Abstract
Background:
We have often encountered adverse events requiring dose reduction and/or discontinuation of nintedanib in patients with idiopathic pulmonary fibrosis.
Objectives:
The objectives of this study were to clarify the incidence of dose reduction and/or discontinuation following the commercialization of nintedanib and to investigate predictors of dose reduction and/or discontinuation of nintedanib at our hospital.
Methods:
We retrospectively identified 25 patients who had received nintedanib 150 mg twice daily at Sendai Kousei Hospital and categorized them into two groups according to whether they had or had not required dose reduction and/or discontinuation and sought to identify predictors of dose reduction and/or discontinuation.
Results:
Seventeen patients developed adverse events, which included diarrhea (n=10, 44%), hepatotoxicity (n=7, 28%), and anorexia (n=2, 16%). No adverse event-related deaths occurred during the study period. Patients who required dose reduction and/or discontinuation were significantly older than those who did not (72 years vs 67 years; P=0.047). Body surface area (BSA) was significantly lower in the group that needed dose reduction and/or discontinuation than in the group that did not (1.63 m2 vs. 1.78 m2; P=0.028). Multivariate logistic regression revealed that the association of low BSA with dose reduction and/or discontinuation was statistically significant.
Conclusions:
A low BSA was associated with dose reduction and/or discontinuation of nintedanib in patients with idiopathic pulmonary fibrosis. Further studies in larger patient samples are needed to validate these findings.
Key words: surface area, discontinuation, dose reduction, idiopathic pulmonary fibrosis, nintedanib
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive, fibrosing interstitial pneumonia that occurs primarily in the elderly population and is characterized by increasing dyspnea and loss of lung function (1-3). IPF is ultimately fatal and has a median survival time of only 2-4 years from diagnosis (3).
Nintedanib is an intracellular tyrosine kinase inhibitor of the receptors for fibroblast growth factor, platelet-derived growth factor, and vascular endothelial growth factor (4, 5) and was approved in Japan for use in IPF in September 2015.
Recently, the results of the two replicate Phase III INPULSIS trials demonstrated that nintedanib slowed disease progression in patients with IPF by significantly reducing the rate of decline in forced vital capacity (FVC) (6). In these trials, the most common adverse events (AEs) were gastrointestinal; these AEs accounted for the majority of discontinuations of nintedanib (6).
We have often encountered AEs that require dose reduction and/or discontinuation of nintedanib. The objectives of this study were to clarify the incidence of dose reduction and/or discontinuation of nintedanib since its commercialization and to identify predictors of dose reduction and/or discontinuation of this agent at our hospital.
Methods
This retrospective study was performed at the Sendai Kousei Hospital. All consecutive patients with IPF newly treated with nintedanib 150 mg twice daily from October 2015 to April 2017 were enrolled. The diagnosis of IPF was made according to the official American Thoracic Society/European Respiratory Society/Japanese Respiratory Society/Latin American Thoracic Association statement of 2011 (3). The clinical severity of AEs was graded based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 (7). The criteria for dose reduction and/or discontinuation of nintedanib were the same as those used in a previous clinical trial (8).
All patients were evaluated by spirometry (CHESTAC-55V; Chest, Tokyo, Japan) in accordance with the American Thoracic Society/European Respiratory Society Task Force guidelines (9).
The study protocol was approved by the Sendai Kousei Hospital Ethics Committee (IRB.30-14) and was carried out in accordance with the Declaration of Helsinki. The requirement for patient consent was waived in view of the retrospective nature of the study and the anonymity of the data.
Statistical analysis
Univariate and multivariate logistic regression analyses were used to identify patient variables related to dose reduction and/or discontinuation of nintedanib. Categorical variables were tested for significance using the chi-squared test, Student’s t-test, Mann-Whitney U test, or Welch’s t-test, as appropriate. Variables identified to be significant prognostic indicators in univariate analysis (P<0.05) were evaluated by forward selection stepwise multivariate logistic regression. A P-value < 0.05 was considered statistically significant. All P-values were two-sided. All statistical analyses were performed using EZR (Saitama Medical Centre, Jichi Medical University, Saitama, Japan), a graphic user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of R commander and is designed to add statistical functions frequently used in biostatistics (10).
