Abstract
Crimean–Congo hemorrhagic fever (CCHF) is a severe human disease with mortality rates of up to 30%. The disease is widespread in Africa, Asia, the Middle East and Eastern Europe. The last few years have seen disease emergence in Spain for the first time and disease re-emergence in other regions of the world after periods of inactivity. Factors, such as climate change, movement of infected ticks, animals, and changes in human activity, are likely to broaden endemic foci. There are therefore concerns that CCHF might emerge in currently nonendemic regions. The absence of approved vaccines or therapies heightens these concerns; thus Crimean–Congo hemorrhagic fever virus (CCHFV) is listed by the World Health Organization as a priority organism. However, the current sporadic nature of CCHF cases may call for targeted vaccination of risk groups as opposed to mass vaccinations. CCHF vaccine development has accelerated in recent years, partly because of the discovery of CCHF animal models. In this review, we discuss CCHF risk groups who are most likely to benefit from vaccine development, the merits and demerits of available CCHF animal models, and the various approaches which have been explored for CCHF vaccine development. Lastly, we present concluding remarks and research areas which can be further explored to enhance the available CCHFV vaccine data.
Keywords: Crimean–Congo hemorrhagic fever virus, orthonairovirus, vaccine development, vaccine vector, virus-like replicon particles, recombinant protein
Introduction
Background
Crimean–Congo hemorrhagic fever virus (CCHFV) is exclusively associated with a virulent disease in humans. In the absence of approved therapeutics or vaccines against the virus, treatment is predominantly supportive. CCHFV possesses a trisegmented negative-sense RNA genome and is classified within the Orthonairovirus genus of the Nairoviridae family. Crimean–Congo hemorrhagic fever (CCHF) was medically recognized in 1944 in the wake of an outbreak involving military personnel stationed in the Crimean peninsula, and the medical condition was named Crimean hemorrhagic fever (CHF).1 A viral etiology and a tick-borne origin for CHF were proposed after Hyalomma marginatum tick filtrates produced the disease in human volunteers and individuals with psychiatric disorders.2 Following the Crimean peninsula outbreak, numerous epidemics of related disease conditions were described in Central Asia, Bulgaria, and the Soviet Union.1 Meanwhile, Dr. Courtois from the Belgian Congo isolated a virus from a febrile teenage boy using newborn mice in 1956, and the virus was designated Congo virus strain V3011.1 The causative agent of CHF was isolated in 1967 in newborn mice after intracerebral inoculation.3,4 Characterization studies of agents responsible for global tick-borne diseases, at the Yale Arbovirus Research Unit, established that the agent causing CHF was antigenically similar to Congo virus strain V3011.5 The names were subsequently combined and the virus named CCHFV.1
CCHFV is sustained in an enzootic cycle encompassing ticks and several vertebrate animals with humans regarded as dead-end hosts. Sources of human infections include bite from an infected tick, close contact with blood or tissue from diseased animals, and CCHF patients. Animals do not display symptoms, but disease in humans progresses through four phases: incubation, prehemorrhagic, hemorrhagic, and convalescence.1 Incubation period depends on the route of transmission; 1–3 days, up to 9 days for tick transmission while after exposure to infected blood or tissues incubation period is mostly 5–6 days and reach up to 13 days.1,6
Geographic distribution
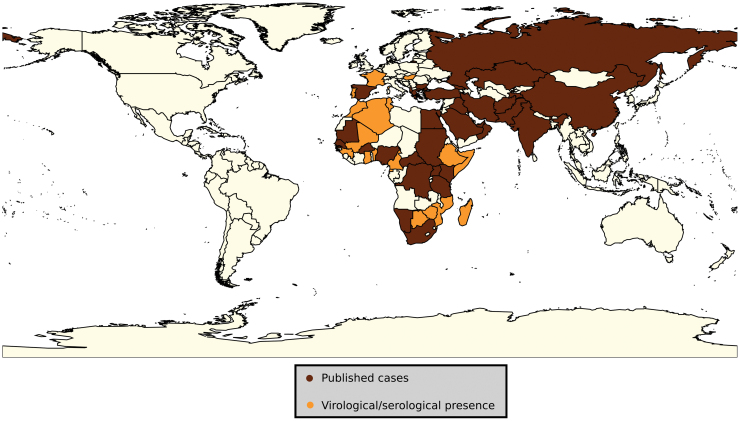
CCHFV is an emerging and re-emerging virus with extensive geographical distribution, as shown in Figure 1. The virus has a constant presence in Africa, the Middle East, Asia, and Eastern Europe. African countries from which CCHF has been reported include Burkina Faso, Central Africa Republic, Democratic Republic of Congo, Egypt, Kenya, Mauritania, Namibia, Nigeria, South Africa, Senegal, Sudan, Tanzania, and Uganda1,7–16 and countries yet to report CCHF but with evidence of viral circulation either from serological surveys or CCHFV isolation from ticks include Algeria, Benin, Cameroon, Equatorial Guinea, Ethiopia, Ghana, Guinea, Mali, Madagascar, Morocco, Mozambique, Niger, Somalia, Tunisia, and Zimbabwe.1,17–30 The presence of CCHFV in Somalia was suggested after evidence of the CCHFV in Hyalomma ticks obtained from Somali cattle and sheep exported to the United Arab Emirates.28 In the Middle East, the disease has been described in Iran, Iraq, Saudi Arabia, Oman, and the United Arab Emirates.31–35 East European countries with described CCHF disease include Albania, Bulgaria, Greece, Kosovo, Turkey, Georgia, and Russia.36–42 Portugal, Hungary, France, and Romania1,43–45 are at risk of CCHF based on serological evidence of viral circulation. Asian countries with described CCHF disease include Afghanistan, Kazakhstan, Pakistan, China, India, Tajikstan, and Uzibekistan.1,46–49
FIG. 1.
Global geographic summary of countries with reported CCHF cases, serological evidence and presence of CCHFV in ticks.1,7–48 CCHF, Crimean–Congo hemorrhagic fever; CCHFV, Crimean–Congo hemorrhagic fever virus.
CCHF cases have over the past few years been described in new regions while the disease has re-emerged in some countries after periods of inactivity. CCHF was first described in Turkey in the year 2000, and the country has the highest incidence of CCHF cases per annum with a mortality rate slightly below 5%.50 Over 1660 cases were reported by Russian Pro-Med between 2005 and 2017.51 The first human case in Iran was reported in 1999, and an increase in incidence has been observed.52,53 A nosocomial outbreak of CCHF at a hospital in India in 2011 stands as the principal case in the country49 and from there on, sporadic cases have been reported.54 The first recorded CCHF cases in Spain were described in 2016 from an adult male (an index case) who most likely acquired infection through a tick bite and a nosocomially acquired infection by a health professional who nursed the index case.55 Even though CCHF has not been described in some countries, the presence of CCHFV in ticks and CCHFV-specific antibodies in wild and domesticated ruminants have been demonstrated.56 CCHFV endemic foci are expected to broaden in the face of climate change,57 human case movement as well as the movement of animals infested with a tick.58 However, in some regions, the observed increase in reported cases could be a product of improved awareness and diagnostic capacity. CCHFV poses a zoonotic risk with public health implications and as such CCHF is a notifiable disease. Nonetheless, with the exception of Turkey reporting more than 50 cases per year,59 CCHF cases are currently sporadic in endemic zones.
CCHF vaccine target population
Considering that CCHF cases are sporadic, global mass vaccinations are unlikely, thus targeting vaccinations would be ideal. People residing in endemic areas who are prone to tick bites, especially Hyalomma ticks, are at risk of acquiring CCHF.60 Development of an efficacious vaccine will prevent infections and possible mortality from the disease in risk groups. CCHF vaccine target population would include farmers dealing with agriculture or animal husbandry. Even though livestock does not develop CCHF, they present with transient viremia. Contact with blood or tissues from infected livestock and bites from infected ticks have been reported as transmitting CCHFV, resulting in outbreaks in farming communities.61 Besides farmers, individuals participating in religious activities such as the Muslim Eid-ul Azha who get exposed to livestock blood and tissue during animal slaughter are at an increased risk of CCHF.62 Veterinarians and abattoir workers stand a high chance of occupational exposure to tick bites or contact with viremic animal blood and thus constitute another risk group. Outdoor activities such as hiking and camping as well as some rural lifestyles where people live in close proximity to livestock pose as risk factors for tick bites and contact with infected animals.63,64
Nosocomial transmission constitutes a major route of CCHFV spread accounting for a large proportion of global CCHF cases,65 often with higher case fatality rates compared with tick bite infections.66 Nosocomial outbreaks often serve as an indicator of unrecognized infections in the general population and seroepidemiological surveys carried in the wake of nosocomial outbreaks reveal prior presence of the virus in the community. Human serum CCHFV titers are high (108–1010 copies/mL) during the prehemorrhagic stage, especially in fatal cases67 and the virus has been detected in urine and saliva.68 The high CCHFV titers put medical professionals in contact with patients at risk and the nonspecific symptoms early in the course of disease exacerbates the risk. Laboratory personnel handling live virus comprise another risk group who could be a target for the CCHF vaccine. Laboratory-acquired infections arising from handling patient samples49,69–71 and during CCHFV research activities70,72 have been reported. Besides humans, livestock could be vaccinated to prevent viremia in animals and subsequent transmission to humans. Vaccinating livestock also prevents them from serving as amplifying hosts although small mammals are considered principal amplifying hosts.
