Abstract
Background:
Pulmonary sarcoidosis patients who get disease progression despite corticosteroid treatment or can’t tolerate corticosteroid required second-line drug. Methotrexate (MTX) is the most widely used in our clinical practice. Data on its safety and efficacy at different doses are still limited, especially for those without folic acid supplements.
Objective:
To report effectiveness of different MTX dosages and tolerability of MTX in pulmonary sarcoidosis without folic acid supplements.
Methods:
A retrospective study on pulmonary sarcoidosis patients receiving MTX therapy with various dose ≥3 months was conducted. The primary outcome was change in high-resolution computed tomography (HRCT) before and after MTX therapy. Other efficacy parameters included SGRQ score, prednisone dose change, discontinuation and relapse-free survival. Response-linked factors and safety outcomes were also analyzed.
Results:
Overall, 49 patients (81.7%) were assessed as MTX responders by HRCT and there was no significant difference in clinical response rate among three groups with different doses. The health-related quality of life (HRQL) of the responders improved obviously, which was evidenced by SGRQ score declining from 16.7(IQR: 7.9-26.4) to 10.7(IQR: 4.8-19.3) (P=0.029). The corticosteroids sparing effect was confirmed in “responders” group (P<0.001). When MTX was discontinued in 11 responders with complete improvement, 2 patients experienced relapses within 15.5 (range: 1-30) months (mean follow-up time of these 11 responders: 13.5±13.0 months). No clinical characteristics were found related to MTX effectiveness. Adverse events occurred in 31.7% of the patients, with gastrointestinal-related being the commonest. Drug discontinuation owing to adverse events occupied 6.7% of the subjects.
Conclusions:
Nearly 80% of the sarcoidosis subjects had well response to MTX. Its effectiveness was irrelevant to the treatment dosages and baseline characteristics. A quite low relapse rate was witnessed in those complete responders discontinuing MTX therapies. The steroid-sparing effect, well drug tolerability and low drug withdrawal rate were observed in these patients even without folic acid supplements in clinical practice.
Key words: sarcoidosis, methotrexate, effectiveness, tolerability
Introduction
Sarcoidosis is a multisystem disease of unknown cause, histologically characterized by the presence of noncaseating granulomas. The most frequently affected sites are the lungs, lymph nodes, skin, although it may occur virtually in any organ. The natural clinical course of this disease was unpredictable, varying from spontaneous resolution to disease progression. It is reported that more than half of patients get remission within 3 years while approximately a third of patients have progressive disease giving rise to significant organ impairment (1). For patients who meet therapy indications, glucocorticoids are the first-line treatment to control their disease. Concerning the severe toxicity of corticosteroids by long-term use, tapering them to the lowest effective dose is needed in these patients. And second-line therapeutics (mainly including antimetabolites and cytotoxic drugs) are generally recommended for patients who experience relapse during tapering corticosteroids or are unresponsive to corticosteroids or intolerant to them.
Methotrexate (MTX) is the most commonly used as a second-line drug for sarcoidosis patients in our clinical practice (2, 3). It was reported that anti-inflammatory properties of MTX might be attributed to the release of adenosine from cells and the inhibition of polyamines (4). Only one randomized controlled trial (RCT) of 24 sarcoidosis patients by Baughman et al. (5) revealed the steroid-sparing effect of MTX in acute sarcoidosis. And the significant steroid-sparing effect, improvement in lung function and safety of MTX on sarcoidosis were found in two retrospective researches (6, 7) and one prospective study (8). The most frequently reported adverse events during MTX therapy were gastrointestinal problems, hepatic abnormalities and infection (6-8). Sarcoidosis patients in the former researches were all given folic acid supplements, but its evidence is lacking in sarcoidosis.
Additionally, until now, researches involving the effect and tolerability of MTX on pulmonary sarcoidosis are still insufficient, especially comparing different MTX dosages in sarcoidosis and analyzing factors related to its efficacy and occurrence of adverse event and prognosis of patients stopping MTX therapy. The present real-world study was conducted to report the effectiveness and safety of different MTX dosages on sarcoidosis patients without taking folic acid supplements in clinical practice and factors related to its effectiveness were investigated.
Methods
Patients
A retrospective single-center research was conducted on 60 subjects given MTX with different dose for pulmonary sarcoidosis from December 2011 to August 2018 in Peking Union Medical College Hospital (PUMCH), Dongcheng District, China. Inclusion criteria were as follows: (1) adult patients (≥18 years old) with the diagnosis of sarcoidosis proven by the biopsy, in accordance with WASOG/ATS/ERS criteria (9); (2) Patients met treatment indications according to WASOG/ATS/ERS criteria (10, 11). Furthermore, they had received MTX therapy for at least 3 months due to failure (refractory or recurrent) or intolerance (side effects or contraindication) of corticosteroid use. Sarcoidosis was considered refractory when the disease was not controlled or even progressive despite corticosteroid treatment at adequate dosage (12) for at least 3 months. Failure of former therapy was assessed and determined by two experienced pulmonologists and one radiologist during patients’ regular clinical care (3). Patients treated by different pulmonologists in our hospital were collected to ensure that patients with various MTX doses were included in this study since different pulmonologists tend to choose different dose of MTX for pulmonary sarcoidosis patients according to their own experience and habits.
Study design
This real-world retrospective study was approved by the Regional Ethics Committee of our hospital (JS-1127/2016). Due to the retrospective nature of the study, informed consent was waived.
1. Data collection
Subjects who met our study inclusion criteria were included for further analysis. We collected the related data of these patients by paper and electronic medical records of our hospital healthcare system. These data included: (1) baseline data involving demographic information, smoking status, comorbidities, disease duration, prednisone dosage and Saint George’s Respiratory Questionnaire (SGRQ) score at baseline, PFTs, HRCT, lung biopsy results, MTX therapeutic dose; (2) data associated with effectiveness assessment including HRCT, SGRQ score and prednisone dose change; (3) information about adverse events, whether discontinued MTX therapy, whether relapse and date of occurrence.
