Abstract
Background:
The role of cytomorphology in differentiating sarcoidosis from tuberculosis is not fully elucidated. Herein, we evaluate the utility of cytological features in differentiating between these two diseases in subjects undergoing endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA).
Methods:
Retrospective analysis of subjects who underwent EBUS-TBNA and had a final diagnosis of sarcoidosis or tuberculosis. The final diagnosis was based on the clinicoradiological features, microbiology and clinical course during follow-up (including response to treatment) at six months. A cytologist blinded to the clinical details and microbiology examined the aspirates. The primary outcome was the diagnostic accuracy of cytologist’s impression to diagnose sarcoidosis as compared to the final diagnosis.
Results:
179 (145 sarcoidosis, 34 tuberculosis) subjects were included. Granuloma was identified in 135 (75.4%) subjects; amongst these, the cytologist made a correct diagnosis in 62.2% cases, misdiagnosed 28.9% cases, and in 8.9% cases differentiating sarcoidosis from tuberculosis was not possible. The sensitivity, specificity, positive and negative predictive values (PPV and NPV) of the cytologist in diagnosing sarcoidosis was 62%, 64%, 90%, and 25%, respectively. The identification of a non-necrotic granuloma, along with a negative TST and the lack of endosonographic features favouring tuberculosis (heterogeneous echotexture and coagulation necrosis sign), provided the best specificity (97%) and PPV (99%) to diagnose sarcoidosis.
Conclusion:
Sarcoidosis cannot be reliably differentiated from tuberculosis based on cytomorphology alone. A combination of clinical features, endosonography, cytology and microbiology is required for accurate diagnosis.
Key words: bronchoscopy, endosonography, EUS, granuloma, tuberculin skin test
Introduction
Sarcoidosis and tuberculosis are the major causes of undiagnosed mediastinal lymphadenopathy in developing countries, including India (1). The differentiation between these two entities is challenging, particularly in high tuberculosis burden countries (2, 3). The exclusion of alternate causes of granuloma, especially tuberculosis, is essential for diagnosing sarcoidosis. Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is the preferred initial modality for the evaluation of undiagnosed intrathoracic lymphadenopathy (4). Although tuberculin skin test (TST) is useful in differentiating sarcoidosis from tuberculosis, the demonstration of Mycobacterium tuberculosis by smear, culture or molecular method provides the most definite evidence of tuberculosis (5). The conventional microbiological techniques have a poor sensitivity; however, the inclusion of Xpert MTB/RIF, has considerably improved the sensitivity of differentiating tuberculosis from sarcoidosis on EBUS-TBNA (6, 7).
The cytological features can be helpful in the diagnosis of granulomatous lymphadenopathy, even when the microbiology results are negative (8, 9). However, most of these studies have been performed on cervical lymph nodes and the differential did not include sarcoidosis (9, 10). The importance of differentiating these two common granulomatous diseases is even more relevant in subjects undergoing EBUS-TBNA for intrathoracic lymphadenopathy, where sarcoidosis is a major consideration. In our practice, cytopathologists diagnose sarcoidosis or tuberculosis based on cytomorphology alone. However, only a few studies have explored the cytomorphologic differences between sarcoidosis and tuberculosis (11-13). Herein, we evaluate the diagnostic performance of cytomorphology in diagnosing sarcoidosis, in patients undergoing EBUS-TBNA.
Methods
This was a retrospective analysis of prospectively collected data from January 2014 to March 2015. The study protocol was approved by the Institute Ethics Committee. A consent waiver was granted due to the retrospective nature of the study and the use of anonymized patient data. A part of the data has been published as an abstract (14) and some of the participants included in the current study were part of a previously published trial (15).
Study subjects: Consecutive subjects who underwent EBUS-TBNA were included if they fulfilled all the following: (i) age >18 years; (ii) enlarged intrathoracic lymph nodes ≥10 mm (short axis) on computed tomography (CT) of the chest; (iii) final diagnosis of sarcoidosis or tuberculosis. We excluded subjects with suspected or known malignancy.
