Abstract
Background:
Takayasu Arteritis (TAK) is a granulomatous large vessel vasculitis that predominantly affects the aorta, major aortic branches and pulmonary arteries resulting in pulselessness. Sarcoidosis is a systemic granulomatous disease of unknown etiology that can affect any organ. Numerous cases of coexistence of both these rare diseases have been described, suggesting that their association may be by more than chance alone.
Objective:
To describe a case of coexistent TAK and sarcoidosis and review the world literature concerning this condition.
Methods:
The clinical presentation and diagnostic approach is described of a woman with TAK who developed sarcoidosis. The world literature was reviewed by searching the PubMed and Google Scholar database for the terms ‘Takayasu arteritis’ and ‘sarcoidosis’; ‘Takayasu arteritis’ and ‘granuloma’; ‘vasculitis’ and ‘sarcoidosis’; and ‘vasculitis’ and ‘granuloma.’ The identified individual articles were reviewed, and the bibliography of these articles were scrutinized to identify more cases. The pertinent clinical features of these cases were summarized.
Result:
A 36-year-old Caucasian woman, who was diagnosed with histologically confirmed TAK at 22 years of age, was referred for evaluation of mediastinal lymphadenopathy. The diagnosis of sarcoidosis was established on histopathology of a mediastinal lymph node biopsy. A literature review identified 23 additional cases of coexisting sarcoidosis and TAK, and the clinical features of these cases is described.
Conclusion:
TAK and sarcoidosis may occur in the same patient. Given the prevalence of these diseases, concomitant development of these two diseases is unlikely to be by chance alone and probably reflects a unifying mechanism. Clinicians should be aware of this association in patients in order to make a timely diagnosis and optimize patient care.
Key words: sarcoidosis, Takayasu arteritis, coexistence
Introduction
Takayasu Arteritis (TAK) is a granulomatous large vessel vasculitis that predominantly affects the aorta, major aortic branches and pulmonary arteries that eventually results in bruits or pulselessness of the affected vessels. The disease is most common in women of childbearing age. Although initially described in Asian countries, the disease has a worldwide distribution with an annual incidence of only 2.6 cases per million in North America (1). Sarcoidosis is a systemic granulomatous disease of unknown cause that can affect every organ, with the lung being most commonly involved (2). There have been numerous reports of both TAK and sarcoidosis developing in the same patient (3-20). We describe a woman with TAK who was subsequently diagnosed with sarcoidosis and review the existing medical literature concerning the concomitant development of these rare diseases in one patient.
Case Report
A 36-year-old Caucasian woman was referred for evaluation of mediastinal lymphadenopathy. At the age of 22, she was diagnosed with an ascending aortic aneurysm and underwent aortic valve replacement and repair of the aneurysm. The pathologic findings and localization were consistent with a diagnosis of TAK (figure 1). She subsequently underwent multiple surgeries for repair of her aortic arch, descending thoracic aorta and for vascular bypass procedures. Over the subsequent 12 years she had been treated with corticosteroids, cyclophosphamide, infliximab, adalimumab, and methotrexate. At age 34, she presented with chest pain and it was suspected that she was having an exacerbation of TAK. Her medication included 10 mg of prednisone daily and 25 mg of methotrexate subcutaneously weekly for management of TAK. A chest computed tomography scan (CT) revealed new mediastinal lymphadenopathy, that was shown to have intense 2-deoxy-2-fluoro-D-glucose (FDG) activity on positron emission tomography scanning (PET). Endobronchial ultrasound (EBUS) with transbronchial needle aspiration (TBNA) of a 4R lymph node revealed many discrete non-necrotizing granulomas some of which contained multinucleated giant cells.
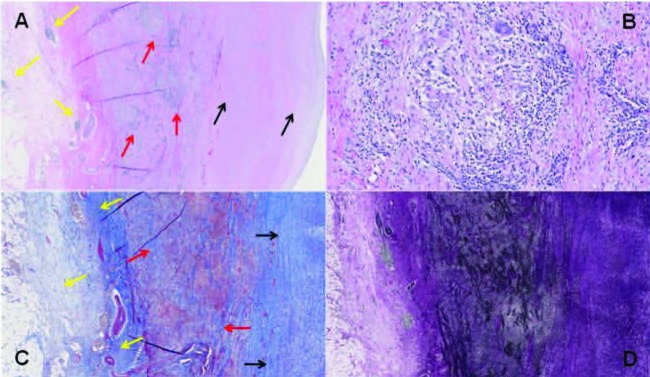
Fig. 1.