Results
Patient characteristics
The patient characteristics are summarized in Table 1. Nineteen (76%) of the 25 patients with IPF enrolled in the study were male and the median patient age was 69 years. The median body surface area (BSA) was estimated to be 1.69 m2 using the Du Bois formula. All patients showed the usual interstitial pneumonia pattern on computed tomography scans. The median %FVC and percent diffusing capacity for lung carbon monoxide (DLCO) at baseline were 67.2% and 52.6%, respectively. The median follow-up duration was 244 days (the data cut-off date was June 30, 2017).
Table 1.
Baseline characteristics of the study population (n=25)
| Characteristic | Value* |
| Age, years | 69 [53-79] |
| Sex (male), n (%) | 19 (76) |
| Body surface area (Du Bois, m2) | 1.69 [1.36-1.79] |
| Findings on CT (UIP pattern), n (%) | 25 (100) |
| Laboratory data | |
| Aspartate aminotransferase (IU/L) | 22.7 (15.0-36.0) |
| Alanine aminotransferase (IU/L) | 20.8 (8.0-47.0) |
| Total bilirubin (mg/dL) | 0.84 (0.29-2.07) |
| Creatinine (mg/dL) | 0.76 (0.39-1.48) |
| Krebs von den Lungen-6 (U/mL) | 1131 (523-2394) |
| Pulmonary function tests | |
| FVC, L (n=23) | 2.19 (0.94-3.84) |
| %FVC, L (n=23) | 67.2 (42.0-112.0) |
| % DLCO, % (n=16) | 52.6 (27.0-89.0) |
| 6MWT | |
| Lowest SpO2, % (n=20) | 86.4 (75-91) |
| Walking distance, m (n=20) | 355.0 (130-465) |
* The values are presented as the median [range] or as the number (percentage). 6MWT, six-minute walk test; CT, computed tomography; DLCO, diffusing capacity of the lungs for carbon monoxide; FVC, forced vital capacity; UIP, usual interstitial pneumonia
Categorization of AEs
Table 2 shows the incidence and nature of the AEs. Seventeen patients developed AEs, consisting of diarrhoea (n=10, 40%), hepatotoxicity (n=7, 28%), and anorexia (n=4, 16%). No AE-related deaths occurred during the study period.
Table 2.
Categorization of adverse events
| n (%) | CTCAE grade, n 1/2/3/4 | |
| Diarrhea | 10 (40) | 3/7/0/0 |
| Hepatotoxicity | 7 (28) | 3/4/0/0 |
| Anorexia | 4 (16) | 2/2/0/0 |
CTCAE, Common Terminology Criteria for Adverse Events
Dose reduction and/or discontinuation of nintedanib
Table 3 shows the reasons for dose reduction and/or discontinuation of nintedanib. Eleven patients required dose reduction because of diarrhoea (n=4) or hepatotoxicity (n=7). Of the 4 patients in whom nintedanib was interrupted, 2 had diarrhoea, 1 had anorexia, and 1 had progression of IPF.
Table 3.
Reasons for dose reduction and/or discontinuation
| n (%) | |
| Dose reduction, n (%) | 11 (44) |
| Diarrhea, n | 4 |
| Hepatotoxicity, n | 7 |
| Discontinuation, n (%) | 4 (16) |
| Diarrhea, n | 2 |
| Anorexia, n | 1 |
| Progression of IPF, n | 1 |
IPF, idiopathic pulmonary fibrosis
Characteristics and examination findings in patients who did and did need dose reduction/discontinuation
The characteristics of patients who did and did not require dose reduction and/or discontinuation are compared in Table 4. There were no significant differences in patient sex, %FVC, %DLCO, 6MWT (six-minute walk test), %min SpO2 during the 6MWT, or any of the laboratory results between the two groups. Patients who required dose reduction and/or discontinuation were significantly older than those who did not (72 years vs 67 years; P=0.047). Furthermore, BSA was significantly smaller in the group that required dose reduction and/or discontinuation than in the group that did not (1.63 m2 vs 1.7 8 m2; P=0.028).
Table 4.