Study protocol: After a thorough clinical history and physical examination, subjects underwent routine laboratory tests (TST, complete blood count, coagulation profile, liver and renal function tests), spirometry, chest radiography and CT of the chest. Intrathoracic lymph nodes and parenchymal abnormalities were assessed with CT scan.
EBUS-TBNA was performed as a day-care procedure in the bronchoscopy suite under moderate sedation (intravenous midazolam and pentazocine targeting a Ramsay sedation score of two). We used the EBUS bronchoscope (BF-UC180F; Olympus Medical, Japan) and a compatible ultrasound image processor (EU-ME1; Olympus Medical, Japan) for performing EBUS-TBNA (1). All subjects received premedication with intramuscular injection of atropine (0.6 mg) and promethazine (25 mg). Nebulized 4% lignocaine (2.5 mL), followed by two puffs of 10% lignocaine spray was administered for topical anaesthesia. 2 mL aliquots of 1% lignocaine was instilled over the vocal cords and the airways using the spray-as-you-go method (16). The EBUS scope was introduced transorally with the subject lying in the supine position. At least two lymph node stations were accessed (with two or more passes from each sampled node). The size, location (as per the IASLC lymph node map) (17) and endosonographic appearance (heterogeneous echotexture and coagulation necrosis sign [CNS]) (18) of the nodes were recorded. TBNA was performed using either a 21G or 22G EBUS-TBNA aspiration needle (Vizishot, NA-201 SX-4021A or NA-201 SX-4022A), under real-time ultrasound guidance (15). We employed continuous suction with a 20 mL VacLoc™ syringe and the catheter was moved back and forth for 15-20 times. Rapid onsite cytologic evaluation (ROSE) was not available. Endobronchial (EBB) and transbronchial lung biopsies (TBLB) were performed for subjects with suspected sarcoidosis.
Processing and reporting of the EBUS-TBNA samples: Smears were prepared from the aspirated material, both air-dried (for May-Grunwald Giemsa staining) and alcohol-fixed (95% alcohol for Ziehl-Neelsen staining to detect acid-fast bacilli [AFB], and haematoxylin-eosin staining). We also transferred the aspirated material in 0.9% sterile saline for mycobacterial culture and Xpert MTB/RIF. A single cytopathologist (NG) who was blinded to the clinical data, biopsies (EBB, TBB), mycobacterial culture, Xpert MTB/RIF, and the final diagnosis, reported the cytomorphological features of the slides. The following features were recorded: (i) adequacy (adequate, if the TBNA slide was diagnostic or showed the presence of numerous lymphocytes); (ii) the presence of granuloma; (iii) granuloma density (<3, 3-6, 7-9, and >9 granulomas per smear); (iv) the presence or absence of necrosis; (v) grading of necrosis, if present (1: focal, 2: intermediate [neither focal nor extensive], 3: extensive); (vi) stain for AFB in cytology slides; and, (vi) the final impression of cytologist in TBNA smears where granulomas could be identified (favour sarcoidosis, favour tuberculosis, indeterminate).
Definitions: The final diagnosis of sarcoidosis was made in subjects with a consistent clinical and radiological presentation after six months of follow-up, when (a) granuloma was demonstrated on either TBNA, TBLB or EBB along with negative AFB, fungal stains, and no growth of mycobacteria on mycobacterial culture; and/or (b) clinical and radiological response after treatment with glucocorticoids or stable disease without treatment (in the absence of an alternate diagnosis) (2). Tuberculosis was diagnosed when two of the following criteria was fulfilled: (a) consistent clinical and radiological presentation; (b) smear positive for AFB and/or culture for Mycobacterium tuberculosis or Xpert MTB/RIF positivity; and, (c) clinicoradiological response to anti-tuberculosis treatment.
Study endpoints: The primary objective was to determine the diagnostic accuracy of the cytologist’s impression to diagnose sarcoidosis on EBUS-TBNA smear, as compared to the final diagnosis and differentiate sarcoidosis from tuberculosis on the basis of cytomorphology. The secondary objectives were to assess the diagnostic performance of various cytomorphologic, endosonographic and clinical features of interest, either alone or in combination.
