Abstract
Sarcoidosis is a granulomatous multisystemic disease of unknown cause most often affecting the lungs, lymph nodes of the pulmonary hilus, eyes, skin, and other structures including central (CNS) or peripheral nervous system (PNS). Isolated neurosarcoidosis is extremely rare. The diagnosis of isolated neurosarcoidosis is challenging because of its rarity, variety of manifestations, and the lack of systemic signs. We report relapsing and remitting isolated intracranial neurosarcoidosis in an 18-year-old male patient who undervent complex diagnostics including cerebral and meninges biopsy. Patient was succesfully treated with corticosteroids.
Key words: isolated neurosarcoidosis, biopsy, treatment
Introduction
Sarcoidosis is a granulomatous multisystemic disease of unknown cause most often affecting the lungs, lymph nodes of the pulmonary hilus, eyes, skin, and other structures including central (CNS) or peripheral nervous system (PNS). Simultaneous affection of CNS or PNS with other systems is estimated to 5-10% of cases (1, 4, 6, 8). Isolated neurosarcoidosis is extremely rare, with an estimated incidence in Caucasians of 0.2/100,000 (2). The diagnosis of isolated neurosarcoidosis is challenging because of its rarity, variety of manifestations, and the lack of systemic signs. To reach a definitive diagnosis of neurosarcoidosis, a biopsy must be obtained in order to demonstrate the typical histological picture of sarcoidosis in the nervous system and to exclude other granulomatous diseases (3, 5). We report relapsing and remitting isolated intracranial neurosarcoidosis in an 18-year-old male patient. Tissue from the frontal lobes and meninges of the patient were taken for biopsy.
Case report
An 18-year-old male patient presented in July 2014 with intensive temporal bilateral headache accompanied by vomitting, photophobia, and dehydration. Neurologic examination revealed quadruhyperreflexia and slowed psychomotoric tempo, without meningeal signs and involvement of the cranial nerves. The patient had a 6-year history of headache, which was unilateral, mild intensity, well controlled with analgetics. Brain MRI imaging performed in 2009 was negative.
Laboratory findings
Laboratory tests showed hyponatremia of 123 mmol/l (136-146 mmol/L). Results were within normal limits for all remaining laboratory tests including hemogram, CRP, basic metabolic panel, autoimmune screens, endocrine laboratory testing, and imunologic status. The results of extensive serum infectious studies including those for VZV, HSV 1,2, CMV, EBV, Chlamydia pneumoniae, Chlamydia trachomatis, Mycoplasma pneumoniae, Borrelia burgdorferi, HIV, Toxoplasma gondii, Aspergillus fumigatus, Cryptococcus neoformans, Candida, rubeola, and syphilis were negative. The tuberculin reaction (Mantoux II) and the QuantiFERON test were both negative. Cerebrospinal fluid (CSF) analysis revealed lymphocytic pleocytosis of 207 lymphocytes, 53 monocytes, and 20 segments, slightly elevated protein levels (0.463 g/l), elevated immunoglobulins (IgM, IgG, IgA), and the presence of oligoclonal bands (Type 3). The results of studies for cerebrospinal fluid infections including bacterial cultivation, cultivation of Mycobacterium tuberculosis, polymerase chain reaction (PCR) for the diagnosis of Herpes simplex virus 1/2, Varicella zoster virus, cytomegalovirus, Epstein-Barr virus, Chlamydia pneumoniae, Chlamydia trachomatis, Mycoplasma pneumoniae, Borrelia burgdorferi, and Neisseria gonorrhoeae were all negative. The immunoregulatory CD4/CD8 index was increased to 5 in CSF. Immunophenotyping examination of the CSF did not indicate B-NHL infiltration of the central nervous system. B-lymphocytes were present minimally and showed no pathologic expression of immunological cell markers. Cytologic examination of CSF revealed no blastic forms of T-lymphocytes.
Imaging and additional investigations
Initial computed tomography (CT) revealed bilateral frontal lobe white matter hypodensity. Subsequent magnetic resonance imaging (MRI) of the head with gadolinium contrast demonstrated extensive confluent bilateral frontal white matter hyperintensities on T2-weighted and FLAIR, extending to the optic tracts, chiasma, and adjacent optic nerves, plus leptomeningeal contrast enhancement in these areas (Figure 1). Retinal examination revealed bilateral papilloedema, which was accentuated in the right eye. The patient was treated with methylprednisolone 3000 mg over 6 days. His headaches receded, and on brain MRI in September 2014, regression of the edema and of the postcontrast enhancement of the leptomenings was seen.
Fig. 1.
