Abstract
Objective:
To identify serum cytokines which predict mortality and/or disease progression in patients with systemic sclerosis, especially with pulmonary involvement.
Methods:
Serum cytokines (IL-6, IL-7, IL-8, IL-10, CCL2, CCL4, TGF-β, TNF-α) were measured in 125 SSc patients, who were recruited and observed in our outpatient clinic. Of these, 60 had pulmonary involvement, classified as either interstitial lung disease (ILD, 43 patients), pulmonary arterial hypertension (PAH, 7 patients) or pulmonary hypertension and ILD (PH-ILD, 10 patients). The association of serum cytokines with clinical features was analysed and their correlation with BAL cytokines measured in a subset of SSc patients with ILD.
Results:
Serum cytokines were detected at different levels: high (TGF-β, median 287.5 pg/ml; CCL2, median 89.7 pg/ml; CCL4, median 104.2 pg/ml), low (IL-6, median 3.2 pg/ml; IL-7 median 2.3 pg/ml; IL-8, median 5.2 pg/ml; TNF-α, median 0 pg/ml but with a bimodal distribution) and very low (IL-10, median 0.4 pg/ml). IL-6 and IL-7 were predictive for death in a Cox regression analysis in all SSc patients as well as in all patients with pulmonary involvement; IL-6 was predictive for mortality in SSc-ILD patients. In a multivariate analysis, cytokine levels could also predict a change in lung function, e.g. IL-7 was a predictor for a decline of diffusion capacity (DLCO) by 20 or 30% in ILD patients. In a subset of ILD patients, serum cytokines were compared to BAL cytokines, but revealed only few correlations.
Conclusion:
In conclusion, the analysis of serum cytokines implicates a role as biomarkers, distinct from BAL.
Key words: systemic sclerosis, cytokines, chemokines, lung involvement, survival
List of abbreviations
- ACA Anti-centromere antibodies
- ACR American College of Rheumatology
- Anti-RNAP III RNA polymerase III
- Anti-Scl70 Anti-topoisomerase
- AUC Area under the curve
- AZA Azathioprine
- BAL Bronchoalveolar lavage
- CCL18 CC-chemokine ligand 18
- CCL2 CC-chemokine ligand 2
- CCL4 CC-chemokine ligand 4
- CI Confidence interval
- CYC Cyclophosphamide
- DLCO Diffusion capacity for carbon monoxide
- EULAR European League Against Rheumatism
- FVC Forced vital capacity
- HR Hazard ratio
- HRCT High resolution computed tomography
- IFN-g Interferon gamma
- IL-10 Interleukin-10
- IL-6 Interleukin-6
- IL-7 Interleukin-7
- IL-8 Interleukin-8
- ILD Interstitial lung disease
- IQR Interquartile range
- MCP-1 Monocyte chemotactic protein 1 (=CCL2)
- MIP-1b Macrophage inflammatory protein 1 beta (=CCL4)
- MMF Mycophenolate mofetil
- mRSS Modified Rodnan skin score
- MTX Methotrexate
- PAH Pulmonary arterial hypertension
- PH Pulmonary hypertension
- PH-ILD Pulmonary hypertension associated with interstitial lung disease
- ROC Receiver operating characteristics
- SD Standard deviation
- SSc Systemic sclerosis
- TGF-b Transforming growth factor beta
- TNF-a Tumor necrosis factor alpha
Introduction
Systemic sclerosis (SSc, scleroderma) is a connective tissue disease characterised by vascular damage, inflammation and progressive fibrosis of the skin and other internal organs (1). The exact etiology of SSc is unknown, current concepts include genetic predisposition, environmental factors and an abnormal immune function (2, 3). At present, it is unclear how inflammation and vasculopathy translate into the later fibrotic stages that cause multiple end-stage organ damage and death (4). The activity and frequency of T cells and monocytes/macrophages is increased in the circulation and tissues of SSc patients. They produce various cytokines, such as interleukin (IL)-8, CCL18, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, IL-6, and transforming growth factor (TGF)-β, that regulate inflammation and tissue fibrosis in SSc and are elevated in the serum of SSc patients (2, 5, 6). In addition, monocyte chemoattractant protein-1 (MCP-1 or CCL2) and macrophage inflammatory protein-1β (MIP-1β or CCL4) have been implicated in the initiating phase and in the perpetuation of the inappropriate fibroblast activation and concomitant extracellular matrix deposition that characterise this disease (7, 8).
Pulmonary involvement, and amongst it interstitial lung disease, has become the main determinant of mortality in systemic sclerosis (9). Because treatment is only efficacious for some patients and harbors side effects (10), there is a need to identify patients whose pulmonary involvement will progress. Bronchoalveolar lavage (BAL) has been evaluated for its prognostic capability with contradicting results (11-13). Even less data are available for a BAL cytokine analysis, but there is some evidence that BAL cytokines could predict clinical worsening (14, 15). In a previous study, our group has identified associations between BAL cytokines and pulmonary involvement: IL-7, IL-4, IL-8, and CCL2 were associated with ILD and of these, IL-4, IL-8, CCL2 were negatively correlated with LFT and CCL2 also with the CT fibrosis score (14).
However, BAL is not a routine procedure and there is scarce knowledge about the correlation of BAL and serum cytokine levels. Hence, we investigated the predictive capacity of the serum cytokines for survival in our SSc cohort, especially in patients with pulmonary involvement.
