Abstract
Introduction:
Besides invasive or non-invasive ventilation, treatment of severe forms of interstitial lung diseases (ILD) includes immunosuppressive medication. In case of refractory organ- or life-threatening courses of disease, cyclophosphamide pulse therapy can serve as a rescue treatment option.
Objectives:
To investigate therapeutic and prognostic effects of cyclophosphamide for the treatment of severe forms of ILD on intensive care unit (ICU) we performed this analysis.
Methods:
Between 2009 and 2017 we identified 14 patients, who were treated on intensive care unit (ICU) with severe forms of ILD. Retrospectively, clinical, radiologic and prognostic data were collected and evaluated.
Results:
Our analysis demonstrated a prognostic impact of cyclophosphamide on the ILD in general. Whereas pulmonary manifestations of both systemic sclerosis (SSc) and ANCA-associated vasculitis had an improved outcome, a reduced overall survival was found for Goodpasture syndrome (GPS), dermatomyositis (DM), cryptogenic organizing pneumonia (COP) and drug reaction with eosinophilia and systemic symptoms (DRESS; p=0.040, logrank test). Besides, additional plasmapheresis and initiation of cyclophosphamide within ten days following initial diagnosis of ILD were associated with improved prognosis.
Conclusion:
Positive prognostic effects of cyclophosphamide pulse therapy in ICU treated patients suffering from severe respiratory failure due to pulmonary manifestations of both SSc and ANCA-associated-vasculitis were observed. Further prognostic and therapeutic data are needed for cyclophosphamide for this indication in order to prevent patients from its toxic side-effects, who most likely will not benefit from its application.
Key words: intensive care medicine, interstitial lung disease, treatment, chemotherapy, computed tomography, exacerbation
Introduction
The term “interstitial lung diseases” (ILD) encompasses both acute and chronic parenchymal lung diseases (1). The origin of ILD can be reactive to toxic agents, idiopathic (i.e. idiopathic interstitial pneumonia) or associated with systemic diseases such as granulomatous disorders, connective tissue diseases (CTD) or vasculitis (2, 3). For the final diagnosis anamnesis, clinical and functional data as well as radiologic ILD patterns and histopathological results are taken into consideration (2, 3, 5-8).
In general, treatment of acute exacerbations and progressive courses of ILDs is difficult. Often, immunosuppressive regimens are initiated with corticosteroids (3, 9). To intensify immunosuppressive treatment, addition of rituximab or cyclophosphamide is recommended only for progressive ILD forms due to either connective tissue disorders (CTD) or to vasculitides (10-12). As a rescue option, the British Thoracic Society (BTS) suggests the application of cyclophosphamide for the treatment of refractory and progressive ILD forms other than idiopathic pulmonary fibrosis (IPF) (13). However, only few data exist upon the prognostic and therapeutic effects of cyclophosphamide in critically ill patients.
For chronic ILD forms, Schupp et al. evaluated the impact of cyclophosphamide pulse therapy in n=26 patients. According to their analysis, prognostic outcome was improved for patients with lymphocytic interstitial pneumonia (LIP) and non-specific interstitial pneumonia (NSIP) following cyclophosphamide application. In contrast, patients with p-ANCA positive vasculitis had the worst prognosis. However, patients who had less than 3 infusions of cyclophosphamide and who were treated on ICU were not included in their study (14).
Since many ICU patients with severe ILD forms require invasive ventilation and sedation (15), it is often impossible to obtain patients’ consent. Consequently, considering toxic side effects (16), the indication to initiate additional cyclophosphamide is met by interdisciplinary teams (7, 8).
To investigate the impact of cyclophosphamide pulse therapy in patients requiring ICU treatment due to respiratory failure caused by severe ILD forms, we performed this retrospective analysis with focus on radiologic ILD patterns and other clinical factors.
Material and Methods
Study population
First, approval of the ethical committee Muenster was obtained (Ref. 2017-599-f-S). In total, n=14,421 ICU patients were treated on our ICUs between 2009 and 2017. Among these patients, we identified n=14 patients suffering from different forms of ILD, who received at least one course of intravenous cyclophosphamide as rescue therapy (Table 1).
Table 1.
