Abstract
Background:
The 6-minute walking test, cardiopulmonary exercise testing, and spirometry are useful tools for evaluation of respiratory impairment and functional capacity in patients with lung disease. Sarcoidosis is a multisystem granulomatous disease of unknown etiology.
Objectives:
Since the pulmonary involvement can affect the quality of life in sarcoidosis patients, this study is aimed to evaluate the tests mentioned above in order to examine the functional capacity of sarcoidosis patients in different stages as well as the cause of exercise intolerance.
Methods:
This cross-sectional study was carried out on 50 Iranian patients with sarcoidosis. Patients were classified into three groups based on the findings of the chest radiography as well as the pulmonary CT scan, reported by an expert radiologist. Pulmonary, cardiac, and activity function have been evaluated in the patients, using cardiopulmonary exercise testing, the 6-minutes walking test, and spirometry.
Results:
In cardiopulmonary exercise testing, percent-predicted peak VO2 (57.75±15.49, p=0.015) and percent-predicted O2 pulse (70.54±17.37, p=0.013) were significantly lower in the third group, in comparison with the others. Also, VE/CO2 (AT) (34.99±5.67, p=0.000) was significantly higher in the third group, in comparison with the other ones. Percent-predicted VO2 showed a strong positive correlation with age (r=0.377, p=0.009), TSH (r= 0.404, p=0.007), and percent-predicted FVC (r=0.443, p=0.002). In addition, O2 pulse had a positive correlation with BMI (r=0.324, p=0.026), percent-predicted FVC (r=0.557, p= 0.000), and percent-predicted FEV1 (r=0.316, p=0.032).
Conclusions:
According to this study, ventilatory limitation, pulmonary involvement, and deconditioning are the main causes of activity limitations in sarcoidosis patients.
Key words: Respiratory function tests (D012129), Cardiopulmonary exercise testing (D005080), 6-minute walk test, Sarcoidosis (D012507), Spirometry (D013147)
Introduction
Sarcoidosis is a heterogeneous, inflammatory, multisystem, granulomatous disease of uncertain etiology (1-4). This disease can affect different organs, but the lungs are involved in more than 90% of the cases. Chest X-ray and spirometry are used to evaluate the extent of pulmonary involvement. Their findings suggest that there is a lack of coordination between the level of breath shortness, spirometry, and chest radiography in sarcoidosis (5). Dyspnea and inability to exercise are affected by several factors including lung involvement and cardiovascular, musculoskeletal, and neurological reasons (6).
Several modalities are available in order to evaluate the functional exercise capacity (7). In recent years, the Six-Minute Walk Test (6MWT) has been used as a prognostic tool in patients with heart failure and pulmonary disease (8). According to The American Thoracic Society guidelines, 6MWT has been found to be a simple, low-cost, renewable, repeatable, and acceptable method which can be applied with minimal facilities. In addition, it has been increasingly used in analyzing the performance of athletic tolerance (9-11). Several studies have evaluated the efficacy of 6MWT in the prediction of mortality rate among patients with chronic pulmonary disease, as well as in those who are candidates of lung transplantation (12, 13).
Cardio-Pulmonary Exercise Testing (CPET) is used to assess the status of the heart, lung, and muscle function (14). Through this test, the functional lung capacity (FLC), amount of consumed oxygen, physical fitness, and respiratory, cardiovascular, or muscular status are estimated by measuring the respiratory gases (15, 16). Since the Pulmonary Function Tests (PFTs) are not reliable enough to predict the functional limitation during exercise in the sarcoidosis patients, CPET can be considered a helpful method for detecting exercise tolerance in such cases (17). Furthermore, some studies have proven the reliability of CPET in the detection of pulmonary gas exchange impairment (PGEI) in early radiographic stages (18, 19).
Since pulmonary involvement is a common presentation of sarcoidosis, this study is aimed to evaluate the pulmonary function of the patients as well as monitoring their pulmonary status in treatment centers. The 6MWT and CPET were used to investigate the functional lung capacity in sarcoidosis patients in different stages and find the correlation between 6MWT and CPET with other clinical markers.
Materials and Methods
Subjects and study design
This research is a cross-sectional study carried out on 50 Iranian patients who were referred to the referral respiratory hospitals of Iran due to dyspnea and exercise limitation. The diagnosis of sarcoidosis was based on biopsy-proven non-caseating epithelioid cell granulomas, according to the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) guidelines (20), clinical features, and radiological findings. The inclusion criteria comprised having a biopsy result proven of sarcoidosis and being more than 18 years old. Those who had any respiratory disorders, recognized muscular disease, tuberculosis, cardiac disease as well as the active/passive smokers were excluded from the study. None of these patients had another relevant medical history or comorbidity.
Demographic data, including age, sex, weight, height, and type of treatment were collected from patient’s records. Clinical and paraclinical tests had also been conducted. Towards this end, levels of 25-hydroxyvitamin D, Hemoglobin concentration (Hb), Thyroid-Stimulating Hormone (TSH), Angiotensin Converting Enzyme (ACE), Calcium (Ca), and Erythrocyte Sedimentation Rate (ESR) had been measured in serum/blood/urine. Pulmonary Artery Pressure (PAP) and Ejection Fraction (EF) had been investigated by echocardiography, and stages of the disease and Pulmonary Artery Size had been measured through computed tomography scan (CT) and chest X-ray (CXR).