Statistical analysis: Data are presented as mean with standard deviation or number with percentage. We used the commercial statistical package SPSS (SPSS for Windows, version 22.0; IBM SPSS Inc; Armonk, NY) for the statistical analysis. Chi-square test and student t-test was used to analyse the differences between categorical and continuous variables, respectively. A p value <0.05 was considered significant. The sensitivity and specificity were calculated using a final diagnosis of tuberculosis and sarcoidosis, respectively. Diagnostic accuracy (by calculating sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV], expressed as percentages with 95% confidence intervals [CI]) of various parameters used to diagnose sarcoidosis was computed. We constructed Bayesian graphs demonstrating the variation in predictive values of a test (along y-axis) in relation to the disease prevalence (x-axis).
Results
We enrolled 179 subjects (mean age of 42.2 years, 59.8% males). A final diagnosis of sarcoidosis and tubercolosis was made in 145 (81%) and 34 (19%) subjects, respectively (Table 1). Granulomas were identified on cytology in 135 (75.4%) subjects (n=113 and n=22 in sarcoidosis and tuberculosis, respectively). Only these 135 subjects were included for the primary outcome analysis. Subjects with tuberculosis were younger (mean age of 34.2 years vs. 44.1 years, p=0.0002), and had a higher proportion of TST positivity (>10 mm; 67.6% vs. 6.2%, p=0.0001). The details of the mediastinal lymph node stations subjected to EBUS-TBNA are described in Table 1. The endosonographic appearance of heterogeneous echotexture (67.6% vs. 22.8%, p<0.0001), and CNS (23.5% vs. 2.8%, p<0.0001) were more frequently encountered in tuberculosis than sarcoidosis. A median of two lymph nodes and an average of two passes from each lymph node were obtained during EBUS-TBNA.
Table 1.
Baseline clinical and endosonographic characteristics of study subjects undergoing endobronchial ultrasound guided transbronchial needle aspiration (EBUS-TBNA) for suspected granulomatous intrathoracic lymphadenopathy
| Total (n=179) | Sarcoidosis (n=145) | Tuberculosis (n=34) | P value | |
| Age in years, mean (SD) | 42.2 (14.2) | 44.1 (13.4) | 34.2 (15.2) | 0.0002 |
| Male gender | 107 (59.8) | 86 (59.3) | 21 (61.8) | 0.48 |
| TST negativity (<10 mm) | 147 (82.1) | 136 (93.8) | 11 (32.4) | 0.0001 |
| Needle gauge | 0.56 | |||
| 21G | 95 (53.1) | 76 (52.4) | 19 (55.9) | |
| 22G | 84 (46.9) | 69 (47.6) | 15 (44.1) | |
| Lymph node stations* sampled during EBUS-TBNA | ||||
| Station 7 | 168 (93.9) | 140 (96.6) | 28 (82) | 0.007 |
| Station 4R | 141 (78.8) | 119 (82.1) | 22 (64.7) | 0.046 |
| Station 4L | 39 (21.8) | 35 (24.1) | 4 (11.8) | 0.18 |
| Station 10R | 5 (2.8) | 5 (3.4) | 0 | 0.73 |
| Station 11R | 21 (11.7) | 18 (12.4) | 3 (8.8) | 0.77 |
| Station 10L | 1 (0.6) | 1 (0.7) | 0 | - |
| Station 11L | 91 (50.8) | 83 (57.2) | 9 (26.5) | 0.0001 |
| Heterogeneous echotexture on EBUS | 56 (31.3) | 33 (22.8) | 23 (67.6) | <0.0001 |
| Coagulation necrosis sign | 12 (6.7) | 4 (2.8) | 8 (23.5) | <0.0001 |
| Central intranodal vessel | 87 (48.6) | 72 (49.7) | 15 (44.1) | 0.57 |
| Number of lymph nodes sampled, median (IQR) | 3 (2-3) | 3 (2-3) | 2 (2-3) | 0.09 |
| Number of passes per node, mean (SD) | 2 (0.5) | 2 (0.5) | 2.4 (0.8) | 0.02 |
| Mean (SD) Duration of procedure, minutes | 23.2 (5.9) | 23.4 (5.8) | 22.1 (6.4) | 0.122 |
All values are presented as number (percentage) unless otherwise stated
CT- computed tomography; EBUS- endobronchial ultrasound; IQR- interquartile range; SD- Standard deviation; TBNA – transbronchial needle aspiration; TST- tuberculin skin test
*As per the IASLC lymph node map (17)
The adequacy of EBUS-TBNA slides, the identification of granuloma on cytology and the number of granulomas per smear were not different in subjects with sarcoidosis and tuberculosis (Table 2). Necrosis was rare in sarcoidosis compared to tuberculosis (6.2% vs. 55.9%); when present, it was always focal (Table 2). Xpert MTB/RIF was available in 130 (72.6%) subjects (16 and 114 subjects with the final diagnosis of tuberculosis and sarcoidosis, respectively). Of these, none in the sarcoidosis group and seven (43.7%) in the tuberculosis group were positive for Xpert MTB/RIF.