Baseline characteristics of the study cohort. Age [years], cyclophosphamide dosage [mg], PaO2/FiO2 ratio [mmHg/%], ventilation period, delay from ILD diagnosis to first cyclophosphamide administration, survival since cyclophosphamide administration and follow-up period [days] are presented as mean with standard deviation (SD) and median with interquartile range (Q1-Q3). Sex, diagnoses, pathologic laboratory values, supportive therapy, ventilation mode, veno-venous extra corporal membrane oxygenation (ECMO), laboratory values and survival status are presented with the absolute and relative (in %) proportions
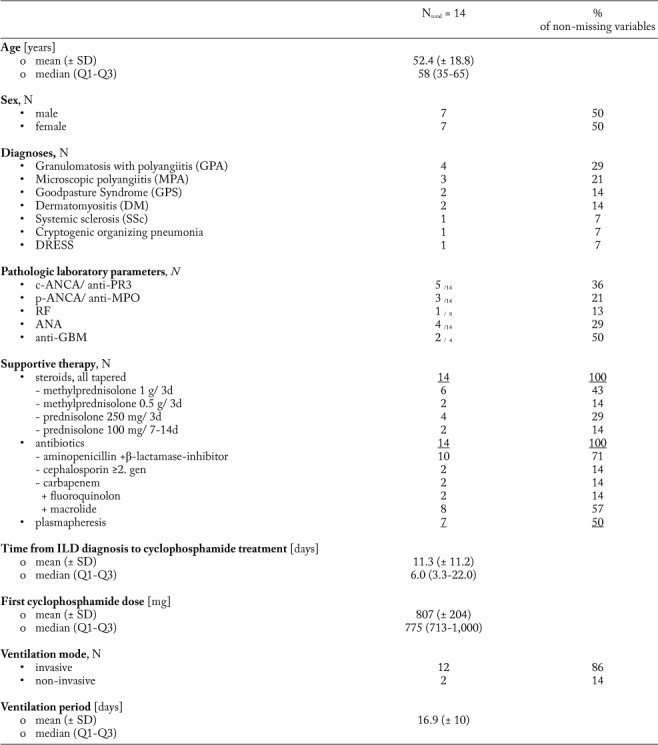
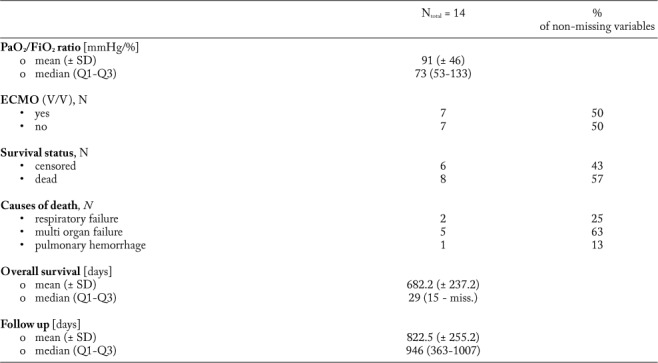
Data collection was performed retrospectively. Besides clinical data, therapeutic information (e.g. cyclophosphamide cycles, dosage, first-line immunosuppression, ventilation mode, ventilation duration, P/F ratio [i.e. Horowitz index=arterial oxygen partial pressure (paO2 in mmHg)/fraction of inhaled oxygen (FiO2 in %)], additional antibiotics, extracorporal membrane oxygenation and plasmapheresis) was gathered, too. Due to varying dosage schedules (mg/kg vs. mg/m2 vs. mg as absolute dosage), all analyses considered the absolute cyclophosphamide dosage. Of interest, cyclophosphamide was given exclusively to patients with severe respiratory failure (as defined by a partial oxygen pressure in [mmHg]/ oxygen saturation in inhaled gas [%]) <200) due to various forms of ILD, who required further immunosuppressive therapy. In contrast, if an infectious origin was suspected, cyclophosphamide was not applied. However, additional antibiotic prophylaxis was initiated before cyclophosphamide was given as prophylactic treatment in all patients.
Radiologic examination
With focus on the radiologic ILD patterns, thoracic computed axial tomography (CAT) scans of the identified patients were categorized as non-specific interstitial pneumonia (NSIP), usual interstitial pneumonia (UIP), organizing pneumonia (OP), diffuse alveolar damage (DAD) and lymphocytic interstitial pneumonia (LIP) (17). To determine the morphologic extent of the underlying disease, the involvement of the affected lobes (i.e. upper left lobe including lingula, lower left lobe, upper right lobe, middle lobe and lower right lobe) was evaluated.
Statistical analyses
Descriptive statistics for mean and standard deviation, median and interquartile range (IQR) as well as frequencies were used to investigate our study population. Two-fold associations between categorical variables were analyzed via Fisher’s exact test or Chi-square test. Continuous variables were tested using either unpaired t-test or Mann-Whitney-U tests depending on the normality of the data. Associations of more than two groups (e.g. diagnosis variable) with categorial and continuous outcomes were analyzed using Chi-square test or Kruskal-Wallis test if applicable.