Based on the reports of expert radiologists, patients were classified into 3 groups in accordance with the findings of the chest radiography as well as the pulmonary CT scan. Group 1 consists of Stage 0 (radiologically normal) and Stage I (bilateral hilar lymphadenopathy without involvement of parenchymal); group 2, Stage II (bilateral hilar lymphadenopathy associated with parenchymal infiltrates) and Stage III (parenchymal infiltration without involvement of hilar lymphadenopathy); and finally group 3, Stage IV (evidence of pulmonary fibrosis) (20).
The study was approved by the ethics committee of Masih Daneshvari Hospital and Shahid Beheshti University of Medical Sciences, and informed consent was obtained from all participants. PFT, 6MWT, and CPET were performed on the same day for each case from July to December 2017.
PFT
Forced vital capacity (FVC) (% predicted), Forced Expiratory Volume in 1s (FEV1) (% predicted), and FEV1/FVC(%) were performed by pneumotachograph (Masterlab, Jaeger, Wurzburg, Germany) at the place of testing.
6MWT
Each patient had remained in a relaxed sitting position for at least 15 minutes before the beginning of the test. According to the mentioned guidelines, the participant walked along a 30-meter, flat, straight hall for 6 minutes (21). None of the patients received oxygen during the test. Heart rate was measured at the beginning as well as the end of the test and the distance walked in 6 minutes was measured as well (22). Peripheral capillary oxygen saturation (SpO2) was monitored continuously and automatically every 30 seconds during the test.
CPET
Patients pedaled at a rate of 50 rpm/min for 3 minutes without resistance (unloaded phase). Work rate was then increased 10-20 watts (W) each minute. Patients were encouraged to take the test for approximately 10 minutes; otherwise, the test was ended due to the symptom limitation including leg pain (which was the most common symptom limitation), chest pain, dyspnea, fatigue, etc. (23) Peripheral capillary oxygen saturation (SpO2), maximum oxygen consumption (VO2 peak), carbon dioxide production (VCO2), minute ventilation (VE), breathing reserve (BR), heart rate reserve (HRR), peak heart rate (HR), oxygen pulse (O2 pulse), and VE/CO2 of anaerobic threshold (AT) were collected by CPET, using Ergostick device of Geratherm company at Masih Daneshvari hospital, according to a standard protocol (23).
Statistical analysis
Statistical analyses were performed using SPSS 22.0 software. The qualitative data were reported as frequency and percentage, and the quantitative variables were reported as means, mean rank, and standard deviation. The Kolmogorov-Smirnov test was used to check the normality of the sample. PFT parameters were compared between groups using ANOVA followed by Tukey-HSD.
Clinical characteristics of the study population were compared among groups using ANOVA parametric testing followed by Tukey-HSD (for age, BMI, vitamin D level, ACE, Hb, ESR, TSH, Ca, and CaU) or Kruskal-Wallis followed by Mann–Whitney U test (for EF, PAP, and mPA). Diagnostic method and distribution of gender among groups were investigated using Fisher’s Exact Test and chi-Square, respectively.
6MWT results were compared between groups using ANOVA parametric testing for HR-0, HR-6, and distance) and Kruskal-Wallis nonparametric testing (for SPO2). CPET results were compared between groups using ANOVA parametric testing (for peak VO2, BR, O2 pulse, and VE/CO2) and Kruskal-Wallis nonparametric testing (for HRR and SPO2). The correlations between parameters were evaluated using Pearson’s correlation coefficient (r). P<0.05 was considered statistically significant.
The mean distribution of VO2 peak (% predicted) and O2 pulse (% predicted) between genders were compared using T-test.
Z-test was used to compare mean SPO2 and HR before and 6 minutes after starting 6MWT.
Results
Subjects
The clinical characteristics of the patients are presented in Table 1. Pulmonary sarcoidosis was classified as stage 0 in six participants (11.8%), stage I in one participant (2%), stage II in 24 participants (47.1%), stage III in nine participants (17.6%), and stage IV in 10 participants (19.6%). Since lung function indices were not statistically different between Stages 0 and I as well as Stages II and III, patients were grouped according to their radiological stages as follow: Group one: Stages 0-I (n=7), Group 2: Stages II–III (n=33), and Group 3: Stage IV (n=10).
Table 1.
