Abstract
Background:
In 2016, the diagnostic criteria for the acute exacerbation (AE) of idiopathic pulmonary fibrosis (IPF) were revised. However, there have been published few clinical reports on AE–IPF published using the new criteria. The aim of this study was to investigate the incidence of, risk factors for, and mortality due to newly defined AE. Moreover, differences between triggered AE and idiopathic AE were investigated.
Methods:
The retrospective study was conducted including all IPF patients diagnosed with surgically-proven usual interstitial pneumonia through multi-disciplinary discussion between January 2006 and December 2015. Data were retrieved from a clinical chart review.
Results:
A total of 107 patients with newly diagnosed 107 IPF patients were included. The cumulative incidence of initial AE were 9.6% at 1 year, 16.8% at 2 years, 23.9% at 3 years, and 37.3% at 4 years after diagnosis. Three risk factors for AE–IPF development were identified: 1) the minimum peripheral ozygen saturation level of ≤88% during the 6-minute walk test at the time of diagnosis; 2) forced vital capacity (FVC) decreasing by ≥10% in 1 year; and 3) diffusion capacity of the lungs for carbon monoxide (DLco) decreasing by ≥15% in 1 year. There were no significant differences in background (excluding C-reactive protein), survival and treatment between patients with triggered AE and those with idiopathic AE.
Conclusions:
The 6-minute walk test and an annual decline in FVC and DLco were predictive factors for AE incidence. The causes of AE–IPF did not affect the prognosis or treatment options in clinical practice.
Key words: acute exacerbation, idiopathic pulmonary fibrosis, risk factors, surgical lung biopsy, usual interstitial pneumonia
Abbreviations
AE acute exacerbation
IPF idiopathic pulmonary fibrosis
FVC forced vital capacity
DLco diffusion capacity of the lungs for carbon monoxide
PaO2 the partial pressure of oxygen in the arterial blood
KL-6 Krebs von den Lungen-6
UIP usual interstitial pneumonia
MDD multi-disciplinary discussion
ATS American Thoracic Society
ERS European Respiratory Society
SpO2 saturation of peripheral oxygen
SP-D surfactant protein D
CRP C-reactive protein
FiO2 fraction of inspired oxygen
P/F PaO2/FiO2.
Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive disease with a poor prognosis. Although IPF progression is typically gradual, new shadows sometimes appear in bilateral lungs during the chronic stage, and an acute exacerbation (AE) can occur, leading to acute respiratory failure. In 2016, the diagnostic criteria for AE–IPF were changed so that acute respiratory failure induced by identified causes (e.g., infections, surgery, and medication) were also included in addition to acute respiratory failure of unknown causes (1). AE due to identified causes was described as triggered AE, AE due to unidentified causes was described as idiopathic AE.
There are few clinical reports on the incidence, prognosis, and treatment of the newly defined AE. In the previous criteria for AE, forced vital capacity (FVC), diffusion capacity of the lung for carbon monoxide (DLco), the partial pressure of oxygen in the arterial blood (PaO2), Krebs von den Lungen-6 (KL-6) level at IPF diagnosis, and FVC decline greater than 10% within 6 months were reported as risk factors for the onset of AE (2-5). Risk factors for the incidence of AE using the new criteria are not well understood. Moreover, the incidence and prognosis of AE remain unknown since the diagnostic criteria have changed. It is not well understood whether there is a difference in patient characteristics, treatment, and prognosis between triggered AE and idiopathic AE in the clinical setting. Therefore, the aim of this study was to investigate the incidence of, risk factors for, and mortality due to newly defined AE. In addition, the treatment and prognosis were compared between triggered AE and idiopathic AE groups.
Methods
Subjects
A retrospective cohort study was conducted including patients with IPF who underwent surgical lung biopsy, who showed a surgically-proven usual interstitial pneumonia (UIP) pattern, and who were diagnosed through multi-disciplinary discussion (MDD) at the Kanagawa Cardiovascular and Respiratory Center between January 2006 and December 2015. IPF diagnoses were based on the 2011 American Thoracic Society (ATS)/European Respiratory Society (ERS) IPF statement (6). Three specialists were involved in MDD: 1) a pulmonologist who specialized in interstitial lung diseases; 2) a chest radiology specialist; and 3) a chest pathology specialist. Patients with cancer, including lung cancer, and those who did not receive regular check-ups at our center after undergoing a surgical lung biopsy were excluded from the study. In addition, the patients for whom the diagnosis was changed to interstitial pneumonia of known causes after the initial diagnoses of IPF (e.g., interstitial pneumonia associated with connective tissue diseases and hypersensitivity pneumonitis) were excluded from the study. The 2016 International Working Group Report on diagnostic criteria for AE-IPF was used to diagnose AE–IPF (1). The protocol for this study was approved by the ethical review board of our center (Research Ethics Committee, Kanagawa Cardiovascular and Respiratory Center, Kanagawa Prefectural Hospital Organization).
Incidence of and risk factors for AE–IPF
Risk factors for the occurence of AE–IPF were examined using data from the time of IPF diagnosis to until one year following diagnosis. The minimum saturation of peripheral oxygen (SpO2) during the 6-minute walk test was set at 88% (7), and the threshold for the difference (ΔSpO2) from the baseline SpO2 to the minimum SpO2 was set at 4% (8). In addition, using previous studies (5) (8), the thresholds for the amount of change on the lung function tests one year following diagnosis were set at a 10% decline for FVC and a 15% decline for DLco.
Prognosis of AE-IPF
The clinical characteristics at AE onset, treatment, and 90-day survival from the onset of AE–IPF were investigated. In cases with multiple incidences of AE–IPF, data from the most recent AE were used in order to compare the history of AE in patients with triggered AE and idiopathic AE.