Materials and methods
Patients
Serum cytokines were measured in 125 SSc patients and all of these fulfilled the ACR/EULAR classification criteria for SSc (16). They were recruited consecutively during routine care through our outpatient clinic/ward and were followed up in our outpatient clinic. Of all 125 patients, 43 had interstitial lung disease (ILD) and 10 patients had ILD and pulmonary hypertension (named PH-ILD). 7 patients had pulmonary arterial hypertension (PAH). After approval of the local ethics committee of the Charité (EA1/013/705), patients were included on the basis of informed consent. For 21 of the patients with interstitial lung disease (ILD), concomitant BAL and serum cytokine analysis was available. Clinical and laboratory characteristics of the enrolled SSc patients are shown in Table 1. Disease duration was defined as the time from onset of the first non-Raynaud’s manifestation of SSc. The presence of anti-centromere antibodies (ACA), anti-topoisomerase I antibodies (Anti-Scl 70) and anti-RNA polymerase III antibodies (Anti-RNAP III) were determined by validated assays. Data were collected at the first visit and during follow-up. Only data from patients with serum cytokine measurements and available clinical evaluations were analysed. Missing items are indicated (e.g. in Table 1).
Table 1.
Clinical characteristics of 125 SSc patients
| Clinical characteristics of patients | No pulmonary involvement (n = 65) | SSc-PAH (n = 7) | SSc PH-ILD (n = 10) | SSc-ILD (n = 43) |
| Female patients (%) | 61 (93.8) | 7 (100) | 8 (80.0) | 37 (86.0) |
| Age (mean±SD) | 59.0±11.6 | 59.7±10.8 | 57.1±15.0 | 55.5±14.4 |
| SSc form (dSSc/lSSc) | 15/50 | 0/7 | 5/5 | 31/12 (p <0.001) |
| Disease duration in years at inclusion/first visit (mean±SD) | 7.5±7.6 | 12.7±10.3 | 3.3±3.2 | 5.7±7.0 |
| Antibody profile (Scl70/ACA/Anti-RNAP III/Other) (n) | 11/42/1/11 | 1/6/0/0 | 4/4/0/2 | 22/8/2/11 (p <0.0001) |
| MRSS (median, IQR) | 6 (3-10) | 9 (5-16) | 6.0 (5-16) |
11 (8-18) (p < 0.0001) |
| Follow-up duration (months,mean±SD) | 69.1±3.2 | 73.9±10.4 |
47.2±9.9 (p = 0.017) |
64.6±4.1 |
| Deceased patiens (%) | 2 (3.0) | 1 (14.3) |
4 (40.0) (p = 0.0023) |
7 (16.3) (p = 0.028) |
| DLCO (% predicted, median, IQR)# | 81.0 (71.1-90.0) |
65.4 (43.6-83.1) (p = 0.018) |
42.7 (22.4-70.3) (p = 0.0005) |
69.2 (57.8-80.8) (p = 0.0003) |
| FVC (% predicted, median, IQR)# | 98.4 (85.0-109.) | 94.5 (88.8-100) |
72.0 (61.3-87.9) (p = 0.0002) |
85.0 (72.6-99.4) (p = 0.0003) |
| DLCO decline >10% (%)* | 33 (50.8) | 5 (71.4) | 4 (40.0) | 23 (53.5) |
| DLCO decline >20% (n,%)* | 14 (21.5) | 3 (42.9) | 3 (30.0) | 13 (30.2) |
| FVC decline >10% (%)* | 6 (9.2) | 2 (28.6) |
4 (40.0) (p<0.024) |
8 (18.6) |
| FVC decline >20% (%)* | 1 (1.5) | 0 (0) | 1 (10.0) |
5 (11.6) (p = 0.036) |
| Glucocorticoid use (%) | 28 (43.1) | 1 (14.3) | 5 (50.0) | 25 (58.1) |
| Immunosuppression (%) (CYC, MTX, AZA, MMF) | 21 (32.3)1 | 4 (57.1) | 6 (60.0) |
35 (81.4) (p<0.0001)2 |
Items are given as frequency (and percentage), mean and standard deviation (SD) for normally distributed data or median and interquartile range. Significant differences to the group without pulmonary involvement were calculated using a student’s t-test for normally distributed data or a Mann-Whitney test, and Fisher’s exact test for categorical data, and are marked in bold. # = only for 124 patients available. * = data only for 122 patients available.
1) Immunosupression was mostly for progressive skin fibrosis, arthritis or myositis.
2) Of the 35 patients with immunosuppression, 14 received cyclophosphamide and 5 mycophenolate mofetil.
Abbreviations: SSc – systemic sclerosis; SSc-PAH – SSc-associated pulmonary arterial hypertension; PH-ILD – SSc-associated interstitial lung disease with pulmonary hypertension; SSc-ILD – SSc-associated interstitial lung disease; dSSc – diffuse systemic sclerosis; lSSc – limited systemic sclerosis; Scl70 – anti-topoisomerase I antibodies; ACA – anti-centromere antibodies; anti-RNAP III – anti-RNA-Polymerase III antibodies; mRSS – modified Rodnan skin score; FVC – forced vital capacity; DLCO – diffusion capacity for carbon monoxide; CYC – cyclophosphamide; MTX – methotrexate; AZA – azathioprine; MMF – mycophenolate mofetil; SD – standard deviation; IQR – interquartile range.