2. Effectiveness and safety outcomes
The effectiveness of MTX on the included subjects who received therapy ≥3 months was assessed by HRCT. Two experienced respiratory specialists and two radiologists compared the chest images before and after MTX therapy in each patient. if the disagreement occurs, the chest images would be evaluated by another experienced radiologist. The chest imaging findings were scored, including bilateral hilar lymphadenopathy and the presence of lung infiltrates, on a scale of 1 to 4 (worsened, stable, partially improved and completely improved). A complete improvement was defined as the complete disappearance of lung lesions, without the occurrence of new lesions. A partial improvement was defined as ≥50% reduction of the pulmonary lesions. Patients who did not fulfill the complete or partial improvement criteria were classified into “non-responders” group. The health-related quality of life (HRQL) of patients was measured by SGRQ. SGRQ score (ranging from 0 to 100) was collected in these subjects, both pre-MTX and post-MTX therapy. Other effectiveness outcomes included corticosteroid sparing at the end of follow-up, discontinuation and relapse-free survival. A relapse was defined as the occurrence of new pulmonary sarcoidosis manifestations or a worsening of existing condition. Additionally, response related factors were also analyzed. Safety outcomes contained adverse events and drug discontinuation owing to adverse event.
Statistical analysis
We analyzed data with SPSS software version 19.0 for Windows (SPSS Inc., Chicago, IL)) and defined two-tailed P<0.05 as statistical significance. Categorical variables were expressed as numbers with percentages and normally distributed continuous variables as means ± SD. For non-normally distributed continuous variables, medians and interquartile range (IQR) were used (like SGRQ score and corticosteroids dosages in this study). Data were compared using Student’s t-test between two groups of measurement data which fulfilled homogeneity of variance (calibration t-test for those not conformed to homogeneity of variance). For non-normally distributed continuous variables and rank variables, data were compared using Mann-Whitney U test (non-parametric tests) for independent samples. Chi-square test was used to compare data of categorical variables and Fisher’s exact test was used as appropriate. Finally, a logistic regression model was performed to identify the factors associated with the efficacy of MTX therapy.
Results
1. Baseline characteristics of the included subjects
A total amount of 60 subjects consistent with our study criteria were included in our research. Baseline characteristics are shown in Table 3. A large number of patients (53/60, 88.3%) were in the pulmonary sarcoidosis stage II or III. More than half of the subjects (37/60, 61.7%) had extra-pulmonary organs involvement, including skin, lymph nodes, eyes, liver or spleen. Mostly, MTX was given to those refractory to corticosteroid (63.3%) or recurrent of the disease (25%). In total, 93.3% of patients (56/60) concomitantly received corticosteroid (mean dosage: 28.8±13.6 mg/day) and none of them had ever been given immunosuppressant for therapy (Table 3).
Table 3.
Baseline characteristics related to treatment effectiveness (“responders” vs. “non-responders” group)
| Baseline characteristics | All patients (n=60) | MTX responders (n = 49) | MTX non-responders (n = 11) | P value |
| Age at start of therapy (years) | 53.3±10.5 | 54.2±10.8 | 49.4±8.4 | 0.168 |
| Male, n (%) | 20 (33.3) | 16 (32.7) | 4 (36.4) | 0.813 |
| BMI (kg/m2) | 24.9±3.7 | 25.3±3.8 | 23.4±3.0 | 0.131 |
| Reason for MTX treatment, n (%) | 0.133 | |||
| Refractory to GCS | 38 (63.3) | 28 (57.1) | 10 (90.9) | |
| Recurrence | 15 (25.0) | 14 (28.6) | 1 (9.1) | |
| Others* | 7 (11.7) | 7 (14.3) | 0 | |
| Duration of treatment (weeks) | 53.1±33.5 | 55.4±34.4 | 42.9±28.8 | 0.267 |
| Smoking status, n (%) | 1.000 | |||
| Former smoker | 2 (3.3) | 2 (4.1) | 0 | |
| Never smoker | 56 (93.3) | 45 (91.8) | 11 (100) | |
| Active smoker | 2 (3.3) | 2 (4.1) | 0 | |
| Disease duration, (years) | 1.9±2.2 | 2.1±2.4 | 1.8±1.7 | 0.677 |
| Chest radiographic staging, n (%) | 0.126 | |||
| I | 4 (6.7) | 3 (6.1) | 1 (9.1) | |
| II | 41 (68.3) | 34 (69.4) | 7 (63.6) | |
| III | 12 (20.0) | 11 (22.4) | 1 (9.1) | |
| IV | 3 (5.0) | 1 (2.1) | 2 (18.2) | |
| Pleura involvement, n (%) | 5 (8.4) | 3 (6.1) | 2 (18.2) | 0.224 |
| Previous therapy, n (%) | 0.478 | |||
| Prednisone | 51 (85.0) | 42 (85.7) | 9 (81.8) | |
| Hydroxychloroquine | 3 (5.0) | 3 (6.1) | 0 | |
| Prednisone+hydroxychloroquine | 6 (10.0) | 4 (8.2) | 2 (18.2) | |
| Extra-pulmonary involvement, n (%) | 37 (61.7) | 30 (61.2) | 7 (63.6) | 1.000 |
| Uveitis | 10 (16.7) | 7 (14.3) | 3 (27.3) | 0.371 |
| Cutaneous | 16 (26.7) | 15 (30.6) | 1 (9.1) | 0.259 |
| Lymph nodes | 14 (23.3) | 10 (20.4) | 4 (36.4) | 0.264 |
| Liver | 1 (1.7) | 1 (2.0) | 0 | 1.000 |
| Spleen | 1 (1.7) | 1 (2.0) | 0 | 1.000 |
| Comorbidity, n (%) | ||||
| Diabetes mellitus | 1 (1.7) | 0 | 1 (9.1) | |
| Hypertension | 4 (6.7) | 2 (4.1) | 2 (18.2) | |
| Coronary artery disease | 1 (1.7) | 1 (2.0) | 0 | |
| GERD | 1 (1.7) | 1 (2.0) | 0 | |
| Biopsy, n (%) | ||||
| Lung | 47 (78.3) | 38 (77.6) | 9 (81.8) | 1.000 |
| Skin | 11 (18.3) | 10 (20.4) | 1 (9.1) | 0.670 |
| Lymph nodes | 2 (3.3) | 1 (9.1) | 1 (2.0) | 0.336 |
GERD: gastroesophageal reflux disease; *: including those who had contraindications for corticosteroid use or refuse to corticosteroid therapy.