Magnetic resonance imaging of subcortical white matter and optic chiasm
A: FLAIR diffuse hyperintensity in the bilateral frontal subcortical white matter extending to the chiasma; B, C: T2-weighted image diffuse hyperintensity in the bilateral frontal subcortical white matter extending to the chiasma; D: Postcontrast enahcement of the lesions
Relapse of the disease
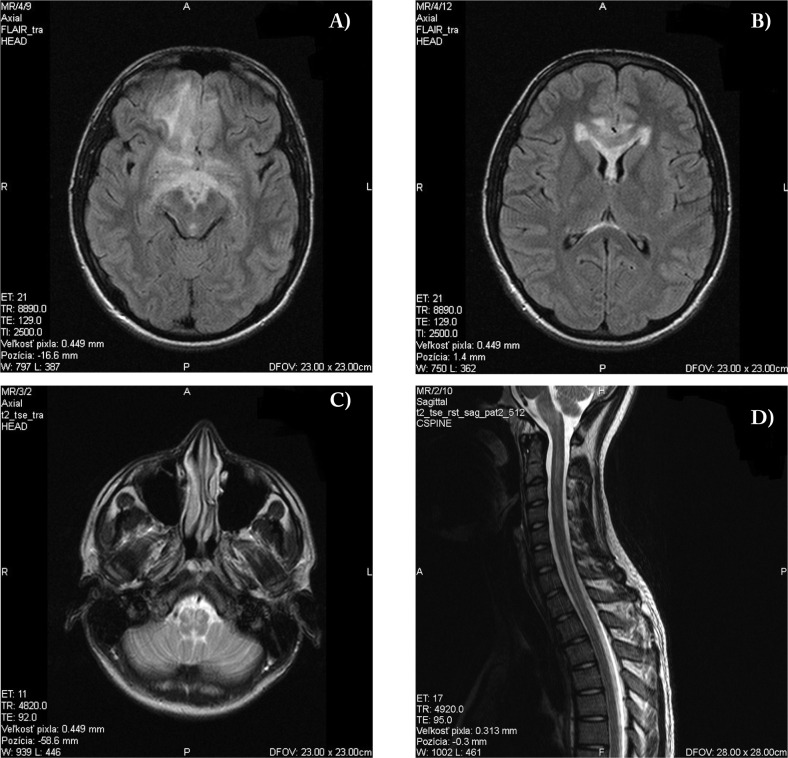
In October 2015, the patient developed a fever of unknown genesis. At that time, he was taking antibiotics. He presented with the same symptoms as those in July 2014, namely intensive bilateral temporal headache associated with vomiting and photophobia, but additionally with blurred vision in the right eye followed by one generalized tonic-clonic seizure. Patient complained on blurred vision on the right eye, other clinical symptoms were identical to initial examination. Retinal examination showed bilateral papilloedema. Brain MRI revealed extensive T2 and FLAIR hyperintensities in the frontobasal area bilaterally, and involvement of the ependyme of the lateral ventricles, corpus callosum, medulla oblongata, and cervical and thoracic parts of the spinal cord. Micronodular postcontrast enhancement was present in these areas (Figure 2). MR angiography of the cerebral arteries was negative. Repeat laboratory examination showed mild leukocytosis of 10,10×109/l without elevation of CRP, hyponatremia of 123 mmol/l (136-146 mmol/l), hypochloremia of 87 mmol/l (95-107 mmol/l), and hypokalemia of 3.4 mmol/l (3.8-5 mmol/l). CSF examination revealed 245 lymphocytes, 25 segments, elevated protein levels of 0.86g/l, elevated immunglobulins (IgM, IgG, IgA), and the presence of oligoclonal bands (Type 3). Extensive autoimmune and microbial screenings were negative once again. EEG examination showed non-specific slow waves in the right frontal lobe with a tendency to spread to the left frontal lobe.
Fig. 2.
Magnetic resonance imaging of subcortical white matter, brainstem, and cervical and thoracic spine
A,B: FLAIR diffuse hyperintensity in the bilateral frontal subcortical white matter with involvement of the ependyma of the lateral ventricles and corpus callosum; C: T2-weighted image involvement of the medulla oblongata; D: T2-weighted image involvment of the cervical and thoracal spine
Biopsy
The biopsy of frontal lobes demonstrated the typical morphology of chronic non-caseating granulomatous inflammation. The granulomas consisted of dominating histiocytes and epitheloid cells with a few intermingled multinucleated giant cells. The intra- and peri-granulomatous lymphocytes were represented exclusively by a CD3+ T-cell population composed of a mixture of slightly prevailing CD4 and less numerous CD8+ T-lymphocytes. Granulomatous lesions in the subleptomeningeal and meningeal areas were associated with the peri-granulomatous fibrous reaction. The biopsy established the diagnosis of neurosarcoidosis (Figure 3, A-E)).
Fig. 3.
