to conduct an analysis of change over time.
Within the Pamphlet group, 13 participants (21%) were eligible for L-Dex measurement. The respective number in the WBMI group was 21 (45%). Among the participants eligible for L-Dex measurement, no statistically significant differences between the Pamphlet and WBMI groups were observed at baseline or in change over the study period (Cohen's d = +0.36).
Although there were few and inconsistent differences between the groups in the observed changes in the outcome variables, as previously noted, there was a statistically significantly lower intervention completion rate in the WBMI group than in the Pamphlet group (77.5% vs. 58.8%, respectively, p = 0.011). Participants in the WBMI group reported that the intervention required statistically significantly more time to complete than did those completing the Pamphlet intervention (WBMI: median = 525 minutes, Pamphlet: median = 60 minutes; p < 0.001). Nevertheless, if completed, participants in the WBMI group were more likely than those in the Pamphlet group to strongly agree that the intervention provided good examples of self-care (p = 0.001). No other statistically significant differences in the evaluations of the interventions were observed (p > 0.05).
Discussion
The groups in the study were similar, as only age differed, with those completing the WBMI being slightly older than those who completed the Pamphlet intervention. Thus, it is unlikely that group differences influenced our findings.
In the Pamphlet group, 77.5% completed that program, compared with 58.8% of the WBMI group. The Pamphlet had an advantage over the WBMI in terms of time required for completion. Participants were required to complete all 12 modules of the WBMI, and it is possible that completion rates might have been higher if they had been able to select only those modules that they perceived as being the most beneficial to them. However, WBMI was perceived as significantly better in offering good examples of self-care than was the Pamphlet.
With the exception of the biobehavioral symptoms (mood), no statistically significant differences between the groups in the reduction of symptoms were apparent between baseline and 1 or 12 months (effect sizes = 0.05–0.28, p > 0.05) based on the LSIDS-A. Thus, the hypothesis related to lower symptom burden in the WBMI group was only partially supported, although the participants reported relatively low levels of all symptoms on study enrollment. It is possible that baseline levels of symptoms were so low that significant improvements would have been difficult to demonstrate.
The hypothesis related to improved psychological well-being in the WBMI group was not supported. The LSIDS-A Biobehavioral cluster and POMS-SF, which contains six subscales assessing tension, depression, anger, vigor, fatigue, and confusion, did not reflect any significant improvements postintervention in the WBMI group relative to the Pamphlet group.
Hypotheses related to improved function and costs in the WBMI were not supported. This finding is probably related to the relatively high function levels at baseline and few documented expenses. These favorable conditions are typical of convenience samples in that higher functioning individuals with less burdensome disease-related problems tend to self-select into research studies. From a statistical perspective, a ceiling effect may explain the findings because it is hard to improve conditions that are already good at baseline. In addition, there was not a sufficient amount of variability in expenses to enable analyses of any changes over time.
The hypothesis related to decreased extracellular fluid in the WBMI was also not supported. A subset of the overall sample participated in arm measurements, so the sample size was small, making it difficult to detect significant improvements.
Comparing the findings in this study with findings from similar studies is difficult because the majority of published intervention trials for BCRL patients focus on manual lymphatic drainage and/or exercise monitored by physical therapists or yoga treatment delivered by yoga instructors with arm volume as a primary outcome.46–48 This study involved Web-based learning, without active contact or interaction with health care professionals. The benefits of this format include convenience for participants and the flexibility of self-paced learning. Although exercise and manual lymphatic drainage were recommended, no supervised exercise program or physical therapy services were involved in this study. Beneficial outcomes of exercise, such as improved function or decreases in pain, have been demonstrated in multiple studies.26 Fu et al. used a 5-minute Web-based program focused on teaching lymphatic self-care drainage that participants reviewed on a daily basis, and their intervention group reported significantly less pain than controls at the 12-week follow-up.26 It is not unusual for interventions of a brief duration to have higher adherence rates, and the length of time and breadth of topics covered in our intervention may have affected completion rates in our WBMI group. Given the greater perceived value of the self-care information in the WBMI group, if participants had been able to select modules of greatest interest, the relevance and completion rates might have been higher. This could have potentially affected outcomes in a more positive manner. It is also possible that studies that do not entail physical engagement in exercise, or other upper body movements such as yoga, may not have effect sizes that are comparable to exercise interventions.