A majority of patients (37/60, 61.7%) were given MTX at the dose of 12.5mg weekly, while 12 patients (20.0%) at 10 mg weekly and 11 patients (18.3%) at 15 mg weekly. There was no significant difference in baseline characteristics, PFTs and chest radiographic staging proportions among three groups with various dosages (Table 1).
Table 1.
Baseline characteristics of three groups with different dosages
| All patients (n=60) | 10 mg/week (n=12) | 12.5 mg/week (n=37) | 15 mg/week (n=11) | P value | |
| Age at start of therapy (years) | 53.3±10.5 | 56.6±9.8 | 52.4±9.1 | 52.9±15.2 | 0.492 |
| Male, n (%) | 20 (33.3) | 4 (33.3) | 11 (29.7) | 5 (45.5) | 0.633 |
| BMI (kg/m2) | 24.9±3.7 | 25.1±3.6 | 25.1±3.5 | 24.3±4.4 | 0.808 |
| PFTs | |||||
| FEV1/FVC (%) | 74.0±8.7 | 72.2±8.0 | 75.1±8.7 | 72.3±9.7 | 0.465 |
| FEV1 (% predicted) | 87.5±15.1 | 91.1±13.6 | 86.8±13.5 | 85.8±21.4 | 0.640 |
| FVC (% predicted) | 96.1±15.8 | 99.1±19.7 | 94.7±13.7 | 97.4±18.6 | 0.670 |
| TLC (% predicted) | 90.2±11.0 | 94.6±13.5 | 89.2±10.0 | 88.8±11.2 | 0.302 |
| DLco (% predicted) | 74.8±10.8 | 76.8±10.3 | 74.0±11.1 | 75.6±10.9 | 0.718 |
| Chest radiographic staging, n (%) | 0.628 | ||||
| I | 4 (6.7) | 1 (8.3) | 3 (8.1) | 0 | |
| II | 41 (68.3) | 7 (58.3) | 26 (70.3) | 8 (81.8) | |
| III | 12 (20.0) | 4 (33.3) | 5 (13.5) | 3 (27.3) | |
| IV | 3 (5.0) | 0 | 3 (8.1) | 0 |
2. Effectiveness of MTX
2.1. The effect of various MTX dosages on HRCT
According to assessment of HRCT changes pre-MTX and post-MTX therapy, 90.9% (10/11) of patients with 15mg weekly were assessed as responders, while 75.0% (9/12) in “10 mg-weekly” group and 81.1% (30/37) in “12.5 mg-weekly” group, with no significant difference among them (P=0.579). Overall, 49 patients (81.7%) were confirmed as MTX responders (Table 2).
Table 2.
The effect of various dosages (10 mg vs. 12.5 mg vs. 15 mg weekly) of MTX therapy on HRCT
| HRCT score after MTX treatment | All patients (n=60) | 10 mg/week (n=12) | 12.5 mg/week (n=37) | 15 mg/week (n=11) | P value |
| 1, n (%) | 1 (1.7) | 0 | 1 (2.7) | 0 | |
| 2, n (%) | 10 (16.7) | 3 (25.0) | 6 (16.2) | 1 (9.1) | |
| 3, n (%) | 32 (53.3) | 8 (66.7) | 18 (48.6) | 9 (81.8) | |
| 4, n (%) | 17 (28.3) | 1 (8.3) | 12 (32.5) | 1 (9.1) | |
| MTX responders, n (%) | 49 (81.7) | 9 (75.0) | 30 (81.1) | 10 (90.9) | 0.579 |
| MTX non-responders, n (%) | 11 (17.4) | 3 (25.0) | 7 (18.9) | 1 (9.1) |
2.2. The effect of MTX on HRQL
The HRQL of patients in “responders” group improved significantly after treatment compared with the baseline value as evidenced by the remarkable decrease of SGRQ score from 16.7 (IQR: 7.9-26.4) to 10.7 (IQR: 4.8-19.3) (P=0.029). While HRQL of the non-responders improved at a lower level after treatment, not reaching the significant difference standard (P=0.554) (Figure 1).
Fig. 1.
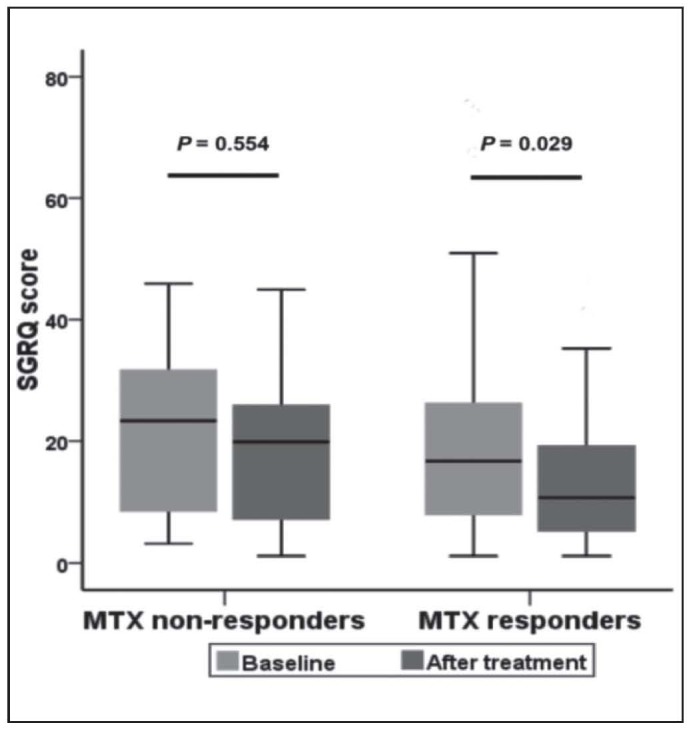
SGRQ before and after MTX treatment in“responders” and “non-responders” group
SGRQ: Saint George’s Respiratory Questionnaire.