Clinical and para clinical characteristics of the study population
| Variable | All Subjects (N=50) | Stages | |||
| 0-I (N=7) | II-III (N=33) | IV (N=10) | P-Value | ||
| Age, yrs | 48.58±8.54 | 47.14±9.96 | 48.88±7.30 | 48.60±11.82 | 0.89 |
| Gender, % | |||||
| Male | 38% | 14.29% | 33.33% | 70.00% | 0.050* |
| Female | 62% | 85.71% | 66.67% | 30.00% | |
| BMI, kg/m2 | 28.68±4.34 | 31.97±4.70 | 28.75±3.66 | 26.52±5.19 | 0.047* |
| Diagnostic method | |||||
| Lung biopsy | 59.1% | 0% | 60.61% | 100% | 0.000* |
| Skin biopsy | 30.6% | 100% | 24.24% | 0% | |
| Neck Lymph nodes biopsy | 10.2% | 0% | 15.15% | 0% | |
| Mean time from diagnosis, yrs | 5.47±5.48 | 5.14±4.14 | 4.48±5.40 | 9.33±5.48 | 0.042* |
| Vit D, ng/ml | 20.75±12.36 | 17.19±10.20 | 22.23±13.61 | 17.98±8.55 | 0.470 |
| Hb, g/dL | 13.85±1.02 | 13.58±1.06 | 13.80±1.05 | 14.23±0.93 | 0.428 |
| TSH, U/Ml | 2.41±1.14 | 2.41±1.27 | 2.53±1.25 | 2.05±0.53 | 0.551 |
| Ca, mg/dl | 9.45±0.58 | 9.32±0.58 | 9.60±0.62 | 9.21±0.42 | 0.207 |
| CaU, mg/day | 150.61±87.92 | 198.50±111.02 | 129.88±27.75 | 159.38±122.52 | 0.600 |
| ACE, U/L | 71.56±35.01 | 86.20±44.35 | 71.44±35.74 | 63.78±28.48 | 0.529 |
| ESR, mm/hr | 23.87±17.99 | 23.86±12.98 | 25.22±22.02 | 20.44±7.75 | 0.805 |
| Echocardiography findings | |||||
| PAP, mmHg | 26.02±8.20 | 23.71±6.21 | 23.24±4.66 | 38.00±9.50 | 0.000* |
| EF, % | 53.91±4.00 | 55.00±2.89 | 55.15±1.97 | 48.56±5.92 | 0.004* |
| CT findings | |||||
| Main Pulmonary Artery Diameter (mPA), mm | 23.78±3.59 | 21.82±2.40 | 23.70±3.33 | 25.61±4.64 | 0.188 |
Data are presented as the mean ± SD or present for 50 patients; BMI=body mass index; Vit D=25-hydroxy vitamin D; Hb=Hemoglobin concentration; TSH=Thyroid-Stimulating Hormone; Ca=Calcium; CaU=Urine calcium level; ACE=Angiotensin Converting Enzyme; ESR=Erythrocyte Sedimentation Rate; PAP= Pulmonary Artery Pressure; EF=Ejection Fraction
The most common diagnostic method for sarcoidosis was lung biopsy, followed by skin biopsy. Biopsy of neck lymph nodes was conducted in five patients.
About 54.4% of patients were receiving Prednisolone, 42.2% Methotrexate, and 3.3% Cyclosporineas the treatment. There was not any significant difference between levels of peak VO2 and receiving Prednisolone, Methotrexate, or Cyclosporineas the treatment.
Females (mean ± SD: 74.30±14.59) reached a higher peak O2 pulse (% predicted) compared with males (mean ± SD: 63.41±20.37) (female vs. male, P= 0.039). VO2 (% predicted) was not significantly different between females and males.
PFT, 6MWT, and CPET results
PFT, 6MWT, and CPET results are detailed in Table 2.
Table 2.
Results of PFT, 6MWT, and CPET
| Variable | All Subjects (N=50) | Stages | |||
| 0-I (N=7) | II-III (N=33) | IV (N=10) | P-Value | ||
| PFT | |||||
| FVC (% predicted) | 79.01±21.53 | 90.00±27.03 | 83.25±16.93 | 58.20±18.47 | 0.001* |
| FEV1 (% predicted) | 73.99±23.96 | 91.29±28.42 | 75.80±21.81 | 56.10±16.92 | 0.007* |
| FEV1/ FVC (%) | 83.98±13.71 | 95.87±16.45 | 82.42±13.11 | 80.80±10.12 | 0.041* |
| 6MWT | |||||
| SPO2-0 | - | 34.29 | 26.97 | 15.50 | 0.010* |
| SPO2-6 | - | 36.07 | 26.62 | 14.40 | 0.007* |
| HR_0 (beats/min) | 86.38±12.07 | 85.29±10.37 | 85.18±9.81 | 91.10±18.68 | 0.393 |
| HR_6 (beats/min) | 124.22±20.37 | 118.00±19.53 | 121.64±18.10 | 137.10±24.55 | 0.072 |
| Distance (meter) | 436.60±92.59 | 465.86±49.73 | 467.73±74.13 | 313.40±66.43 | 0.000* |
| CPET | |||||
| Peak VO2 | 1.16±0.35 | 1.05±0.30 | 1.19±0.32 | 1.05±0.40 | 0.399 |
| peakVO2 (% predicted) | 57.75±15.49 | 50.50±8.17 | 61.81±13.95 | 47.40±17.51 | 0.015* |
| VO2/kg (ml/kg/min) | 14.89±4.34 | 12.17±3.73 | 15.48±3.97 | 13.63±4.27 | 0.129 |
| VE | 52.63±15.30 | 43.33±10.84 | 53.06±13.92 | 54.60±19.84 | 0.304 |
| VE(% predicted) | 58.25±15.65 | 45.67±9.81 | 61.65±14.16 | 54.90±19.95 | 0.055 |
| VE/VCO2 (AT) | 34.99±5.67 | 33.52±4.81 | 33.36±4.08 | 41.28±6.63 | 0.000* |
| HR peak | 143.21±20.60 | 131.17±21.37 | 145.47±17.34 | 140.50±26.67 | 0.286 |
| HR% | 84.81±11.64 | 75.50±9.01 | 86.80±10.15 | 83.00±14.79 | 0.080 |
| HRR (beats/min) | - | 35.00 | 21.77 | 26.95 | 0.086 |
| O2 pulse | 8.20±2.37 | 8.07±1.80 | 8.40±2.39 | 7.37±2.59 | 0.496 |
| O2 pulse (% predicted) | 70.54±17.37 | 76.17±20.06 | 73.77±14.66 | 56.30±18.57 | 0.013* |
| BR (breaths/min) (%) | 36.16±23.18 | 34.73±15.57 | 37.37±23.06 | 32.35±29.45 | 0.838 |
Data are presented as the mean ± SD for all parameters except SPO2-0, SPO2-6, and HRR which are presented as mean rank in each group. Asterisk indicates Significant (P<0.05); PFT=Pulmonary Function Tests; FVC=forced vital capacity; FEV1=forced expiratory volume in one second; 6MWT=6 min Walking Test; SPO2-0=oxygen saturation of 0 min; SPO2-6=oxygen saturation after 6MWT; HR=Heart Rate; Distance=traveled in 6MWT; CPET=Cardiopulmonary exercise testing; Peak VO2=peak oxygen uptake; VO2/kg=Volume of oxygen per kilogramme of body weight per minute; VE=Minute Ventilation; VE/VCO2(AT)=ventilatory equivalent for carbon dioxide at anaerobic threshold; HRR=Heart Rate Reserve; O2 pulse=oxygen pulse; BR=Breathing reserve
Since there were no significant differences between the SPO2 of 6MWT and CPET tests, only the result of 6MWT is expressed (Table 2). SPO2-0, SPO2-6, and the distance were lower in group three in comparison with other groups (Table 3). On the other hand, except in group one, SPO2 of all patients had decreased after six minutes. HR of patients in minute 0 and six in group three were higher, although this difference was not statistically significant (Table 2). HR had increased significantly after six minutes (P<0.000).
