Abstract
Purpose:
Mortality of acute exacerbation of idiopathic pulmonary fibrosis is high, and it remains unknown whether cyclophosphamide is an effective treatment for this condition.
Objectives:
This study compared the effects of cyclophosphamide combined with systemic glucocorticoids with those of systemic glucocorticoids alone.
Methods:
Using the Diagnosis Procedure Combination database in Japan, adult patients with idiopathic pulmonary fibrosis who had received high-dose methylprednisolone and mechanical ventilation at admission from July 1, 2010, to March 31, 2014, were identified. Instrumental variable analyses based on a hospital preference for cyclophosphamide were performed to compare in-hospital outcomes.
Results:
Eligible patients (n=1847) were divided into the methylprednisolone plus cyclophosphamide group (n=104) and the methylprednisolone alone group (n=1743). The results of an instrumental variable analysis detected no significant differences between the groups with respect to in-hospital mortality (odds ratio, 1.11; 95% confidence interval, 0.19-6.43), ventilator-free days (difference, 2.2; 95% confidence interval, −2.6 to 7.0).
Conclusions:
In a Japanese inpatient database study analyzing outcomes from patients with acute exacerbation idiopathic pulmonary fibrosis receiving systemic glucocorticoids, the addition of cyclophosphamide was not associated with improved in-hospital mortality and ventilator-free days.
Key words: idiopathic pulmonary fibrosis, mortality, cyclophosphamide, glucocorticoids
Introduction
Idiopathic pulmonary fibrosis (IPF) is a specific form of chronic, progressive fibrotic interstitial pneumonia of unknown cause. Acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) is defined as respiratory deterioration in less than 1 month (1). Respiratory failure caused by AE-IPF is associated with high in-hospital mortality (55.6%-80%) (2-5). In particular, studies have shown that the mortality of patients with AE-IPF requiring mechanical ventilation is 81.8%-94% (6, 7).
Treatment of AE-IPF has not been established, and only anecdotal treatment reports exist. International evidenced-based guidelines weakly recommend a standard therapy for AE-IPF of administrating systemic glucocorticoids, including methylprednisolone at a dosage of 1 g per day intravenously for 3 days (8). The international evidenced-base guidelines do not comment on the use of other immunosuppressant agents combined with glucocorticoids owing to a lack of conclusive results for the combination treatment (8). It remains controversial whether cyclophosphamide combined with methylprednisolone is effective for patients with IPF (9-11). Previous studies have reported that cyclophosphamide combined with high-dose methylprednisolone is potentially effective for patients with AE-IPF (12, 13). However, the patients in those studies had connective tissue diseases. To date, there has been no study comparing high-dose methylprednisolone plus cyclophosphamide with high-dose methylprednisolone alone as therapy for patients with AE-IPF, and thus it remains unknown whether cyclophosphamide would have an additive effect with high-dose methylprednisolone in these patients.
Therefore, the aim of the present study was to use data from a national inpatient database in Japan to compare the effectiveness of the administration of cyclophosphamide combined with systemic glucocorticoids to that of systemic glucocorticoids alone for reducing the mortality of AE-IPF.
Methods
Data source
Inpatient data were extracted from the Japanese Diagnosis Procedure Combination database. More than 1,000 hospitals voluntarily contribute to the database, which includes data from approximately 7 million inpatients, representing approximately 50% of all discharges from acute care hospitals in Japan. The data used in the present study included the hospital identification number; patient sex and age; body weight and height; consciousness level on admission; dates of hospitalization and discharge; main diagnoses, pre-existing comorbidities on admission, and complications that occurred during hospitalization, which were coded with the International Classification of Diseases, tenth revision (ICD-10) codes and text in Japanese; surgical and nonsurgical procedures and dates of procedures performed; dates and doses of drugs or blood products administered during the hospitalization; and discharge status.