Granulomatous aortitis consistent with Takayasu disease. A: Ascending aorta wall (H&E, 20x) showing medial dominant inflammation (red arrows), foci of adventitial inflammation (yellow arrows), and fibrointimal expansion (black arrows). B. Focus of medial predominant inflammation at high power (H&E, 200x) comprised of foci of non-necrotizing granulomas with frequent multinucleated giant cells in a background of mostly lymphocytes and plasma cells. C. Fibrous expansion of adventitia (yellow arrows), medial fibrosis (red arrows), and fibrous expansion of intima (black arrows), as highlighted blue with trichrome stain (40x). D: Marked disruption of the medial elastic layers that are highlighted black with VVG elastin stain (40x)
The patient was lost to follow up and re-presented at age 36 complaining of night sweats for the previous 6 months. She was receiving prednisone 10 mg daily and methotrexate 25 mg subcutaneously weekly. She denied any fever, weight loss, cough, sputum production, palpitations, orthopnea, or chest pain. She had no history of tuberculosis, significant tuberculosis exposure or significant travel history. Physical examination revealed normal vital signs and no abnormalities. Laboratory data revealed a normal lactate dehydrogenase, normal C-reactive protein and negative interferon gamma release assay for ESAT-6, CFP-10 and TB7.7 tuberculous antigens. A chest CT demonstrated mediastinal lymphadenopathy that had enlarged compared to her prior scan 2 years earlier. A PET scan revealed hypermetabolic activity in the mediastinal and new non- mediastinal lymph node groups (figure 2). No other organ involvement was seen. Because of the concern for infection and lymphoma, She underwent a second bronchoscopy with bronchoalveolar lavage (BAL) and EBUS with TBNA of a mediastinal lymph node (station 7). The BAL showed CD4/CD8 ratio of 7:1. The histopathology of the lymph node showed non-necrotizing granulomas consistent with sarcoidosis (figure 3). She was continued on methotrexate and prednisone with a plan to start tocilizumab for refractory TAK, however she failed to return for further care.
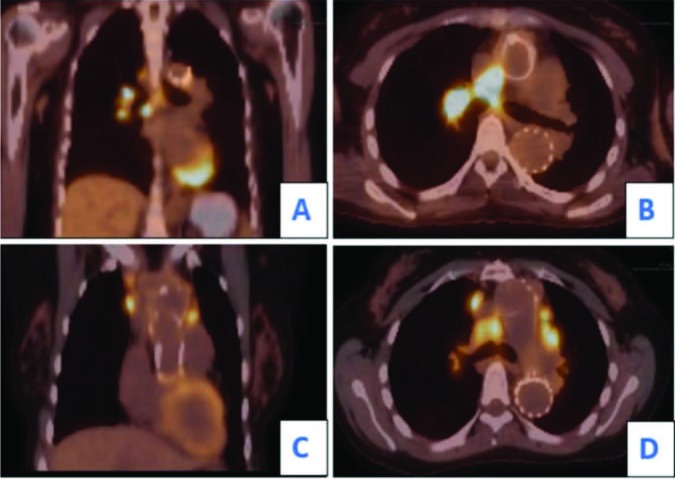
Fig. 2.
PET scan demonstrating hypermetabolic lymph nodes. In the upper panel, coronal (A) and axial (B) views at the age of 34 years showed FDG avid hilar and predominantly right mediastinal lymph nodes. On the PET scan obtained at the age of 36 (lower panel), coronal (C) and axial (D) sections revealed new areas of FDG uptake including, right anterior mediastinal, internal mammary, and left prevascular lymph nodes. There was also hypermetabolic activity involving right sub pectoral paraesophageal lymph nodes (not shown). The hilar lymphadenopathy with intense FDG uptake persisted
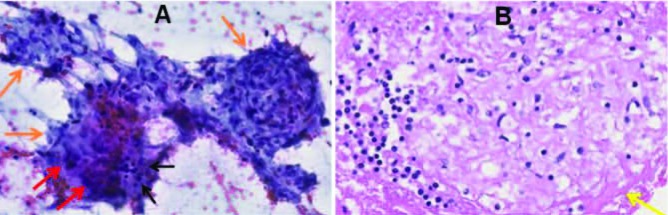
Fig. 3.