2.3. Effect of MTX on daily corticosteroid dosages
Corticosteroid remission was obtained in patients of “MTX responders” group with a statistically significant decrease in the daily dose from 30 (IQR: 15-40) to 10 (IQR: 10-15) mg/day within an average duration of 55.4 weeks (P<0.001) (Figure 2, Table 3). Daily corticosteroid dose was also dropped after MTX treatment in “non-responders” group from 20 (IQR: 17.5-35) to 15 (IQR: 15-20) mg/day within a mean duration of 42.9 weeks, though without statistical significance (P=0.083).
Fig. 2.
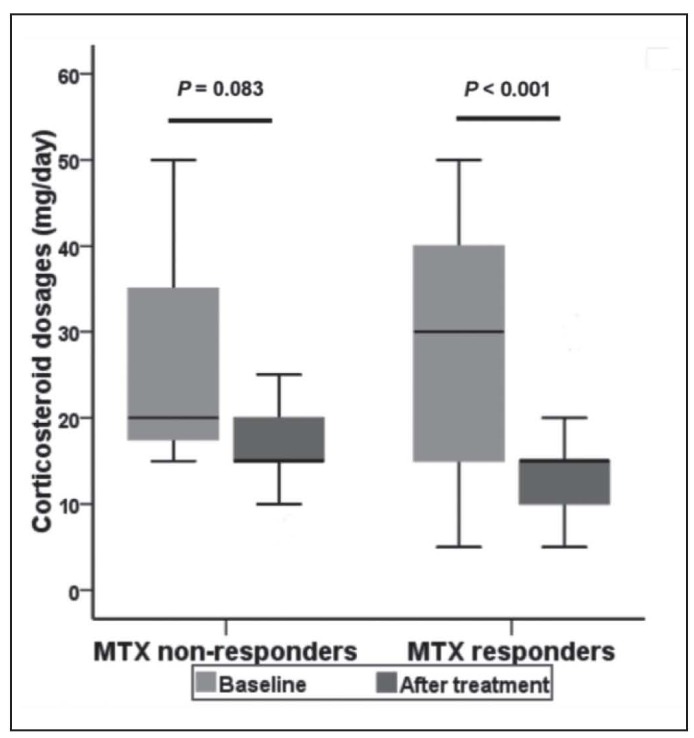
Effect of MTX on daily corticosteroid dosages
2.4. Therapy discontinuation and relapse
In total, MTX therapy was stopped in 17 patients (17/60, 28.3%) with a mean treatment duration of 54.6 weeks, containing 13 subjects in “responders” group (3 due to adverse events and 10 because of complete disappearance of lung lesions) and 4 in “non-responders” group (1 due to adverse events and 3 because of therapy failure). 4 patients in “non-responders” group were given azathioprine (AZA), cyclophosphamide, mycophenolate mofetil, tacrolimus respectively. Only one of these 4 patients remarkably improved after 1.5 years of AZA treatment and the other three remained unchanged. Disease relapses were observed in 2 patients (2/11, 18.2%) in “responders” group who achieved complete improvement (1 stopped due to adverse events and 10 because of complete disappearance of lung lesions), with a mean follow-up time of 13.5 months (range: 1-30). These two patients were given MTX therapy again. And their prognosis needed further follow-up.
3. Baseline characteristics of two groups (“responders” and “non-responders”) and investigation of response-linked factors
Baseline characteristics of 60 subjects on the basis of the response to MTX were presented in Table 3. All the included subjects had biopsy-proven sarcoidosis and a larger proportion of them got the biopsy tissue from lung (78.3%) or skin (18.3%). The mean duration of therapy for the enrolled subjects was 53.1±33.5 weeks. The responders had longer MTX treatment duration than the non-responders (55.4±34.4 weeks vs. 42.9±28.8 weeks), though with no statistically significant difference (P=0.267). Meanwhile, the responders were older than the non-responders, with 54.2±10.8 years and 49.4±8.4 years respectively (P=0.168). The proportion of subjects at different chest radiographic stages of pulmonary sarcoidosis had no significant difference between “responders” and “non-responders” group (P=0.126). 18.2% (2/11) of the patients in the “non-responders” group had pleura involvement, higher than the “responders” group (6.1%, 3/49) (P=0.224). No other significant differences between these two groups (“MTX responders” vs. “MTX non-responders”) were observed in any other baseline parameters, including sex, BMI, disease duration, smoking status and so on (Table 3).
HRCT performance of almost half proportion of “responders” group (59.2%) was only ground glass opacity (GGO) or nodular change/parenchymal masses, while combinations of several kinds of HRCT changes (54.6%) represented the most frequently in “non-responders” group, though without a statistically significant difference (P=0.627). Meanwhile, no significant difference in the other baseline indexes (PFTs and SGRQ score) was revealed between these groups (Table 4).
Table 4.
Baseline HRCT, PFTs and SGRQ
| All patients (n=60) | MTX responders (n=49) | MTX non-responders (n=11) | P value | |
| HRCT performance, n (%) | 0.627 | |||
| Only hilar and ediastinal lymphadenopathy | 4 (6.7) | 3 (6.1) | 1 (9.1) | |
| GGO | 8 (13.3) | 7 (14.3) | 1 (9.1) | |
| Nodular change/parenchymal masses | 25 (41.7) | 22 (44.9) | 3 (27.2) | |
| GGO and nodular | 6 (10.0) | 5 (10.2) | 1 (9.1) | |
| Others* | 17 (28.3) | 12 (24.5) | 5 (45.5) | |
| PFTs | ||||
| FEV1/FVC (%) | 74.0±8.7 | 73.9±9.0 | 74.1±7.6 | 0.957 |
| FEV1 (% predicted) | 87.5±15.1 | 87.6±15.1 | 87.0±15.2 | 0.910 |
| FVC (% predicted) | 96.1±15.8 | 96.2±16.1 | 95.8±14.8 | 0.940 |
| TLC (% predicted) | 90.2±11.0 | 89.7±10.6 | 92.1±12.9 | 0.529 |
| DLco (% predicted) | 74.8±10.8 | 75.1±8.8 | 73.8±17.8 | 0.729 |
| SGRQ score | 17.1(IQR: 7.9-26.9) | 16.7(IQR: 7.9-26.4) | 23.3(IQR: 8.4-31.4) | 0.731 |
GGO: Ground glass opacities; Others*: more than 2 changes including GGO, nodular change, thickening of the bronchovascular bundles, parenchymal bands or fibrosis; PFTs, pulmonary function tests; FEV1: forced expiratory volume in one second; FVC, forced vital capacity; TLC: total lung capacity; DLco, diffusion capacity of carbon monoxide
In the multivariate logistic regression analysis, no parameter (age (OR=1.001, 95% CI, 0.916-1.093, P=0.984), sex (OR=0.244, 95% CI, 0.034-1.777, P=0.164), BMI (OR=1.203, 95% CI, 0.921-1.571, P=0.175), chest radiographic stage (OR=4.828, 95% CI, 0.628-37.087, P=0.130), whether extra-pulmonary organ involvement (OR=0.414, 95% CI, 0.072-2.380, P=0.323), duration of the disease (OR=1.765, 95% CI, 0.737-4.226, P=0.202), MTX therapy period (OR=1.072, 95% CI, 0.944-1.218, P=0.281) was found significantly associated with the clinical response to MTX.