CCHF animal models
Animal models for CCHF have recently been described. Previously, besides humans, the only other vertebrate known to be susceptible to CCHFV were newborn mice and rats. The immature immune systems of these infant rodents meant these animals could not serve as models. CCHF infections in immunocompetent animals result in transient viremia and absence of noticeable symptoms.73,74 Experimental infection of knockout mice with CCHFV displayed some disease signs and physiological changes, which parallel findings in humans, although differences are found in rapidity of disease onset and level of lethality. The first animal models described lacked signaling either to all the three types of interferons (STAT-1−/−)75 or type 1 interferon (IFNAR−/−).76 Besides the knockout mice, a mouse model whose type 1 interferon system is temporarily suppressed IS has been described.77 The knockout models and the (IS) model are permissive to infection, succumbing to CCHFV infection within 5 days. STAT-1 knockout mice present with leukopenia, thrombocytopenia, elevated levels of serum hepatic alanine aminotransferase, and proinflammatory cytokines.75 CCHFV RNA is widespread in tissues of STAT-1−/− and IFNAR−/− mouse models.75,76 Additionally, a humanized mouse model was prepared by injecting NSG-SGM3 mice with CD34+ human stem cells.78 The humanized model displayed different disease patterns when inoculated with CCHF strain from Oman and Turkey. Lethal outcomes and neurological disease were only observed with the Turkish strain.78 A nonhuman primate model was described in 2018. Cynomolgus macaque infected with the Kosova Hoti CCHFV strain developed disease patterns and outcomes characteristic of CCHF human cases.79 In an independent comparative investigation in Cynomolgus macaque, animals infected with the CCHFV Afghanistan strain (Afg09-2990) and the Kosova Hoti CCHFV strain developed a clinical picture, laboratory clinical chemistry, and hematological parameters, as well as serum cytokine levels commonly seen in humans.80 However, all the 12 animals recovered, unlike the infection studies involving the Kosova Hoti strain, in which only 25% (1/4) of the animals did not meet euthanasia criteria. The nonhuman primate model has provided evidence regarding the ability of CCHFV to replicate and persist in the testes of monkeys, opening the possibility of sexual transmission.81
Vaccines Against CCHF
Bulgarian vaccine
Even though there is not a globally recognized vaccine for CCHFV, there is a vaccine that has been in use in Bulgaria since 1974. The Bulgarian vaccine originated in the Union of Soviet Socialist Republics (USSR). This is an inactivated vaccine prepared from brain tissue of CCHFV infected newborn mice. Inactivation of the CCHFV particles was brought about by a combination of chloroform and heat treatment. The vaccine is administered subcutaneously multiple times in risk groups who are more than 16 years old. Between 1974 and 1996, there was a precipitous drop in cases (from 1105 to 279) reported to the Bulgarian Ministry of Health and fewer than 20 cases reported per annum after 1996.82 Remarkably, there were no reported cases from military and laboratory personnel who were vaccinated.82 Nevertheless, the decrease in reported Bulgarian cases could have been independent of vaccine efficacy but a product of other factors. The observed reduction could have been attributed to a change in CCHFV epidemiology and ecology in the absence of deliberate intervention.83 Increased CCHFV awareness could have led to behavior change thus reduced tick exposure or a different case definition and reporting following the introduction of vaccine.83
Although the mouse brain-derived vaccine has been in use in Bulgaria, the vaccine is not a viable option for widespread global use largely due to safety concerns and lack of efficacy trials. Propagating CCHFV in brain tissue of newborn mice requires biosafety level 4 (BSL-4) facilities. International approval of this vaccine is unlikely because of safety concerns surrounding the mouse neural tissue content, which has potential to cause autoimmune and allergic responses84 and the requirement for high containment facilities for propagation. Furthermore, the Bulgarian vaccine requires multiple immunizations with vaccinations every 5 years to preserve immunity. Individuals below the age of 16 do not qualify to receive the vaccine and leaves a fraction of the population without immunity to the virus. Apart from that, the efficacy of the Bulgarian vaccine is yet to be demonstrated in clinical trials.
CCHFV Protein Targets for Vaccine Development
Recent, CCHF vaccine development has focused on the viral glycoproteins and the nucleoprotein (NP) even though the immune correlates of protection are yet to be described. The investigated CCHFV proteins are recombinant proteins produced either in vitro or in vivo. In vitro proteins were produced in cell cultures, purified, and vaccinated into animal models while in vivo protein production utilized vectors, which were used to deliver genes encoding CCHFV antigens facilitating endogenous protein production. In situ antigen generation is desirable since proteins acquire post-translational modifications similar to natural infections.85
Nucleoprotein
The role of the CCHFV NP in protection against infection and clearance of viruses is not known. However, the NP possesses features which make the antigen ideal for CCHFV vaccine development. The protein is produced in large amounts during infection and is highly immunogenic containing B and T cell epitopes.86,87 Besides that, the NP amino acid sequence shows the least variation88 thus, an NP-based vaccine is expected to offer protection against the diverse CCHFV strains. Recently, complete protection in knockout mice against CCHFV challenge infection has been reported after vaccination with NP-based vaccines using different expression systems.89–90 Even though the NP is an internal protein and is not expected to induce neutralizing antibodies, the NP is released from infected cells91 thus can interact with antibodies forming immune complexes capable of antiviral activity.92
Glycoproteins
The CCHFV M segment encodes a polyprotein glycoprotein precursor (GPC), which is post-translationally processed to intermediate glycoproteins (pre-Gn and pre-Gc). Further processing of the intermediary glycoproteins yields envelope glycoproteins Gn and Gc, nonstructural M protein (NSM) as well as secreted nonstructural proteins (GP160, GP85, and GP38) and mucin-like domain.93–95 The secreted GP38 has been demonstrated to localize to viral and cellular membranes of cells expressing the M segment.96 The glycoproteins Gn and Gc have been largely considered as the antigen of choice for the CCHFV vaccine chiefly because they are located on the surface of virus particles and hence considered responsible for inducing neutralizing antibodies. To this end, monoclonal neutralizing antibodies against the pre-Gn and Gc glycoproteins were described.97 Recently all the neutralizing antibodies previously reported to target the pre-Gn interacted with the GP38 and none interacted with the Gn.96 mAb-13G8, a GP38 specific non-neutralizing monoclonal antibody protected IFNAR−/− mice against lethal infection, whereas Gc-specific neutralizing antibodies could not offer protection96 despite demonstrating in vitro virus neutralization.97 Passive administration of mAb-13G8 (homologous to the IbAr 10200 CCHFV strain) in IFNAR−/− mice displayed limited protection against a heterologous CCHFV Afg09-2990 strain.96 The diversity of the M segment, especially the region encoding the nonstructural proteins, has long been suspected to impact crossreactivity and ultimately, the neutralization ability against heterologous strains. One factor contributing to the diversity of the M segment is genetic reassortment. The consequence of reassortment on viral aspects, such as replication, transmission, virulence, and immunogenicity is yet to be fully investigated.
CCHF vaccine candidates
The search for a CCHF vaccine has accelerated in recent years, and this has been partly attributed to the discovery of animal models for CCHF. Before the recognition of the animal models, there have been few attempts described in literature and vaccine efficacy studies were not performed. Investigated CCHF vaccine approaches include subunit antigen preparations, genetically modified plants, as well as DNA and viral vectors expressing CCHFV antigens, transcriptionally competent virus-like particles (VLPs), messenger RNA (mRNA) vaccine, and inactivated whole CCHFV particles.98–105 A summary of the investigated CCHFV vaccine approaches and outcomes is outlined below.
Subunit vaccines
Using insect expression technology, the ectodomains of the CCHFV envelope glycoproteins Gn and Gc from the IbAr 10200 strain were expressed in Drosophila Schneider 2 (S2) cells and purified. The Gn and Gc proteins formulated with the Sigma Adjuvant system separately elicited antibodies with neutralizing capacity in vitro after intraperitoneal vaccination in STAT129 mice using a prime and boost strategy (Table 1). However, the elicited in vitro neutralizing antibodies could not offer protection following subcutaneous CCHFV challenge infection.98
Table 1.