Overall survival was defined as time (days) between first cyclophosphamide application on intensive care unit and death or censoring. Survival time was compared between groups using Log rank tests and visualized by Kaplan Meier plots. Both, data collection and calculations were performed using IBM SPSS Statistics® version 24 (released 2016, IBM Corp., Armonk, NY, USA). The local significance level was set to 0.05. An adjustment to multiplicity was not determined as the analysis was explorative.
Results
The study population is characterized in Table 1. The majority of the investigated patients (n=12) displayed autoimmune features. Regardless of the underlying disease, mean time between the first diagnosis of interstitial lung disease and the ICU admission was 4.1 (± 8.3) days. Due to respiratory failure (P/F ratio average 90.6 (± 45.6)) on admission, supportive ventilation (i.e. invasive ventilation in n=12 patients; non-invasive ventilation in n=2 patients) was applied in all patients for a period of 16.9 days (± 10.0 days) independent from the underlying disease (p= .134, Kruskal-Wallis test). Additional extracorporal membrane oxygenation (ECMO) support was applied in n=7 patients.
With regard to systemic medication, all patients received both corticosteroids and antibiotics prior to cyclophosphamide pulse therapy. The time between first ILD diagnosis and the initiation of cyclophosphamide therapy ranged between 1 and 35 days (mean 11.3 days, median 6 days). The applied absolute dosages of cyclophosphamide varied from 400 mg up to 1.000 mg (mean 807.1 mg). In addition, plasmapheresis was performed in five patients concurrently to the first application of cyclophosphamide. In two cases cyclophosphamide was given after or at least one week prior to plasmapheresis.
Overall survival for cyclophosphamide therapy is demonstrated in Figure 1. Cyclophosphamide pulse therapy was associated with an increased overall survival in one patient with systemic sclerosis and in patients with ANCA-associated vasculitis [i.e. microscopic polyangiitis; MPA, granulomatosis with polyangiitis; GPA, eosinophilic granulomatosis with polyangiitis; EGPA (10)]. Whereas median overall survival in patients with underlying Goodpasture syndrome (GPS) was 29 days, patients suffering from Dermatomyositis (DM), Drug reaction with eosinophilia and systemic symptoms (DRESS) and cryptogenic organizing pneumonia (COP) achieved less than 10 days. Of interest, one PR3 positive GPS patient died within 29 days, whereas one ANCA-negative GPS patient was still alive after 1007 days.
Fig. 1.
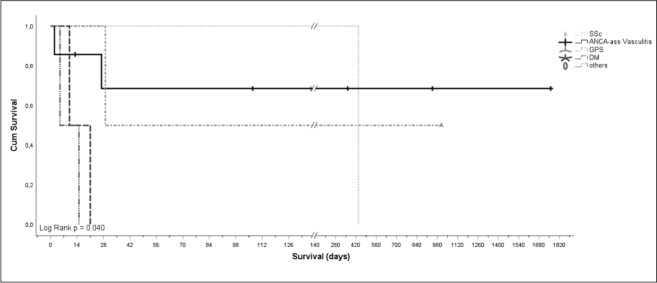
Overall survival following first application of cyclophosphamide [days] stratified for ANCA-associated vasculitis (i.e. GPA, MPA), GPS, DM, SSc and other ILD forms (Log Rank p=0.040).
Legend: SSc: Systemic sclerosis, n=1; ANCA-ass vasculitis: Anti-neutrophil cytoplasmic antibodies-associated vasculitis (i.e. Granulomatosis with polyangiitis (GPA), Microscopic polyangiitis (MPA) and Eosinophilic granulomatosis with polyangiitis (EGPA)), n=7; GPS: Goodpasture syndrome, n=2; DM: Dermatomyositis, n=2; others, n=2, containing COP: Cryptogen organizing pneumonia, and DRESS: Drug reaction with eosinophilia and systemic symptoms. Survival axis scale [days] is split (indicated by //) after 140 days and then shows greater intersections.