4. Adverse reactions and drug discontinuation
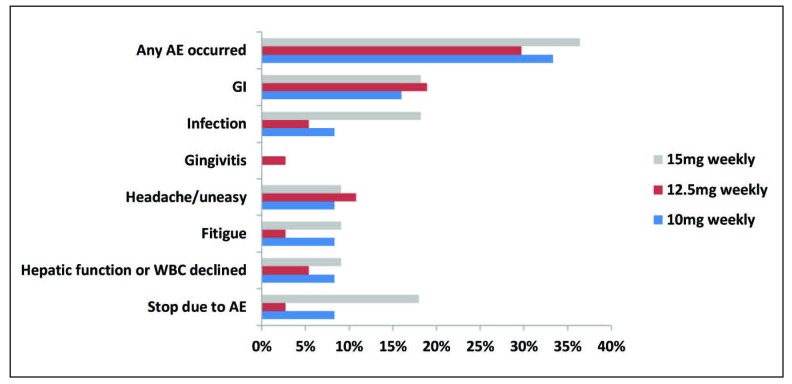
Totally, adverse events were reported in 19 subjects (19/60, 31.7%) with sarcoidosis. The most frequently reported side effects were gastrointestinal-related (11/60, 18.3%), headache/uneasy (6/60, 10.0%) and infection (5/60, 8.3%). Nausea and abdominal distension occurred the most often in gastrointestinal-related adverse events. Among 5 patients who got the infection during MTX therapy period, 3 patients experienced pulmonary infection. Laboratory abnormalities including aminotransferase elevations or WBC level declined were noted in 4 patients (4/60, 6.7%). No statistically significant differences in the occurrence of adverse events were observed between “responders” group and “non-responders” group (Table 5). MTX therapy was stopped due to adverse events in 4 patients (4/60, 6.7%), with 3 (3/49, 6.1%) in “responders” group and 1 (1/11, 9.1%) in “non-responders” group (P=0.566). The detailed information was presented in Table 5. There were no differences in the occurrence of these side effects among three subgroups with different dosage of MTX (10 mg/week, 12.5mg/week and 15mg/week) (P>0.05) (Figure 3).
Table 5.
Reported side effects during MTX treatment
| Adverse event, n (%) | All patients (n = 60) | MTX responders (n = 49) | MTX non-responders (n = 11) | P value |
| Adverse event occurred | 19 (31.7) | 15 (30.6) | 4 (36.4) | 0.990 |
| Gastrointestinal-related | 11 (18.3) | 9 (18.4) | 2 (18.2) | 1.000 |
| Nausea, n (%) | 6 (10.0) | 5 (10.2) | 1 (9.1) | 1.000 |
| Epigastric discomfort, n (%) | 2 (3.3) | 2 (4.1) | 0 | 1.000 |
| Anorexia, n (%) | 3 (5.0) | 2 (4.1) | 1 (9.1) | 0.462 |
| Abdominal distension, n (%) | 4 (6.7) | 4 (8.2) | 0 | 1.000 |
| Gingivitis, n (%) | 1 (1.7) | 1 (2.0) | 0 | 1.000 |
| Infection, n (%) | 5 (8.3) | 4 (8.2) | 1 (9.1) | 0.395 |
| Pulmonary | 3 (5.0) | 3 (6.1) | 0 | |
| The upper respiratory tract | 1 (1.7) | 0 | 1 (9.1) | |
| Oral cavity | 1 (1.7) | 1 (2.0) | 0 | |
| Headache/uneasy, n (%) | 6 (10.0) | 5 (10.2) | 1 (9.1) | 1.000 |
| Fatigue, n (%) | 3 (5.0) | 1 (2.0) | 2 (18.2) | 0.084 |
| Hepatic function or WBC declined, n (%) | 4 (6.7) | 4 (8.2) | 0 | 1.000 |
| Aminotransferase elevations, n (%) | 3 (5.0) | 3 (6.1) | 0 | 1.000 |
| WBC level declined, n (%) | 1 (1.7) | 1 (2.0) | 0 | 1.000 |
| Drug discontinuation due to AEs, n (%) | 4 (6.7) | 3 (6.1) | 1 (9.1) | 0.566 |
AEs: adverse events.
Fig. 3.
Adverse events of various dose of MTX
No significant difference was found in every kind of event among three groups (P>0.05).
AE: adverse event; Skin: skin-related adverse event; GI: gastrointestinal related adverse event.