Approaches in the Development of CCHFV Vaccines
| Vaccine type | CCHFV antigena | Mouse model | Doseb | Antibody response | T cell response | Challengec | Efficacy, % survival | Reference |
|---|---|---|---|---|---|---|---|---|
| Recombinant protein | Gc-e ectodomain (adjuvanted) Gn ectodomain (adjuvanted) Gc-eΔ ectodomain (adjuvanted) |
STAT-1−/− STAT-1−/− STAT-1−/− |
1.4 μg (i.p.) day 0 and 21 15 μg (i.p.) day 0 and 21 7.5 μg (i.p.) day 0 and 21 |
Yesd Yesd Yesd |
NT NT NT |
100 pfu CCHFV IbAr 10200 strain (s.c.) day 42 100 pfu CCHFV IbAr 10200 strain (s.c.) day 42 100 pfu CCHFV IbAr 10200 strain (s.c.) day 42 |
0% 0% 0% |
98 98 98 |
| Transgenic plants | Gn and Gc (Iranian strain) | BALB/c BALB/c BALB/c BALB/c BALB/c |
Fed leaves 10 μg 5 × (1-week intervals) Fed roots 10 μg 5 × (1-week intervals) Fed leaves 10 μg 4 × (1-week intervals) and injected 5 μg Gn/Gc Fed roots 10 μg 4 × (1-week intervals) and injected 5 μg Gn/Gc Bulgarian vaccine, injected four doses at 2-week intervals (s.c.) |
Yese Yese Yese Yese Yese |
NT NT NT NT NT |
NT NT NT NT NT |
NT NT NT NT NT |
99 99 99 99 99 |
| Virus-like replicon particles | GPC, L, and NP (IbAr 10200 L, NP, and Oman-1998 GPC ) GPC, L, and NP (IbAr 10200 L, NP, and Oman-1998 GPC) GPC, L, and NP (IbAr 10200 L, NP, and Oman-1998 GPC) Gn, Gc, and NP |
IFNAR−/− IFNAR−/− IFNAR−/− IFNAR−/− IFNAR−/− |
High dose (105 TCID50) (s.c.)f Low dose (103 TCID50) (s.c.)f 105 TCID50 (s.c.)f 105 TCID50 (s.c.)f 106 VLPs/mouse (i.p.) day 0, 28, and 49 |
Yese Yese Yese Yese Yesd |
NT NT NT NT Yes |
100 TCID50 recombinant CCHFV-IbAr 10200 (s.c.) day 32 100 TCID50 recombinant CCHFV-IbAr 10200 (s.c.) day 32 100 TCID50 CCHFV Oman-199723179 (s.c.) day 28 100 TCID50 CCHFV Turkey-200406546 (s.c.) day 28 400 FFU CCHFV strain IbAr 10200 (i.p.) day 91 |
100% 78% 100% 100% 40% |
109 109 110 110 100 |
| DNA | GPC Gn, Gc, and NP GPC GPC NP (Ank-2 strain) NP (Ank-2 strain) NP (Ank-2 strain) |
BALB/c BALB/c IFNAR−/− IFNAR−/− IS C57BL/6 BALB/c and IFNAR−/− BALB/c and IFNAR−/− BALB/c and IFNAR−/− |
10 μg (g.g.) day 0, 28, 56, and 84g 2.5 μg (g.g.) day 0, 28, 56, and 84h 50 μg (i.d.) day 0, 28, and 49 25 μg (i.m.) day 0, 21, and 42 25 μg (i.m.) day 0, 21, and 42 50 μg (i.m.) day 0 and 14 pV-N13 (50 μg) (i.m.) day 0 and 14 pV-N13 (40 μg) + pCD24 (10 μg) (i.m.) day 0 and 14 |
Yesd Yesd Yesd Yesd Yesd Yesi Yesi Yesi |
NT NT Yes NT NT Yes Yes Yes |
NT NT 400 FFU CCHFV strain IbAr 10200 (i.p.) day 91 100 pfu CCHFV strain IbAr 10200 (i.p.) day 70 100 pfu CCHFV strain IbAr 10200 (i.p.) day 70 1000 TCID50 of Ank-2 strain (i.p.) day 28 1000 TCID50 of Ank-2 strain (i.p.) day 28 1000 TCID50 of Ank-2 strain (i.p.) day 28 |
NT NT 100% 71% 60% 75% *50% 100% 100% |
113 113 100 77 77 90 89 89 |
| mRNA | NP | IFNα/βR−/− |
25 μg (i.m.)f 25 μg (i.m.) day 0, day 14 |
Yesi Yesi |
Yes Yes |
1000 TCID50 of Ank-2 strain (i.p.) day 42 1000 TCID50 of Ank-2 strain (i.p.) day 56 |
50% 100% |
104 104 |
| MVA vector Recombinant adenovirus type 5 Recombinant vesicular stomatitis virus Recombinant BoHV-4 Recombinant adenovirus type 5 Formalin inactivated vaccine |
GPC GPC NP NP NP (3010 strain) NP (3010 strain) NP NP GPC GPC NP (Ank-2 strain) NP (Ank-2 strain) Whole virus particle (Turkey-Kelkit06 strain) |
IFNα/βR−/− 129Sv/Ev IFNα/βR−/− 129Sv/Ev IFNα/βR-/ 129Sv/Ev IFNAR−/− IFNAR−/− STAT-1−/− STAT-1−/− BALB/c and IFNAR−/− BALB/c and IFNAR−/− IFNAR−/− IFNAR−/− IFNAR−/− |
107 pfu MVA-GP (i.m.) day 0 and 14 107 pfu MVA-GP (i.m.) day 0 and 14 107 pfu MVA-NP10200 (i.m.) day 0 and 14 107 pfu MVA-NP10200 (i.m.) day 0 and 14 107 pfu MVA-NP3010 (i.m.) day 0 and 14 107 pfu MVA-NP3010 (i.m.) day 0 and 14 1.25 × 107 ifu (i.m.)f 1.25 × 107 IFU (i.m.) day 0 and 108 IFU (i.n.) day 28 107 pfu (i.p.)f 107 pfu (i.p.) day 0 and 14 100 TCID50 (i.p.) day 0 and 14 100 TCID50 (i.p.) day 0 and 14 5 μg (i.p.) day 0, 21 and 42 20 μg (i.p.) day 0, 21 and 42 40 μg (i.p.) day 0, 21 and 42 |
Yese Yese Yese Yese Yese Yese NT Yese Yesd Yesd Yesi Yesi Yesd Yesd Yesd |
Yes Yes Yes Yes Yes Yes NT NT NT NT Yes Yes NT NT NT |
200 TCID50 CCHFV virus strain IbAr 10200 (i.d.) day 28 Not challenged 200 TCID50 CCHF virus strain IbAr 10200 (i.d.) day 28 Not challenged Not challenged Not challenged 50 TCID50 CCHFV strain IbAr 10200 (s.c.) day 28 50 TCID50 CCHFV strain IbAr 10200 (s.c.) day 56 50 pfu of CCHFV strain Turkey200406546 (i.p.) day 35 50 pfu of CCHFV strain Turkey200406546 (i.p.) day 35 1000 TCID50 of Ank-2 strain (i.p.) day 28 1000 TCID50 of Ank-2 strain (i.p.) day 28 1000 PPFU Turkey-Kelkit06 strain (i.p.) day 56 1000 PPFU Turkey-Kelkit06 strain (i.p.) day 56 1000 PPFU Turkey-Kelkit06 strain (i.p.) day 56 |
100% N/A 0% N/A N/A N/A 33% 78% 100% 100% 100% 75%j 100% 50%j 60% 80% 80% |
101 101 102 102 102 102 103 103 116 116 90 90 105 105 105 |
All vaccine candidates were based on IbAr 10200 strain unless otherwise stated.
Vaccine dose, timing, and route of inoculation, i.d.; i.p.; i.m.; i.n.; s.c.; g.g.
CCHFV challenge strain, dose, route, and timing.
Neutralizing antibodies in vitro.
Antibody ability to neutralize in vitro not assessed.
Single-dose administered.
Mice immunized with 10 μg of CCHF DNA vaccine.
Mice immunized with 2.5 μg of each of Rift Valley fever virus, CCHF, Hantaan virus and tick-borne encephalitis virus DNA vaccine.
Non-neutralizing antibodies in vitro.
Antibody passive and T cell adoptive transfer experiment.
BoHV-4, bovine herpesvirus type 4; Crimean–Congo hemorrhagic fever; CCHFV, Crimean–Congo hemorrhagic fever virus; ffu, focus-forming units; g.g., gene gun; GPC, glycoprotein precursor; i.d., intradermal; ifu, infectious units; i.m., intramuscular; i.n., intranasal; i.p., intraperitoneal; IFU, infectious units; IS, transiently suppressed type 1 interferon system; L, RNA-dependent RNA polymerase; mRNA, messenger RNA; MVA, modified Vaccinia Ankara virus; NP, nucleoprotein; NT, not tested; pfu, plaque-forming unit; PPFU, pseudo plaque-forming unit; s.c., subcutaneous.
Plant-based vaccines
Genetically engineered plants can express foreign antigen for vaccine development purposes. Approaches for foreign gene expression in transgenic plants include stable transgenic plants, use of viral vectors for transient expression, and the chloroplast expression system.106,107 The nonrequirement of the cold chain for the recombinant proteins and production of abundant biologically active proteins relatively inexpensively makes plant-based vaccines appealing especially for developing countries.
Vertebrate animals, particularly domestic animals, are significant in CCHFV transmission cycle. Reducing viral amplification in vertebrates could decrease CCHFV transmission to humans. Genetically modified tobacco plants expressing the envelope glycoproteins Gn and Gc from an Iranian strain were fed to BALB/c mice (Table 1). The Gn and Gc glycoproteins were genetically engineered to form one reading frame. Immunized mice elicited CCHFV-specific anti-Gn/Gc IgG and IgA antibodies in serum and fecal material. End boost groups induced higher endpoint antibody titers (1:32768) compared with the fed groups (1:256). Interestingly, fecal pellets had higher IgA endpoint titer (1:512) compared with serum (1:256).99 The neutralizing potential of the antibodies was not assessed, and challenge studies were not performed.