Histopathology of meningeal and of a frontal lobe biopsy
A: Overview of the biopsy specimen showing two granulomatous lesions in the subleptomeningeal area (HE staining, 4x); B: Detail of Fig. A. showing the morphology of the granuloma (HE staining, 10x); C: Intraparenchymatous granuloma composed of epitheloid cells, surrounded by lymphocytic reaction (HE staining, 20x); D: The peri- and intra-granulomatous lymphocytes are represented by CD3+ T-cell (immunohistochemical staining of CD3 antigen, 10x); E: Detection of CD68 antigen showing positivity in the intra-granulomatous histiocytes (immunohistochemical staining of CD68 antigen, 20x)
Treatment
Throughout the period of illness, no signs of the involvement of any organs other than the brain were seen. An HRCT scan of the thorax, pulmonary function tests, ophthalmological examination, hand radiographs, and abdominal ultrasonogram were all negative. Our patient met the diagnostic criteria for isolated neurosarcoidosis. Glucocorticoid therapy with prednisolone at 40 mg daily and anti-epileptic therapy with karbamazepine was initiated. The patient was treated with prednisolone for 19 months with slow detraction of the dose. The patient complained on occasional headaches. On follow-up MRI scans (4/2016, 10/2017), a marked regression of the hyperintensities and postcontrast enhancement could be seen. Small hyperintensities were still present in frontal lobes, medulla oblongata, and cervical spine with minimal postcontrast enhancement (Figure 4).
Fig. 4.
Magnetic resonance imaging after 19 months of corticosteroid therapy
A, B: T2-weighted image marked regression of hyperintensities in the frontal lobes, corpus callosum, and the ependym of the lateral ventricles; C: FLAIR small hyperintensities are still present in the frontal lobes; D: Minimal postcontrast enhacement, T1-weighted image
Discussion
Based on the MRI findings, the clinical picture, and the laboratory findings, we were able to exclude inflammatory, neoplastic (especially lymphoma, metastasis), and infectious etiologies. The good response to intravenous corticosteroids, in addition to the negative extensive infectious studies, excluded infectious etiology but, on the other hand, led us to suspect aseptic meningoencephalomyelitis, neurosarcoidosis, another autoimmune disease, or CNS lymphoma. An important factor to be aware of is the transient but profound response of CNS lymphoma to the use of glucocorticoids (e.g., dexamethasone and prednisolone) (10). More recently, an increase has been reported in the incidence of sporadic primary CNS lymphomas in immunocompetent individuals; this is particularly seen in older patients (50-80 years of age). The vast majority (>90%) of primary CNS lymphoma are of B-cell in origin. Patients with primary CNS lymphoma present similarly to patients with other central nervous system mass lesions, with symptoms and signs of raised intracranial pressure, focal neurological deficit, and seizures. They can present as solitary (60-70%) or multiple (30-40%) lesions with a predilection for the periventricular white matter, although they can also arise in the cortex or deep gray matter (13, 16). The examination of the CSF including immunophenotyping and cytologic investigations did not establish B-cell lymphoproliferation, the blastic transformation of lymphocytes, or the presence of tumor cells in our patient. The patient’s age was also not characteristic for the manifestation of lymphoma. The definitive diagnosis was established after a biopsy. In the present case study, non-caseating granulomatous inflammation was evident in the pathologic analysis. When granulomatous inflammation is discovered through biopsy, other possible etiologies that can cause granulomatous inflammation need to be considered, because it is not specific for sarcoidosis. Tuberculosis, parasitic, fungal infections, and vasculitis should be excluded (12). Our patient fulfilled the diagnostic criteria for the diagnosis of definitive neurosarcoidosis, namely the typical histologic picture of sarcoidosis in the nervous system and exclusion of other granulomatous diseases. Patient presented with optic neuropathy, headache, and one generalised epileptic seizure. According to the literature, the most common symptomps for neurosarcoidosis are cranial nerve deficits (50%) and headache (30%). The facial nerve and the optic nerve are the most frequently affected. Other symptoms that can be present are seizures (10%), sensory and motor deficits (10%), neuropsychological deficits (10%), hydrocephalus (5%), and hypothalamic and pituitary dysfunction (5). The most common anatomic sites of symptomatic involvement are the cranial nerves, the meninges, the brain parenchyma, the spinal cord and its meninges, the hypothalamic-pituitary axis, and the peripheral nerves (14). The MRI picture of our patient, showed white matter hyperintensities on T2-weighted and FLAIR imaging extending to the optic tracts, chiasm, optic nerves, brainstem, and spine, plus leptomeningeal contrast enhancement confirmed the diagnosis of neurosarcoidosis. Lymphocytosis, elevated protein levels, and positive oligoclonal bands were present in our patient; these symptoms are suggestive, but not specific for sarcoidosis (7, 14). Isolated neurosarcoidosis is reported to have a better clinical prognosis compared with systemic neurosarcoidosis. A good clinical course could thus be a typical feature of isolated neurosarcoidosis (7).