Table 3.
Comparing Variables’ P-Values between groups
| Parameters/Stages | P-Value | ||
| 0-I vs II–III | 0-I vs IV | II–III vs IV | |
| Peak VO2(%predicted) | 0.188 | 0.907 | 0.021* |
| O2 pulse (%predicted) | 0.942 | 0.056 | 0.013* |
| VE/VCO2(AT) | 0.997 | 0.008* | 0.000* |
| SpO2-0 | 0.372 | 0.002* | 0.000* |
| SpO2-6 | 0.082 | 0.011* | 0.014* |
| FVC(%predicted) | 0.671 | 0.004* | 0.002* |
| FEV1(%predicted) | 0.220 | 0.006* | 0.044* |
| FEV1/FVC % | 0.044* | 0.060 | 0.937 |
| Distance, meter | 0.998 | 0.000* | 0.000* |
*Asterisk indicates Significant (P<0.05); Peak VO2=peak oxygen uptake; O2 pulse=oxygen pulse; VE=Minute Ventilation; SPO2-0=Peripheral capillary oxygen saturation of minute 0; SPO2-6=Peripheral capillary oxygen saturation of minute 6, FVC=forced vital capacity; FEV1=forced expiratory volume in one second; Distance=traveled in 6MWT
Correlations
The correlation of two variables of VO2 (% predicted) and O2 pulse (% predicted) with Age, BMI, TSH, ACE, ESR, Vit D, Hb, Ca, CaU, PAP, EF, main pulmonary artery diameter, PFT parameters, and distance had been evaluated (Table 4).
Table 4.
The correlation between VO2(%predicted) and O2 Pulse(%predicted) with Age, Gender, BMI, TSH, ACE, ESR, Vit D, Hb, PAP, EF, Main Pulmonary Artery Diameter, PFT results and distance
| PeakVO2(% predicted) | O2pulse(% predicted) | ||||
| p | r | p | r | ||
| AGE | 0.009 | 0.377** | 0.051 | 0.287 | |
| BMI | 0.675 | 0.063 | 0.026 | 0.324* | |
| TSH | 0.007 | 0.404** | 0.072 | 0.274 | |
| ACE | 0.130 | 0.261 | 0.410 | 0.144 | |
| ESR | 0.635 | 0.082 | 0.686 | 0.070 | |
| Vit D | 0.145 | 0.218 | 0.909 | -0.017 | |
| Hb | 0.447 | -0.116 | 0.917 | 0.016 | |
| CA | 0.161 | 0.242 | 0.513 | 0.114 | |
| CAU | 0.974 | -0.009 | 0.670 | -0.112 | |
| PAP | 0.203 | -0.191 | 0.186 | -0.199 | |
| EF | 0.653 | 0.068 | 0.110 | 0.239 | |
| Main Pulmonary Artery Diameter | 0.314 | -0.152 | 0.119 | -0.233 | |
| PFT | |||||
| FVC(% predicted) | 0.002 | 0.443** | 0.000 | 0.557** | |
| FEV1(% predicted) | 0.339 | 0.144 | 0.032 | 0.316* | |
| FEV1/FVC % | 0.423 | -0.120 | 0.751 | 0.048 | |
| 6MWD | 0.058 | 0.278 | 0.011 | 0.369* | |
*Correlation is significant at the 0.05 level (2-tailed)
**Correlation is significant at the 0.01 level (2-tailed)
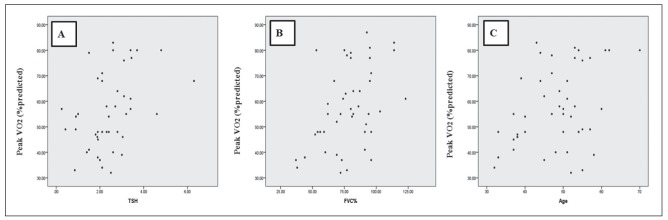
VO2 (% predicted) showed a strong positive correlation with age, TSH, and FVC (% predicted) (Figure 1). There was not any correlation between VO2 (% predicted) and BMI, ACE, ESR, Vitamin D, Hb, Ca, CaU, PAP, EF, main pulmonary artery diameter, FEV(% predicted), FEV1/FVC %, and distance.