Clinical assessments
A complete medical history, physical examination and laboratory evaluation were obtained in each patient. Clinical assessment of skin sclerosis measured by modified Rodnan skin score (mRSS) was performed concurrently with blood sampling. SSc patients were classified according to the presence/absence of internal organ involvement at first visit, in which cytokine expression was measured, and during the follow up period (mean period 66.16±26.38 months). All patients were assessed for signs of lung fibrosis with pulmonary function tests and high-resolution computed tomography (HRCT) scans. Pulmonary fibrosis was defined by evidence of fibrosis such as bibasilar fibrosis on chest radiograms or HRCT scans or both. Pulmonary function tests included measurement of forced vital capacity (FVC) by flow-volume spirometry and pulmonary diffusing capacity for carbon monoxide (DLCO) by single-breath test (Jaeger GmbH, Würzburg, Germany). Values for FVC and DLCO were expressed as percentages of predictive normal values adjusted for age, sex, and height. Pre-capillary pulmonary hypertension (PH) was defined as estimated right ventricular systolic pressure ≥ 25 mmHg at right heart catheterization and a wegde pressure ≤ 15 mmHg; in patients without ILD, pulmonary hypertension was defined as pulmonary arterial hypertension (PAH). Clinical and laboratory characteristics on the basis of pulmonary involvement of the enrolled SSc patients are shown in Table 1. Development of lung function and mortality was assessed during follow-up.
Serum cytokines and chemokine assays
Fresh venous blood samples were taken at the SSc patients’ first visit. Samples were centrifuged shortly after clot formation. All serum samples were stored at −70°C prior to assays. For the detection of cytokines and chemokine concentrations, a commercially available bioplex system was used as previously described (14) for selected cytokines (IL-6, IL-7, IL-8, IL-10, CCL2, CCL4, TNF-α, and TGFβ1).
Statistical analysis
Descriptive statistics for continuous variables are presented either by the mean and standard deviation (SD) or median and interquartile range, where appropriate, categorical variables as frequency (and percentage). Significant differences to the group without pulmonary involvement were determined using a student’s t-test for normally distributed data or otherwise a Mann-Whitney test, and Fisher’s exact test for categorical data. The Cox proportional hazards model was used to evaluate predictors of time to death, displayed with respective hazard ratio (HR) and 95% confidence interval (CI). Parameters assumed to be associated with an improvement or decline of lung function were assessed with univariate and, if statistically significant, subsequent multivariate logistic regression. Improvement or decline of lung function was categorized as follows: improvement was defined by an increase of FVC or DLCO over 10% (of absolute predicted values), and a decline of FVC and DLCO was determined to be over 10%, 20% or 30% (of absolute predicted values). P values less than 0.05 were considered significant. Only for those cytokines, that were significantly associated with an outcome in the Cox model, receiver operating characteristic curves (ROC) with the area under the curve (AUC) were calculated to determine discriminative power of cytokine levels for predicting mortality or increase/decline of lung function parameters. Cut-offs were determined by the minimal distance of the ROC to the graph coordinates x=0,y=1. All calculations were performed using IBM SPSS Statistics version 20 and Graphpad Prism 6.0.
Results
Clinical characteristics of SSc patients
All patients were classified according to the presence of pulmonary involvement at the first visit and during the follow up period (Table 1). Patients with SSc-ILD had the highest proportion with diffuse SSc and anti-Scl70 positivity. According to pulmonary involvement, patients with SSc-ILD and PH-ILD had a lower FVC and DLCO. A decline >10% in FVC was most frequent in PH-ILD patients whereas a decline >20% was most frequent in ILD patients; in addition, immunosuppressive treatment was used more often in ILD. SSc-ILD and PH-ILD patients had the highest mortality during follow-up.
Serum cytokines in a cohort of 125 SSc patients
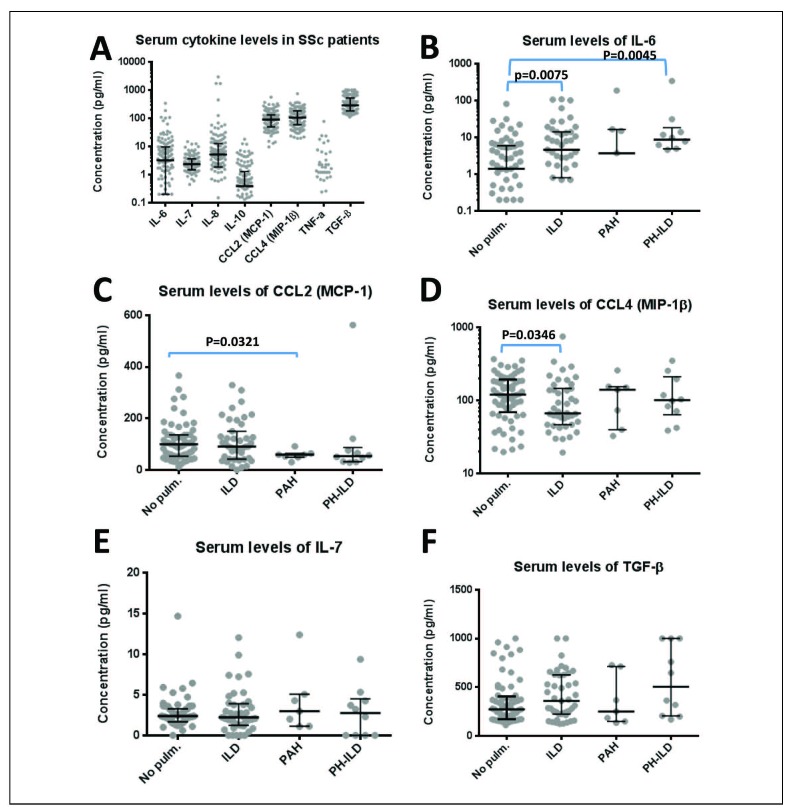