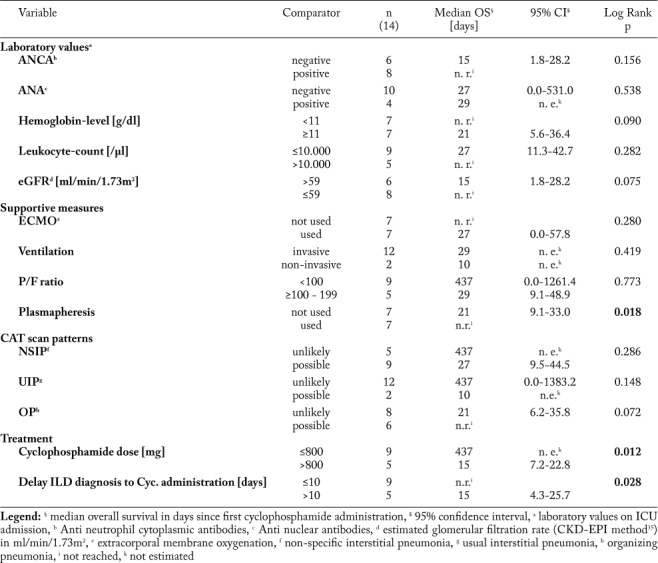
Cyclophosphamide doses >800 mg absolute were associated with a reduced overall survival (p=0.012, log rank test; Table 3). Yet, considering confounders, hemoglobin count, positivity for ANA or ANCA, impaired renal function (eGFR ≤59 ml/min/1.73m2), ECMO application, ventilation mode, P/F ratio, plasmapheresis or radiologic ILD patterns did not alter cyclophosphamide dosage (p>0.05, Mann-Whitney-U test; Supplementary Table S1).
Table 3.
Univariate prognostic models depending on laboratory values, supportive therapies, CAT scan patterns and first absolute cyclophosphamide dosage. Additional plasmapheresis, early cyclophosphamide administration (≤10 days to ILD diagnosis) and an absolute first cyclophosphamide dose ≤800 mg were associated with improved overall survival in ILD patients treated with cyclophosphamide on ICU
Supplementary Table S1.
Mann Whitney U test analysis for equal variances of not normally distributed absolute cyclophosphamide dose regarding laboratory values, supportive therapeutic measures and CAT scan patterns.
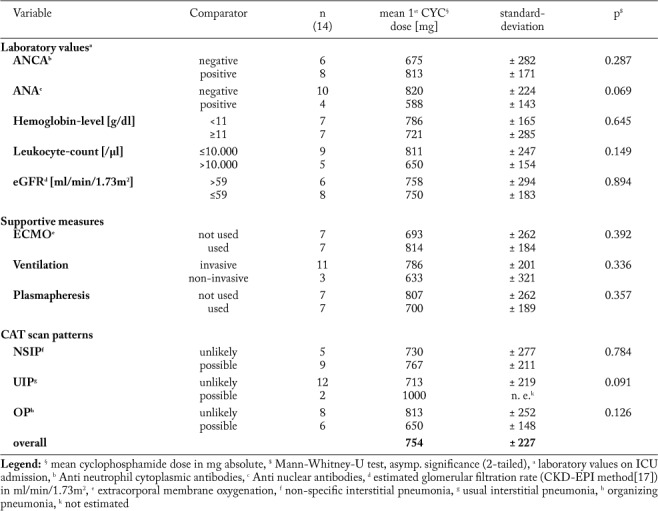
Regarding ILD patterns, we found possible non-specific interstitial pneumonia (NSIP) pattern in n=9 patients, usual interstitial pneumonia (UIP) pattern in n=2 patients and an organizing pneumonia (OP) pattern in n=6 patients. Of note, multiple radiographic patterns were observed in four patients (Table 2). For none of the radiologic patterns a significant prognostic effect was found (p>0.05, log rank test; Table 3). In n=12 cases all pulmonary lobes were affected by ILD, whereas in two patients only two respectively four lobes were affected. Of interest, overall survival did not correlate with the morphologic extent of ILD on chest CT scan (p=0.557, log rank test) or severity of pulmonary failure. Furthermore no differences were found for anti-neutrophil cytoplasmic antibodies, anti-nuclear antibodies, rheumatoid factors, anti-glomerular basal membrane antibodies and blood count (p>0.05, log rank test). Impaired renal function (eGFR in ml/min/1.73 m2 cutoff >59 vs. ≤59) as well as anemia (hemoglobin level in g/dl cutoff ≥11 vs. < 11) or leukocytosis (in cells/µl cutoff >10.000 vs. ≤10.000) was not associated with improved or reduced prognosis (p>0.050, log rank test). Among the investigated patients, n=7 patients died on ICU due to the fulminant course of disease. N=2 patients died due to respiratory failure, n=4 died due to multiple organ failure (MOF) and one patient died due to pulmonary hemorrhage. Of interest, one patient died 12 months later following initial ICU admission due to non-septic MOF.
Table 2.