Fig. 1.
Relationship of Peak VO2 (% predicted) with Thyroid-Stimulating Hormone (r=0.404; P=0.007) (panel A); FVC(% predicted) (r=0.443; P=0.002) (panel B) and age (r=0.377; P=0.009) (panel C). Correlation was determined using Pearson’s correlation coefficient (r). P<0.05 was considered statistically significant (2-tailed)
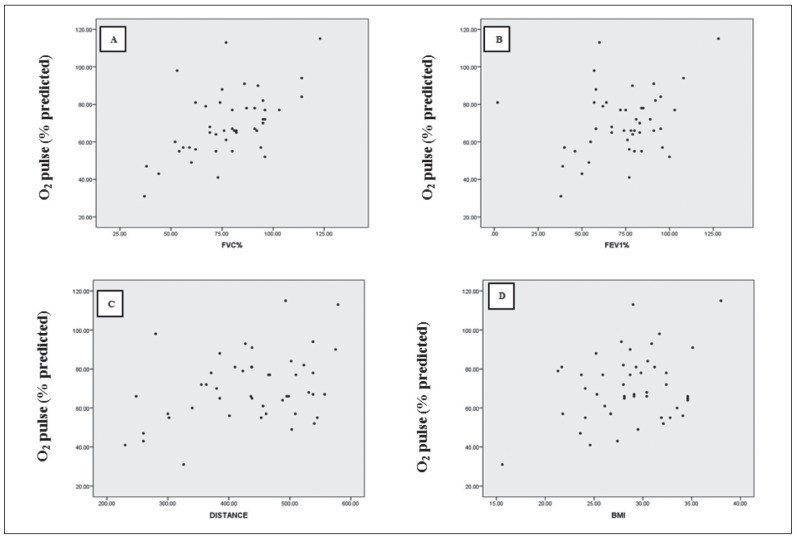
There was a moderate bivariate correlation between O2 Pulse (% predicted) and BMI; and FEV1 (% predicted) and 6MWD. In addition, a strong positive correlation has been seen between O2 Pulse (% predicted) and FVC (% predicted) (Figure 2). There was not any correlation between O2 Pulse (% predicted) and Age, TSH, ACE, ESR, Vitamin D, Hb, Ca, CaU, PAP, EF, main pulmonary artery diameter, and FEV1/FVC %.
Fig. 2.
Relationship of O2 pulse (% predicted) with FVC (% predicted) (r=0.557; P=0.000) (panel A); FEV1 (% predicted) (r=0.316; p=0.032) (panel B); distance (r=0.369; P=0.011) (panel C) and BMI (r=0.324; P=0.026) (panel D). Correlation was determined using Pearson’s correlation coefficient (r). P<0.05 was considered statistically significant, with the exception of the correlation between O2 pulse (% predicted) and FVC (% predicted) which significance was considered as P<0.01 (2-tailed)
Discussion
Due to the effects of sarcoidosis on multiple organs including lungs, heart, and rarely musculoskeletal system (5, 24) in addition to its effects on the patients’ activity performance, there is a real need to evaluate the pulmonary function and activity performance in this disease. This cross-sectional study is aimed to assess the role of CPET, the 6MWT, and spirometry test as the monitoring tools in sarcoidosis patients.
Findings of the current study, achieved through CPET, the 6MWT, and spirometry, revealed intolerance of sarcoidosis patients through CPET in advanced stages.
Percent-predicted VO2 and O2 pulse and their correlation were compared in different stages. The relationship between the above-mentioned parameters, clinical and paraclinical characteristics, and other parameters, such as the 6MWT, CPET, and PFT, have also been investigated in sarcoidosis patients.
The third group had reached a lower percent-predicted peak VO2, percent-predicted O2 pulse, and PFT in comparison with other groups. These findings are consistent with previous studies, in which the mentioned parameters decreased as well (Wallaert, 2011 #119) (25-28). Therefore, the assessments of percent-predicted VO2 and O2 are considered effective tools to predict the pulmonary and physical functioning in patients with sarcoidosis, especially in advanced stages. A lower percent-predicted peak VO2 in the third group, in comparison with other groups, may be due to the severity of the disease in the constituents. Percent-predicted peak VO2 was higher in group two, in comparison with group one. This contradictory result may either be related to the deconditioning in patients of group one or due to other reasons which are not related to cardio-pulmonary diseases. This finding is consistent with previous studies, in which the said parameter was also decreased in advanced stages (26-29).
Since VO2/kg (ml/kg/min) depends on percent-predicted peak VO2, we expected significant decreases in VO2/kg (ml/kg/min) in the third group, in comparison with other groups. However, VO2/kg (ml/kg/min) was not significantly lower in the third group. This is justified by the simultaneous reduction of the mean BMI at stage IV. Both mean BMI and percent-predicted peak VO2 decreased in the third group, in comparison with other groups. Therefore, VO2/kg in the third group was not different from the others. The decrease in BMI, in the third group, has been associated with increased breathing problems, due to which more energy is spent on breathing, or it may be because of the decreased appetite in patients with chronic diseases.