Discussion
In this single-center retrospective study, we mainly analyzed the effectiveness and safety of MTX on pulmonary sarcoidosis patient refractory to or intolerant of corticosteroid therapy. Our mean follow-up duration was 15.7 months (range: 3-63). More than 90% of the included patients had previously been treated with corticosteroid, without improvement or with relapse during corticosteroid tapering. Around 80% of sarcoidosis subjects were accessed as responders by HRCT. More than half of the patients received MTX at the dose of 12.5mg weekly and others at 10 mg or 15 mg weekly. No statistically significant difference was noted in the response to MTX among various dosages. One earlier prospective study of 50 sarcoidosis patients by Goljan-Geremek and colleagues (8) observed a dose-related effect of MTX in their cohort, showing that MTX total dose was significantly higher in the “MTX responders” group compared to the “non-responders” group. However, the treatment duration was significantly longer in the “MTX responders” group of this study and the weekly dose-related effect of MTX on sarcoidosis was not for further analysis. Because the data related to the number of responders in different weekly doses of MTX were provided, we continued the statistical analysis of the relationship between MTX efficacy and various weekly dosages, revealing that no statistically significant difference (P=0.084) was observed in the proportion of responders on different MTX dosages (10 mg/week vs. 15 mg/week). This result was in coincidence with our study. The recommended initial dosage of oral MTX in sarcoidosis was 5–15 mg weekly by WASOG (13). Until now, no researches have been conducted to compare efficacy on different weekly dosages of MTX in sarcoidosis patients. Studies in rheumatic arthritis (RA) comparing different dosages of oral MTX showed dose-dependent efficacy in resistant RA patients (14). While the conclusion of dose-related effect of MTX was inconsistent in early RA patients (15, 16). More researches with large sample are needed for further analysis of the dosage related response to MTX on sarcoidosis patients.
In contrast to the present results, the literature has shown a lower response rate with improvement in only 55% of the subjects (8). The longer-lasting disease duration (12.34±20.49 years) and lower PFTs of the included subjects in that literature might explain this.
In addition to improvements in HRCT, MTX was also effective in lowering SGRQ score. Little studies had paid attention to the HRQL in sarcoidosis patients receiving MTX therapy. Only one research (8) in 2014 showed that 6-minute walk test (6MWT) improved from 531±106 m to 546±106 m and Borg score decreased from 1.22±1.9 to 0.75±1.3 in sarcoidosis patients given MTX, though without significant difference. And our study reported that HRQL of patients improved after MTX treatment as evidenced by the decline of SGRQ score both in “responders” group and “non-responders” group. This study showed that MTX had steroid-sparing potency both in “responders” group (from 30 (IQR: 15-40) to 10 (IQR: 10-15) mg/day within an average duration of 55.4 weeks) and “non-responders” group (from 20 (IQR: 17.5-35) to 15 (IQR: 15-20) mg/day within a mean duration of 42.9 weeks), though only with statistical significance in the former one. This was in coincidence with the earlier researches (7, 17).
Several studies have attempted to investigate the factors in association with response to drug therapy in sarcoidosis. The former studies had found that pulmonary involvement was associated with poorer response to anti-tumor necrosis factor antagonists (18) or Acthar gel (19). However, studies concerning the factors associated with responsiveness to MTX in pulmonary sarcoidosis are rare. Only one (8) reported that pulmonary sarcoidosis patients who benefited from MTX therapy were those with initially impaired volume and capacity parameters. However, in our research, no relation between PFTs at baseline and the effectiveness of MTX in pulmonary sarcoidosis was found. The same results were observed in other parameters including age, sex, BMI, chest radiographic stage, whether extra-pulmonary organ involvement, duration of the disease, smoking status, MTX therapy period.
In addition to the above baseline characteristics, some laboratory results were also analyzed in the previous studies. One research (20) of 114 sarcoidosis patients reported that high baseline levels of serum angiotensin converting enzyme (sACE) correlated significantly with lung function improvement after MTX treatment, and the level of sACE decreased obviously after treatment. But sACE was still under debate because various researches reported conflicting conclusions. Until now, no definite evidence was provided for the use of sACE in the chest radiographic stage, monitoring of pulmonary sarcoidosis and predicting response to therapy (21-23). Furthermore, one study of 306 sarcoidosis patients by Doubkova et al. (24) showed that a higher CD4 to CD8 ratio (CD4/CD8) in the BALF was significantly related to spontaneous resolution. Another study (25) reported the same result that untreated patients with radiological improvement had higher numbers of CD4 cells and a higher CD4/CD8 in BALF than patients without improvement. A high CD4/CD8 was also related to better response to therapy (23). However, the lack of laboratory results in our study may not allow such kind of analysis and the relationship between these parameters and clinical responsiveness to MTX are needed to be analyzed in the future researches.
Responders to MTX were at risk of relapse after drug discontinuation. In our study, 2 relapses occurred in 11 responders with complete improvement (18.2%) who interrupted therapy with an average follow-up time of 13.5 months (range: 1-30). Owing to the limited sample, we were not able to do analysis to determine predictive factors for relapses. Therefore, larger sample size and longer follow-up time will be needed for further analysis. And 2 relapsed subjects were given MTX therapy again and the efficacy needed further assessment.
In the earlier researches, the sarcoidosis patients were given 5 mg folic acid weekly (7, 8). The recommendation on the prescription of folic acid during MTX treatment for sarcoidosis was based on some researches from rheumatoid arthritis (26, 27). However, until now, no studies on folic acid supplement during MTX treatment for sarcoidosis have been available. Therefore, considering shortness of evidence for folic acid supplements, our study focused on the safety of MTX on sarcoidosis without folic acid intake. It revealed that the occurrence rate of adverse events was 31.7% in our study with mean treatment duration of 53.1 weeks. The most frequently reported adverse events were gastrointestinal-related (11/60, 18.3%) and infection (5/60, 8.3%), obviously lower than the earlier research (7). And the occurrence rate of hepatic abnormalities in our study was dramatically lower than the previous data (8). However, long-term follow-up data on the safety of MTX were from rheumatoid arthritis (26), showing the lower morality (28) and discontinuation rate than other antirheumatic drugs owing to toxicity (29, 30). No studies with long-term follow-up data on safety profile of MTX for sarcoidosis was found.
There were several limitations to this study. First, due to the retrospective design of this study, nonstandardization of follow-up inevitably occurs, which might lead to missed side effects. But we have tried to minimize the loss of data through thorough investigation of patients’ electronic and paper medical records. Second, no objective global assessment tools have been established to accurately assess sarcoidosis patients’ response to a particular therapy (31). Furthermore, a large proportion of patients in our real-world clinical practice did not have regular lung function tests and most of them might take these tests in different hospitals, giving rise to the failure to analyze effectiveness by PFTs at different times according to one standardization. Third, laboratory results like sACE and CD4/CD8 in BALF were not available and analyzed in this study, so more researches are needed to investigate their relationship with response to MTX.