Table 2.
EBUS-TBNA cytology features of subjects with granulomatous intrathoracic lymphadenopathy
| Cytology parameter, n (%) | Total (n=179) | Sarcoidosis (n=145) | Tuberculosis (n=34) | P value |
| Adequacy | 165 (92.2) | 132 (91) | 33 (97.1) | 0.16 |
| Granuloma identified | 135 (75.4) | 113 (77.9) | 22 (64.7) | 0.69 |
| Absence of hemorrhage | 56 (31.3) | 36 (24.8) | 20 (58.8) | 0.002 |
| Number of granulomas per smear* | 0.11 | |||
| <3 | 23 (17) | 17 (15) | 6 (27.3) | |
| 3-6 | 19 (14.1) | 19 (16.8) | 0 | |
| 7-9 | 31 (23) | 27 (23.9) | 4 (18.2) | |
| >9 | 62 (45.9) | 50 (44.2) | 12 (54.5) | |
| Necrosis | 0.0001 | |||
| None | 151 (84.4) | 136 (93.8) | 15 (44.1) | |
| Grade 1 (Focal) | 16 (8.9) | 9 (6.2) | 7 (20.6) | |
| Grade 2 (Intermediate) | 9 (5) | 0 | 9 (26.5) | |
| Grade 3 (Extensive) | 3 (1.7) | 0 | 3 (8.8) | |
| AFB positivity** | 15 (8.4) | 0 | 15 (44.2) | 0.0001 |
| Cytologist’s final impression based on the morphology (including AFB stain)* | 0.003 | |||
| Correct diagnosis | 84 (62.2) | 70 (61.9) | 14 (63.6) | |
| Misdiagnosis | 39 (28.9) | 34 (30.1) | 5 (22.7) | |
| Indeterminate | 12 (8.9) | 9 (8) | 3 (13.1) | |
AFB – acid-fast bacilli; EBUS-TBNA – endobronchial ultrasound guided transbronchial needle aspiration
*n=135 (subjects having granuloma) was used as the denominator to calculate these percentages
**AFB smear performed on the EBUS-TBNA aspirate slides by the cytologist
Primary outcome: On the basis of cytomorphology of granuloma (n=135), the cytologist was able to make a correct diagnosis in 84 (62.2%) cases, and misdiagnosis occurred in 39 (28.9%). The differentiation between tuberculosis and sarcoidosis was indeterminate on cytology in 12 (8.9%). The sensitivity, specificity, PPV, and NPV of the cytologist’s impression to diagnose sarcoidosis was 62%, 64%, 90% and 25%, respectively (Table 3).
Table 3.