Percent VE was unexpectedly higher in group two, in comparison with group one, although this difference was not statistically significant. This finding poses the possibility of higher effort made by the patients of the second group, and also revealed that subjects in this group had performed the test for a longer period. It is obvious that subjects in the first group failed to continue the test, because of irrelevant reasons to ventilation and cardiovascular defects. As expected, BR decreased in the third group. However, this difference was not statistically significant, which may be due to the small sample size in the third group.
In the current study, VE/VCO2 (AT) increased significantly in stage IV, which was completely proportional to the severity of the disease in the third group. This finding was in agreement with the studies published by Kallianos (29) and Wallaert (25). Since the increased VE/VCO2 (AT) is a gas exchange abnormally, this parameter could be used as a prognostic factor in chronic heart failure (29). Therefore, probably it can also be used as a predictor of mortality in sarcoidosis.
A decrease in percent-predicted peak VO2 is correlated with a decrease of percent-predicted FVC in all cases and is in agreement with Lopes (27) and Wallaert (25) studies. This decrease correlates with the severity of the disease at advanced stages.
Two parameters of HR and SPO2 are measurable using both the 6MWT and CPET tests with no significant difference. So, the 6MWT, due to lower costs and easiness to perform, is more suitable to use instead of CPET for evaluating the parameters in advanced stages. This finding is in agreement with the American Thoracic Society guidelines for the 6MWT that find this test cheaper, easier to perform, more tolerable, and more reproducible, compared to the other tests (9-11).
To mention the 6MWT’s weakness, in addition to that it is not possible to calculate the patients’ VCO2 and VE promptly, it also does not provide us with the cause of intolerance. On the other word, the reason behind stopping the test is not being defined by the 6MWT. For instance, in CPET after the interruption caused by exhaustion, it can be determined that it was due to deconditioning, but in the 6MWT we cannot be sure whether it is because of the exhaustion or respiratory reasons.
Although HR peak and percent-predicted HR were unexpectedly decreased in the third group, in comparison with the second one, this difference was not statistically significant. This incompatible result may either be related to the inability of cases in the third group to continue the test for a longer time or lower effort of these patients to continue the test.
If the halt in performing CPET will be related to cardiac insufficiency, in spite of the common belief that HRR will decrease, it may even increase in the said situation. Similarly, we witnessed that HRR had more escalation in the third group in comparison to the second group. The Lack of increase in HRR in higher stages can be explained by the inability of the patients in achieving tachycardia due to cardiac conductive disease.
The VO2 /HR ratio, which is usually named as “oxygen pulse” (7, 30), significantly decreased in advanced stages. This finding was consistent with the results of Wallaert et al. (25) in which patients at stage IV had reached a lower O2 pulse compared with those of the other stages. We also found a correlation between the 6MWD and O2 pulse. Our study revealed that percent-predicted O2 pulse was influenced by gender, BMI, percent-predicted FVC, and percent-predicted FEV1. Therefore, percent-predicted O2 pulse could be a prognostic factor in sarcoidosis.
In the current study, as expected, SPO2 decreased in the third group. The decrease of SPO2 is correlated with the severity of the disease. Therefore, evaluating the SPO2 can be considered a convenient way to predict the mortality in sarcoidosis patients. In group one, the increase of SPO2 after walking for six minutes was related to the increases in heart rate after physical activity.
The 6MWD decreases in many patients with sarcoidosis (31). Since the 6MWD is correlated with several factors (e.g., FVC and oxygen saturation), it seems that these parameters can be used to evaluate the functional status of patients with sarcoidosis. In the current study, the distance walked during six minutes was significantly less in patients at stage IV, which was consistent with the results of our last study (32) as well as the studies by Alhamad et al. (33) and Wallaert et al. (25).
We found that percent-predicted peak VO2 was affected by age and TSH levels. This was in accordance with that of Artur et al. study, explaining that VO2 is an age-related parameter (34). Regarding TSH levels, our result is inconsistent with that of Ittermann study (35). Sarcoidosis is known as one of the causes of subclinical hypothyroidism (36), and we also concluded that percent-predicted peak VO2 which was affected by multiple factors, is affected by TSH levels as well.
Our study revealed a correlation between percent FVC and percent peak VO2, which is consistent with Wallaert et al. (25) and Karetzky et al. (37) results; introducing percent FVC as a major significant predictor of percent peak VO2. However, it is in contrast with Matthews study, also demonstrating the lack of correlation between PFT and percent peak VO2 (38).
Since 25(OH) Vit D deficiency is known as a predicting factor, regarding the course of chronic sarcoidosis (39), we expected to witness the relationship between percent-predicted VO2 and percent-predicted O2 pulse with the mentioned biomarker; but it was not found as a contributing factor in this regard. This is likely to be due to the epidemy of moderate to severe vitamin D deficiency in Iran (40, 41).
Despite a reverse correlation that was seen between the chronicity of sarcoidosis and ACE levels (42) among the Iranian population, we did not find any significant correlation between ACE levels, percent-predicted VO2, and O2 pulse. ACE levels were lower in advanced stages, but the lack of a significant association may be due to the small sample size in our third group.