Virus-like replicon particles
Using reverse genetics, a transcriptionally active virus-like particle (tc-VLP) system, has been developed.108 Structurally, the VLPs consists of a genome like a reporter encapsidated by the CCHFV NP and L protein enclosed in a membrane displaying Gn and Gc proteins on the surface. Thus, VLPs have morphology and protein antigenicity resembling native CCHF viruses. Vaccinating three times intraperitoneally with the tc-VLP displaying the envelope glycoproteins (Gn and Gc) from the CCHFV IbAr 10200 strain on their surface was accompanied by a strong induction of in vitro neutralizing antibodies in an IFNAR−/− mice model, which protected 40% of the challenged mice100 (Table 1). Cytokine analysis before challenge infection demonstrated induction of Th2-type immunity, whereas postchallenge cytokine analysis could not be performed.100
A virus-like replicon particle (VRP) vaccine candidate based on IbAr 10200 strain but with the GPC sequence from the Oman-1998 strain provided complete protection against lethal challenge following a single high dose (105 TCID50 of VRP) subcutaneous vaccination in IFNAR−/− mouse model.109 In contrast, a low dose (103 TCID50 of VRP) vaccination protected seven out of nine mice (Table 1). In a related study, the VRP candidate vaccine provided complete protection against challenge with each of the CCHFV IbAr 10200 strain, the CCHFV-Turkey strain, and the CCHFV-Oman-97 strain110 (Table 1).
DNA vaccines
DNA vaccines do provide an attractive alternative for emerging and re-emerging pathogens such as CCHFV. DNA vaccines are temperature stable and can be designed to incorporate specific immunogenic viral proteins desired for immunization. Depending on the immune correlates of protection, genetic vaccines can be tailored to raise either type 1 T-helper (Th) or type 2 Th cell responses.111 DNA immunization also allows for the differentiation between natural infections and vaccine-induced responses since specific antigens are selected. The mechanisms of inducing cytotoxic T lymphocyte-mediated adaptive immunity are similar for DNA vaccines and live attenuated vaccines, but the risk of reverting to virulence associated with the latter is eliminated in DNA vaccines.112 A DNA-based vector designed to deliver the GPC of the IbAr 10200 CCHFV strain was first described in 2006. The CCHFV vaccine construct was either delivered three times individually or coadministered with DNA vectored vaccine constructs for Hantaan and Rift Valley fever viruses encoding the GPC and tick-borne encephalitis virus encoding the premembrane and envelope genes (Table 1). Fifty percent of the vaccinated BALB/c mice either with the CCHFV DNA vaccine or combined with other vaccine constructs developed in vitro neutralizing antibodies.113 Induction of cell-mediated immune responses was not evaluated and challenge studies were not performed. Intradermal immunization (three times) of a DNA vector encoding the mature CCHFV envelope glycoproteins (Gn and Gc) and the NP of the IbAr 10200 strain, elicited antibody and T cell immune responses, which protected IFNAR−/− mice against lethal CCHFV challenge100 (Table 1). Curiously, mice which received a VLP construct in the same study presented with higher in vitro neutralizing antibodies compared with the CCHFV DNA vaccine but protection was partial. These findings imply that neutralizing antibodies are not the sole correlate of CCHFV protection.
Vaccine immunogenicity and efficacy studies in IFNAR−/− and interferon receptor antibody transiently suppressed (IS) mouse models were compared after intramuscular electroporation of a DNA expression vector encoding the entire GPC of the IbAr 10200 CCHFV strain77 (Table 1). Intraperitoneal (i.p.) route for the challenge was chosen based on previous observations that the i.p. route displayed the most rapid disease onset compared with the intramuscular, intranasal, and subcutaneous routes.114 Antibody responses in the two mouse models reflected a predominant Th1 response and the IS mouse model had a significantly lower Th1/Th2 ratio indicating balanced antibody responses with the immunocompetent mice.77 Although a higher survival rate after the lethal challenge was observed in the IFNAR−/− model, with 71.4% (5 out 7 animals) compared with the IS model, with 60% (6 out 10 animals), this was not statistically different. Significantly, complete protection was not achieved in both mouse models.77
Vaccinating IFNAR−/− mice twice with a DNA vector encoding the complete NP of the CCHFV Turkey-Kelkit06 or codelivery of the DNA vectors encoding the complete NP of the CCHFV Turkey-Kelkit06 and the cluster differentiation 24 (CD24), protected animals from lethal challenge infection89 (Table 1). Codelivery of CD24 and NP significantly enhanced induction of Th1 and Th2 cytokines as well as antibody responses, although the elicited antibodies lacked neutralization ability. Intramuscular administration of a DNA vector encoding the complete NP gene of the CCHFV Turkey-Kelkit06 produced 75% protection from lethal challenge infection, whereas a 50% survival rate was observed in the antibody passive transfer and T cell adoptive transfer experiment90 (Table 1). Despite the induction of high antibody titers and protective efficacy in animal models, elicited NP-specific antibodies in both studies could not neutralize CCHFV in vitro. Absence of neutralizing antibodies should not diminish the NP as a potential vaccine candidate because non-neutralizing antibodies can be protective by promoting phagocytosis, complement, or antibody-dependent cellular cytotoxicity.115
mRNA vaccine
A naked conventional CCHF mRNA vaccine has been described. The vaccine expresses the NP of the CCHF Ank-2 strain flanked by a 5′ cap (antireverse cap analog), 3′-polyA tail, 3′ and 5′ untranslated regions to enhance stability and translation of the construct. Intramuscular vaccination of IFNAR−/− using a prime boost approach provided 100% protection following viral challenge while a single dose of the vaccine construct conferred 50% protection104 (Table 1).
Viral vectored vaccines
Recombinant viruses have been extensively investigated as vectors in gene therapy and gene delivery for vaccine development. Viral expression systems explored for CCHF vaccine development comprise the modified Vaccinia Ankara virus (MVA),101,102 recombinant vesicular stomatitis virus (rVSV),116 recombinant adenovirus type 5 (AdV-5),90,103 and recombinant bovine herpesvirus type 4 (BoHV-4).90 The MVA platform was used to deliver the GPC and the NP of the IbAr 10200 CCHFV strain. The N-termini of the GPC and NP were fused to the human tissue plasminogen activator leader sequence, whereas a V5 epitope tag was fused to the C-termini. An mH5 promoter was selected to drive gene transcription. The MVA-based vaccine constructs were administered two times intramuscularly (Table 1) and the construct designed to encode the GPC-induced in vitro neutralizing antibodies and T cell responses and complete protection in IFNAR−/− mice101 after intradermal lethal challenge. Although the MVA-delivered NP induced humoral and cellular immune responses in vaccinated mice, the immune responses could not protect animals from lethal challenge infection.102 A replication-competent recombinant VSV encoding the CCHFV GPC gene of the IbAr 10200 CCHFV strain yielded 100% protection in a STAT-1−/− mouse model following single intraperitoneal immunization, while a replication-deficient VSV construct did not confer protection from intraperitoneally administered lethal virus challenge116 (Table 1). The replication-competent construct developed nonsynonymous single nucleotide polymorphisms (SNPs), and two of these SNPs were nonsense mutations, which resulted in the truncation of part of the C-terminal tail of the Gc protein. Interestingly, although 100% protection was observed with the prime and boost group, the prime group elicited higher IgG and in vitro neutralizing antibody titers compared with the boost group at the endpoint. The clinical data and immunohistochemistry analysis of the spleen and liver of study animals suggested higher CCHFV replication in the prime group compared with the boost group thus viral challenge may have served as a heterologous booster for the prime group.116 A CCHFV NP-based candidate vaccine based on the human adenovirus 5 encoding the NP of the CCHFV strain IbAr 10200 partially protected IFNAR−/− mice against virus challenge103 (Table 1). A prime-boost strategy improved protection and resulted in reduced clinical signs compared with single-dose vaccination. IFNAR−/− mice immunized intraperitoneally with a recombinant AdV-5 encoding the NP from the Turkey-Kelkit06 strain survived challenge with CCHFV Ank-2 strain, and half of the mice survived a lethal challenge in the antibody passive transfer and T cell adoptive transfer experiment90 (Table 1).
In the face of safety challenges and antivector immunity posed by commonly used viral expression platforms, there is a need to explore new platforms. BoHV-4 possesses features such as a less complex genome compared with other herpesviruses coupled by large package size, easy growth in cell culture, limited or no pathogenicity or oncogenicity,117 and availability of an animal model (rabbit),118 which makes it a good candidate. A BoHV-4 vector encoding the full-length NP of the CCHFV Turkey-Kelkit06 strain utilizing a prime and boost strategy provided complete protection of IFNAR−/− mice against lethal challenge infection, and partial protection was observed in the antibody passive transfer and T cell adoptive transfer experiment90 (Table 1).
Inactivated vaccines
The CCHFV Turkey-Kelkit06 strain was propagated in cell culture, harvested, and inactivated by formaldehyde to prepare an inactivated vaccine. The vaccine was mixed with the Imject Alum adjuvant and delivered intraperitoneally using a prime, boost, and boost strategy105 in IFNAR−/− mice (Table 1). Vaccine doses of 5, 20, and 40 μg were investigated. In immunogenicity studies, the 5 μg dose group induced the lowest levels of in vitro neutralizing antibody titers and the increase in antibody titer was dose dependent. Despite differences in the levels of neutralizing antibodies, similar survival rate (80%) was observed with the 20 and 40 μg dose groups in IFNAR−/− mice following lethal challenge infection. The effect of the vaccine on the T cell immune response was not evaluated.