Diagnostic accuracy of various parameters in diagnosing sarcoidosis among subjects undergoing EBUS-TBNA for granulomatous lymphadenopathy
| Parameter | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
| Primary outcome* | ||||
| Diagnosis of sarcoidosis based on the cytomorphology of granuloma | 62 (52-71) | 64 (41-83) | 90 (81-95) | 25 (14-38) |
| Secondary outcomes** | ||||
| TST negativity (10 mm or less) | 94 (89-97) | 68 (49-83) | 93 (87-96) | 72 (53-86) |
| Absence of necrosis on cytology | 94 (89-97) | 56 (74-93) | 90 (84-94) | 68 (48-84) |
| Microbiology | ||||
| Negative Mycobacterial culture | 100 (97-100) | 44 (27-62) | 88 (83-93) | 100 (78-100) |
| Negative Xpert MTB/RIF# | 100 (97-100) | 44 (20-70) | 93 (87-97) | 100 (59-100) |
| EBUS-related characters | ||||
| Absence of heterogeneous echotexture or CNS in any node | 68 (49-83) | 77 (69-83) | 91(84-95) | 40 (27-54) |
| Combination of various parameters | ||||
| Identification of granuloma and negative TST | 72 (64-80) | 79 (62-91) | 94 (88-97) | 40 (28-53) |
| Non-necrotic granuloma and a negative TST | 71 (63-78) | 85 (69-95) | 95 (90-98) | 41 (29-53) |
| Identification of granuloma and a negative stain for AFB | 77 (69-83) | 68 (49-83) | 91 (84-95) | 40 (28-54) |
| Absence of endosonographic features suggestive of tuberculosis¶ and a negative TST | 72 (64-80) | 88 (73-97) | 96 (91-99) | 43 (31-55) |
| Presence of non-necrotic granuloma, negative TST and no endosonographic features suggestive of tuberculosis¶ | 55 (47-63) | 97 (85-100) | 99 (93-100) | 34 (24-44) |
AFB – acid fast bacilli; CI – confidence interval; CNS – coagulation necrosis sign; EBUS- endobronchial ultrasound; NPV – negative predictive value; PPV – positive predictive value; TBNA – transbronchial needle aspiration; TST – tuberculin skin test
Subjects with granuloma on cytology* (n=135) or the entire study cohort** (n=179) were used to calculate the primary and secondary outcomes, respectively (unless otherwise mentioned)
¶Heterogeneous echotexture and coagulation necrosis sign
#49 subjects in whom Xpert MTB/RIF was not available were excluded for this analysis
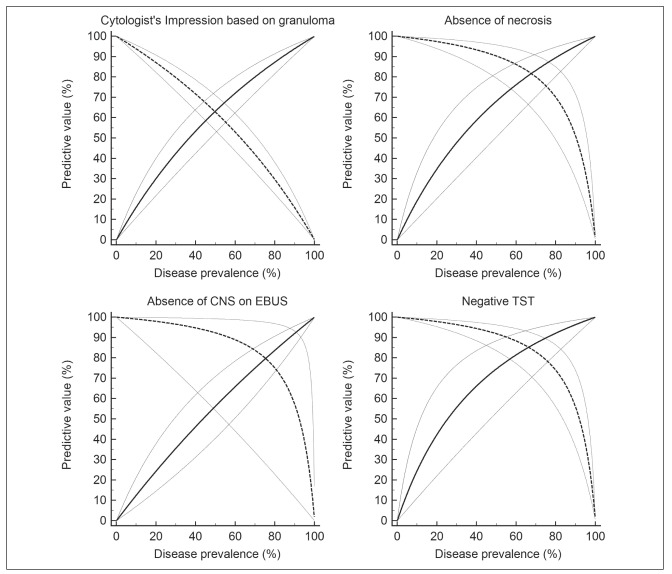
Secondary objectives: In subjects with sarcoidosis, mycobacteria were not demonstrated by either conventional microbiological investigations (smear or culture) or Xpert MTB/Rif, thereby yielding a sensitivity of 100%. The specificity was however low (44%) due to lack of demonstration of mycobacteria in a large number of cases of tuberculosis (Table 3). A negative TST, absence of necrosis on cytology, and the absence of CNS during EBUS were other useful features suggesting the diagnosis of sarcoidosis (Table 3). The mere identification of a granuloma had a poor specificity (35%) to diagnose sarcoidosis. However, the identification of a granuloma and the presence of a negative TST improved the specificity to 79% (Table 3). The presence of a non-necrotic granuloma, negative TST and the lack of endosonographic findings favouring tuberculosis (heterogeneous node and CNS) provided the best specificity (97%) and PPV (99%) to diagnose sarcoidosis. The positive predictive value of the various tests decreased significantly with decreasing prevalence of sarcoidosis (Figure 1).