Serum cytokines could arbitrarily be grouped into three categories: high (TGF-β, median 287.5 pg/ml; CCL2, median 89.7 pg/ml; CCL4, median 104.2 pg/ml), low (IL-6, median 3.2 pg/ml; IL-7 median 2.3 pg/ml; IL-8, median 5.2 pg/ml; TNF-α, median 0 pg/ml, due to a bimodal distribution where most patients had undetectable levels of TNF-α) and very low (IL-10, median 0.4 pg/ml; Figure 1A). Patients with ILD and PH-ILD had higher serum levels of IL-6 than patients without pulmonary involvement (p=0.0075 and p=0.0045 respectively, Figure 1B), whereas CCL2 was lower in patients with PAH (p=0.0321, Figure 1C) and CCL4 was lower in ILD patients (p=0.0346, Figure 1D). Only a subset of patients had detectable levels of TNF-α, but there was no association between this cytokine and clinical features. Serum levels of IL-7 and TGF-β were not different between the patient subgroups (Figure 1E and 1F); however, 44.2% of SSc-ILD patients were above the 75th percentile of the TGF-β level of patients without pulmonary involvement (406 pg/ml; p= 0.04). There were no statistically significant differences in serum cytokine levels between lSSc and dSSc patients within the subgroups of pulmonary involvement (data not shown).
Fig. 1.
Serum cytokines of 125 SSc patients. Fig. 1A: Serum cytokines measured are displayed as median and interquartile range. Due to the high frequency of values below the detection limit which are not shown, for IL-10 and TNF-alfa the IQR cannot be displayed. Serum cytokines split up for patients without pulmonary involvement (No pulm.), SSc-associated interstitial lung disease (ILD), SSc-associated pulmonary arterial hypertension (PAH) and SSc-associated interstitial lung disease with pulmonary hypertension (PH-ILD) are displayed for IL-6 (Fig. 1B), CCL2 (MCP-1, Fig. 1C), CCL4 (MIP-1β, Fig. 1D), IL-7 (Fig. 1E) and TGF-β (Fig. 1F). Significant differences between the SSc subgroups were calculated using the Mann-Whitney test and are indicated.
Predictors of death
Of the SSc patients 125 patients in the cohort, we registered 14 deaths, of whom 7 were deemed SSc-related by the treating physicians, in 6 patients there was no information on this and 1 patient died breast cancer. In all patients, as well as in the subgroup with pulmonary involvement (n=60), in patients with PAH (n=7) or PH-ILD (n=10) or in patients with ILD (n=43), we calculated independently predictive factors for time to death with a Cox regression analysis using serum cytokine levels and clinical features (gender, age, SSc form, anti-Scl-70 and anti-centromere positivity, mRSS, DLCO, FVC, ILD and/or pulmonary hypertension at inclusion). In the whole cohort, the presence of pulmonary hypertension at inclusion was the strongest predictor of time to death (HR 84.4, 95% CI 9.4-759.3), but a higher mRSS and a lower FVC were also associated with a higher likelihood of death (Table 2). IL-6 and IL-7 were also both predictive in this population (HR 1.017, 95% CI 1.008-1.026 and HR 1.256, 95% CI 1.056-1.495 respectively). These two cytokines were also able to predict mortality in the subset of patients with pulmonary involvement (HR 1.010, 95% CI 1.002-1.017 and HR 1.379, 95% CI 1.139-1.671 respectively), in addition to pulmonary hypertension at inclusion and a lower FVC (Table 2). No predictive items could be found for PAH and PH-ILD patients. However, in ILD patients, IL-6 was an independent factor associated with mortality (HR 1.033, 95% CI 1.011-1.055), next to a lower FVC (Table 2).
Table 2.
Cox regression analysis of predictive items for death
| Group | Variable | Cox-Regression Analysis | ||
| P value | HR | 95% CI | ||
| Whole cohort | IL-6 | <0.0001 | 1.017 | 1.008 – 1.026 |
| IL-7 | 0.010 | 1.256 | 1.056 – 1.495 | |
| Pulmonary hypertension at inclusion | <0.0001 | 84.38 | 9.377 – 759.314 | |
| mRSS | 0.035 | 1.102 | 1.007 – 1.207 | |
| FVC | 0.003 | 0.959 | 0.934 – 0.986 | |
| All patients with pulmonary involvement | IL-6 | 0.012 | 1.010 | 1.002 – 1.017 |
| IL-7 | 0.001 | 1.379 | 1.139 – 1.671 | |
| Pulmonary hypertension at inclusion | <0.0001 | 48.512 | 6.039 – 389.679 | |
| FVC | 0.001 | 0.934 | 0.910 – 0.977 | |
| PAH/PH-ILD | None | |||
| ILD | IL-6 | 0.003 | 1.033 | 1.011 – 1.055 |
| FVC | 0.002 | 0.933 | 0.894 – 0.975 | |
Clinical items (gender, age, SSc form, anti-Scl-70 and anti-centromere positivity, mRSS, DLCO, FVC, lung fibrosis and/or pulmonary hypertension at inclusion) and serum cytokines were included in a Cox regression analysis in order to calculate independently predictive factors for death. Only significant items are given with the hazard ratio (HR) and the 95% confidence interval (95% CI).