Study collective
| No. | Sex | Age | Diagn. | Ventilation mode | Ventilation days | P/F ratio | ECMO | Plasmapheresis | CAT scan pattern | Survival status | Overall survival [days] |
| 1 | M | 55 | GPA | IV | 26 | 64,4 | Yes | Yes | NSIP+ UIP- OP+ | Alive | 946 |
| 2 | F | 61 | DM | IV | 18 | 58,8 | No | No | NSIP+ UIP- OP- | Dead | 21 |
| 3 | M | 80 | GPS | IV | 30 | 141,6 | Yes | Yes | NSIP+ UIP- OP+ | Dead | 29 |
| 4 | M | 64 | MPA | IV | 2 | 69,7 | No | Yes | NSIP+ UIP- OP+ | Alive | 363 |
| 5 | M | 60 | MPA | IV | 6 | 40,2 | Yes | Yes | NSIP+ UIP- OP- | Alive | 107 |
| 6 | F | 51 | SSc | NIV | 15 | 46,1 | No | No | NSIP- UIP- OP- | Dead | 437 |
| 7 | M | 36 | DRESS | IV | 15 | 54,7 | Yes | No | NSIP- UIP- OP- | Dead | 15 |
| 8 | F | 67 | DM | NIV | 18 | 99,7 | No | No | NSIP+ UIP+ OP- | Dead | 10 |
| 9 | M | 61 | COP | IV | 28 | 130,0 | Yes | No | NSIP+ UIP- OP+ | Dead | 5 |
| 10 | F | 31 | GPA | IV | 8 | 130,0 | No | No | NSIP- UIP- OP- | Alive | 13 |
| 11 | M | 19 | GPS | IV | 9 | 169,2 | No | Yes | NSIP- UIP- OP+ | Alive | 1007 |
| 12 | F | 56 | MPA | IV | 24 | 152,8 | Yes | No | NSIP+ UIP+ OP- | Dead | 27 |
| 13 | F | 72 | GPA | IV | 32 | 76,0 | No | Yes | NSIP- UIP- OP+ | Alive | 1760 |
| 14 | M | 20 | GPA | IV | 5 | 35,0 | Yes | Yes | NSIP+ UIP- OP- | Dead | 2 |
Legend: Sex category (M = male, F = female), Age [years], ventilation mode (IV = invasive ventilation, NIV = non-invasive ventilation), absolute period of ventilation [days], the P/F ratio (i.e. partial oxygen pressure in [mmHg]/ oxygen saturation in inhaled gas [%]), the use of ECMO and Plasmapheresis, CAT scan patterns, regarding NSIP, UIP and OP as possible (+) or unlikely (-), survival status (alive/ dead) and survival since first cyclophosphamide administration [days]. Of interest, patient no. 10 was lost-to-follow-up after 13 days and discharge from hospital
Additional plasmapheresis (p=0.018, log rank test) and application of cyclophosphamide within ten days following first ILD diagnosis (p=0.028, log rank test) had a positive effect on prognosis (Table 3). Moreover, we observed that plasmapheresis was performed more often in patients with hemoglobin levels below 11 g/dl (p=0.029, Fisher’s exact test) independent from ANCA- or ANA-positivity, leukocyte count, renal impairment, ECMO use, ventilation mode and radiographic ILD pattern (Fisher’s exact test p>0.05 in all calculations, data not shown).
Discussion
Treatment of acute respiratory failure due to various ILD forms is challenging. The therapeutic armamentarium includes supportive ventilation, extracorporal membrane oxygenation, plasmapheresis, antibiotics and immunosuppressive medication. Corticosteroids are often applied as first immunosuppression (10, 13, 18-21). However, there are patients, who require more intensified immunosuppressive regimens to overcome refractory courses of disease. Here, cyclophosphamide or rituximab can serve as therapeutic alternatives especially for ANCA-associated vasculitis (10, 20, 21) or for CTDs (11). With regard to cyclophosphamide, the recommended dosage is 15 mg/kg body weight every 2 to 3 weeks in combination with corticosteroids (22).
Schupp et al. evaluated the prognostic impact of intravenous cyclophosphamide pulsed therapy in n=26 patients with chronic ILD forms, who did not require ICU treatment. Patients were followed up for 18 months. According to their analysis, beneficial effects were demonstrated for patients with both LIP-patterns and NSIP-patterns, whereas p-ANCA associated ILDs did not benefit from additional cyclophosphamide pulsed therapy (14).