Fig. 1.
Figure 1: Graph showing the relation between positive (solid line) and negative (dotted lines) predictive values of various study parameters to diagnose sarcoidosis (y-axis), with varying prevalence (along the x-axis). The graphs also show the 95% confidence interval for the PPV and NPV (lighter colour)
Discussion
The results of our study indicate that it is difficult to differentiate sarcoidosis from tuberculosis, solely based on cytomorphology. The absence of necrosis on cytology and a negative TST were useful features in diagnosing sarcoidosis. Necrosis was rare in sarcoidosis, and when present, it was never extensive. Although several findings shown in the current study are known, we reinforce the results using strict definitions for the final diagnosis, a large sample size, and the blinding of the cytologist to clinical and microbiological details (culture and Xpert MTB/RIF).
Granulomatous inflammation is a feature common to both tuberculosis and sarcoidosis. However, the identification of granuloma is neither sufficiently sensitive nor specific. A previous study investigating the rate of monocytopoiesis and monocyte recruitment in the granulomas, described the granuloma in tuberculosis to be “high turnover”, and sarcoidosis to be a “low turnover” granulomas (19). The high turnover and the increased macrophage destruction could explain the frequent occurrence of necrosis in tuberculous granulomas. In our study, the absence of necrosis had a high sensitivity for diagnosing sarcoidosis (94%) and its presence was distinctly uncommon (6% of sarcoidosis had necrosis in a study of bronchial biopsies) (20). However, it lacked specificity (56% in our study) as several patients with tuberculosis present with non-necrotic granuloma. In a study of 212 tubercular lymphadenitis, three major morphologies were observed on fine-needle aspiration cytology (FNAC) that correlated with the bacillary load. These included epithelioid granuloma without necrosis (stain for AFB was positive in 5% of these cases), granuloma with necrosis (AFB-positive in 60.8%), and necrosis alone in the absence of granuloma (AFB-positive in 77.1%) (21). Thus, the most specific feature for tuberculosis on cytomorphology (necrosis) seem to correlate well with microbiology. The cytologic features are seldom helpful in the subset of cases (non-necrotic granuloma with negative microbiological investigations), where differentiation of sarcoidosis from tuberculosis is difficult. This explains the diagnostic challenge faced by the cytologist, as also shown in the current study.
While the ultrastructural (using an electron microscopy) size and shape of nuclei in the inflammatory cells of the granuloma have been shown to be different in sarcoidosis and tuberculosis (22), they are unlikely to be useful in routine practice. In a small study, the cellular composition (using monoclonal antibodies against surface markers of lymphocytes/macrophages) of both sarcoid and tubercular granulomas have also been shown to be similar (11). Further, most of the evidence on cytomorphologic differentiation is from an era where molecular techniques to diagnose or exclude tuberculosis were not in vogue (20). In a recent study of 49 subjects undergoing EBUS-TBNA, cytomorphology alone was unable to differentiate sarcoidosis from tuberculosis (12), an observation similar to ours. Nevertheless, we found that certain features in cytology (necrosis), and a combination of cytologic features with TST are useful.
What does the current study add? We have systematically evaluated and provided the diagnostic accuracy of the various cytology parameters in diagnosing sarcoidosis among subjects undergoing EBUS-TBNA for suspected granulomatous lymphadenopathy. Misdiagnosing tuberculosis as sarcoidosis could be disastrous, particularly in high tuberculosis endemic region (in our study, 20% with suspected granulomatous lymphadenopathy had tuberculosis). The diagnostic accuracy of the cytology findings and EBUS signs would vary widely with varying prevalence of tuberculosis (and sarcoidosis).