Conclusion
The present study has demonstrated efficiency of MTX with various dosages in pulmonary sarcoidosis as evidenced by the improvement of HRCT and HRQL. The steroid-sparing effect of MTX was observed both in “responders” group and “non-responders” group. No baseline characteristics were found in association with response to MTX. Additionally, this was the first study revealing the well tolerability of MTX therapy without folic acid supplements. Relapse rate was low after MTX withdrawal while relapse-linked factors are still needed to be analyzed and illuminated.
Authors’ contributions:
Chuling Fang, Qian Zhang and Zuojun Xu contributed to the construction of the database and the study design; Chuling Fang, Qian Zhang, Na Wang and Xiaoyan Jing contributed to the data collection; Chuling Fang, Qian Zhang and Zuojun Xu contributed to the data analysis; Chuling Fang contributed to the draft of the manuscript; and all authors contributed to the revision and final approval of the manuscript.
Funding:
The research leading to these results has received funding from National Key Technologies R & D Program Precision Medicine Research (No.2016YFC0905700) and the National Natural Science Foundation of China (No.81670061).
References
- 1.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. [Journal Article; Research Support, N.I.H., Extramural; Review]. 2007. 2007-11-22;357(21):2153–65. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Nunes H. Therapy for sarcoidosis: evidence-based recommendations. Expert Rev Clin Immunol. [Journal Article; Review]. 2012. 2012-01-01;8(1):95–103. doi: 10.1586/eci.11.84. [DOI] [PubMed] [Google Scholar]
- 3.Schutt AC, Bullington WM, Judson MA. Pharmacotherapy for pulmonary sarcoidosis: a Delphi consensus study. Respir Med. [Journal Article]. 2010. 2010-05-01;104(5):717–23. [Google Scholar]
- 4.Chan ES, Cronstein BN. Methotrexate--how does it really work? Nat Rev Rheumatol. [Review]. 2010. 2010-03-01;6(3):175–8. doi: 10.1038/nrrheum.2010.5. [DOI] [PubMed] [Google Scholar]
- 5.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. [Clinical Trial; Journal Article; Randomized Controlled Trial]. 2000. 2000-03-01;17(1):60–6. [PubMed] [Google Scholar]
- 6.Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med. [Clinical Trial; Journal Article]. 1995. 1995-04-24;155(8):846–51. [PubMed] [Google Scholar]
- 7.Vorselaars A, Wuyts WA, Vorselaars V, Zanen P, Deneer V, Veltkamp M, Thomeer M, van Moorsel C, Grutters JC. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. CHEST. [Comparative Study; Journal Article; Multicenter Study]. 2013. 2013-09-01;144(3):805–12. doi: 10.1378/chest.12-1728. [DOI] [PubMed] [Google Scholar]
- 8.Goljan-Geremek A, Bednarek M, Franczuk M, Puscinska E, Nowinski A, Czystowska M, Kaminski D, Korzybski D, Stoklosa A, Kowalska A, Wojda E, Sliwinski P, Burakowska B, Ptak J, Baranska I, Drygalska A, Malek G, Bestry I, Wesolowski S, Kram M, Gorecka D. Methotrexate as a single agent for treating pulmonary sarcoidosis: a single centre real-life prospective study. Pneumonol Alergol Pol. [Clinical Trial; Journal Article]. 2014. 2014-01-20;82(6):518–33. doi: 10.5603/PiAP.2014.0069. [DOI] [PubMed] [Google Scholar]
- 9.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. [Consensus Development Conference; Journal Article; Review]. 1999. 1999-09-01;16(2):149–73. [PubMed] [Google Scholar]
- 10.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, Eklund A, Kitaichi M, Lynch J, Rizzato G, Rose C, Selroos O, Semenzato G, Sharma OP. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. [Consensus Development Conference; Journal Article; Review]. 1999. 1999-09-01;16(2):149–73. [PubMed] [Google Scholar]
- 11.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. [Journal Article; Research Support, N.I.H., Extramural; Review]. 2011. 2011-03-01;183(5):573–81. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korsten P, Strohmayer K, Baughman RP, Sweiss NJ. Refractory pulmonary sarcoidosis - proposal of a definition and recommendations for the diagnostic and therapeutic approach. Clin Pulm Med. [Journal Article]. 2016. 2016-03-01;23(2):67–75. doi: 10.1097/CPM.0000000000000136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cremers JP, Drent M, Bast A, Shigemitsu H, Baughman RP, Valeyre D, Sweiss NJ, Jansen TL. Multinational evidence-based World Association of Sarcoidosis and Other Granulomatous Disorders recommendations for the use of methotrexate in sarcoidosis: integrating systematic literature research and expert opinion of sarcoidologists worldwide. Curr Opin Pulm Med. [Journal Article; Research Support, Non-U.S. Gov’t; Review]. 2013. 2013-09-01;19(5):545–61. doi: 10.1097/MCP.0b013e3283642a7a. [DOI] [PubMed] [Google Scholar]
- 14.Furst DE, Koehnke R, Burmeister LF, Kohler J, Cargill I. Increasing methotrexate effect with increasing dose in the treatment of resistant rheumatoid arthritis. J Rheumatol. [Clinical Trial; Journal Article; Randomized Controlled Trial; Research Support, Non-U.S. Gov’t; Research Support, U.S. Gov’t, P.H.S.]. 1989. 1989-03-01;16(3):313–20. [PubMed] [Google Scholar]