Endobronchial ultrasound (EBUS) guided fine needle aspiration of a mediastinal lymph node demonstrates multiple discrete granulomas. A: Photomicrograph from a Papanicolaou stained smear (200x) demonstrates three discrete granulomas (orange arrows) comprised of clusters of epithelioid histiocytes with admixed mature lymphocytes (black arrows), and multinucleated giant histiocytes (red arrows). The granulomas are a frequent and dominant finding in the aspirate smears. No background necrosis is present. B: Image of an H&E stained cell block slide (400x) with a non-necrotizing granuloma comprised of a cluster of epithelioid histiocytes admixed with lymphocytes and peripheral pink hyaline fibrosis (yellow arrow)
Discussion
We have described a patient with concomitant TAK and sarcoidosis. Both diseases were confirmed both by a compatible clinical presentation as well as histological evaluation of involved tissues.
Including the present case, we have identified 25 confirmed cases of concomitant TAK associated with sarcoidosis reported in the literature, which are shown in Table 1 and summarized in Table 2. The majority of patients were female (88%), as would be expected given the female predilection of both diseases. The distribution of organ involvement is described in Table 3. Thoracic involvement was seen in 15/25 (60%) and extrathoracic involvement was seen in 8/25 (32%). In 2 cases the site was not specified. The survival of patients was high in this cohort, with only one death associated with sarcoidosis and one associated with TAK.
Table 1.
Sarcoidosis and Takayasu Arteritis, Literature Review
| Case Report | Year | Age | Sex | Race | First Diagnosed Condition | Sarcoidosis Site | Treatment | Survival |
| Betancourt et al. (3) | 2019 | 32 | F | NS | Takayasu | Mediastinal LN | Sponteneous resolution | Survived |
| Chapelon-Abric et al. (4) | 2018 | 19 54 53 27 52 25 30 |
F F F F F F F |
Black Black Caucasian Black Caucasian Caucasian NS |
Simultaneous Sarcoidosis Sarcoidosis Sarcoidosis Takayasu Simultaneous Sarcoidosis |
Lung Eye, Kidney Skin, Joint Lung, Exocrine glands Skin, Joint, Eye Skin, Joint Skin, Joint |
CS CS, MTX CS CS CS, MTX, INFLX, TOC CS CS, MTX, INFLX |
Survived Survived Survived Survived Survived Survived Survived |
| Rhanim et al. (5) | 2016 | 24 | F | Caucasian | Simultaneous | Mediastinal LN | CS | Survived |
| Ri et al. (6) | 2015 | 67 | F | Asian | Simultaneous | Skin | Topical CS | Survived |
| Liu et al. (7) | 2015 | 29 | F | Asian | Sarcoidosis | Lung, Mediastinal LN | CS, MTX | Survived |
| Chougule et al. (8) | 2014 | 38 | F | Indian | Takayasu | Lung | NS | Death due to TAK (ruptured aneurysm) |
| Izumikawa et al. (9) | 2011 | 24 | M | Asian | Takayasu | Lung, Mediastinal LN | CS | Survived |
| Hamzaoui et al. (10) | 2011 | 34 | F | Black | Sarcoidosis | Lung, LN, Salivary Gland, Liver | CS, MTX | Survived |
| Ishii et al. (11) | 2011 | 72 | M | Asian | Simultaneous | LN, Lung | NS | Death due to sarcoidosis |
| Mi Hye Im et al. (12) | 2011 | 55 | F | Asian | Simultaneous | Lung, Eye, LN | CS | Survived |
| Vaurs et al. (13) | 2009 | M | Caucasian | Sarcoidosis | Lung, Skin | CS, MTX | Survived | |
| Rafiq et al. (14) | 2007 | 41 | F | NS | Takayasu | Mediastinal LN | CS | Survived |
| Kokturk et al. (15) | 2007 | 30 | F | NS | Sarcoidosis | Lung, Mediastinal LN | CS, AZA | Survived |
| Robaday et al. (16) | 2005 | 26 | F | Caucasian | Sarcoidosis | NS | CS | Survived |
| Korkmaz et al. (17) | 1999 | 29 | F | NS | Sarcoidosis | Bone, Tendon, Muscle | CS, AZA | Survived |
| Schapiro et al. (18) | 1994 | 42 | F | NS | Sarcoidosis | Lung, Skin, Mediastinal LN | CS, CYC | Survived |
| Rose et al. (19) | 1990 | 17 | F | NS | Sarcoidosis | NS | NS | Not reported |
| Weiler et al. (20) | 2000 | 39 | F | Caucasian | Sarcoidosis | Joint, Skin, Eye, LN | Surgery, CS | Survived |
| Present Case | 2018 | 37 | F | Caucasian | Takayasu | Mediastinal LN | CS, MTX, INFLX, ADA, CYC | Survived |
| Survival | 22/24 (92%) |
NS: not specified in the article; CS: corticosteroid; MTX: methotrexate; AZA: azathioprine; LN: lymph node; TOC: tocilizumab; INFLX: infliximab; ADA: adalimumab; CYC: cyclophosphamide
Table 2.