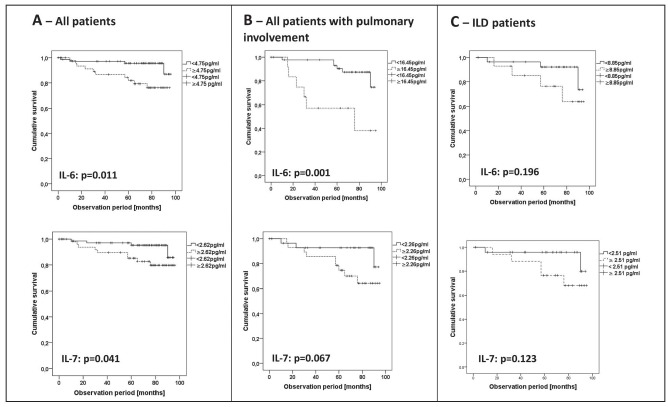
With cytokine cut-off levels calculated by a ROC analysis, Kaplan-Meier survival curves were generated for IL-6 and IL-7 in the respective level subsets (Figure 2). IL-6 and IL-7 cut-offs were able to define subgroups with a different mortality risk in the whole cohort (cut-off 4.75 pg/ml, AUC 0.724 and cut-off 2.62 pg/ml, AUC 0.686, respectively; Figure 2A). Furthermore, in the group of patients that had both IL-6 levels above 4.75pg/ml and IL-7 levels above 2.62 pg/ml there were more deaths (24.3%) than in the group who had only one cytokine above the cut-off or none (4.6%, p= 0.01). IL-6 was also a distinctive feature in all patients with pulmonary involvement (cut-off 16.45 pg/ml, AUC 0.693), whereas there was only a trend for IL-7 (Figure 2B). The Kaplan-Meier analysis in ILD patients showed no statistically significant difference of IL-6 or IL-7 subsets (cut-off IL-6 8.85 pg/ml, AUC 0.653; cut-off IL-7 2.51 pg/ml, AUC 0.700, both Figure 2C).
Fig. 2.
Predictive capacity of cytokine levels IL-7 and IL-6 for survival in different cohorts.
Kaplan-Meier survival curves are shown according to cytokine cut-off levels determined by ROC analysis in different cohorts, restricted to cytokines that were predictive for time to death in Cox regression analysis. Significance is given by the log-rank test comparing survival curves. A) All patients (whole cohort). B) All patients with pulmonary involvement. C) Patients with ILD.
Multivariate analysis of cytokines predictive for improvement or decline of lung function
Improvement or decline of lung function was documented during follow-up (see Table 1 for follow-up times). Amongst cytokines and clinical features (gender, age, SSc form, anti-Scl-70 and anti-centromere positivity, mRSS, DLCO, FVC, ILD and/or pulmonary hypertension at inclusion) we identified the following predictive factors: In SSc patients without pulmonary involvement, lower CCL2 and younger age at inclusion were associated with an increase in FVC >10% (HR 0.972, 95% CI 0.947-0.997 and HR 0.903, 95% CI 0.820-0.994, respectively; Table 3); whereas a higher age at inclusion was associated with a decline in FVC >10% (HR 1.107, 95% CI 1.003-1.222; Table 3). The cut-off level associated with an increase of FVC > 10% for CCL2 was calculated with a separate ROC analysis (cut-off 60.4 pg/ml, AUC 0.722). Both female sex and lower IL-10 levels (cut off 0.16 pg/ml, AUC 0.729) were associated with a decline of DLCO>20% in this population (HR 0.13.364, 95% CI 1.267-140.934 and HR 0.064, 95% CI 0.006-0.744, respectively, Table 3). In patients with pulmonary involvement, TGF-β (cut-off 360.7 pg/ml, AUC 0.661) was the only factor associated with lung function change: it was inversely associated with a decline in DLCO >20% (HR 0.997, 95% CI 0.995-1.000, Table 3). In patients with ILD, IL-7 was the only predictive factor for lung function change as it was associated with a decline of DLCO >20% (HR 1.591, 95% CI 1.078-2.349) at a cut-off level of 2.2 pg/ml (AUC 0.713) and with a decline >30% (HR 1.996, 95% CI 1.078-3.695) at a cut-off level of 3.9 pg/ml (AUC 0.726; Table 3).
Table 3.
Multivariate analysis of predictive items for improvement or deterioration of lung function
| Group | Variable | Factor | Multivariate analysis | ||
| P value | HR | 95% CI | |||
| No pulmonary involvement | FVC increase > 10% | CCL2 (MCP-1) | 0.028 | 0.972 | 0.947-0.997 |
| Age at inclusion | 0.038 | 0.903 | 0.820-0.994 | ||
| FVC decline > 10% | Age at inclusion | 0.044 | 1.107 | 1.003-1.222 | |
| DLCO decline > 20% | Female sex | 0.031 | 13.364 | 1.267-140.934 | |
| IL-10 | 0.028 | 0.064 | 0.006-0.744 | ||
| All patients with pulmonary involvement | DLCO decline > 20% | TGF-b | 0.041 | 0.997 | 0.995-1.000 |
| ILD | DLCO decline > 20% | IL-7 | 0.019 | 1.591 | 1.078-2.349 |
| DLCO decline > 30% | IL-7* | 0.028 | 1.996 | 1.078-3.695 | |
Clinical items (gender, age, SSc form, anti-Scl-70 and anti-centromere positivity, mRSS, DLCO, FVC, lung fibrosis, pulmonary hypertension at inclusion) and serum cytokines were included in a multivariate analysis in order to calculate independently predictive factors for improvement or deterioration of lung function (e.g. decline of FVC or DLCO by 10, 20 or 30%). Only significant items are given with the hazard ratio (HR) and the 95% confidence interval (95% CI). Items were calculated with two methods (forward and backward inclusion), items marked with an asterisk (*) were calculated by both models.