Future Directions and Concluding Remarks
Interferon-deficient mice and the Cynomolgus macaque CCHF animal models have allowed significant advancements in vaccine development.75–80 The impact of type I and/or type II interferon deficiency on CCHF vaccine-induced adaptive immune responses, however, deserves further evaluation. The disease spectrum in the Cynomolgus macaque depicts disease states seen in humans.79 Despite issues around variability in observed disease outcomes,81 the cost, and size of the animals, this immunocompetent animal model will be valuable in the development of CCHFV therapies. The addition of a humanized mouse model, which previously exhibited strain-specific virulence by producing different disease outcomes by a CCHFV Turkish and an Oman strain78 would be a valuable addition to evaluate the interplay between pathogenicity and immunogenicity.
Currently, immune responses conferring protection following CCHFV infection have not yet been described. CCHFV-neutralizing antibodies are likely produced against the Gn and Gc glycoproteins, which bind target cells,97 thus vaccine attempts focused on the M segment. Studies have demonstrated the absence of correlation between in vitro neutralization and protection in the available mouse models.89,90,98,100 Future studies should probe and delineate factors responsible for the observed differences between in vitro neutralization and protective efficacy. CCHFV is genetically diverse and the concern is whether a single vaccine can protect against global CCHFV strains. So far, efficacy studies against genetic strains have only been investigated for a VRP vaccine candidate.110 Recently, NP-based vaccine candidates have also resulted in complete protection in knockout mice despite the absence of in vitro neutralizing antibodies.89,90 The NP has thus proved to be an important vaccine target. Since B and T cell epitopes have been mapped on the glycoproteins and NPs, efforts can be directed in developing multiepitope-based vaccines. Epitope-based vaccines would be one way to develop an effective vaccine against the diverse CCHFV strains by selecting multiple antigenic epitopes. The design of epitope-based vaccines allows B or cytotoxic T lymphocyte (CTL) epitopes to be linked together in series with helper T lymphocyte epitopes ensuring CD4 T cells and pathogen-derived molecules are appropriately primed facilitating robust humoral and CTL responses.119 One characteristic of an ideal vaccine is that it should confer long-term sterilizing immunity after single administration. Despite several vaccine strategies providing complete protection of knockout mice after viral challenge (Table 1), protective single-dose regimens have been achieved by a rVSV-based vaccine expressing the GPC116 and a VRP vaccine,109,110 whereas the rest were administered using a prime/boost approach. Additionally, mice administered with the VRP vaccine did not develop clinical disease signs and CCHFV RNA was not detected in tissues at study end point. While complete protection has been achieved using a single-dose regimen, none of the available candidate vaccines has been evaluated for their ability to induce long-term immunity. Immune responses against either the NP or the GPC have been protective in efficacy studies necessitating the investigation of the role of both proteins in vaccine development. A thorough dissection of immune responses generated by the NP and GPC whether singly or in combination can help unmask the immune correlates of protection.
The use of genetic adjuvants to enhance immune responses in CCHF vaccine development has been sparsely investigated. Plasmid-expressing cytokines such as interferon-γ, interleukin (IL)-2, IL-12, Granulocyte/macrophage colony-stimulating factor,120–122 chemokines MIP-1α and RANTES,123,124 and ICAM-1, CD40L, and CD80/86 costimulatory molecules,125–127 have been investigated as genetic adjuvants in vivo with promising results in different settings. In the sole CCHF vaccine study using genetic adjuvants described in the literature, the CD24 costimulatory molecule was codelivered with the CCHFV NP. CD24 enhanced antibody and cytokine responses, although this was not translated to protective efficacy studies.89
The prototype IbAr 10200 CCHFV strain has mostly been used in vaccine studies. This prototype CCHFV strain was discovered in a tick128 and its virulence in humans is unknown. The NP from IbAr 10200 and AP92 strains did not antagonize interferon response in vitro as did the Hoti strain.129 In a study by Zivcec and colleagues,130 VLP-bearing glycoproteins from the IbAr 10200 CCHF strain displayed the reduced capacity to enter monocyte-derived macrophages. The effect in stimulating immune responses although remains to be elucidated. Considering the diversity of the CCHFV glycoproteins, it will be interesting to evaluate if differences in amino acid sequences between various global CCHFV strains does affect immunogenicity.
The utility of a BoHV-4 viral vector in comparison to an AdV-5 and a DNA vector in NP-based vaccine development was evaluated.90 BoHV-4 persists in monocytes and macrophages. Persistence in white blood cells could eliminate the need for booster doses for antigens delivered by BoHV-4. Besides that, delivering antigens by the BoHV-4 vector can enhance antigen presentation since the virus persists in monocytes and macrophages, which are antigen-presenting cells.90 Even though similar protection rates in knockout mice were obtained with vaccine constructs delivered with the BoHV-4 and AdV-5 vectors, the advantages offered by the BoHV-4 vector needs to be further explored in detail. The role played by the various expression systems in shaping CCHFV immune responses in animal models warrant investigation.
Since animals, particularly livestock, play an important role in CCHFV transmission cycle, the ability of vaccines to prevent viremia in livestock could reduce the rate of transmission to humans. CCHF human vaccine development has accelerated in recent years, and some vaccine studies have reported promising results in animal models. Whether these results can be translated to human clinical trials remains to be seen. Vaccine design and efficacy can be further enhanced by the delineation of correlates of CCHF protection which up to now have remained an enigma.
Acknowledgment
The authors thank Mr. P.A. Bester for his assistance in drawing Figure 1.
Abbreviations Used
- AdV-5
adenovirus type 5
- BoHV-4
bovine herpesvirus type 4
- BSL-4
biosafety level 4
- CCHF
Crimean–Congo hemorrhagic fever
- CCHFV
Crimean–Congo hemorrhagic fever virus
- CD24
cluster differentiation 24
- CHF
Crimean hemorrhagic fever
- CTL
cytotoxic T lymphocyte
- g.g.
gene gun
- GPC
glycoprotein precursor
- i.d.
intradermal
- i.m.
intramuscular
- i.n.
intranasal
- IP
intraperitoneal
- IS
transiently suppressed type 1 interferon system
- mRNA
messenger RNA
- MVA
modified Vaccinia Ankara virus
- NP
nucleoprotein
- rVSV
recombinant vesicular stomatitis virus
- s.c.
subcutaneous
- SNPs
single nucleotide polymorphisms
- Th
T-helper
- USSR
Union of Soviet Socialist Republics
- VLP
virus-like particle
- VRP
virus-like replicon particle
Authors Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Research Foundation South African Research Chairs Initiative (SARChI), Vector-borne and zoonotic pathogen research. Grant number: U 98346.
Cite this article as: Tipih T, Burt FJ (2020) Crimean-Congo hemorrhagic fever virus: advances in vaccine development, BioResearch Open Access 9:1, 137–150, DOI: 10.1089/biores.2019.0057.