Clinical characteristics of patients
| Variable | N | |
| Age (years), Mean ± SD, Median | 25 | 37 ± 15, 33 |
| Gender (Female) | 22 | 88% |
| Race Black Caucasian Asian Indian Unreported |
5 8 5 1 6 |
20% 32% 20% 4% 24% |
| Initial Diagnosis Sarcoidosis Takayasu arteritis Simultaneous |
13 6 6 |
52% 24% 24% |
Table 3.
Sites of sarcoidosis involvement in patients with Sarcoidosis and Takayasu Arteritis
| Sarcoidosis Site | N | % |
| Pulmonary (Lung and Mediastinal LN) | 15 | 60% |
| Extrapulmonary | 8 | 32% |
| Unreported | 2 | 8% |
| Number of organs involved | N | % |
| One organ involved | 7 | 30% |
| More than one organ involved | 16 | 70% |
| Number of organs involved per patient Mean ± SD, median | 2.04±٠.88, 2 | |
While 25 cases are identified here, it is possible that there may be additional cases of concomitant sarcoidosis and TAK that may have possibly been falsely described as sarcoidosis-induced vasculitis. A systemic granulomatous vasculitis secondary to sarcoidosis is extremely uncommon. The sarcoid granulomas can affect all three layers of the vessel wall and focal destruction of elastic tissue is often seen in such cases (21), which may result in pulseless extremities due to involvement of medium and large size vessels. Fernandes et al. (2000) described three patients with sarcoidosis who subsequently developed pulselessness. Arteriograms showed stenosis or aneurysms of the aorta and its main branches, and histopathology revealed multinucleated giant cells (22). While the authors attributed these findings to sarcoid-induced vasculitis, these histopathologic findings are consistent with TAK.
In the United States, the prevalence of TAK is 2.6 per million (1), and the prevalence of sarcoidosis is 60 per 100,000 adults (23). Therefore, if the development of TAK and sarcoidosis were independent events, the prevalence of individuals developing TAK and sarcoidosis concomitantly in their lifetime would be 1.56/100,000,000. Since 25 cases have been reported in the world literature since 1990, we suspect that concomitant development of these diseases in one patient did not occur by chance alone and relates to some unifying mechanism.
TAK patients who develop granulomatous inflammation may not meet the diagnostic criteria for sarcoidosis. One postulation for this observed association may be that in a fraction of these cases, the granulomatous inflammation may have been the result of TAK, and not have represented true sarcoidosis. In addition, although it is controversial (24), many require evidence of granulomatous inflammation to be present in at least two organs for the diagnosis of sarcoidosis to be secure (25). In particular, granulomas isolated to the mediastinal lymph nodes may represent a nonspecific reaction to another process, such as a connective tissue disease (26) as suggested by a previous review of cases with concomitant “sarcoidosis” and connective tissue diseases (26). As sarcoidosis is a disease of unknown etiology and CTD may cause granulomatous inflammation, it is possible that the granulomatous inflammation may be induced by TAK rather than the development of sarcoidosis. In our review, we found that in 7 of the 23 patients (30%), only one organ was clinically involved with sarcoidosis (granuloma in single organ): lung (2), mediastinal lymph node (4), and skin (1). These data suggest that a portion, albeit not the entirety of the report cases of patients with concomitant TAK and sarcoidosis may have only had the former disease. This might explain how some of these patients, such as ours, developed enlarging lymphadenopathy on immunosuppressive therapy that is usually effective for sarcoidosis. It is possible that some of these patients did truly have concomitant TAK and sarcoidosis, but such immunosuppressive therapy for TAK delayed or modified the presentation of sarcoidosis.
In TAK induced granulomatous inflammation, it would be expected that TAK would develop prior or at the same time that the patients developed sarcoidosis. The data in Table 4 support this postulation. Patients with the occurrence of single organ granuloma were being diagnosed after or simultaneously with TAK, suggesting that these patients may not, in fact, have systemic sarcoidosis, but instead possibly a local granulomatous reaction in response to TAK. The association was statistically significant (p=0.0032). However, this postulation would only explain a number of the reported cases, as half of the patients presented with sarcoidosis and systemic granuloma prior to the diagnosis of TAK. Therefore, we suspect that there is a true unifying mechanism to explain the concomitant development of these two diseases in the reported cases.