Our findings support the fact that a randomized trial such as this is feasible in the targeted population and further research in this area is needed. Issues identified, such as ceiling effect and completion rates, must be addressed in future studies. Thus, testing a modified version of this WMBI is warranted. Indicated modifications include screening for highly symptomatic patients to reduce the ceiling effect, using a different stress outcome measure, and shortening videos for each component. Empowering patients to choose to complete a minimum number of modules (e.g., 6) that they feel are the most relevant to them versus all modules should also be considered.
Conclusions
Patients with BCRL encounter a wide range of psychological and physical challenges on a daily basis. Our intervention attempted to cover the broad range of topics that could be relevant to these patients, such as diet and exercise strategies, dealing with negative emotions and stress, body image changes, uncertainty, and enhancing social support. In developing a program that tried to meet a wide range of educational needs, we developed a lengthy program that may be better absorbed in selective chunks based on individual preferences. Feedback from participants was positive on actual content, and we suggest this program's usefulness as an online reference for BCRL patients deserves exploration.
Acknowledgments
The authors would like to acknowledge Melissa Adair, RN and Jeff Gordon, PhD for their assistance with this study. This work was supported by a Research Scholar Grant, RSG-13-022-01-CPPB from the American Cancer Society. The project described was supported by CTSA award number UL1 TR002243 and UL1 TR000445 from the National Center for Advancing Translational Sciences. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Author Disclosure Statement
The authors have no known conflicts of interest.
References
- 1. Pinto M, Gimigliano F, Tatangelo F, et al. Upper limb function and quality of life in breast cancer related lymphedema: A cross-sectional study. Eur J Phys Rehabil Med 2013;49:665–673 [PubMed] [Google Scholar]
- 2. DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol 2013;14:500–515 [DOI] [PubMed] [Google Scholar]
- 3. Dominick SA, Natarajan L, Pierce JP, Madanat H, Madlensky L. The psychosocial impact of lymphedema-related distress among breast cancer survivors in the WHEL Study. Psychooncology 2014;23:1049–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherman KA, Miller SM, Roussi P, Taylor A. Factors predicting adherence to risk management behaviors of women at increased risk for developing lymphedema. Support Care Cancer 2015;23:61–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fu MR, Ridner SH, Hu SH, Stewart BR, Cormier JN, Armer JM. Psychosocial impact of lymphedema: A systematic review of literature from 2004 to 2011. Psychooncology 2013;22:1466–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gill A, Chakraborty A, Selby D. What is symptom burden: A qualitative exploration of patient definitions. J Palliat Care 2012;28:83–89 [PubMed] [Google Scholar]
- 7. Ridner SH, Bonner CM, Deng J, Sinclair VG. Voices from the shadows: Living with lymphedema. Cancer Nurs 2012;35:E18–E26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Khan F, Amatya B, Pallant JF, Rajapaksa I. Factors associated with long-term functional outcomes and psychological sequelae in women after breast cancer. Breast 2012;21:314–320 [DOI] [PubMed] [Google Scholar]
- 9. Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer 2005;13:904–911 [DOI] [PubMed] [Google Scholar]
- 10. Fu MR. Women at work with breast cancer-related lymphoedema. J Lymphoedema 2008;3:20. [PMC free article] [PubMed] [Google Scholar]