In our study, we focused on those ILD patients who received cyclophosphamide as part of a rescue immunosuppressive treatment on ICU to overcome respiratory failure. We found superior treatment effects of cyclophosphamide in n=7 patients (50%) suffering from ANCA-associated ILDs compared to other ILDs. Similar observations were reported by Novack et al. in 1971 (23). A possible explanation for the results of Schupp et al. might be due to the fact, that in chronic (stable) ILD the possible life threatening side effects of cyclophosphamide exceed the benefit. Since plasmapheresis was applied in five out of seven patients (71%) with ANCA-associated vasculitis, it is difficult to interpret the sole effect of cyclophosphamide in this context.
For sure, one major limitation of our study is its small study population. However, it is difficult to perform prospective studies with larger study populations with focus on the risk-benefit analysis for cyclophosphamide for this indication. Among n=14,421 ICU patients treated between 2009 and 2017 on our ICUs, we identified only n=14 ILD patients, who were treated with cyclophosphamide due to this indication. To our knowledge, the underlying study is the first study, which investigates the impact of cyclophosphamide for critically ill ILD patients suffering severe respiratory failure and requiring ICU therapy.
Acute treatment of various ILD forms is summarized in Figure 2. As demonstrated, the application of cyclophosphamide represents a therapeutic alternative for ILD forms. Whereas cyclophosphamide is recommended as first line treatment of SSc (20, 21, 24) and ANCA-associated vasculitis (10), there is less evidence for RA, DM and GPS. Here, cyclophosphamide could be an alternative option for refractory courses (19, 25, 26). Even though favorable effects of cyclophosphamide are reported for the treatment of pulmonary manifestations of GPS (18), there are still patients who died due to respiratory failure (27-29). Likewise, cyclophosphamide application had positive effects on lung function in some DM patients, still the prognosis in general is poor (19, 30). Even more evidence for the application of cyclophosphamide in COP is lacking. Whereas, corticosteroids are recommended for COP treatment (13, 31), less data exist upon the intensified immunosuppression with azathioprine, cyclosporine A or cyclophosphamide (13). With respect to the rather low incidence, one review recommended corticosteroids and intravenous immunoglobulins following the withdrawal of potential causal agents as treatment option for DRESS syndrome (32), whereas additional cyclophosphamide was only suggested by one study (33).
Fig. 2.
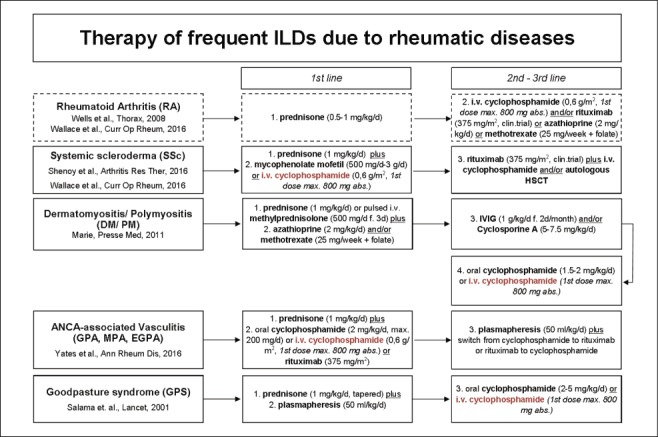
Possible therapeutic options for the therapy of interstitial lung diseases (ILDs)
Against this background, we evaluated the therapeutic impact of cyclophosphamide for severe respiratory failure due to various ILD forms. Corresponding to the published recommendations, we observed positive effects of cyclophosphamide in those ILD patients with systemic sclerosis (SSc) (20) and ANCA-associated vasculitis (10). Especially in these patients, early administration of cyclophosphamide within ten days and a starting dosage of less than 800 mg had favorable effects. In consideration of the toxic side effects (e.g. anemia, thrombocytopenia, leukocytopenia, hemorrhage cystitis) of cyclophosphamide further studies should investigate the optimal dosage (34). Without doubt, additional plasmapheresis (10) might have improved the clinical status. Since patients suffering from DM/PM did not benefit from additional cyclophosphamide therapy in our analysis, we cannot support its use for this specific indication.
In conclusion cyclophosphamide pulse therapy for the treatment of respiratory failure due to ILD can be a beneficial therapy option for some patients with underlying SSc and ANCA-associated vasculitis. When considering cyclophosphamide in these patients, treatment should be initiated early, the dosage should be below 800 mg and plasmapheresis should be added. However, there is less evidence for the application of cyclophosphamide in those patients with various ILD patterns due to GPS, DM, COP or DRESS. In consideration of the available literature and our analysis we cannot support the application of cyclophosphamide as first line treatment for ILD forms other than ANCA-positive vasculitis or SSc associated ILDs. Even though cyclophosphamide is still applied for this indication, it is one of the most toxic routinely prescribed immunosuppressive agents (13). Consequently, the identification of those patients who might benefit from this therapy is crucial. Here, the investigation of larger study collectives with focus on ICU patients with severe ILD forms could serve for further therapeutic stratification. Whether less toxic agents or targeted therapies will be available in future is unclear.