- 15.Verstappen SM, Jacobs JW, van der Veen MJ, Heurkens AH, Schenk Y, ter Borg EJ, Blaauw AA, Bijlsma JW. Intensive treatment with methotrexate in early rheumatoid arthritis: aiming for remission. Computer Assisted Management in Early Rheumatoid Arthritis (CAMERA, an open-label strategy trial) Ann Rheum Dis. [Journal Article; Multicenter Study; Randomized Controlled Trial]. 2007. 2007-11-01;66(11):1443–9. doi: 10.1136/ard.2007.071092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergstra SA, Allaart CF, Stijnen T, Landewe R. Meta-Regression of a Dose-Response Relationship of Methotrexate in Mono- and Combination Therapy in Disease-Modifying Antirheumatic Drug-Naive Early Rheumatoid Arthritis Patients. Arthritis Care Res (Hoboken). [Journal Article; Meta-Analysis; Review]. 2017. 2017-10-01;69(10):1473–83. doi: 10.1002/acr.23164. [DOI] [PubMed] [Google Scholar]
- 17.Baughman RP, Winget DB, Lower EE. Methotrexate is steroid sparing in acute sarcoidosis: results of a double blind, randomized trial. Sarcoidosis Vasc Diffuse Lung Dis. [Clinical Trial; Journal Article; Randomized Controlled Trial]. 2000. 2000-03-01;17(1):60–6. [PubMed] [Google Scholar]
- 18.Jamilloux Y, Cohen-Aubart F, Chapelon-Abric C, Maucort-Boulch D, Marquet A, Perard L, Bouillet L, Deroux A, Abad S, Bielefeld P, Bouvry D, Andre M, Noel N, Bienvenu B, Proux A, Vukusic S, Bodaghi B, Sarrot-Reynauld F, Iwaz J, Amoura Z, Broussolle C, Cacoub P, Saadoun D, Valeyre D, Seve P. Efficacy and safety of tumor necrosis factor antagonists in refractory sarcoidosis: A multicenter study of 132 patients. Semin Arthritis Rheum. [Journal Article; Multicenter Study]. 2017. 2017-10-01;47(2):288–94. doi: 10.1016/j.semarthrit.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. [Journal Article; Multicenter Study]. 2016. 2016-01-01;110:66–72. doi: 10.1016/j.rmed.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Vorselaars AD, van Moorsel CH, Zanen P, Ruven HJ, Claessen AM, van Velzen-Blad H, Grutters JC. ACE and sIL-2R correlate with lung function improvement in sarcoidosis during methotrexate therapy. Respir Med. [Journal Article; Observational Study]. 2015. 2015-02-01;109(2):279–85. doi: 10.1016/j.rmed.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Bradley B, Branley HM, Egan JJ, Greaves MS, Hansell DM, Harrison NK, Hirani N, Hubbard R, Lake F, Millar AB, Wallace WA, Wells AU, Whyte MK, Wilsher ML. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. THORAX. [Journal Article; Practice Guideline]. 2008. 2008-09-01;63(Suppl 5):v1–58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 22.Keir G, Wells AU. Assessing pulmonary disease and response to therapy: which test? Semin Respir Crit Care Med. [Journal Article; Review]. 2010. 2010-08-01;31(4):409–18. doi: 10.1055/s-0030-1262209. [DOI] [PubMed] [Google Scholar]
- 23.Baughman RP, Fernandez M, Bosken CH, Mantil J, Hurtubise P. Comparison of gallium-67 scanning, bronchoalveolar lavage, and serum angiotensin-converting enzyme levels in pulmonary sarcoidosis. Predicting response to therapy. Am Rev Respir Dis. [Comparative Study; Journal Article; Research Support, U.S. Gov’t, P.H.S.]. 1984. 1984-05-01;129(5):676–81. doi: 10.1164/arrd.1984.129.5.676. [DOI] [PubMed] [Google Scholar]
- 24.Doubkova M, Pospisil Z, Skrickova J, Doubek M. Prognostic markers of sarcoidosis: an analysis of patients from everyday pneumological practice. Clin Respir J. [Journal Article]. 2015. 2015-10-01;9(4):443–9. doi: 10.1111/crj.12160. [DOI] [PubMed] [Google Scholar]
- 25.Verstraeten A, Demedts M, Verwilghen J, van den Eeckhout A, Marien G, Lacquet LM, Ceuppens JL. Predictive value of bronchoalveolar lavage in pulmonary sarcoidosis. Chest. [Journal Article]. 1990. 1990-09-01;98(3):560–7. doi: 10.1378/chest.98.3.560. [DOI] [PubMed] [Google Scholar]
- 26.Visser K, Katchamart W, Loza E, Martinez-Lopez JA, Salliot C, Trudeau J, Bombardier C, Carmona L, van der Heijde D, Bijlsma JW, Boumpas DT, Canhao H, Edwards CJ, Hamuryudan V, Kvien TK, Leeb BF, Martin-Mola EM, Mielants H, Muller-Ladner U, Murphy G, Ostergaard M, Pereira IA, Ramos-Remus C, Valentini G, Zochling J, Dougados M. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. [Consensus Development Conference; Journal Article; Multicenter Study; Research Support, Non-U.S. Gov’t]. 2009. 2009-07-01;68(7):1086–93. doi: 10.1136/ard.2008.094474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shea B, Swinden MV, Tanjong GE, Ortiz Z, Katchamart W, Rader T, Bombardier C, Wells GA, Tugwell P. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev. [Journal Article; Research Support, Non-U.S. Gov’t; Review]. 2013. 2013-05-31;(5):D951. doi: 10.1002/14651858.CD000951.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi HK, Hernan MA, Seeger JD, Robins JM, Wolfe F. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. [Journal Article; Research Support, Non-U.S. Gov’t; Research Support, U.S. Gov’t, P.H.S.]. 2002. 2002-04-06;359(9313):1173–7. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 29.Yazici Y, Sokka T, Kautiainen H, Swearingen C, Kulman I, Pincus T. Long term safety of methotrexate in routine clinical care: discontinuation is unusual and rarely the result of laboratory abnormalities. Ann Rheum Dis. [Journal Article; Research Support, Non-U.S. Gov’t; Research Support, U.S. Gov’t, P.H.S.]. 2005. 2005-02-01;64(2):207–11. doi: 10.1136/ard.2004.023408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salliot C, van der Heijde D. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis. [Journal Article; Meta-Analysis; Research Support, Non-U.S. Gov’t; Review]. 2009. 2009-07-01;68(7):1100–4. doi: 10.1136/ard.2008.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spagnolo P, Rossi G, Trisolini R, Sverzellati N, Baughman RP, Wells AU. Pulmonary sarcoidosis. Lancet Respir Med. [Journal Article; Review; Research Support, Non-U.S. Gov’t]. 2018. 2018-05-01;6(5):389–402. doi: 10.1016/S2213-2600(18)30064-X. [DOI] [PubMed] [Google Scholar]