Table 4.
Relationship between sarcoidosis diagnosed before TAK and the presence of single organ versus systemic granuloma
| Single organ granuloma | Systemic granuloma | Total | |
| Sarcoidosis diagnosed first | 0 | 11 | 11 |
| TAK diagnosed first | 7 | 5 | 12 |
| Total | 7 | 16 | 23 |
Fisher’s exact test, P= 0.0032
Site of sarcoidosis not specified in two of the 25 reported cases.
Conclusion
We report a case of concomitant sarcoidosis and TAK and report on the world literature. Given the prevalence of these diseases, concomitant development of these two diseases is unlikely to be by chance alone and probably reflects a unifying mechanism. Some of the cases may have been misdiagnosed as having sarcoidosis, but this would only explain a small percentage of them. Clinicians should be aware of this association in patients in order to make a timely diagnosis and optimize patient care.
Summary of conflict of interest of the authors:
BKS doesn’t have any conflict of interest. SLB has no any conflict of interest. LAF doesn’t have any conflict of interest. MAJ is a consultant for Biogen and Institution grant support from Novartis, Mallinckrodt and aTyr.
References
- 1.Hall S, Barr W, Lie JT, Stanson AW, Kazmier FJ, Hunder CG. Takayasu arteritis: a study of 32 North American patients. Medicine (Baltimore) 1985 Mar;64(2):89–99. [PubMed] [Google Scholar]
- 2.Judson MA. The Clinical Features of Sarcoidosis: A Comprehensive Review. Clin Rev Allergy Immunol. 2015 Aug;49(1):63–78. doi: 10.1007/s12016-014-8450-y. [DOI] [PubMed] [Google Scholar]
- 3.Betancourt BY, Ahlman MA, Grayson PC. Sarcoidosis Concomitant With Takayasu Arteritis, Identified by Advanced Molecular Imaging. Arthritis Rheumatol. 2019 Jun 1;71(6):990–990. doi: 10.1002/art.40847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapelon-Abric C, Saadoun D, Marie I, Comarmond C, Desbois AC, Domont F, et al. Sarcoidosis with Takayasu arteritis: a model of overlapping granulomatosis. A report of seven cases and literature review. Int J Rheum Dis. 2018 Mar;21(3):740–745. doi: 10.1111/1756-185X.13137. [DOI] [PubMed] [Google Scholar]
- 5.Rhanim A, Marc K. Association of sarcoidosis and Takayasu’s disease: A case report. Rev Pneumol Clin. 2016 Aug;72(4):255–258. doi: 10.1016/j.pneumo.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Ri G, Yoshikawa E, Shigekiyo T, Ishii R, Okamoto Y, Kakita K, et al. Takayasu arteritis and ulcerative cutaneous sarcoidosis. Intern Med. 2015;54(9):1075–80. doi: 10.2169/internalmedicine.54.3345. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Li S, Cao J, Wang YX, Bi YL, Xu ZJ, et al. Concurrence of sarcoidosis and Takayasu aortitis. Chin Med J (Engl) 2015 Mar;128(6):851–852. doi: 10.4103/0366-6999.152694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chougule A, Bal A, Das A, Jain S, Bahl A. Uncommon associations and catastrophic manifestation in Takayasu arteritis: an autopsy case report. Cardiovasc Pathol. 2014 Sep-Oct;23(5):313–316. doi: 10.1016/j.carpath.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Izumikawa K, Motoi N, Takaya H, Miyamoto A, Eishi Y, Yoshimura K, et al. A case of concurrent sarcoidosis, aortitis syndrome and Crohn’s disease. Intern Med. 2011;50(23):2915–2917. doi: 10.2169/internalmedicine.50.5298. [DOI] [PubMed] [Google Scholar]
- 10.Hamzaoui A, Salem R, Klii R, Harzallah O, Berriche O, Golli M, et al. Co-existing sarcoidosis and Takayasu arteritis: report of a case. Int Arch Med. 2011 Feb;23(4):9. doi: 10.1186/1755-7682-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishii A, Hoshii Y, Nakashima T, Umemoto S, Nakamura H, Tanaka N, et al. Sarcoidosis with pulmonary hypertension exacerbated by Takayasu-like large vessel vasculitis. Pathol Int. 2011 Sep;61(9):546–550. doi: 10.1111/j.1440-1827.2011.02703.x. [DOI] [PubMed] [Google Scholar]