Comparison of characteristics between the group that required dose reduction and/or discontinuation and the group that did not
| Variable | Dose reduction and/or discontinuation (n=15) | No dose reduction and/or discontinuation (n=10) | P-value |
| Male sex, n (%) | 10 (67%) | 9 (90%) | 0.390* |
| Age, years | 72 [58-79] | 67 [53-72] | 0.047† |
| Body surface area, m2 | 1.63 [1.36-1.95] | 1.78 [1.55-1.95] | 0.028† |
| %FVC, % | 65.6 [42.0-78.0] (n=13) | 69.4 [45.0-112.0] | 0.877‡ |
| %DLCO, % | 51.8 [40.0-70.0] (n=6) | 53.1 [45.0-89.0] | 0.897† |
| 6MWT, m | 339 [130-415] (n=10) | 370 [305-465] | 0.705‡ |
| %min SpO2 during 6MWT | 86.3 [80-90] (n=10) | 86.5 [75-91] | 0.760‡ |
| Krebs von den Lungen-6 | 974 [523-1520] | 1368 [656-2394] | 0.081§ |
| Albumin | 3.9 [3.1-4.4] | 4.0 [3.5-4.6] | 0.328† |
| Serum creatinine | 0.76 [0.39-1.48] | 0.76 [0.56-0.95] | 0.853§ |
| Total bilirubin | 0.81 [0.29-2.07] | 0.88 [0.38-1.62] | 1.000‡ |
| Aspartate aminotransferase | 22.5 [15.0-36.0] | 23.1 [15.0-26.0] | 0.738‡ |
| Alanine aminotransferase | 19.5 [8.0-47.0] | 22.6 [10.0-26.3] | 0.374‡ |
FVC, forced vital capacity; 6MWT, six-minute walk test; min SpO2, minimal oxygen saturation measured by pulse oximetry.
*Chi-squared test.
†Student’s t-test.
‡Mann-Whitney U test.
§Welch’s t-test.
In univariate analysis, older age and a low BSA were significant predictors of dose reduction and/or discontinuation of nintedanib. In multivariate analysis, the only independent predictor of a treatment response was low BSA (odds ratio 0.53, 95% confidence interval 0.29-0.97, P=0.040; Table 5).
Table 5.
Multivariate analysis of predictors of dose reduction and/or discontinuation of nintedanib (n = 25)
| Variable | OR | 95% CI | P-value* |
| Body surface area | 0.53 | 0.29-0.97 | 0.040 |
*Result calculated by logistic regression. CI, confidence interval; OR, odds ratio
Figure 1 shows the results of the receiver-operating characteristic (ROC) curve analysis used to determine the cut-off values for BSA. The area under the curve was 0.757 (95% confidence interval 0.56-0.95) and the cut-off value for which sensitivity and specificity was maximal was 1.650 m2 (67.0% sensitivity and 80.0% specificity).
Fig. 1.
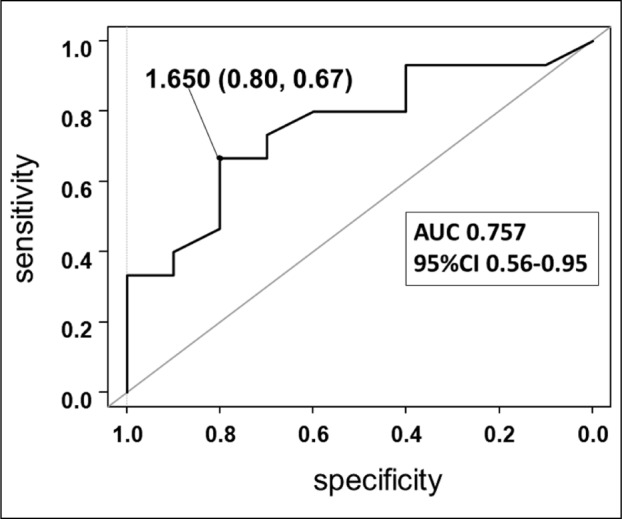
Receiver-operating characteristic curve analysis used to determine the cut-off values for body surface area. The area under the curve was 0.757 (95% confidence interval 0.56-0.95) and the cut-off value for which sensitivity and specificity was maximal was 1.650 m2 (67.0% sensitivity and 80.0% specificity)
Discussion
This single-center, retrospective study sought to identify predictors of dose reduction and/or discontinuation of nintedanib in patients with IPF. These predictors have hitherto been unknown. Multivariate analysis revealed that low BSA was the only independent predictor of dose reduction and/or discontinuation of nintedanib 150 mg twice daily. To the best of our knowledge, this is the first study to report this association.
Seven of our 25 patients developed hepatotoxicity, which was grade 2 in 4 cases and grade 1 in 3 cases. No patient developed hepatotoxicity that was more severe than grade 3. In all cases, the hepatotoxicity was completely reversible with dose reduction. Therefore, it may be unnecessary to hesitate when administering nintedanib because of concerns over hepatotoxicity. However, close monitoring and appropriate management according to the guide for appropriate use of nintedanib was required.