- 11. Shigaki CL, Madsen R, Wanchai A, Stewart BR, Armer JM. Upper extremity lymphedema: Presence and effect on functioning five years after breast cancer treatment. Rehabil Psychol 2013;58:342–349 [DOI] [PubMed] [Google Scholar]
- 12. Viehoff PB, Gielink PDC, Damstra RJ, Heerkens YF, van Ravensberg DC, Neumann MHA. Functioning in lymphedema from the patients’ perspective using the International Classification of Functioning, Disability and health (ICF) as a reference. Acta Oncol 2015;54:411–421 [DOI] [PubMed] [Google Scholar]
- 13. Viehoff PB, Potijk F, Damstra RJ, et al. Identification of relevant ICF (International Classification of Functioning, Disability and Health) categories in lymphedema patients: A cross-sectional study. Acta Oncol 2015;54:1218–1224 [DOI] [PubMed] [Google Scholar]
- 14. Armer J, Fu MR, Wainstock JM, Zagar E, Jacobs LK. Lymphedema following breast cancer treatment, including sentinel lymph node biopsy. Lymphology 2004;37:73–91 [PubMed] [Google Scholar]
- 15. Ostby PL, Armer JM. Complexities of adherence and post-cancer lymphedema management. J Pers Med 2015;5:370–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taha SA, Matheson K, Anisman H. Everyday experiences of women posttreatment after breast cancer: The role of uncertainty, hassles, uplifts, and coping on depressive symptoms. J Psychosoc Oncol 2012;30:359–379 [DOI] [PubMed] [Google Scholar]
- 17. Alcorso J, Sherman KA. Factors associated with psychological distress in women with breast cancer-related lymphoedema. Psychooncology 2016;25:865–872 [DOI] [PubMed] [Google Scholar]
- 18. Winch CJ, Sherman KA, Koelmeyer LA, Smith KM, Mackie H, Boyages J. Sexual concerns of women diagnosed with breast cancer-related lymphedema. Support Care Cancer 2015;23:3481–3491 [DOI] [PubMed] [Google Scholar]
- 19. Thomas R, Hamilton R. Illustrating the (in)visible: Understanding the impact of loss in adults living with secondary lymphedema after cancer. Int J Qual Stud Health Well-Being 2014;9:24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Armer JM, Hulett JM, Bernas M, Ostby P, Stewart BR, Cormier JN. Best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Curr Breast Cancer Rep 2013;5:134–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brayton KM, Hirsch AT O B rien PJ, Cheville A, Karaca-Mandic P, Rockson SG. Lymphedema prevalence and treatment benefits in cancer: Impact of a therapeutic intervention on health outcomes and costs. PLoS One 2014;9:e114597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmitz KH, DiSipio T, Gordon LG, Hayes SC. Adverse breast cancer treatment effects: The economic case for making rehabilitative programs standard of care. Support Care Cancer 2015;23:1807–1817 [DOI] [PubMed] [Google Scholar]
- 23. Shih YC, Xu Y, Cormier JN, et al. Incidence, treatment costs, and complications of lymphedema after breast cancer among women of working age: A 2-year follow-up study. J Clin Oncol 2009;27:2007–2014 [DOI] [PubMed] [Google Scholar]
- 24. Fu MR. Cancer survivors' views of lymphoedema management. J Lymphoedema 2010;5:39–48 [PMC free article] [PubMed] [Google Scholar]
- 25. Brown JC, Cheville AL, Tchou JC, Harris SR, Schmitz KH. Prescription and adherence to lymphedema self-care modalities among women with breast cancer-related lymphedema. Support Care Cancer 2014;22:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fu MR, Axelrod D, Guth AA, et al. Usability and feasibility of health IT interventions to enhance Self-Care for Lymphedema Symptom Management in breast cancer survivors. Internet Interv 2016;5:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnson-Turbes A, Schlueter D, Moore AR, Buchanan ND, Fairley TL. Evaluation of a web-based program for African American young breast cancer survivors. Am J Prev Med 2015;49:S543–S549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wheelock AE, Bock MA, Martin EL, et al. SIS.NET: A randomized controlled trial evaluating a web-based system for symptom management after treatment of breast cancer. Cancer 2015;121:893–899 [DOI] [PubMed] [Google Scholar]