Authorship Statement:
LHS and MM designed the study. LHS, ABS and GE performed research, collected data and analyzed the data. LHS and ABS wrote the paper. FR, AK and JS collected data and gave relevant input upon treatment on ICU. JPH and CS performed retrospective CAT scan analysis. JAE detected cyclophosphamide treated patients on ICU and gave relevant input on cyclophosphamide dosage and treatment schedules. DG planned, controlled and supported the statistical analysis. PJB, GL, MM, LHS and HB gave relevant input on underlying disease. Every author read and approved the corrected manuscript.
References
- 1.Travis WD, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demedts M, Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur Respir J. 2002;19:794–6. doi: 10.1183/09031936.02.00492002. [DOI] [PubMed] [Google Scholar]
- 3.Fischer A, Du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380:689–698. doi: 10.1016/S0140-6736(12)61079-4. [DOI] [PubMed] [Google Scholar]
- 4.Sambataro G, et al. State of the art in interstitial pneumonia with autoimmune features: a systematic review on retrospective studies and suggestions for further advances. Eur Respir Rev an Off J Eur Respir Soc 27. 2018;148:170139. doi: 10.1183/16000617.0139-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischer A, Lee JS, Cottin V. Interstitial lung disease evaluation: Detecting connective tissue disease. Respiration. 2015;90:177–184. doi: 10.1159/000440665. [DOI] [PubMed] [Google Scholar]
- 6.Robles-Perez A, Molina-Molina M. Treatment considerations of lung involvement in rheumatologic disease. Respiration. 2015;90:265–274. doi: 10.1159/000441238. [DOI] [PubMed] [Google Scholar]
- 7.Tomassetti S, Piciucchi S, Tantalocco P, Dubini A, Poletti V. The multidisciplinary approach in the diagnosis of idiopathic pulmonary fibrosis: A patient case-based review. Eur Respir Rev. 2015;24:69–77. doi: 10.1183/09059180.00011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonini M, Fiorenzano G. Exertional dyspnoea in interstitial lung diseases: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev. 2017;26:235–8. doi: 10.1183/16000617.0099-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathai SC, Danoff SK. Management of interstitial lung disease associated with connective tissue disease. BMJ. 2016 doi: 10.1136/bmj.h6819. h6819. doi:10.1136/bmj.h6819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates M, et al. EULAR/ERA-EDTA recommendations for the management of ANCA-associated vasculitis. Ann Rheum Dis. 2016;75:1583–1594. doi: 10.1136/annrheumdis-2016-209133. [DOI] [PubMed] [Google Scholar]
- 11.Saunders P, et al. Rituximab versus cyclophosphamide for the treatment of connective tissue disease-associated interstitial lung disease (RECITAL): study protocol for a randomised controlled trial. Trials. 2017;18:275. doi: 10.1186/s13063-017-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone JH, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363:221–32. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wells AU, Hirani N. Interstitial lung disease guideline. Thorax. 2008;63:v1–v58. doi: 10.1136/thx.2008.101691. [DOI] [PubMed] [Google Scholar]
- 14.Schupp JC, Köhler T, Müller-Quernheim J. Usefulness of Cyclophosphamide Pulse Therapy in Interstitial Lung Diseases. Respiration. 2016;91:296–301. doi: 10.1159/000445031. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, et al. Prone position ventilation support for acute exacerbation of interstitial lung disease? Clin Respir J. 2017;12:1732–1380. doi: 10.1111/crj.12665. doi:10.1111/crj.12665. [DOI] [PubMed] [Google Scholar]
- 16.Hengstler JG, et al. Induction of DNA crosslinks and DNA strand lesions by cyclophosphamide after activation by cytochrome P450 2B1. Mutat Res - Fundam Mol Mech Mutagen. 1997;373:215–223. doi: 10.1016/s0027-5107(96)00200-x. [DOI] [PubMed] [Google Scholar]