Consciousness level on admission was evaluated using Japan Coma Scale scores (14, 15), which is widely used in Japan, and its assessment is well correlated with the Glasgow Coma Scale assessment (16).
The Institutional Review Board of The University of Tokyo approved this study. Informed consent was waived because of the anonymous nature of the data.
Patient selection
This study used data from July 1, 2010, to March 31, 2014. The inclusion criteria were patients aged ≥15 years who were diagnosed as having idiopathic pulmonary fibrosis (ICD-10 codes: J84.1, J84.8, and J84.9) and who received mechanical ventilation within 1 day after admission. The patients were divided into two groups: (1) those who received cyclophosphamide 500 to 1,000 mg per day intravenously for 1 day and methylprednisolone 1 gram per day intravenously for 3 days within 5 days after admission (termed the methylprednisolone plus cyclophosphamide group); (2) those who received methylprednisolone 500 to 1,000 mg per day intravenously for 3 days within 4 days after admission (methylprednisolone alone group).
Baseline characteristics
Baseline characteristics included the following: age; sex; Hugh-Jones classification on admission (17); consciousness level on admission; Charlson comorbidity index (CCI); smoking index (packs per year); past history of diabetes mellitus, chronic kidney disease, lung cancer, chronic obstructive pulmonary disease, and congestive heart failure; and use of cotrimoxazole, azithromycin (18), continuous renal replacement therapy, and noradrenaline within 1 day after admission. Patients were categorized into five age groups: 15-40, 41-60, 61-70, 71-80, and ≥81 years old. The CCI was classified into five groups: 0, 1, 2, 3-5, and ≥6 points. The smoking index was categorized into five groups: 0, 1-20, 21-40, 41-60, and ≥61 packs per year.
Outcomes
The primary outcome was in-hospital mortality. The secondary outcome was ventilator-free days (VFDs) (19), incidence of sepsis (ICD-10 codes: A32.7, A39.4, A40.x, and A41.x), and incidence of mycosis (ICD-10 codes: B37.1, B37.5-B37.8, B44.0,B44.1, B45.0, B45.1, B45.7, B45.9, B48.7, B49, and J17.2).
Statistical analysis
Because some values were missing for the Hugh-Jones classification, smoking index, and CCI, a multiple imputation procedure was performed to replace each missing value with a set of submitted plausible values by creating 20 filled-in complete datasets using a Markov chain Monte Carlo algorithm known as chained equations imputation (20). The multiple imputation method assumes that data are missing at random and that any systemic differences between the missing and observed values can be explained by differences in the observed data (21, 22).
An instrumental variable (IV) analysis was also performed. Unmeasured confounders can lead to incorrect inferences regarding the effectiveness of different treatments. The IV analysis can theoretically balance both measured and unmeasured confounders between two groups (23, 24). A hospital preference for cyclophosphamide was selected as an IV, because use of cyclophosphamide depended on physician preference. When hospitals are strongly consistent in whether or not they use cyclophosphamide to treat AE-IPF, it is assumed that the decision to administer the drug may be made independently of an individual patient’s background. In such a situation, a hospital preference for cyclophosphamide may have acted as an IV, thereby setting the stage for a “natural experiment” that allowed an unbiased estimate of the risk of AE-IPF, even if unmeasured confounders existed (25, 26). An IV analysis assumes that patient hospital choice is made independently of the hospital’s choice of a specific drug, and the hospital’s use of the drug is independent of the outcomes. The number of patients with AE-IPF who received cyclophosphamide in each hospital was counted, and then the average number of patients with AE-IPF who received cyclophosphamide among all the hospitals was calculated. Hospitals with more than the average number of cyclophosphamide users were defined as hospitals with a preference for cyclophosphamide. Hospitals with less than the average number of cyclophosphamide users were defined as hospitals without a preference for cyclophosphamide. To assess the validity of hospital preference as an IV, we confirmed that hospital preference was highly correlated to the receipt of cyclophosphamide (F statistic >10) (25). We examined hospital preference was not associated with outcomes.