Finally, our study has a few limitations. This was a single center study and all the slides were reported by a single experienced cytologist. Inter-observer correlation between cytologists was not evaluated. The EBUS findings of CNS and heterogeneous echotexture are frequently encountered in malignancies, another major indication for EBUS-TBNA. This would further alter the diagnostic accuracy of sonographic appearances in the real-world scenario. We primarily discuss the cytology findings, while in routine clinical practice the imaging findings (symmetric lymphadenopathy, presence of necrosis, and others), and involvement of extrapulmonary sites can provide additional clues to differentiate sarcoidosis and tuberculosis. We did find a significantly higher proportion of subjects with sarcoidosis having involvement of lymph node stations 11L, 4R and 7. Unfortunately, we have not systematically recorded and evaluated these differences in the current study. However, radiological appearance may be identical in both these diseases, and in fact, in a randomized trial of subjects with suspected sarcoidosis (based on imaging and clinical features), tuberculosis was diagnosed in 5.3% (15). Further, the results of our study cannot be extrapolated to immunocompromised hosts (such as HIV-AIDS) with suspected granulomatous adenopathy, where the morphologic features on cytology might be different.
In conclusion, cytomorphology alone is insufficient in differentiating sarcoidosis from tuberculosis in subjects undergoing EBUS-TBNA. A combination of microbiological, cytomorphological features (especially necrosis), TST, and endosonographic characteristics are helpful in the diagnosis of granulomatous mediastinal adenopathy.
References
- 1.Dhooria S, Sehgal IS, Gupta N, Aggarwal AN, Behera D, Agarwal R. Diagnostic Yield and Complications of EBUS-TBNA Performed Under Bronchoscopist-directed Conscious Sedation: Single Center Experience of 1004 Subjects. J Bronchology Interv Pulmonol. 2017;24(1):7–14. doi: 10.1097/LBR.0000000000000332. [DOI] [PubMed] [Google Scholar]
- 2.Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16(2):149–173. [PubMed] [Google Scholar]
- 3.Jindal SK, Gupta D, Aggarwal AN. Sarcoidosis in developing countries. Curr Opin Pulm Med. 2000;6(5):448–454. doi: 10.1097/00063198-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Dhooria S, Sehgal IS, Aggarwal AN, Agarwal R. Convex-probe Endobronchial Ultrasound: A Decade of Progress. Indian J Chest Dis Allied Sci. 2016;58(1):21–35. [PubMed] [Google Scholar]
- 5.Gupta D, Agarwal R, Aggarwal AN, Jindal SK. Sarcoidosis and tuberculosis: the same disease with different manifestations or similar manifestations of different disorders. Curr Opin Pulm Med. 2012;18(5):506–516. doi: 10.1097/MCP.0b013e3283560809. [DOI] [PubMed] [Google Scholar]
- 6.Dhooria S, Gupta N, Bal A, et al. Role of Xpert MTB/RIF in differentiating tuberculosis from sarcoidosis in patients with mediastinal lymphadenopathy undergoing EBUS-TBNA: a study of 147 patients. Sarcoidosis Vasc Diffuse Lung Dis. 2016;33(3):258–266. [PubMed] [Google Scholar]
- 7.Dhasmana DJ, Ross C, Bradley CJ, et al. Performance of Xpert MTB/RIF in the diagnosis of tuberculous mediastinal lymphadenopathy by endobronchial ultrasound. Ann Am Thorac Soc. 2014;11(3):392–396. doi: 10.1513/AnnalsATS.201308-250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asimacopoulos EP, Berry M, Garfield B, et al. The diagnostic efficacy of fine-needle aspiration using cytology and culture in tuberculous lymphadenitis. Int J Tuberc Lung Dis. 2010;14(1):93–98. [PubMed] [Google Scholar]