Comparison of cytokine levels in BAL and serum
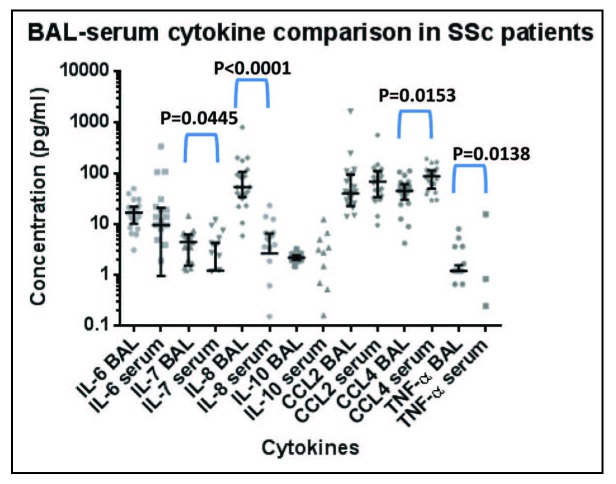
In 21 SSc patients with ILD, concomitant measurements of cytokine levels in BAL and in serum were compared (Figure 3). IL-7 and IL-8 were significantly higher in BAL (p=0.0445 and p<0.0001 respectively), whereas CCL4 (p=0.0153) and TNF-α (p=0.0138) were significantly lower in BAL than in serum. There were only few and weak to moderate correlations of BAL and serum cytokines: Serum IL-8 correlated with BAL IL-8 (r=0.574, p=0.006) and BAL TNF-α (r=0.521, p=0.022), whereas serum TGF-β correlated with the BAL cytokines IL-7 (r=0.626, p=0.002), IL-10 (r=0.466, p=0.039), CCL2 (r=0.488, p=0.021) and TNF-α (r=0.570, p=0.009). As reported earlier, TGF-β was below the detection range in BAL samples (14).
Fig. 3.
Cytokine levels in BAL and serum of 21 SSc-ILD patients. BAL and serum cytokines measured at the same time point are displayed as median and interquartile range; due to the high frequency of values below the detection limit not done for serum IL-10 and TNF-α. Significant differences were calculated using a Wilcoxon signed rank test. Statistically significant differences are indicated.
Discussion
Serum cytokines were studied in a large SSc cohort with all forms of pulmonary involvement, even without lung disease. Different tests revealed both IL-6 and IL-7 as predicting factors for mortality in patients with different subtypes of pulmonary involvement. The predictive value of IL-6 for mortality in SSc patients with ILD could be confirmed, which was also found by De Lauretis et al. and anti-IL-6 treatment with tocilizumab has been shown to halt progression of lung fibrosis (17-19). In addition, IL-6 also predicted the mortality for a mixed cohort of SSc patients with different forms of pulmonary involvement and we identified IL-7 as a new marker for the prediction of mortality in SSc patients in general as well as all SSc patients with pulmonary involvement. In addition, IL-7 was predictive for the decline of DLCO in SSc-ILD patients. When comparing predicting factors for worsening of lung disease with those recently published to be associated with worsening of skin fibrosis, there is only little overlap (apart from the items that have only been included in one analysis or the other) (20). We found gender to be predictive for both worsening of skin and lung fibrosis. However, whereas disease duration played a major role in the prediction of skin fibrosis, the age at baseline was more important for the deterioration of lung function.
Compared to patients without pulmonary involvement, IL-6 was found to be elevated in ILD patients, but even more so in PH-ILD patients, whereas CCL4 was lower in ILD patients. This is contradictory to other reports where CCL4 was significantly increased in patients with SSc, regardless of the disease subtype and stage, and correlated with the presence of pulmonary fibrosis (15, 21). Serum CCL2 was lower in patients with PAH and had no predictive value for patients with lung involvement in our cohort. This was also unexpected because CCL2 is a chemokine that was amongst the top 20 up-regulated genes in SSc-PAH lungs in a different investigation and was predictive for lung function decline in two different cohorts (22, 23). However, the analyses of BAL cellularity and cytokine levels in SSc patients that have been hitherto published, did not always take into account the various subtypes of pulmonary involvement (ILD, PH/PAH, PH-ILD) and this may explain some of the new findings.