A two-stage residual inclusion estimation framework of the IV analysis was used (27, 28). The residual inclusion approach has been shown to generate more consistent and less biased estimates for a variety of nonlinear models. In the first stage model, the association between receipt of cyclophosphamide and hospital preference for cyclophosphamide was measured, with adjustment for patient level covariates. From this model, the raw residual for each patient was determined by calculating the difference between the model-predicted probability of receiving cyclophosphamide and the actual treatment received. The residuals were then included as an additional covariate in the second-stage model. In the second-stage model, the association between treatment and outcomes was estimated, adjusting for covariates. All IV analyses were performed using robust standard errors.
A sensitivity analysis was performed to confirm the correctness of the inclusion criteria for AE-IPF (1). First, patients who had not received a computed tomography (CT) scan within 1 day after admission were excluded. Second, patients who had not received a CT scan within 1 day after admission and with the use of furosemide within 1 day after admission were excluded.
Continuous variables are presented as an average along with the standard deviation or the median with the interquartile range. Categorical variables are presented as the number with a proportion. In the unadjusted comparisons, averages of continuous variables were compared using t-tests, and proportions of categorical variables using χ2 tests.
A P value <0.05 was considered to indicate statistical significance. All statistical analyses were performed using STATA/MP version 14.0 software (STATA Corp, College Station, TX, USA).
Results
During the analyzed period, we identified 12,992 patients who received methylprednisolone at a dose of 500 to 1,000 mg per day for 3 days within 4 days after admission (Figure 1). Among them, 1,847 patients were eligible for the present study, including 104 patients administered cyclophosphamide and 1,743 patients without cyclophosphamide administration.
Fig. 1.
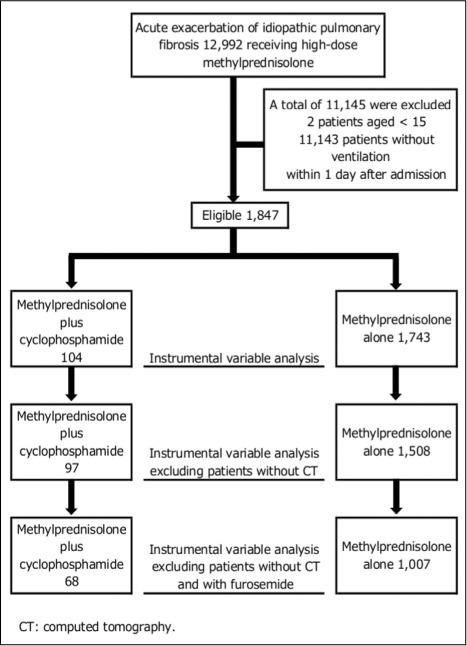
Patient selection
Values were missing for smoking status, Hugh-Jones classification, and CCI (15.4%, 23.6%, and 30.0%, respectively; Table 1). Patient backgrounds in the methylprednisolone plus cyclophosphamide group were significantly different from those in the methylprednisolone alone group with respect to Hugh-Jones classification. Patients in the methylprednisolone plus cyclophosphamide group received more cotrimoxazole within 1 day after admission than those in the methylprednisolone alone group (31.7% vs. 18.0%, P=0.0005).
Table 1.