- 17.de Lauretis A, Veeraraghavan S, Renzoni E. Review Series: Aspects of Interstitial lung disease: Connective tissue disease-associated interstitial lung disease: How does it differ from IPF? How should the clinical approach differ? Chron Respir Dis. 2011;8:53–82. doi: 10.1177/1479972310393758. [DOI] [PubMed] [Google Scholar]
- 18.Salama AD, Levy JB, Lightstone L, Pusey CD. Goodpasture’s disease. Lancet. 2001;358:917–920. doi: 10.1016/S0140-6736(01)06077-9. [DOI] [PubMed] [Google Scholar]
- 19.Marie I, Mouthon L. Therapy of polymyositis and dermatomyositis. Autoimmun Rev. 2011;11:6–13. doi: 10.1016/j.autrev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Wallace B, Vummidi D, Khanna D. Management of connective tissue diseases associated interstitial lung disease. Curr Opin Rheumatol. 2016;28:236–245. doi: 10.1097/BOR.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shenoy PD, Bavaliya M, Sashidharan S, Nalianda K, Sreenath S. Cyclophosphamide versus mycophenolate mofetil in scleroderma interstitial lung disease (SSc-ILD) as induction therapy: a single-centre, retrospective analysis. Arthritis Res Ther. 2016;18:123. doi: 10.1186/s13075-016-1015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Groot K, et al. Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody-associated vasculitis: a randomized trial. Ann Intern Med. 2009;150:670–80. doi: 10.7326/0003-4819-150-10-200905190-00004. [DOI] [PubMed] [Google Scholar]
- 23.Novack SN, Pearson CM. Cyclophosphamide Therapy in Wegener’s Granulomatosis. N Engl J Med. 1971;284:938–942. doi: 10.1056/NEJM197104292841703. [DOI] [PubMed] [Google Scholar]
- 24.Tashkin DP, et al. Cyclophosphamide versus Placebo in Scleroderma Lung Disease. N Engl J Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 25.Kurita T, Yasuda S, Amengual O, Atsumi T. The efficacy of calcineurin inhibitors for the treatment of interstitial lung disease associated with polymyositis/dermatomyositis. Lupus. 2015;24:3–9. doi: 10.1177/0961203314554849. [DOI] [PubMed] [Google Scholar]
- 26.Shimojima Y, Ishii W, Matsuda M, Kishida D, Ikeda S. Effective Use of Calcineurin Inhibitor in Combination Therapy for Interstitial Lung Disease in Patients With Dermatomyositis and Polymyositis. JCR J Clin Rheumatol. 2017;23:87–93. doi: 10.1097/RHU.0000000000000487. [DOI] [PubMed] [Google Scholar]
- 27.Salam N, Rezki H, Fadili W, Hachim K, Ramdani B. Goodpasture’s syndrome - four case reports. Saudi J Kidney Dis Transpl. 2007;18:235–8. [PubMed] [Google Scholar]
- 28.Uezu Y, Kiyatake I, Tokuyama K. A case of Goodpasture’s syndrome with massive pulmonary hemorrhage ameliorated by cyclophosphamide pulse therapy. Nihon Jinzo Gakkai Shi. 1999;41:499–504. [PubMed] [Google Scholar]
- 29.Kojima K, et al. Two cases of Goodpasture’s syndrome--clinicopathological studies and relapse. Nihon Jinzo Gakkai Shi. 1993;35:89–96. [PubMed] [Google Scholar]
- 30.Kameda H, Takeuchi T. Recent advances in the treatment of interstitial lung disease in patients with polymyositis/dermatomyositis. Endocrine Metab Immune Disord - Drug Targets. 2006;6:409–415. doi: 10.2174/187153006779025775. [DOI] [PubMed] [Google Scholar]
- 31.Lazor R, et al. Cryptogenic organizing pneumonia. Characteristics of relapses in a series of 48 patients. The Groupe d’Etudes et de Recherche sur les Maladles ‘Orphelines’ Pulmonaires (GERM”O”P) Am J Respir Crit Care Med. 2000;162:571–7. doi: 10.1164/ajrccm.162.2.9909015. [DOI] [PubMed] [Google Scholar]
- 32.Cacoub P, et al. The DRESS syndrome: A literature review. Am J Med. 2011;124:588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Laban E, et al. Cyclophosphamide Therapy for Corticoresistant Drug Reaction With Eosinophilia and Systemic Symptoms (DRESS) Syndrome in a Patient With Severe Kidney and Eye Involvement and Epstein-Barr Virus Reactivation. Am J Kidney Dis. 2010;55:e11–e14. doi: 10.1053/j.ajkd.2009.10.054. [DOI] [PubMed] [Google Scholar]
- 34.Jayne D. Review article: Progress of treatment in ANCA-associated vasculitis. Nephrology. 2009;14:42–48. doi: 10.1111/j.1440-1797.2009.01101.x. [DOI] [PubMed] [Google Scholar]
- 35.Levey AS, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