The incidence of dose reduction in the present study was considerably higher than that in the INPULSIS trials (44% vs 26.5%-29.2%). The reason for the higher incidence in our study is unclear but could be related to physical or ethnic differences in the study populations. Ikeda et al reported an association of low BSA with hepatotoxicity in patients with IPF receiving nintedanib 150 mg twice daily (11). Of note, the present study included only Japanese patients. A sub-analysis of the INPULSIS trials revealed that elevated hepatic enzymes of any CTCAE grade were more common in the Japanese population than in the non-Japanese population (39.5% vs 10.1%; P<0.001); however, the incidence of elevation of aspartate aminotransferase and/or alanine aminotransferase to a CTCAE grade ≥2 was not significantly different between the Japanese and non-Japanese populations (6.6% and 4.8%; P=0.572) (12).
Sixteen percent of our patients required discontinuation of nintedanib. In the INPULSIS trials, nintedanib was discontinued in 18.8%-21.0% of patients. We speculate that the reason for this low incidence of discontinuation was prompt dose interruption when hepatotoxicity developed. Furthermore, diarrhoea could be controlled by anti-diarrheal medication.
In patients with a small body habitus, particularly Japanese and Eastern Asian patients with a BSA <1.65m2, a good option would be to start nintedanib at a dose of 100 mg twice daily and then increase the dose to 150 mg twice daily if safety permits. However, the effectiveness of low-dose nintedanib in Japanese patients is unclear. In the TOMORROW trial, low-dose nintedanib was not demonstrated to be effective; however, that study did not contain Japanese patients (13) and dose reduction was not evaluated in the INPULSIS trials (6). Therefore, the data on dose reduction of nintedanib in the Japanese population are inconclusive.
This study has some limitations in that it had a retrospective single-center design and included a small number of patients. Furthermore, the observation period was too short to assess long-term safety.
In summary, a low BSA was associated with dose reduction and/or discontinuation of nintedanib 150 mg twice daily in patients with IPF. Further studies in larger patient samples are needed to validate these findings.
Acknowledgements
The authors thank Associate Professor Masataka Taguri of Yokohama City University School of Medicine for his statistical evaluation.
Competing interests:
Dr. Kimura reports lecture fees from Boehringer Ingelheim.
Dr. Sugawara reports lecture fees from Boehringer Ingelheim.
References
- 1.Fell CD, Martinez FJ, Liu LX, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181:832–837. doi: 10.1164/rccm.200906-0959OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalysnyk L, Cid-Ruzafa J, Rotella P, Esser D. Incidence and prevalence of idiopathic pulmonary fibrosis: review of the literature. Eur Respir Rev. 2012;21:355–361. doi: 10.1183/09059180.00002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G, Collard HR, Egan JJ, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hilberg F, Roth GJ, Krssak M, et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res. 2008;68:4774–4782. doi: 10.1158/0008-5472.CAN-07-6307. [DOI] [PubMed] [Google Scholar]
- 5.Wollin L, Maillet I, Quesniaux V, Holweg A, Ryffel B. Antifibrotic and anti-inflammatory activity of the tyrosine kinase inhibitor nintedanib in experimental models of lung fibrosis. J Pharmacol Exp Ther. 2014;349:209–220. doi: 10.1124/jpet.113.208223. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, du Bois RM, Raghu G, et al. INPULSIS Trial Investigators. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 7.Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. 2016 http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40 . Accessed: 1 March, 2016. [Google Scholar]
- 8.Corte T, Bonella F, Crestani B, et al. Safety, tolerability and appropriate use of nintedanib in idiopathic pulmonary fibrosis. Respir Res. 2015;16:116. doi: 10.1186/s12931-015-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur. Respir. J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda S, Sekine A, Baba T, et al. Low body surface area predicts hepatotoxicity of nintedanib in patients with idiopathic pulmonary fibrosis. Sci Rep. 2017;7:10811. doi: 10.1038/s41598-017-11321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pharmaceuticals and Medical Devices Agency, Japan. [The examination report for nintedanib-ethanesulfonate] http://www.pmda.go.jp/PmdaSearch/iyakuDetail/GeneralList/3999039M1 . in Japanese. Accessed: 3 July 2015. [Google Scholar]
- 13.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]