Baseline characteristics at admission
| Variable | Methylprednisolone alone (n=1,743) | Methylprednisolone plus cyclophosphamide (n=104) | P value |
| Sex, n (%) | |||
| male | 1,182 (67.8) | 72 (69.2) | 0.77 |
| Age, years, n (%) | |||
| 15-40 | 9 (0.5) | 0 0.0 | 0.30 |
| 41-60 | 112 (6.4) | 9 (8.7) | |
| 61-70 | 384 (22.0) | 29 (27.9) | |
| 71-80 | 751 (43.1) | 45 (43.3) | |
| ≥81 | 487 (27.9) | 21 (20.2) | |
| Smoking index (packs per year), n (%) | |||
| 0 | 779 (44.7) | 50 (48.1) | 0.72 |
| 1-20 | 143 (8.2) | 9 (8.7) | |
| 21-40 | 237 (13.6) | 12 (11.5) | |
| 41-60 | 179 (10.3) | 14 (13.5) | |
| ≥61 | 134 (7.7) | 5 (4.8) | |
| missing | 271 (15.5) | 14 (13.5) | |
| Hugh-Jones classification, n (%) | |||
| 1 | 54 (3.1) | 3 (2.9) | 0.04 |
| 2 | 82 (4.7) | 5 (4.8) | |
| 3 | 107 (6.1) | 2 (1.9) | |
| 4 | 198 (11.4) | 6 (5.8) | |
| 5 | 903 (51.8) | 52 (50.0) | |
| missing | 399 (22.9) | 36 (34.6) | |
| Charlson comorbidity index, n (%) | |||
| 0 | 316 (18.1) | 20 (19.2) | 0.38 |
| 1 | 321 (18.4) | 17 (16.3) | |
| 2 | 384 (22.0) | 17 (16.3) | |
| 3-5 | 119 (6.8) | 7 (6.7) | |
| ≥6 | 88 (5.0) | 3 (2.9) | |
| missing | 515 (29.5) | 40 (38.5) | |
| Japan coma scale, n (%) | |||
| 0 (alert) | 1,273 (73.0) | 85 (81.7) | 0.17 |
| 1-digit (dizziness) | 283 (16.2) | 14 (13.5) | |
| 2-digit (somnolence) | 87 (5.0) | 3 (2.9) | |
| 3-digit (coma) | 100 (5.7) | 2 (1.9) | |
| Lung cancer, n (%) | 72 (4.1) | 5 (4.8) | 0.73 |
| Chronic obstructive pulmonary disease, n (%) | 140 (8.0) | 7 (6.7) | 0.63 |
| Congestive heart failure, n (%) | 142 (8.1) | 3 (2.9) | 0.06 |
| Diabetes mellitus, n (%) | 425 (24.4) | 17 (16.3) | 0.06 |
| Chronic kidney disease, n (%) | 46 (2.6) | 3 (2.9) | 0.85 |
| Noradrenaline, n (%) | 162 (9.3) | 10 (9.6) | 0.92 |
| Azithromycin, n (%) | 272 (15.6) | 9 (8.7) | 0.06 |
| Cotrimoxazole, n (%) | 313 (18.0) | 33 (31.7) | 0.0005 |
| Continuous renal replacement therapy, n (%) | 24 (1.4) | 3 (2.9) | 0.22 |
The overall in-hospital mortality was 48.6% (897/1847). Unadjusted in-hospital mortality was significantly higher in the methylprednisolone plus cyclophosphamide group than in the methylprednisolone alone group (64.4% vs 47.6%, P=0.0009). In the unadjusted comparison, VFDs in the methylprednisolone plus cyclophosphamide group were significantly lower than those in the methylprednisolone alone group (6.7 days vs. 10.4 days, P=0.0008). In the unadjusted comparison, there were no significant differences between the groups in incidence of sepsis and mycosis (6.7% vs, 3.5%, P=0.09; 3.9% vs. 2.1%, P=0.24, respectively).
The average number of patients with AE-IPF was 1.7 per year. The hospital preference for cyclophosphamide was highly associated with actual receipt of cyclophosphamide (F statistic=73.2), whereas the hospital preference for cyclophosphamide was not significantly associated with death (coefficient, −0.07; 95% confidence interval [CI], −0.32 to 0.18), VFDs (0.14; 95% CI, −0.51 to 0.80), incidence of sepsis (0.20; 95% CI, −0.33 to 0.74), or incidence of mycosis (0.21; 95% CI, −0.50 to 0.92).