As far as we know, the correlation of PAP, EF, main pulmonary artery size, percent-predicted VO2, and percent-predicted O2 pulse were evaluated in sarcoidosis patients. For the first time, through the current study we encountered lack of the association between the mentioned parameters, percent-predicted VO2, and percent-predicted O2 pulse and this may be probably due to the test sites which are situated at 1700 meters above sea level. Future parallel studies in lower areas may clarify this issue.
Since PAP increased significantly in the third group, it can be used as a predictor of disease severity in sarcoidosis. Considering that EF had a significant decrease in the stage IV patients, a decrement in EF can also be considered as a predictor of disease severity in sarcoidosis patients.
It is noteworthy that we did not classify our patients according to the type of pulmonary involvement. Although patients in the third group clearly had fibrosis involvement in their lungs, some cases may have had pulmonary vascular involvement, which undoubtedly has an effect on the results. This should be mentioned as the limitation of our study.
In conclusion, CPET revealed restriction in exercise capacity to a similar extent, disregarding the radiological stage in patients with sarcoidosis, while the 6MWT represents clinical weakness only in those with the most advanced disease.
Another finding of the current study is that pulmonary constraint is the main cause of activity limitation in sarcoidosis patients. However, it should be stated that deconditioning is as effective as the pulmonary constraint in activity limitation in sarcoidosis. The mentioned point is particularly crucial in the first stages of the disease because patients still do not have ventilation disorder. We recommend the use of non-pharmacological treatments and rehabilitation for them in order to tackle the deconditioning and furthermore improving the patients’ dyspnea. It should be considered that dyspnea is not always caused by ventilatory problems urging us to prescribe immunosuppressors leading to worsening of the deconditioning.
In further studies, we are planning to start rehabilitation and physical exercise for patients with deconditioning and rerun the tests and investigate the impact on dyspnea.
Acknowledgments
The authors wish to thank all the sarcoidosis patients and all laboratory specialists who helped to get results of better quality.
References
- 1.Costabel U. Sarcoidosis: clinical update. European Respiratory Journal. 2001;18(32 suppl):56s–68s. [PubMed] [Google Scholar]
- 2.Judson M, et al. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases: official journal of WASOG. 1999;16(1):75–86. [PubMed] [Google Scholar]
- 3.Semenzato G. ACCESS: A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases: official journal of WASOG. 2005;22(2):83–86. [PubMed] [Google Scholar]
- 4.Francis D, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2slope and peak VO2. European Heart Journal. 2000;21(2):154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 5.Baughman RP, et al. Clinical characteristics of patients in a case control study of sarcoidosis. American journal of respiratory and critical care medicine. 2001;164(10):1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 6.Meek PM, et al. Dyspnea: mechanisms, assessment, and management: a consensus statement. American Journal of Respiratory and Critical Care Medicine. 1999;159(1):321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 7.Wasserman K, et al. Vol. 3. Lippincott Williams & Wilkins Philadelphia; 2005. Principles of exercise testing and interpretation. [Google Scholar]
- 8.Kotloff RM, et al. Comparison of short-term functional outcomes following unilateral and bilateral lung volume reduction surgery. Chest. 1998;113(4):890–895. doi: 10.1378/chest.113.4.890. [DOI] [PubMed] [Google Scholar]
- 9.Enright PL. The six-minute walk test. Respiratory care. 2003;48(8):783–785. [PubMed] [Google Scholar]
- 10.Sciurba F, et al. Six-minute walk distance in chronic obstructive pulmonary disease: reproducibility and effect of walking course layout and length. American Journal of Respiratory and Critical Care Medicine. 2003;167(11):1522–1527. doi: 10.1164/rccm.200203-166OC. [DOI] [PubMed] [Google Scholar]
- 11.Li AM, et al. Standard reference for the six-minute-walk test in healthy children aged 7 to 16 years. American Journal of Respiratory and Critical Care Medicine. 2007;176(2):174–180. doi: 10.1164/rccm.200607-883OC. [DOI] [PubMed] [Google Scholar]
- 12.Miyamoto S, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension: comparison with cardiopulmonary exercise testing. American journal of respiratory and critical care medicine. 2000;161(2):487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 13.VanWagner LB, et al. Use of six-minute walk test to measure functional capacity after liver transplantation. Physical therapy. 2016;96(9):1456–1467. doi: 10.2522/ptj.20150376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller A, et al. Cardiorespiratory responses to incremental exercise in sarcoidosis patients with normal spirometry. Chest. 1995;107(2):323–329. doi: 10.1378/chest.107.2.323. [DOI] [PubMed] [Google Scholar]
- 15.Risk C, Epler GR, Gaensler E. Exercise alveolar-arterial oxygen pressure difference in interstitial lung disease. Chest. 1984;85(1):69–74. doi: 10.1378/chest.85.1.69. [DOI] [PubMed] [Google Scholar]