References
- 1. Hoogstraal H. The epidemiology of tick-borne Crimean-Congo heamorrhagic fever virus in Asia, Europe and Africa. J Med Entomol. 1979;15:307–417 [DOI] [PubMed] [Google Scholar]
- 2. Chumakov MP. On 30 years of investigation of Crimean hemorrhagic fever. Tr. Inst. Polio Virusn. Entsefalitov Akad. Med. Nauk SSSR 22, 5–18 (in Russian; in English, NAMRU3-T950). In: Whitehouse CA. Crimean-Congo hemorrhagic fever. Antivir Res. 2004:145–16015550268 [Google Scholar]
- 3. Butenko AM, Chumakov MP, Rubin VN, et al. Isolation and investigation of Astrakhan strain (“Drozdov”) of Crimean hemorrhagic fever virus and data on serodiagnosis of this infection. Mater. 15 Nauchn. Sess. Inst. Polio Virus Entsefalitov (Moscow) 3, 88–90 (in Russian; in English, NAMRU3-T866). In: Whitehouse CA. Crimean-Congo hemorrhagic fever. Antivir Res. 2004:145–16015550268 [Google Scholar]
- 4. Chumakov MP, Butenko AM, Shalunova NV, et al. New data on the virus causing Crimean hemorrhagic fever (CHF). Vopr. Virusol. 13, 377 (in Russian; in English, NAMRU3-T596). In: Whitehouse CA. Crimean-Congo hemorrhagic fever. Antivir Res. 2004:145–16015550268 [Google Scholar]
- 5. Simpson DI, Knight EM, Courtois G, et al. Congo virus: a hitherto undescribed occurring In Africa. I. Human isolations—clinical notes. East Afr Med J. 1967;44:86–92 [PubMed] [Google Scholar]
- 6. World Health Organization. Crimean-Congo haemorrhagic fever. 2013. Available at: https://www.who.int/news-room/fact-sheets/detail/crimean-congo-haemorrhagic-fever (accessed November3, 2019)
- 7. Watts DM, Ksiazek TG, Linthicum KJ, et al. Crimean-Congo hemorrhagic fever. In: The Arboviruses: Epidemiology and Ecology, volume 2. Monath TP. (ed.). CRC Press: Boca Raton, FL; pp. 177–260; 1988 [Google Scholar]
- 8. Georges AJ, Gonzalez JP. Could Crimea-Congo haemorrhagic fever be a biohazard in the Central African Republic? Trans R Soc Trop Med Hyg. 1986;80:994–995 [DOI] [PubMed] [Google Scholar]
- 9. El-Bahnasawy MM, Sabah AA, Saleh HA, et al. The tick-borne Crimean-Congo hemorrhagic fever in Africa, Asia, Europe, and America: what about Egypt? J Egypt Soc Parasitol. 2012;42:373–384 [DOI] [PubMed] [Google Scholar]
- 10. Dunster L, Dunster M, Ofula V, et al. First documentation of human Crimean-Congo hemorrhagic fever, Kenya. Emerg Infect Dis. 2002;8:1005–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saluzzo JF, Aubry P, McCormick J, et al. Haemorrhagic fever caused by Crimean Congo haemorrhagic fever virus in Mauritania. Trans R Soc Trop Med Hyg. 1985;79:268. [DOI] [PubMed] [Google Scholar]
- 12. Bukbuk DN, Dowall SD, Lewandowski K, et al. Serological and virological evidence of Crimean-Congo haemorrhagic fever virus circulation in the human population of Borno State, Northeastern Nigeria. PLoS Negl Trop Dis. 2016;10:e0005126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gear JH, Thomson PD, Hopp M, et al. Congo-Crimean haemorrhagic fever in South Africa. Report of a fatal case in the Transvaal. S Afr Med J. 1982;62:576–580 [PubMed] [Google Scholar]
- 14. Nabeth P, Thior M, Faye O, et al. Human Crimean-Congo hemorrhagic fever, Sénégal. Emerg Infect Dis. 2004;10:1881–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aradaib IE, Erickson BR, Mustafa ME, et al. Nosocomial outbreak of Crimean-Congo hemorrhagic fever, Sudan. Emerg Infect Dis. 2010;16:837–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Simpson DI, Knight EM, Courtois G, et al. Congo Virus: a hitherto undescribed occurring in Africa. I. Human isolations—clinical notes. East Afr Med J. 1967;44:86–92 [PubMed] [Google Scholar]
- 17. Kautman M, Tiar G, Papa A, et al. AP92-like Crimean-Congo hemorrhagic fever virus in Hyalomma aegyptium Ticks, Algeria. Emerg Infect Dis. 2016;22:354–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson ML, Gonzalez J-P, LeGuenno B, et al. Epidemiology of Crimean-Congo hemorrhagic fever in Senegal: temporal and spatial patterns. Arch Virol. 1990;Suppl 1:323–340 [Google Scholar]
- 19. Sadeuh-Mba SA, Yonga Wansi GM, Demanou M, et al. Serological evidence of rift valley fever Phlebovirus and Crimean-Congo hemorrhagic fever orthonairovirus infections among pygmies in the east region of Cameroon. Virol J. 2018;15:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gonzalez JP, Josse R, Johnson ED, et al. Antibody prevalence against haemorrhagic fever viruses in randomized representative Central African populations. Res Virol. 1989;140:319–331 [DOI] [PubMed] [Google Scholar]
- 21. Akuffo R, Brandful JAM, Zayed A, et al. Crimean-Congo hemorrhagic fever virus in livestock ticks a and animal handler seroprevalence at an abattoir in Ghana. BMC Infect Dis. 2016;16:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Butenko AM. Arbovirus circulation in the Republic of Guinea. Med Parazitol (Mosk). 1996:40–45 [PubMed] [Google Scholar]
- 23. Maiga O, Sas MA, Rosenke K, et al. Serosurvey of Crimean–Congo hemrrhagic fever virus in cattle, Mali, West Africa. Am J Trop Med Hyg. 2017;96:1341–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathiot CC, Fontenille D, Georges AJ, et al. Antibodies to haemorrhagic fever viruses in Madagascar populations. Trans R Soc Trop Med Hyg. 1989;83:407–409 [DOI] [PubMed] [Google Scholar]
- 25. Palomar AM, Portillo A, Santibáñez P, et al. Crimean-Congo hemorrhagic fever virus in ticks from migratory birds, Morocco. Emerg Infect Dis. 2013;19:260–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Muianga AF, Watson R, Varghese A, et al. First serological evidence of Crimean-Congo haemorrhagic fever in febrile patients in Mozambique. Int J Infect Dis. 2017;62:119–123 [DOI] [PubMed] [Google Scholar]
- 27. Mariner JC, Morrill J, Ksiazek TG. Antibodies to hemorrhagic fever viruses in domestic livestock in Niger: Rift Valley fever and Crimean-Congo hemorrhagic fever. Am J Trop Med Hyg. 1995;53:217–221 [DOI] [PubMed] [Google Scholar]
- 28. Khan AS, Maupin GO, Rollin PE, et al. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994–1995. Am J Trop Med Hyg. 1997;57:519–525 [DOI] [PubMed] [Google Scholar]
- 29. Wasfi F, Dowall S, Ghabbari T, et al. Sero-epidemiological survey of Crimean-Congo hemorrhagic fever virus in Tunisia. Parasite. 2016;23:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shepherd AJ, Swanepoel R, Shepherd SP, et al. Antibody to Crimean-Congo hemorrhagic fever virus in wild mammals from southern Africa. Am J Trop Med Hyg. 1987;36:133–142 [DOI] [PubMed] [Google Scholar]
- 31. Chinikar S, Mazaheri V, Mirahmadi R, et al. A serological survey in suspected human patients of Crimean-Congo hemorrhagic fever in Iran by determination of IgM-specific ELISA method during 2000–2004. Arch Iranian Med. 2005;8:52 – 55 [Google Scholar]
- 32. Tantawi HH, Al-Moslih MI, Al-Janabi NY, et al. Crimean-Congo haemorrhagic fever virus in Iraq: isolation, identification and electron microscopy. Acta Virol. 1980; 24:464–467 [PubMed] [Google Scholar]
- 33. El Azazy OM, Scrimgeour EM. Crimean-Congo haemorrhagic fever virus infection in the western province of Saudi Arabia. Trans R Soc Trop Med Hyg. 1997;91:275–278 [DOI] [PubMed] [Google Scholar]
- 34. Schwarz TF, Nitschko H, Jäger G, et al. Crimean-Congo haemorrhagic fever in Oman. Lancet. 1995;346:1230. [DOI] [PubMed] [Google Scholar]
- 35. Mohamed Al Dabal L, Rahimi Shahmirzadi MR, Baderldin S, et al. An outbreak of Crimean-Congo hemorrhagic fever in the United Arab Emirates, 1994–1995. Am J Trop Med Hyg. 1997;57:519–525 [DOI] [PubMed] [Google Scholar]
- 36. Papa A, Bino S, Llagami A, et al. Crimean-Congo hemorrhagic fever in Albania, 2001. Eur J Clin Microbiol Infect Dis. 2002;21:603–606 [DOI] [PubMed] [Google Scholar]
- 37. Papa A, Christova I, Papadimitriou E, et al. Crimean-Congo hemorrhagic fever in Bulgaria. Emerg Infect Dis. 2004;10:1465–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Maltezou HC, Papa A, Tsiodras S, et al. Crimean-Congo hemorrhagic fever in Greece: a public health perspective. Int J Infect Dis. 2009;13:713–716 [DOI] [PubMed] [Google Scholar]
- 39. Drosten C, Minnak D, Emmerich P, et al. Crimean-Congo hemorrhagic fever in Kosovo. J Clin Microbiol. 2002;40:1122–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karti SS, Odabasi Z, Korten V, et al. Crimean-Congo hemorrhagic fever in Turkey. Emerg Infect Dis. 2004;10:1379–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zakhashvili K, Tsertsvadze N, Chikviladze T, et al. Crimean-Congo hemorrhagic fever in man, Republic of Georgia, 2009. Emerg Infect Dis. 2010;16:1326–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Onishchenko GG. Infectious diseases in natural reservoirs: epidemic situation and morbidity in the Russian Federation and prophylactic measures. Zh Mikrobiol Epidemiol Immunobiol. 2001;:22–28 [PubMed] [Google Scholar]
- 43. Filipe AR, Calisher CH, Lazuick J. Antibodies to Congo-Crimean haemorrhagic fever, Dhori, Thogoto and Bhanja viruses in southern Portugal. Acta Virol. 1985;29:324–328 [PubMed] [Google Scholar]
- 44. Horvath LB. Precipitating antibodies to Crimean haemorrhagic fever virus in human sera collected in Hungary. Acta Microbiol Acad Sci Hung. 1976;23:331–335 [PubMed] [Google Scholar]