In the IV analysis, no significant difference was detected between the methylprednisolone plus cyclophosphamide group and the methylprednisolone alone group with respect to in-hospital mortality (odds ratio [OR], 1.11; 95% CI, 0.19-6.43; Table 2). There were also no significant differences between the groups with respect to VFDs (difference, 2.2; 95% CI, −2.6 to 7.0), incidence of sepsis (OR, 6.68; 95% CI, 0.12-379), or incidence of mycosis (OR, 5.93; 95% CI, 0.05-665; Tables 3, 4).
Table 2.
Comparison of in-hospital mortality between the methylprednisolone plus cyclophosphamide and methylprednisolone alone groups
| OR* | 95% CI† | P value | |
| Unadjusted | 1.99 | 1.32 - 3.01 | 0.0009 |
| Instrumental variable analysis | 1.11 | 0.19 - 6.43 | 0.91 |
| Instrumental variable analysis, patients with CT‡ | 1.44 | 0.21 - 9.97 | 0.71 |
| Instrumental variable analysis, patients with CT, without furosemide | 0.95 | 0.04 - 23.97 | 0.97 |
*OR, odds ratio
†CI, confidence interval
‡CT, computed tomography
Table 3.
Comparison of ventilator-free days between the methylprednisolone plus cyclophosphamide and methylprednisolone alone groups
| Difference | 95% CI* | P value | |
| Unadjusted | −3.7 | −5.9 - −1.6 | 0.0008 |
| Instrumental variable analysis | 2.2 | −2.6 - 7.0 | 0.37 |
| Instrumental variable analysis, patients with CT† | 3.8 | −1.1 - 8.7 | 0.13 |
| Instrumental variable analysis, patients with CT, without furosemide | 5.8 | −5.2 - 16.8 | 0.30 |
*CI, confidence interval
†CT, computed tomography
Table 4.
Comparison of incidence of sepsis or mycosis between the methylprednisolone plus cyclophosphamide and methylprednisolone alone groups
| OR* | 95% CI† | P value | |
| Sepsis | |||
| Unadjusted | 1.99 | 0.89 - 4.47 | 0.10 |
| Instrumental variable analysis | 6.68 | 0.12 - 379 | 0.36 |
| Instrumental variable analysis, patients with CT‡ | 6.02 | 0.12 - 309 | 0.37 |
| Instrumental variable analysis, patients with CT, without furosemide | 1.90 | 0.00 - 1060 | 0.84 |
| Mycosis | |||
| Unadjusted | 1.84 | 0.64 - 5.28 | 0.25 |
| Instrumental variable analysis | 5.93 | 0.05 - 665 | 0.46 |
| Instrumental variable analysis, patients with CT | 4.82 | 0.05 - 488 | 0.50 |
| Instrumental variable analysis, patients with CT, without furosemide | 1.70 | 0.01 - 532 | 0.86 |
*OR, odds ratio
†CI, confidence interval
‡CT, computed tomography
The numbers of patients having a CT scan within 1 day after admission in the methylprednisolone plus cyclophosphamide and methylprednisolone alone groups were 97 and 1,508, respectively. The F statistic was 67.3, and the hospital preference for cyclophosphamide treatment was not significantly associated with death (coefficient, −0.05; 95% CI, −0.32 to 0.22), VFDs (0.32; 95% CI, −0.41 to 1.06), incidence of sepsis (0.23; 95% CI, −0.31 to 0.78) or incidence of mycosis (0.24; 95% CI, −0.47 to 0.95). There were no significant differences between the groups for in-hospital mortality (OR, 1.44; 95% CI, 0.21-9.97), VFDs (difference, 3.8; 95% CI, −1.1 to 8.7), incidence of sepsis (OR, 6.02; 95% CI, 0.12-309), or incidence of mycosis (OR, 4.82; 95% CI, 0.05-488). The numbers of patients having CT scans within 1 day after admission and without furosemide within 1 day after admission in the groups were 68 and 1,007, respectively. The F statistic was 20.3, and the hospital preference for cyclophosphamide was not significantly associated with death (coefficient, −0.25; 95% CI, −0.75 to 0.25), VFDs (0.57; 95% CI, −0.69 to 1.84), incidence of sepsis (−0.02; 95% CI, −1.20 to 1.15), or incidence of mycosis (0.52; 95% CI, −0.78 to 1.81). No significant differences between the groups were detected for in-hospital mortality (OR, 0.95; 95% CI, 0.04-23.97), VFDs (difference, 5.8; 95% CI, −5.2 to 16.8), incidence of sepsis (OR, 1.90; 95% CI, 0.00-1060), or incidence of mycosis (OR, 1.70; 95% CI, 0.01-532).