- 9.Wright CA, van der Burg M, Geiger D, Noordzij JG, Burgess SM, Marais BJ. Diagnosing mycobacterial lymphadenitis in children using fine needle aspiration biopsy: cytomorphology, ZN staining and autofluorescence - making more of less. Diagn Cytopathol. 2008;36(4):245–251. doi: 10.1002/dc.20788. [DOI] [PubMed] [Google Scholar]
- 10.Mittal P, Handa U, Mohan H, Gupta V. Comparative evaluation of fine needle aspiration cytology, culture, and PCR in diagnosis of tuberculous lymphadenitis. Diagn Cytopathol. 2011;39(11):822–826. doi: 10.1002/dc.21472. [DOI] [PubMed] [Google Scholar]
- 11.van den Oprd JJ, de Wolf-Peeters C, Facchetti F, Desmet VJ. Cellular composition of hypersensitivity-type granulomas: immunohistochemical analysis of tuberculous and sarcoidal lymphadenitis. Hum Pathol. 1984;15(6):559–565. doi: 10.1016/s0046-8177(84)80010-6. [DOI] [PubMed] [Google Scholar]
- 12.Kaur G, Dhamija A, Augustine J, Bakshi P, Verma K. Can cytomorphology of granulomas distinguish sarcoidosis from tuberculosis? Retrospective study of endobronchial ultrasound guided transbronchial needle aspirate of 49 granulomatous lymph nodes. Cytojournal. 2013;10:19. doi: 10.4103/1742-6413.119008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berzosa M, Tsukayama DT, Davies SF, et al. Endoscopic ultrasound-guided fine-needle aspiration for the diagnosis of extra-pulmonary tuberculosis. Int J Tuberc Lung Dis. 2010;14(5):578–584. [PubMed] [Google Scholar]
- 14.Gupta N, Muthu V, Agarwal R, Dhooria S. Role of EBUS-TBNA in the Diagnosis of Tuberculosis and Sarcoidosis. J Cytol. 2019;36(2):128–130. doi: 10.4103/JOC.JOC_150_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muthu V, Gupta N, Dhooria S, et al. A Prospective, Randomized, Double-Blind Trial Comparing the Diagnostic Yield of 21- and 22-Gauge Aspiration Needles for Performing Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in Sarcoidosis. Chest. 2016;149(4):1111–1113. doi: 10.1016/j.chest.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Kaur H, Dhooria S, Aggarwal AN, Gupta D, Behera D, Agarwal R. A Randomized Trial of 1% vs 2% Lignocaine by the Spray-as-You-Go Technique for Topical Anesthesia During Flexible Bronchoscopy. Chest. 2015;148(3):739–745. doi: 10.1378/chest.15-0022. [DOI] [PubMed] [Google Scholar]
- 17.Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol. 2009;4(5):568–577. doi: 10.1097/JTO.0b013e3181a0d82e. [DOI] [PubMed] [Google Scholar]
- 18.Dhooria S, Agarwal R, Aggarwal AN, Bal A, Gupta N, Gupta D. Differentiating tuberculosis from sarcoidosis by sonographic characteristics of lymph nodes on endobronchial ultrasonography: a study of 165 patients. J Thorac Cardiovasc Surg. 2014;148(2):662–667. doi: 10.1016/j.jtcvs.2014.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Schmitt E, Meuret G, Stix L. Monocyte recruitment in tuberculosis and sarcoidosis. Br J Haematol. 1977;35(1):11–17. doi: 10.1111/j.1365-2141.1977.tb00557.x. [DOI] [PubMed] [Google Scholar]
- 20.Danila E, Zurauskas E. Diagnostic value of epithelioid cell granulomas in bronchoscopic biopsies. Intern Med. 2008;47(24):2121–2126. doi: 10.2169/internalmedicine.47.1452. [DOI] [PubMed] [Google Scholar]
- 21.Masilamani S, Arul P, Akshatha C. Correlation of cytomorphological patterns and acid-fast Bacilli positivity in tuberculous lymphadenitis in a rural population of southern India. J Nat Sci Biol Med. 2015;6(Suppl 1):S134–138. doi: 10.4103/0976-9668.166121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tosi P, Miracco C, Luzi P, Cintorino M, Kraft R, Cottier H. Morphometric distinction of granulomas in tuberculosis and sarcoidosis. Difference in nuclear profiles. Anal Quant Cytol Histol. 1986;8(3):233–240. [PubMed] [Google Scholar]