- 45. Ceianu CS, Panculescu-Gatej RI, Coudrier D, et al. First serologic evidence for the circulation of Crimean-Congo hemorrhagic fever virus in Romania. Vector Borne Zoonotic Dis. 2012;12:718–721 [DOI] [PubMed] [Google Scholar]
- 46. Mustafa ML, Ayazi E, Mohareb E, et al. Crimean-Congo hemorrhagic fever, Afghanistan, 2009. Emerg Infect Dis. 2011;17:1940–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Burney MI, Ghafoor A, Saleen M, et al. Nosocomial outbreak of viral hemorrhagic fever caused by Crimean hemorrhagic fever-Congo virus in Pakistan, January 1976. Am J Trop Med Hyg. 1980;29:941–947 [DOI] [PubMed] [Google Scholar]
- 48. Yen YC, Kong LX, Lee L, et al. Characteristics of Crimean-Congo hemorrhagic fever virus (Xinjiang strain) in China. Am J Trop Med Hyg. 1985;34:1179–1182 [PubMed] [Google Scholar]
- 49. Mishra AC, Mehta M, Mourya DT, et al. Crimean-Congo haemorrhagic fever in India. Lancet. 2011;378:372. [DOI] [PubMed] [Google Scholar]
- 50. Leblebicioglu H, Ozaras R, Irmak H, et al. Crimean-Congo hemorrhagic fever in Turkey: current status and future challenges. Antiviral Res. 2016;126:21–34 [DOI] [PubMed] [Google Scholar]
- 51. Rakhmanova NA, Melnik V, Pshenichnaya N, et al. Crimean-Congo hemorrhagic fever (CCHF) cases reported by Russian ProMED. Int J Infect Dis. 2018;73:188 [Google Scholar]
- 52. Papa A, Weber F, Hewson R, et al. Meeting report: first international conference on Crimean-Congo hemorrhagic fever. Antivir Res. 2015;120:57–65 [DOI] [PubMed] [Google Scholar]
- 53. Shahhosseini N, Chinikar S, Shams E, et al. Crimean-Congo hemorrhagic fever cases in the North of Iran have three distinct origins. Virusdisease. 2017;28:50–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yadav PD, Patil DY, Shete AM, et al. Nosocomial infection of CCHF among healthcare workers in Rajasthan, India. BMC Infect Dis. 2016;16:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, et al. Autochthonous Crimean-Congo hemorrhagic fever in Spain. N Engl J Med. 2017;377:154–161 [DOI] [PubMed] [Google Scholar]
- 56. Bente DA, Forrester NL, Watts DM, et al. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir Res. 2013;100:159–189 [DOI] [PubMed] [Google Scholar]
- 57. Estrada-Pena A, Palomar AM, Santibanez P, et al. Crimean-Congo hemorrhagic fever virus in ticks, Southwestern Europe, 2010. Emerg Infect Dis. 2012;18:179–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Spengler JR, Bergeron E, Spiropoulou CF. Crimean-Congo hemorrhagic fever and expansion from endemic regions. Curr Opin Virol. 2019;34:70–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. World Health Organization. Geographic distribution of Crimean-Congo haemorrhagic fever. 2017. Available at: https://www.who.int/emergencies/diseases/crimean-congo-haemorrhagic-fever/Global_CCHFRisk_2017.jpg?ua=1 (accessed November4, 2019)
- 60. Whitehouse CA. Risk groups and control measures for Crimean-Congo hemorrhagic fever In: Crimean-Congo Hemorrhagic Fever. Ergonul O, Whitehouse CA (eds.). Springer: Dordrecht, Netherlands; pp. 273–280; 2007 [Google Scholar]
- 61. Bakir M, Ugurlu M, Dokuzoguz B, et al. Crimean-Congo haemorrhagic fever outbreak in Middle Anatolia: a multicentre study of clinical features and outcome measures. J Med Microbiol. 2005;4:385–389 [DOI] [PubMed] [Google Scholar]
- 62. Leblebicioglu H, Sunbul M, Memish ZA, et al. Consensus report: preventive measures for Crimean-Congo hemorrhagic fever during Eid-al-Adha festival. Int J Infect Dis. 2015;38:9–15 [DOI] [PubMed] [Google Scholar]
- 63. Ergonul O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis. 2006;6:203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mourya DT, Yadav PD, Gurav YK, et al. Crimean Congo hemorrhagic fever serosurvey in humans for identifying high-risk populations and high-risk areas in the endemic state of Gujarat, India. BMC Infect Dis. 2019;19:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vorou R, Pierroutsakos IN, Maltezou HC. Crimean-Congo heamorrhagic fever. Curr Opin Infect Dis. 2007;20:495–500 [DOI] [PubMed] [Google Scholar]
- 66. Whitehouse CA. Crimean-Congo hemorrhagic fever. Antivir Res. 2004;145–160 [DOI] [PubMed] [Google Scholar]
- 67. Kaya S, Elaldi N, Kubar A, et al. Sequential determination of serum viral titers, virus-specific IgG antibodies, and TNF-α, IL-6, IL-10, and IFN-γ levels in patients with Crimean-Congo hemorrhagic fever. BMC Infect Dis. 2014;14:416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bodur H, Akinci E, Ongürü P, et al. Detection of Crimean-Congo hemorrhagic fever virus genome in saliva and urine. Int J Infect Dis. 2010;14:e247–e249 [DOI] [PubMed] [Google Scholar]
- 69. Swanepoel R, Shepherd AJ, Leman PA, et al. Epidemiologic and clinical features of Crimean–Congo hemorrhagic fever in southern Africa. Am J Trop Med Hyg. 1987;36:120–132 [DOI] [PubMed] [Google Scholar]
- 70. Gaidamovich SY, Butenko A M, Leschinskaya HV. Human laboratory acquired arbo-, arena-, and hantavirus infections. Appl Biosaf. 2000;5:5–11 [Google Scholar]
- 71. Weidmann M, Avsic-Zupanc T, Bino S, et al. Biosafety standards for working with Crimean-Congo hemorrhagic fever virus. J Gen Virol. 2016;97:2799–2808 [DOI] [PubMed] [Google Scholar]
- 72. Karimov SK, Kiryushchenko TV, Reformatskaya AF, et al. A case of a laboratory infection with the Crimean hemorrhagic fever virus. Zh Mikrobiol Epidemiol Immunobiol. 1975;5:136–137 [PubMed] [Google Scholar]
- 73. Shepherd AJ, Swanepoel R, Leman PA. Antibody response in Crimean–Congo hemorrhagic fever. Rev Infect Dis. 1989;11:S801–S806 [DOI] [PubMed] [Google Scholar]
- 74. Smirnova SE. A comparative study of the Crimean hemorrhagic fever-Congo group of viruses. Arch Virol. 1979;62:137–143 [DOI] [PubMed] [Google Scholar]
- 75. Bente DA, Alimonti JB, Shieh WJ, et al. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J Virol. 2010;84:11089–11100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bereczky S, Lindegren G, Karlberg H, et al. Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J Gen Virol. 2010;91:1473–1477 [DOI] [PubMed] [Google Scholar]
- 77. Garrison AR, Shoemaker CJ, Golden JW, et al. A DNA vaccine for Crimean-Congo hemorrhagic fever protects against disease and death in two lethal mouse models. PLoS Negl Trop Dis. 2017;11:e0005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Spengler JR, Kelly Keating M, McElroy AK, et al. Crimean-Congo hemorrhagic fever in humanized mice reveals glial cells as primary targets of neurological infection. J Infect Dis. 2017;216:1386–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Haddock E, Feldmann F, Hawman DW, et al. A cynomolgus macaque model for Crimean–Congo haemorrhagic fever. Nat Microbiol. 2018;3:556–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Smith DR, Shoemaker CJ, Zeng X, et al. Persistent Crimean-Congo hemorrhagic fever virus infection in the testes and within granulomas of nonhuman primates with latent tuberculosis. PLOS Pathog. 2019;15:e1008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Garrison AR, Smith DR, Golden JW. Animal models for Crimean-Congo hemorrhagic fever human disease. Viruses. 2019;11:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Christova I, Kovacheva T, Georgieva D, et al. Vaccine against Congo-Crimean haemorhagic fever virus—Bulgarian input in fighting the disease. Probl Infect Parasit Dis. 2010;37:7–8 [Google Scholar]
- 83. Keshtkar-Jahromia M, Kuhn JH, Christova I, et al. Crimean-Congo hemorrhagic fever: current and future prospects of vaccines and therapies. Antivir Res. 2011;90:85–92 [DOI] [PubMed] [Google Scholar]
- 84. Hemachudha T, Griffin DE, Giffels JJ, et al. Myelin basic protein as an encephalitogen in encephalomyelitis and polyneuritis following rabies vaccination. N Engl J Med. 1987;316:369–374 [DOI] [PubMed] [Google Scholar]
- 85. Bins AD, van den Berg JH, Oosterhuis K, et al. Recent advances towards the clinical application of DNA vaccines. Neth J Med. 2013;71:1–9 [PubMed] [Google Scholar]
- 86. Moming A, Tuoken D, Yue X, et al. Mapping of B-cell epitopes on the N- terminal and C-terminal segment of nucleocapsid protein from Crimean-Congo hemorrhagic fever virus. PLoS One. 2018;13:e0204264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Goedhals D, Paweska JT, Burt FJ. Long-lived CD8+ T cell responses following Crimean-Congo haemorrhagic fever virus infection. PLoS Negl Trop Dis. 2017;11:e0006149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hewson R, Chamberlain J, Mioulet V, et al. Crimean-Congo haemorrhagic fever virus: sequence analysis of the small RNA segments from a collection of viruses worldwide. Virus Res. 2004;102:185–189 [DOI] [PubMed] [Google Scholar]