Discussion
This study used data obtained from a Japanese national inpatient database to compare the effectiveness of high-dose methylprednisolone plus cyclophosphamide with high-dose methylprednisolone alone for treating patients with AE-IPF. Our IV analysis showed no significant difference between the two treatment groups for in-hospital mortality, VFDs, incidence of sepsis, or incidence of mycosis.
Two previous studies showed that cyclophosphamide was potentially effective for treating patients with AE-IPF (12, 13). However, those studies were limited by having no control group and small sample sizes (n=11, 17, respectively). Despite the high mortality associated with AE-IPF, treatment of the condition remains uncertain. International evidence-based guidelines weakly recommend administration of systemic corticosteroids and do not judge whether other medications are effective for AE-IPF because of a lack of evidence regarding the combined treatment (8). Furthermore, a recent review examining AE-IPF reported that studies investigating treatment of AE-IPF were mostly small and uncontrolled and could not adjust for confounders (1).
The advantage of the present study was that we performed an IV analysis, and this analysis generated pseudo-randomization adjusting for unmeasured and measured confounders. We found no significant difference for in-hospital mortality or VFDs between the methylprednisolone plus cyclophosphamide group and methylprednisolone alone group. One potential reason for this may be that cyclophosphamide had no effect on AE-IPF. Previous studies have reported that cyclophosphamide has no effect on patients with IPF (9, 10). Another potential reason for the lack of differences is that no currently known medication, including corticosteroids and other immunosuppressant agents, may be effective against AE-IPF because AE-IPF is a severe condition with a rapid progression. Although cyclophosphamide suppresses the immune system with depressing bone marrow, the present study showed that there were no significant differences in incidence of sepsis or mycosis between the cyclophosphamide users and non-users. One potential reason for this may be that patients receiving cyclophosphamide died before cyclophosphamide had time to suppress their immune systems.
This study has several limitations. First, the database did not include detailed data on patients’ physical conditions, laboratory examinations, and other tests, such as respiratory rates, partial pressure of arterial oxygen/fraction of inspired oxygen ratios, lactate dehydrogenase levels, serum KL-6 levels, and CT imaging results (29). We could therefore not obtain information on the severity of IPF. Moreover, it is possible that patients receiving cyclophosphamide were treated more aggressively. We therefore used instrumental variable analysis to account for these unmeasured confounders. Furthermore, previous studies have shown that the prognosis of suspected AE-IPF is similar to that of AE-IPF (4, 5). The second limitation is that it cannot be proven that our IV analysis fully addressed unmeasured confounders (30). However, we conducted sensitivity analyses based on revised diagnostic criteria for AE-IPF, and the results of the sensitivity analysis were similar to those in the primary analysis. Furthermore, the Japanese Diagnosis Procedure Combination database has been well validated and can serve as a relatively accurate substitute for clinical data although any administrative data have some limitations to the recorded data (31). Third, it is unknown whether our results can be applied to patients with AE-IPF who are not using mechanical ventilation.