The cytokine IL-7 has not yet attracted much attention in patients with systemic sclerosis. IL-7 targets T and B cells as well as innate immune cells and can be found locally elevated in other autoimmune diseases, such as rheumatoid arthritis (24). Systemically, IL-7 has been found to be elevated in sarcoidosis (25), while there are several studies indicating lower serum IL-7 levels in rheumatoid arthritis (24, 26) as well as in systemic sclerosis (27)compared to healthy controls; these IL-7 levels were comparable to our findings in the serum. However, there is contradictory evidence to its function: on the one hand, IL-7 drives autoimmunity in a mouse model by expansion of self-reactive lymphocytes (28, 29), whereas, on the other hand, it decreases pulmonary fibrosis, as measured by pulmonary hydroxyproline and collagen content, in a different mouse model (30). TGF-β is known to stimulate fibroblast proliferation and collagen deposition. In the same mouse model (30), IL-7 administration also decreased TGF-β production. In our patients, we have seen no correlation of serum IL-7 and TGF-β (data not shown). In rheumatoid arthritis, IL-7 influenced homing and angiogenesis by monocytes, partly via TNF-α and CCL2 (31); again there was no significant correlations in our cohort between the two cytokines (data not shown).
This might be interesting for further investigations, as monocytes are increasingly recognised to play an important role in the pathogenesis of SSc (32).
In addition, we have compared BAL and serum cytokines in a smaller subset of ILD patients and found cytokine levels in BAL in general to be either comparable or higher. Although this has also been reported by other groups for some cytokines such as IL-6 and IL-8 (33, 34), there is no such comprehensive comparison published. The correlation of serum IL-8 and TGF-β with BAL cytokines was only weak to moderate. IL-7 was significantly lower in serum than in BAL, but was also strongly associated with ILD in our previous study (14), confirming our present data. However, the commonalities and differences in cytokine levels imply a local cytokine production associated with ILD that is paralleled by a rise in serum cytokines, as well as distinct compartments with different levels for other cytokines. This is exemplified by the striking difference between serum and BAL TGF-β levels.
An important limitation of the present study, besides it being a retrospective analysis, is the fact that the majority of patients received immunosuppressive therapies at the time of cytokine/chemokine measurement for different indications such as progressing lung or skin fibrosis, but also myositis and arthritis (e.g. 14 patients had cyclophosphamide and 5 patients mycophenolate mofetil in the ILD group). This, together with the significant inter-individual variation of cytokine levels may explain some findings that are contradictory to other reports.
Conclusions
In summary, our study could confirm IL-6 as an important biomarker for pulmonary involvement and identify IL-7 as a new biomarker. Our findings extend earlier work on the association of serum cytokines with clinical manifestations and survival. IL-6 and IL-7 may be interesting biomarkers for further trials, possibly allowing for risk stratification of SSc patients with pulmonary involvement.
Author’s contributions:
M.O.B. and M.R. collected data, analysed the data and wrote the manuscript. K.S. collected data for the manuscript. D.H. supported the statistical analyses and revised the manuscript. A.R. collected data for the manuscript. M.M. collected data and wrote part of/improved the manuscript. C.M. contributed data. R.E. and G.R.B. improved the manuscript and G.R. formed the concept, wrote and improved the manuscript and supervised.
Ethical standards:
The study was approved by the local ethics committee and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments and included written consent of each patient.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Gu YS, Kong J, Cheema GS, Keen CL, Wick G, Gershwin ME. The immunobiology of systemic sclerosis. Semin Arthritis Rheum. 2008;38:132–60. doi: 10.1016/j.semarthrit.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. The Journal of clinical investigation. 2007;117:557–67. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katsumoto TR, Whitfield ML, Connolly MK. The pathogenesis of systemic sclerosis. Annu Rev Pathol. 2011;6:509–37. doi: 10.1146/annurev-pathol-011110-130312. [DOI] [PubMed] [Google Scholar]
- 5.Gunther J, Kill A, Becker MO, et al. Angiotensin receptor type 1 and endothelin receptor type A on immune cells mediate migration and the expression of IL-8 and CCL18 when stimulated by autoantibodies from systemic sclerosis patients. Arthritis Res Ther. 2014;16:R65. doi: 10.1186/ar4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schupp J, Becker M, Gunther J, Muller-Quernheim J, Riemekasten G, Prasse A. Serum CCL18 is predictive for lung disease progression and mortality in systemic sclerosis. Eur Respir J. 2014;43:1530–2. doi: 10.1183/09031936.00131713. [DOI] [PubMed] [Google Scholar]
- 7.Distler JH, Jungel A, Caretto D, et al. Monocyte chemoattractant protein 1 released from glycosaminoglycans mediates its profibrotic effects in systemic sclerosis via the release of interleukin-4 from T cells. Arthritis Rheum. 2006;54:214–25. doi: 10.1002/art.21497. [DOI] [PubMed] [Google Scholar]