- 89. Aligholipour Farzani T, Hanifehnezhad A, Földes K, et al. Co-delivery effect of CD24 on the Immunogenicity and lethal challenge protection of a DNA vector expressing nucleocapsid protein of Crimean congo hemorrhagic fever virus. Viruses. 2019;11:pii: E75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Aligholipour Farzani T, Földes K, Hanifehnezhad A, et al. Bovine herpesvirus type 4 (BoHV-4) vector delivering nucleocapsid protein of Crimean-congo hemorrhagic fever virus induces comparable protective immunity against lethal challenge in IFNα/β/γR−/− mice models. Viruses. 2019;11:pii: E237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhou ZR, Wang ML, Deng F, et al. Production of CCHF virus-like particle by a baculovirus-insect cell expression system. Virol Sin. 2011;26:338–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. LaMere MW, Lam HT, Moquin A, et al. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol. 2011;186:4331–4339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Altamura LA, Bertolotti-Ciarlet A, Teigler J, et al. Identification of a novel C-terminal cleavage of Crimean-Congo hemorrhagic fever virus PreGN that leads to generation of an NSM protein. J Virol. 2007;81:6632–6642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sanchez AJ, Vincent MJ, Erickson BR, et al. Crimean-congo hemorrhagic fever virus glycoprotein precursor is cleaved by Furin-like and SKI-1 proteases to generate a novel 38-kilodalton glycoprotein. J Virol. 2006;80:514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sanchez AJ, Vincent MJ, Nichol ST. Characterization of the glycoproteins of Crimean-Congo hemorrhagic fever virus. J Virol. 2002;76:7263–7275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Golden JW, Shoemaker CJ, Lindquist ME, et al. GP38-targeting monoclonal antibodies protect adult mice against lethal Crimean-Congo hemorrhagic fever virus infection. Sci Adv. 2019;5:eaaw9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bertolotti-Ciarlet A, Smith J, Strecker K, et al. Cellular localization and antigenic characterization of Crimean-Congo hemorrhagic fever virus glycoproteins. J Virol. 2005;79:6152–6161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kortekaas J, Vloet RP, McAuley AJ, et al. Crimean-Congo hemorrhagic fever virus subunit vaccines induce high levels of neutralizing antibodies but no protection in STAT1 knockout mice. Vector Borne Zoonotic Dis. 2015;15:759–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Ghiasi SM, Salmanian AH, Chinikar S, et al. Mice orally immunized with a transgenic plant expressing the glycoprotein of Crimean-Congo hemorrhagic fever virus. Clin Vaccine Immunol. 2011;18:2031–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hinkula J, Devignot S, Åkerström S, et al. Immunization with DNA plasmids coding for Crimean-Congo hemorrhagic fever virus capsid and envelope proteins and/or virus-like particles induces protection and survival in challenged mice. J Virol. 2017;91:e02076-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Buttigieg KR, Dowall SD, Findlay-Wilson S, et al. A novel vaccine against Crimean-Congo haemorrhagic fever protects 100% of animals against lethal challenge in a mouse model. PLoS One. 2014;9:e91516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Dowall SD, Buttigieg KR, Findlay-Wilson SJ, et al. A Crimean-Congo hemorrhagic fever (CCHF) viral vaccine expressing nucleoprotein is immunogenic but fails to confer protection against lethal disease. Hum Vaccines Immunother. 2016;12:519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zivcec M, Safronetz D, Scott DP, et al. Nucleocapsid protein-based vaccine provides protection in mice against lethal Crimean-Congo hemorrhagic fever virus challenge. PLoS Negl Trop Dis. 2018;12:e0006628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Aligholipour Farzani T, Földes K, Ergünay K, et al. Immunological analysis of a CCHFV mRNA vaccine candidate in mouse models. Vaccines (Basel). 2019;7:pii: E115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Canakoglu N, Berber E, Tonbak S, et al. Immunization of knock-out α/β interferon receptor mice against high lethal dose of Crimean-Congo hemorrhagic fever virus with a cell culture based vaccine. PLoS Neglected Trop Dis. 2015;9:e0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Mason HS, Arntzen CJ. Transgenic plants as vaccine production systems. Trends Biotechnol. 1995;13:388–392 [DOI] [PubMed] [Google Scholar]
- 107. Skarjinskaia M, Ruby K, Araujo A, et al. Hairy roots as a vaccine production and delivery system. Adv Biochem Eng Biotechnol. 2013;134:115–134 [DOI] [PubMed] [Google Scholar]
- 108. Devignot S, Bergeron E, Nichol S, et al. A virus-like particle system identifies the endonuclease domain of Crimean-Congo hemorrhagic fever virus. J Virol. 2015;89:5957–5967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Scholte FEM, Spengler JR, Welch SR, et al. Single-dose replicon particle vaccine provides complete protection against Crimean-Congo hemorrhagic fever virus in mice. Emerg Microbes Infect. 2019;8:575–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Spengler JR, Welch SR, Scholte FEM, et al. Heterologous protection against Crimean-Congo hemorrhagic fever in mice after a single dose of replicon particle vaccine. Antiviral Res. 2019;170:104573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Robinson HL, Pertmer TM. DNA vaccines for viral infections: basic studies and applications. Adv Virus Res. 2000;55:1–74 [DOI] [PubMed] [Google Scholar]
- 112. Flingai S, Czerwonko M, Goodman J, et al. Synthetic DNA vaccines: improved vaccine potency by electroporation and co-delivered genetic adjuvants. Front Immunol. 2013;4:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Spik K, Shurtleff A, McElroy AK, et al. Immunogenicity of combination DNA vaccines for Rift valley virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine. 2006;24:4657–4666 [DOI] [PubMed] [Google Scholar]
- 114. Zivcec M, Safronetz D, Scott D, et al. Lethal Crimean-Congo hemorrhagic fever virus infection in interferon α/β receptor knockout mice is associated with high viral loads, proinflammatory responses, and coagulopathy. J Infect Dis. 2013;207:1909–1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: the two extremes of a wide spectrum. Nat Rev Immunol. 2006;6:231–243 [DOI] [PubMed] [Google Scholar]
- 116. Rodriguez SE, Cross RW, Fenton KA, et al. Vesicular stomatitis virus-based vaccine protects mice against Crimean-Congo hemorrhagic fever. Sci Rep. 2019;9:7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Donofrio G, Sartori C, Ravanetti L, et al. Establishment of a bovine herpesvirus 4 based vector expressing a secreted form of the bovine viral diarrhoea virus structural glycoprotein E2 for immunization purposes. BMC Biotechnol. 2007;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Thiry E, Bublot M, Dubuisson J, et al. Molecular biology of bovine herpesvirus type 4. Vet Microbiol. 1992;33:79–92 [DOI] [PubMed] [Google Scholar]
- 119. Wang Y, Alahdal M, Ye J, et al. Inhibition of RM-1 prostate carcinoma and eliciting robust immune responses in the mouse model by using VEGF-M2-GnRH3-hinge-MVP vaccine. Genes Immun. 2018:1–10 [DOI] [PubMed] [Google Scholar]
- 120. Kim JJ, Yang JS, Montaner L, et al. Coimmunization with IFN-gamma or IL-2, but not IL-13 or IL-4 cDNA can enhance Th1-type DNA vaccine-induced immune responses in vivo. J Interferon Cytokine Res. 2000;20:311–319 [DOI] [PubMed] [Google Scholar]
- 121. Chattergoon MA, Saulino V, Shames JP, et al. Co-immunization with plasmid IL-12 generates a strong T-cell memory response in mice. Vaccine. 2004;22:1744–1750 [DOI] [PubMed] [Google Scholar]
- 122. Kim JJ, Yang JS, Lee DJ, et al. Macrophage colony-stimulating factor can modulate immune responses and attract dendritic cells in vivo. Hum Gene Ther. 2000;11:305–321 [DOI] [PubMed] [Google Scholar]
- 123. Kim JJ, Nottingham LK, Sin JI, et al. CD8 positive T cells influence antigen-specific immune responses through the expression of chemokines. J Clin Invest. 1998;102:1112–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Sin J, Kim JJ, Pachuk C, et al. DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD4+ T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J Virol. 2000;74:11173–11180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Kim JJ, Tsai A, Nottingham LK, et al. Intracellular adhesion molecule-1 modulates beta-chemokines and directly costimulates T cells in vivo. J Clin Invest. 1999;103:869–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Sin JI, Kim JJ, Zhang D, et al. Modulation of cellular responses by plasmid CD40L: CD40L plasmid vectors enhance antigen-specific helper T cell type 1 CD4+ T cell-mediated protective immunity against herpes simplex virus type 2 in vivo. Hum Gene Ther. 2001;12:1091–1102 [DOI] [PubMed] [Google Scholar]
- 127. Agadjanyan MG, Chattergoon MA, Holterman MJ, et al. Costimulatory molecule immune enhancement in a plasmid vaccine model is regulated in part through the Ig constant-like domain of CD80/86. J Immunol. 2003;171:4311–4319 [DOI] [PubMed] [Google Scholar]
- 128. Causey OR, Kemp GE, Madbouly MH, et al. Congo virus from domestic livestock, African hedgehog, and arthropods in Nigeria. Am J Trop Med Hyg. 1970;19:846–850 [DOI] [PubMed] [Google Scholar]
- 129. Fajs L, Resman K, Avšič-Županc T. Crimean-Congo hemorrhagic fever virus nucleoprotein suppresses IFN-beta-promoter-mediated gene expression. Arch Virol. 2014;159:345–348 [DOI] [PubMed] [Google Scholar]
- 130. Zivcec M, Metcalfe MG, Albariño CG, et al. Assessment of inhibitors of pathogenic Crimean-Congo hemorrhagic fever virus strains using virus-like particles. PLoS Negl Trop Dis. 2015;9:e0004259. [DOI] [PMC free article] [PubMed] [Google Scholar]