Conclusions
Despite these limitations, our IV analysis using a Japanese inpatient database showed that the administration of cyclophosphamide to patients with AE-IPF who were also receiving systemic corticosteroids was not associated with improved in-hospital mortality or VFDs. Further prospective studies will be required to confirm the effect of cyclophosphamide in the treatment of AE-IPF.
Authorship:
KF contributed to the study design and data acquisition. SA, HM, and HY performed the statistical analyses and produced the first draft of the manuscript. All authors commented on the manuscript and approved the final version.
Funding:
This work was supported by grants for Research on Policy Planning and Evaluation from the Ministry of Health, Labour and Welfare, Japan (grant numbers H29-Policy-Designated-009, H27-Policy-Strategy-011); Ministry of Education, Culture, Sports, Science and Technology, Japan (grant numbers 17H04141); and the Japan Agency for Medical Research and Development (AMED). The funders had no role in the execution of this study or interpretation of the results.
Disclosure Statement:
HY and KF received grant support from the Japanese government. The funders had no role in the execution of this study or interpretation of the results. All authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis an international working group report. Am J Respir Crit Care Med. 2016;194(3):265–75. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 2.Song JW, Hong S-B, Lim C-M, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–63. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 3.Usui Y, Kaga A, Sakai F, et al. A cohort study of mortality predictors in patients with acute exacerbation of chronic fibrosing interstitial pneumonia. BMJ Open. 2013;3:e002971. doi: 10.1136/bmjopen-2013-002971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huie TJ, Olson AL, Cosgrove GP, et al. A detailed evaluation of acute respiratory decline in patients with fibrotic lung disease: Aetiology and outcomes. Respirology. 2010;15(6):909–17. doi: 10.1111/j.1440-1843.2010.01774.x. [DOI] [PubMed] [Google Scholar]
- 5.Collard HR, Yow E, Richeldi L, Anstrom KJ, Glazer C. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res. 2013;14:73. doi: 10.1186/1465-9921-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mallick S. Outcome of patients with idiopathic pulmonary fibrosis (IPF) ventilated in intensive care unit. Respir Med. 2008;102(10):1355–9. doi: 10.1016/j.rmed.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: Frequency and clinical features. Eur Respir J. 2006;27:143–50. doi: 10.1183/09031936.06.00114004. [DOI] [PubMed] [Google Scholar]
- 8.Raghu G, Collard HR, Egan JJ, et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collard HR, Ryu JH, Douglas WW, et al. Combined corticosteroid and cyclophosphamide therapy does not alter survival in idiopathic pulmonary fibrosis. Chest. 2004;125(6):2169–74. doi: 10.1378/chest.125.6.2169. [DOI] [PubMed] [Google Scholar]
- 10.Johnson MA, Kwan S, Snell NJ, Nunn AJ, Darbyshire JH, Turner-Warwick M. Randomised controlled trial comparing prednisolone alone with cyclophosphamide and low dose prednisolone in combination in cryptogenic fibrosing alveolitis. Thorax. 1989 Jan;44:280–8. doi: 10.1136/thx.44.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pereira CAC, Malheiros T, Coletta EM, et al. Survival in idiopathic pulmonary fibrosis - Cytotoxic agents compared to corticosteroids. Respir Med. 2006;100(2):340–7. doi: 10.1016/j.rmed.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Novelli L, Ruggiero R, Giacomi F De, et al. Corticosteroid and cyclophosphamide in acute exacerbation of idiopathic pulmonary fibrosis : a single center experience and literature review. Sarcoidosis Vasc Diffus Lung Dis. 2016;33:385–91. [PubMed] [Google Scholar]