- 8.Yanaba K, Komura K, Kodera M, et al. Serum levels of monocyte chemotactic protein-3/CCL7 are raised in patients with systemic sclerosis: association with extent of skin sclerosis and severity of pulmonary fibrosis. Ann Rheum Dis. 2006;65:124–6. doi: 10.1136/ard.2005.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972-2002. Ann Rheum Dis. 2007;66:940–4. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tashkin DP, Elashoff R, Clements PJ, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 11.Krause A, Hohberg B, Heine F, John M, Burmester GR, Witt C. Cytokines derived from alveolar macrophages induce fever after bronchoscopy and bronchoalveolar lavage. American journal of respiratory and critical care medicine. 1997;155:1793–7. doi: 10.1164/ajrccm.155.5.9154894. [DOI] [PubMed] [Google Scholar]
- 12.Goh NS, Veeraraghavan S, Desai SR, et al. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum. 2007;56:2005–12. doi: 10.1002/art.22696. [DOI] [PubMed] [Google Scholar]
- 13.Strange C, Bolster MB, Roth MD, et al. Bronchoalveolar lavage and response to cyclophosphamide in scleroderma interstitial lung disease. American journal of respiratory and critical care medicine. 2008;177:91–8. doi: 10.1164/rccm.200705-655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt K, Martinez-Gamboa L, Meier S, et al. Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res Ther. 2009;11:R111. doi: 10.1186/ar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prasse A, Pechkovsky DV, Toews GB, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56:1685–93. doi: 10.1002/art.22559. [DOI] [PubMed] [Google Scholar]
- 16.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis. 2013;72:1747–55. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 17.De Lauretis A, Sestini P, Pantelidis P, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol. 2013;40:435–46. doi: 10.3899/jrheum.120725. [DOI] [PubMed] [Google Scholar]
- 18.Khanna D, Denton CP, Jahreis A, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387:2630–40. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 19.Khanna D, Denton CP, Lin CJF, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate) Ann Rheum Dis. 2018;77:212–20. doi: 10.1136/annrheumdis-2017-211682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maurer B, Graf N, Michel BA, et al. Prediction of worsening of skin fibrosis in patients with diffuse cutaneous systemic sclerosis using the EUSTAR database. Ann Rheum Dis. 2015;74:1124–31. doi: 10.1136/annrheumdis-2014-205226. [DOI] [PubMed] [Google Scholar]
- 21.Codullo V, Baldwin HM, Singh MD, et al. An investigation of the inflammatory cytokine and chemokine network in systemic sclerosis. Ann Rheum Dis. 2011;70:1115–21. doi: 10.1136/ard.2010.137349. [DOI] [PubMed] [Google Scholar]
- 22.Hsu E, Shi H, Jordan RM, Lyons-Weiler J, Pilewski JM, Feghali-Bostwick CA. Lung tissues in patients with systemic sclerosis have gene expression patterns unique to pulmonary fibrosis and pulmonary hypertension. Arthritis Rheum. 2011;63:783–94. doi: 10.1002/art.30159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu M, Baron M, Pedroza C, et al. CCL2 in the Circulation Predicts Long-Term Progression of Interstitial Lung Disease in Patients With Early Systemic Sclerosis: Data From Two Independent Cohorts. Arthritis Rheumatol. 2017;69:1871–8. doi: 10.1002/art.40171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Churchman SM, Ponchel F. Interleukin-7 in rheumatoid arthritis. Rheumatology (Oxford) 2008;47:753–9. doi: 10.1093/rheumatology/ken053. [DOI] [PubMed] [Google Scholar]
- 25.Patterson KC, Franek BS, Muller-Quernheim J, Sperling AI, Sweiss NJ, Niewold TB. Circulating cytokines in sarcoidosis: phenotype-specific alterations for fibrotic and non-fibrotic pulmonary disease. Cytokine. 2013;61:906–11. doi: 10.1016/j.cyto.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponchel F, Verburg RJ, Bingham SJ, et al. Interleukin-7 deficiency in rheumatoid arthritis: consequences for therapy-induced lymphopenia. Arthritis Res Ther. 2005;7:R80–92. doi: 10.1186/ar1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Makino T, Fukushima S, Wakasugi S, Ihn H. Decreased serum IL-7 levels in patients with systemic sclerosis. Clin Exp Rheumatol. 2009;27:68–9. [PubMed] [Google Scholar]
- 28.Calzascia T, Pellegrini M, Lin A, et al. CD4 T cells, lymphopenia, and IL-7 in a multistep pathway to autoimmunity. Proc Natl Acad Sci U S A. 2008;105:2999–3004. doi: 10.1073/pnas.0712135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathak K. The expanding role of IL-7 and thymic stromal lymphopoietin as therapeutic target for rheumatoid arthritis. Expert opinion on therapeutic targets. 2014;18:581–94. doi: 10.1517/14728222.2014.893295. [DOI] [PubMed] [Google Scholar]
- 30.Huang M, Sharma S, Zhu LX, et al. IL-7 inhibits fibroblast TGF-beta production and signaling in pulmonary fibrosis. J Clin Invest. 2002;109:931–7. doi: 10.1172/JCI14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z, Kim SJ, Chamberlain ND, et al. The novel role of IL-7 ligation to IL-7 receptor in myeloid cells of rheumatoid arthritis and collagen-induced arthritis. J Immunol. 2013;190:5256–66. doi: 10.4049/jimmunol.1201675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kania G, Rudnik M, Distler O. Involvement of the myeloid cell compartment in fibrogenesis and systemic sclerosis. Nat Rev Rheumatol. 2019;15:288–302. doi: 10.1038/s41584-019-0212-z. [DOI] [PubMed] [Google Scholar]
- 33.Crestani B, Seta N, De Bandt M, et al. Interleukin 6 secretion by monocytes and alveolar macrophages in systemic sclerosis with lung involvement. Am J Respir Crit Care Med. 1994;149:1260–5. doi: 10.1164/ajrccm.149.5.8173768. [DOI] [PubMed] [Google Scholar]
- 34.Hesselstrand R, Wildt M, Bozovic G, et al. Biomarkers from bronchoalveolar lavage fluid in systemic sclerosis patients with interstitial lung disease relate to severity of lung fibrosis. Respiratory medicine. 2013;107:1079–86. doi: 10.1016/j.rmed.2013.03.015. [DOI] [PubMed] [Google Scholar]