- 16.Brådvik I, et al. Lung mechanics and gas exchange during exercise in pulmonary sarcoidosis. Chest. 1991;99(3):572–578. doi: 10.1378/chest.99.3.572. [DOI] [PubMed] [Google Scholar]
- 17.Marcellis RG, et al. Is there an added value of cardiopulmonary exercise testing in sarcoidosis patients? Lung. 2013;191(1):43–52. doi: 10.1007/s00408-012-9432-6. [DOI] [PubMed] [Google Scholar]
- 18.Barros WG, et al. Clinical, radiographic and functional predictors of pulmonary gas exchange impairment at moderate exercise in patients with sarcoidosis. Respiration. 2004;71(4):367–373. doi: 10.1159/000079641. [DOI] [PubMed] [Google Scholar]
- 19.Athos L, Mohler JG, Sharma OP. Exercise testing in the physiologic assessment of sarcoidosis. Annals of the New York Academy of Sciences. 1986;465(1):491–501. doi: 10.1111/j.1749-6632.1986.tb18526.x. [DOI] [PubMed] [Google Scholar]
- 20.Hutchinson J. Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ER. Am J Respir Crit Care Med. 1999;160(736):55. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 21.Statement, A guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 22.Prefaut C, et al. Exercise-induced arterial hypoxaemia in athletes. Sports Medicine. 2000;30(1):47–61. doi: 10.2165/00007256-200030010-00005. [DOI] [PubMed] [Google Scholar]
- 23.Society AT. ATS/ACCP statement on cardiopulmonary exercise testing. American journal of respiratory and critical care medicine. 2003;167(2):211. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 24.Silverstein A, Siltzbach LE. Muscle involvement in sarcoidosis: asymptomatic, myositis, and myopathy. Archives of neurology. 1969;21(3):235–241. doi: 10.1001/archneur.1969.00480150025002. [DOI] [PubMed] [Google Scholar]
- 25.Wallaert B, et al. Reduction of maximal oxygen uptake in sarcoidosis: relationship with disease severity. Respiration. 2011;82(6):501–508. doi: 10.1159/000330050. [DOI] [PubMed] [Google Scholar]
- 26.Pilzak K, et al. Physical Functioning and Symptoms of Chronic Fatigue in Sarcoidosis Patients. 2017 doi: 10.1007/5584_2017_85. [DOI] [PubMed] [Google Scholar]
- 27.Lopes A, et al. Cardiopulmonary exercise testing variables as predictors of long-term outcome in thoracic sarcoidosis. Brazilian Journal of Medical and Biological Research. 2012;45(3):256–263. doi: 10.1590/S0100-879X2012007500018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sietsema KE, et al. Abnormal oxygen uptake responses to exercise in patients with mild pulmonary sarcoidosis. Chest. 1992;102(3):838–845. doi: 10.1378/chest.102.3.838. [DOI] [PubMed] [Google Scholar]
- 29.Kallianos A, et al. Reduction of exercise capacity in sarcoidosis in relation to disease severity. Patient preference and adherence. 2015;9:1179. doi: 10.2147/PPA.S86465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jones NL. WB Saunders Company; 1997. Clinical exercise testing. [Google Scholar]
- 31.Baughman RP, Sparkman BK, Lower EE. Six-minute walk test and health status assessment in sarcoidosis. Chest Journal. 2007;132(1):207–213. doi: 10.1378/chest.06-2822. [DOI] [PubMed] [Google Scholar]
- 32.Samadi K, et al. Six-Minute Walking Test (6MWT) Results Assessment in Pulmonary Sarcoidosis atients. J Pulm Respir Med. 2016;6(341):2. [Google Scholar]
- 33.Alhamad EH. The six-minute walk test in patients with pulmonary sarcoidosis. Annals of thoracic medicine. 2009;4(2):60. doi: 10.4103/1817-1737.49414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herdy AH, Uhlendorf D. Reference values for cardiopulmonary exercise testing for sedentary and active men and women. Arquivos brasileiros de cardiologia. 2011;96(1):54–59. doi: 10.1590/s0066-782x2010005000155. [DOI] [PubMed] [Google Scholar]
- 35.Ittermann T, et al. Serum thyroid-stimulating hormone levels are not associated with exercise capacity and lung function parameters in two population-based studies. BMC pulmonary medicine. 2014;14(1):145. doi: 10.1186/1471-2466-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Biondi B, Cooper DS. The clinical significance of subclinical thyroid dysfunction. Endocrine reviews. 2007;29(1):76–131. doi: 10.1210/er.2006-0043. [DOI] [PubMed] [Google Scholar]
- 37.Karetzky M, McDonough M. Exercise and resting pulmonary function in sarcoidosis. Sarcoidosis, vasculitis, and diffuse lung diseases: official journal of WASOG/World Association of Sarcoidosis and Other Granulomatous Disorders. 1996;13(1):43–49. [PubMed] [Google Scholar]
- 38.Matthews JI, Hooper RG. Exercise testing in pulmonary sarcoidosis. Chest. 1983;83(1):75–81. doi: 10.1378/chest.83.1.75. [DOI] [PubMed] [Google Scholar]
- 39.Kiani A, et al. Association Between Vitamin D Deficiencies in Sarcoidosis with Disease Activity, Course of Disease and Stages of Lung Involvements. Journal of Medical Biochemistry. doi: 10.1515/jomb-2017-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heshmat R, et al. Vitamin D deficiency in Iran: A multi-center study among different urban areas. Iran J Public Health. 2008;(37 suppl) [Google Scholar]
- 41.Ebrahimi M, et al. Prevalence of vitamin D deficiency among Iranian adolescents. Journal of Pediatric Endocrinology and Metabolism. 2014;27(7-8):595–602. doi: 10.1515/jpem-2013-0428. [DOI] [PubMed] [Google Scholar]
- 42.Kahkouee S, et al. Serum ACE level in sarcoidosis patients with typical and atypical HRCT manifestation. Polish journal of radiology. 2016;81:458. doi: 10.12659/PJR.897708. [DOI] [PMC free article] [PubMed] [Google Scholar]