References
- 1.Baughman RP, Teirstein AS, Judson MA, et al. Case Control Etiologic Study of Sarcoidosis (ACCESS) research group. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164(10, Pt 1):1885. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 2.Brinar VV, Habek M. Isolated central nervous system sarcoidosis: A great mimicker. Clin Neurol Neurosurg. 2008;110:939–42. doi: 10.1016/j.clineuro.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 3.Carlson ML, White JR, Jr, Espahbodi M, et al. Cranial base manifestations of neurosarcoidosis: a review of 305 patients. Otol Neurotol. 2015;36(01):156–166. doi: 10.1097/MAO.0000000000000501. [DOI] [PubMed] [Google Scholar]
- 4.Daan Fritz, Diederik van de Beek, Matthijs C. Brouwer. 2016. Clinical features, treatment and outcome in neurosarcoidosis: systematic review and meta-analysis. BMC Neurology. 2016;16:220. doi: 10.1186/s12883-016-0741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel A. Culver, DO1 Manuel L. Ribeiro Neto, MD1 Brandon P. Moss, MD2 Mary A. Willis, MD2. 2017. Neurosarcoidosis. Semin Respir Crit Care Med. 2017;38:499–513. doi: 10.1055/s-0037-1604165. [DOI] [PubMed] [Google Scholar]
- 6.Kantorová E, Kurča E, De Riggo J, Šutovský J, Michalik J, Nosáľ V, Rozborilová E, Hamžík J, Hladká M, Plank J. 2008. Neurosarkoidóza: zriedkavý prípad sarkoidózy krčnej miechy - kazuistika. Cesk Slov Neurol N. 2008;71/104(5):588–591. [Google Scholar]
- 7.Shimizu K, Yuki K, Sadatomo T, Kaoro K. Isolated neurosarcoidosis presenting with multiple cranial nerve palsies. Surg Neurol Int. 2016;7:44. doi: 10.4103/2152-7806.180765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krenzlin H, Jussen D, Musahl D, Scheil-Bertram S, Wernecke K, Horn P. 2015. A Rare Case of Isolated Cerebral Sarcoidosis Presenting as Suprasellar Mass Lesion with Salt-Wasting Hypopituitarism. J Neurol Surg Rep. 2015 Jul;76(1):e140–e145. doi: 10.1055/s-0035-1549310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leonhard SE, Fritz D, Eftimov F, van der Kooi AJ, van de Beek D, Brouwer MC. Neurosarcoidosis in a tertiary referral center: acrosssectional cohort study. Medicine (Baltimore) 2016;95(14):e3277. doi: 10.1097/MD.0000000000003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muzio B, Gailard F, et al. Primary CNS lymphoma. https://radiopaedia.org/articles/primary-cns-lymphoma . [Google Scholar]
- 11.Nozaki K, Scott TF, Sohn M, Judson MA. Isolated neurosarcoidosis: case series in 2 sarcoidosis centers. Neurologist. 2012;18(06):373–377. doi: 10.1097/NRL.0b013e3182704d04. [DOI] [PubMed] [Google Scholar]
- 12.Nowak DA, Widenka DC. 2001. Neurosarcoidosis: a review of its intracranial manifestation. J Neurol. 2001 May;248(5):363–72. doi: 10.1007/s004150170175. [DOI] [PubMed] [Google Scholar]
- 13.Okita Y, Narita Y, Miyakita Y, Ohno M, Fukushima S, Maeshima A, et al. 2012. Long-term follow-up of vanishing tumors in the brain: How should a lesion mimicking primary CNS lymphoma be managed. Clin Neurol Neurosurg. 2012;114:1217–21. doi: 10.1016/j.clineuro.2012.02.053. [DOI] [PubMed] [Google Scholar]
- 14.Smith JK, Matheus MG, Castillo M. Imaging manifestations of neurosarcoidosis. AJR Am J Roentgenol. 2004;182:289–95. doi: 10.2214/ajr.182.2.1820289. [DOI] [PubMed] [Google Scholar]
- 15.Wegener S, Linnebank M, Martin R, Valavanis A, Weller M. 2015 Clinically Isolated Neurosarcoidosis: A Recommended Diagnostic Path. Eur Neurol. 2015;73(1-2):71–7. doi: 10.1159/000366199. [DOI] [PubMed] [Google Scholar]
- 16.Yi JQ, Lin TY, He YJ, Huang HQ, Xia ZJ, Xia YF, Xu RH, Guo Y, Guan ZZ. Primary central nervous system lymphoma--a report of 32 cases with literature review. Ai Zheng. 2006 Apr;25(4):476–80. [PubMed] [Google Scholar]