- 13.Ota M, Iwasaki Y, Harada H, Sasaki O, Nagafuchi Y, Nakachi S, et al. Efficacy of intensive immunosuppression in exacerbated rheumatoid arthritis-associated interstitial lung disease. Mod Rheumatol. 2016 May;7595:1–7. doi: 10.3109/14397595.2016.1173816. [DOI] [PubMed] [Google Scholar]
- 14.Ohta T, Waga S, Handa H, et al. New grading of level of disordered consciousness (author’s translation) No shinkei geka. 1974;2:623–7. [PubMed] [Google Scholar]
- 15.Shigematsu K, Nakano H, Watanabe Y. The eye response test alone is sufficient to predict stroke outcome—reintroduction of Japan Coma Scale: a cohort study. BMJ Open. 2013;3:e002736. doi: 10.1136/bmjopen-2013-002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ono K, Wada K, Takahara T, Shirotani T. Indications for Computed Tomography in Patients With Mild Head Injury. Neurol Med Chir. 2007;47:291–8. doi: 10.2176/nmc.47.291. [DOI] [PubMed] [Google Scholar]
- 17.Fletcher CM. The clinical diagnosis of pulmonary emphysema; an experimental study. Proc R Soc Med. 1952;45(9):577–84. [PubMed] [Google Scholar]
- 18.Oda K, Yatera K, Fujino Y, et al. Efficacy of concurrent treatments in idiopathic pulmonary fibrosis patients with a rapid progression of respiratory failure: an analysis of a national administrative database in Japan. BMC Pulm Med. 2016;16(1):91. doi: 10.1186/s12890-016-0253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoenfeld DA, Bernard GR. Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–7. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Stat Med. 1991;10(4):585–98. doi: 10.1002/sim.4780100410. [DOI] [PubMed] [Google Scholar]
- 21.Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393. doi: 10.1136/bmj.b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li P, Stuart EA, Allison DB. Multiple Imputation: A Flexible Tool for Handling Missing Data. JAMA. 2015;314(18):1966–7. doi: 10.1001/jama.2015.15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newhouse JP, McClellan M. Econometrics in outcomes research: the use of instrumental variables. Annu Rev Public Health. 1998;19(1):17–34. doi: 10.1146/annurev.publhealth.19.1.17. [DOI] [PubMed] [Google Scholar]
- 24.Iwashyna TJ, Kennedy EH. Instrumental variable analyses: Exploiting natural randomness to understand causal mechanisms. Ann Am Thorac Soc. 2013;10(3):255–60. doi: 10.1513/AnnalsATS.201303-054FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staiger D, Stock J. Instrumental Variables Regression with Weak Instruments. Econometrica. 1997;65(3):557–86. [Google Scholar]
- 26.Stukel TA, Fisher ES, Wennberg DE, Alter DA, Vermeulen MJ. Analysis of Observational Studies in the Presence of Treatment Selection Bias. Jama. 2007;297(3):278–85. doi: 10.1001/jama.297.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Terza JV, Basu A, Rathouz PJ. Two-stage residual inclusion estimation: Addressing endogeneity in health econometric modeling. J Health Econ. 2008;27(3):531–43. doi: 10.1016/j.jhealeco.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terza JV, Bradford WD, Dismuke CE. The use of linear instrumental variables methods in health services research and health economics: A cautionary note. Health Serv Res. 2008;43(3):1102–20. doi: 10.1111/j.1475-6773.2007.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kishaba T, Tamaki H, Shimaoka Y, Fukuyama H, Yamashiro S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung. 2014;192(1):141–9. doi: 10.1007/s00408-013-9530-0. [DOI] [PubMed] [Google Scholar]
- 30.Harris KM, Remler DK. Who is the marginal patient? Understanding instrumental variables estimates of treatment effects. Health Serv Res. 1998;33(5 Pt 1):1337–60. [PMC free article] [PubMed] [Google Scholar]
- 31.Yamana H, Moriwaki M, Horiguchi H, Kodan M, Fushimi K, Yasunaga H. Validity of diagnoses, procedures, and laboratory data in Japanese administrative data. J Epidemiol. 2017;27(10):476–82. doi: 10.1016/j.je.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