- 12.Mi Hye Im, Jeong JW, Jin KA, Byung HL, Yun SC. Takayasu Arteritis Associated with Sarcoidosis: A Case Report. J Korean Soc Radiol. 2011;65(5):487–490. [Google Scholar]
- 13.Vaurs C, Ammoury A, Cordel N, Lamant L, Chaufour X, Paul C. Large-vessel granulomatous vasculitis during the course of sarcoidosis: Takayasu’s arteritis? Ann Dermatol Venereol. 2009 Dec;136(12):890–893. doi: 10.1016/j.annder.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Rafiq I, Nadig V, Freeman LJ. Sarcoidosis, microvascular angina and aortitis: New dimensions of the ‘Takayasu syndrome’ - A case report. Int J Angiol. 2007 Fall;16(3):113–114. doi: 10.1055/s-0031-1278261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokturk N, Turktas H, Ozturk MA, Aksoy H, Atasever T. The role of positron emission tomography for the diagnosis and follow up of a patient with sarcoidosis and Takayasu arteritis. South Med J. 2007 Mar;100(3):331. doi: 10.1097/SMJ.0b013e318030ef88. [DOI] [PubMed] [Google Scholar]
- 16.Robaday S, Hervé F, Cailleux N, Dominique S, Levesque H, Marie I. Association of sarcoidosis and Takayasu’s arteritis: an additional case report. Rev Med Interne. 2005 Oct;26(10):816–9. doi: 10.1016/j.revmed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 17.Korkmaz C, Efe B, Tel N, Kabukçuoglu S, Erenoglu E. Sarcoidosis with palpable nodular myositis, periostitis and large-vessel vasculitis stimulating Takayasu’s arteritis. Rheumatology (Oxford) 1999 Mar;38(3):287–288. doi: 10.1093/rheumatology/38.3.287. [DOI] [PubMed] [Google Scholar]
- 18.Schapiro JM, Shpitzer S, Pinkhas J, Sidi Y, Arber N. Sarcoidosis as the initial manifestation of Takayasu’s arteritis. J Med. 1994;25(1-2):21–128. [PubMed] [Google Scholar]
- 19.Rose CD, Eichenfield AH, Goldsmith DP, Athreya BH. Early onset sarcoidosis with aortitis--”juvenile systemic granulomatosis?”. J Rheumatol. 1990 Jan;17(1):102–106. [PubMed] [Google Scholar]
- 20.Weiler V, Redtenbacher S, Bancher C, Fischer MB, Smolen JS. Concurrence of sarcoidosis and aortitis: case report and review of the literature. Ann Rheum Dis. 2000 Nov;59(11):850–853. doi: 10.1136/ard.59.11.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen Y. Pathology of Sarcoidosis. 8 Seminars in Respiratory and Critical Care Medicine. 2007 Nov;28(1) doi: 10.1055/s-2007-970332. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes S, Singsen BH, Hoffman GS. Sarcoidosis and Systemic Vasculitis. Semin Arthritis Rheum. 2000 Aug;30(1):33–46. doi: 10.1053/sarh.2000.8364. [DOI] [PubMed] [Google Scholar]
- 23.Baughman RP, Field S, Costabel U, Crystal RG, Culver DA, Drent M, et al. Sarcoidosis in America: analysis based on health care use. Ann Am Thorac Soc. 2016;13:1244–1252. doi: 10.1513/AnnalsATS.201511-760OC. [DOI] [PubMed] [Google Scholar]
- 24.Judson MA, Baughman RP. How many organs need to be involved to diagnose sarcoidosis: An unanswered question that, hopefully, will become irrelevant. Sarcoidosis Vasculitis Diffuse Lung Disease. 2014;31:6–7. [PubMed] [Google Scholar]
- 25.Hunninghake GW, Costabel U, Ando M, Baughman R, Cordier JF, du Bois R, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 1999;16(2):149–73. [PubMed] [Google Scholar]
- 26.Judson MA, Shapiro L, Freitas S, Polychronopoulos VS, Highland KB. Concomitant sarcoidosis and a connective tissue disease: review of the clinical findings and postulations concerning their association. Respir Med. 2013;107:1453–9. doi: 10.1016/j.rmed.2013.01.004. [DOI] [PubMed] [Google Scholar]