- 29. Lazarus RS, Folkman S: Coping and adaptation. In: Gentry WD, ed. The handbook of behavioral medicine. New York, NY: Guilford, 1984:282–325 [Google Scholar]
- 30. Rhoten BA, Radina ME, Adair M, Sinclair V, Ridner SH. Hide and seek: Body image-related issues for breast cancer survivors with lymphedema. J Womens Health Issues Care 2015;4:1–7 [Google Scholar]
- 31. Ridner SH, Rhoten BA, Radina ME, Adair M, Bush-Foster S, Sinclair V. Breast cancer survivors' perspectives of critical lymphedema self-care support needs. Support Care Cancer 2016;24:2743–2750 [DOI] [PubMed] [Google Scholar]
- 32. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Warshaw R. Guide to understanding lymphedema [pamphlet], 3rd ed. Ardmore, PA: Living Beyond Breast Cancer, 2011 [Google Scholar]
- 34. Ridner SH, Dietrich MS. Development and validation of the Lymphedema Symptom and Intensity Survey-Arm. Support Care Cancer 2015;23:3103–3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shacham S. A shortened version of the Profile of Mood States. J Pers Assess 1983;47:305–306 [DOI] [PubMed] [Google Scholar]
- 36. Beaton DE, Wright JG, Katz JN. Development of the QuickDASH: Comparison of three item-reduction approaches. J Bone Joint Surg Am 2005;87:1038–1046 [DOI] [PubMed] [Google Scholar]
- 37. Carver CS, Pozo C, Harris SD, et al. How coping mediates the effect of optimism on distress: A study of women with early stage breast cancer. J Pers Soc Psychol 1993;65:375–390 [DOI] [PubMed] [Google Scholar]
- 38. Cohen S, Williamson GM. Perceived stress in a probability sample of the United States. In: Spacapan S, Oskamp S, eds. The social psychology of health: The Claremont Symposium on applied social psychology. Newbury Park, CA: Sage Publications, 1988:31–68 [Google Scholar]
- 39. Wallston KA, Osborn CY, Wagner LJ, Hilker KA. The Perceived Medical Condition Self-Management Scale applied to persons with HIV/AIDS. J Health Psychol 2011;16:109–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wallston KA, Rothman RL, Cherrington A. Psychometric properties of the Perceived Diabetes Self-Management Scale (PDSMS). J Behav Med 2007;30:395–401 [DOI] [PubMed] [Google Scholar]
- 41. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–714 [DOI] [PubMed] [Google Scholar]
- 42. Cook RF, Billings DW, Hersch RK, Back AS, Hendrickson A. A field test of a web-based workplace health promotion program to improve dietary practices, reduce stress, and increase physical activity: Randomized controlled trial. J Med Internet Res 2007;9:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cornish BH, Chapman M, Hirst C, et al. Early diagnosis of lymphedema using multiple frequency bioimpedance. Lymphology 2001;34:2–11 [PubMed] [Google Scholar]
- 44. Ward LC, Czerniec S, Kilbreath SL. Quantitative bioimpedance spectroscopy for the assessment of lymphoedema. Breast Cancer Res Treat 2009;117:541–547 [DOI] [PubMed] [Google Scholar]
- 45. IBM SPSS Statistics for Windows [computer program]. Version 25.0. Armonk, NY: IBM Corp., 2017 [Google Scholar]
- 46. Jonsson C, Johansson K. The effects of pole walking on arm lymphedema and cardiovascular fitness in women treated for breast cancer: A pilot and feasibility study. Physiother Theory Pract 2014;30:236–242 [DOI] [PubMed] [Google Scholar]
- 47. Loudon A, Barnett T, Piller N, Immink MA, Visentin D, Williams AD. The effects of yoga on shoulder and spinal actions for women with breast cancer-related lymphoedema of the arm: A randomised controlled pilot study. BMC Complement Altern Med 2016;16:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: A randomised controlled pilot-trial. BMC Complement Altern Med 2014;14:214. [DOI] [PMC free article] [PubMed] [Google Scholar]