Statistical analysis
Kaplan-Meier curves and the log-rank test were used to analyze survival. A Cox proportional hazards regression analysis was used to analyze the risk factors for the incidence of AE–IPF. First, a univariate analysis was conducted to elicit significant factors, which were then inserted into covariates. Next, a multivariate analysis was conducted using stepwise regression. Comparison between the two groups was done using the Mann-Whitney’ U test. Fisher’s exact test was used in the test of the crosstab. A p-value of <0.05 was considered statistically significant. Excel Statistics (Social Survey Research Information Co., Ltd.) was used for the analyses.
Results
Subject characteristics
Overall, 145 patients were diagnosed with UIP by surgical lung biopsy between January 2006 and December 2015. A total of 38 patients were excluded: 7 patients with cancer; 11 patients who were referred to another facility after surgical lung biopsy and thus not available for follow-up; 13 patients who were determined to be non-IPF through MDD; and 7 patients whose diagnosis was changed from IPF to interstitial pneumonia of known causes (6 patients with connective tissue disease-associated interstitial pneumonia and 1 patient with hypersensitivity pneumonitis) during the observation period. Finally, 107 patients were included in the retrospective cohort analysis (Figure 1). The mean observation period was 3.9 years, and the baseline characteristics at the time of IPF diagnosis are shown in Table 1. Of the included patients, 77% were male, the mean age was 66.9 years, 25 were non-smokers (23%), and 16 patients had a family history (15%). Average KL-6 and surfactant protein D (SP-D) levels were rather elevated (1147 U/mL [152–400 U/mL] and 219 ng/mL [0–109.9 ng/mL]) respectively. The mean FVC % predicted and DLco % predicted were 85.3% and 82.1%, respectively. The 6-minute walk test was conducted under room air conditions for all patients, and the mean distance was 458 meters. The mean minimum SpO2 was 91% during the 6-minute walk test, and the mean SpO2 decline from baseline was 5.3%. While the patients exhibited normal lung function, they experienced diminished exercise tolerance.
Fig. 1.
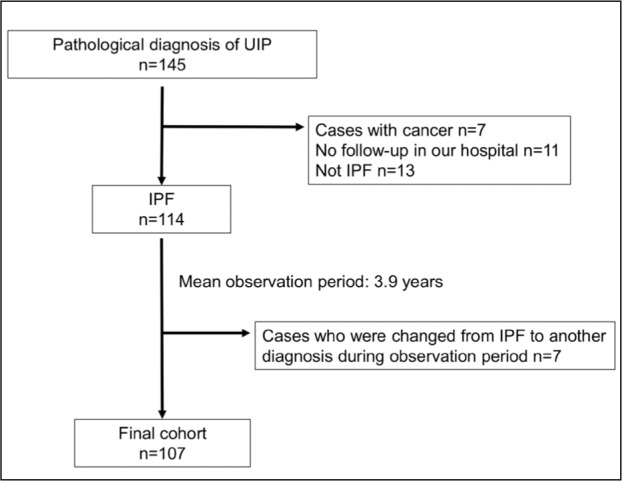
Flow of this study. UIP: usual interstitial pneumonia, IPF: idiopathic pulmonary fibrosis
Table 1.
Baseline characteristics at the time of IPF diagnosis
| Characteristics | |
| Subjects | 107 |
| Male | 82 |
| Age (yrs) | 66.9±7.1 |
| Smoking history | |
| Never smoke | 25 |
| Current smoker | 3 |
| Ex-smoker | 69 |
| Pack-year | 40.2±25.5 |
| Surgical lung biopsy (Yes) | 107 |
| Familial interstitial lung diseases (Yes) | 16 |
| Blood tests (n=107) | |
| KL-6 (U/mL) | 1147±826 |
| SP-D (ng/mL) | 219±139 |
| LDH (U/L) | 225±43 |
| CRP (mg/dL) | 0.40±1.81 |
| PaO2 (Torr) | 84.9±8.3 |
| Pulmonary function (n=107) | |
| FVC (L) | 2.82±0.79 |
| FVC %pred (%) | 85.3±17.0 |
| FEV1/FVC (%) | 79.0±6.5 |
| DLco %pred (%) | 82.1±19.3 |
| DLco (mL/min/mm Hg) | 14.8±3.9 |
| 6-minute walk test (n=95) | |
| Distance (meter) | 458±82 |
| Minimum SpO2 (%) | 91±4 |
| Bronchoalveolar lavage fluid findings (n=81) | |
| Total cell count (×10^5) | 2.07±1.55 |
| Macrophages (%) | 78.2±18.0 |
| Lymphocytes (%) | 16.9±16.8 |
| Neutrophils (%) | 3.4±5.0 |
| Eosinophils (%) | 1.6±1.9 |
Data are presented as n or mean±standard deviation. Definition of abbreviations: IPF: idiopathic pulmonary fibrosis, KL-6: Krebs von den Lungen-6, SP-D: surfactant protein-D, LDH: lactate dehydrogenase, CRP: C-reactive protein, PaO2: partial pressure of oxygen in arterial blood, FVC: forced vital capacity, %pred: % predicted, FEV1: forced expiratory volume in 1 second, DLco: diffusion capacity of the lung for carbon monoxide, SpO2: arterial oxygen saturation measured by pulse oximetry.
Prognosis and causes of death
The 50% survival rate from the time of IPF diagnosis was 5.6 years. The main causes of death were AE (41%), chronic respiratory failure (41%), sudden death (9%), and lung cancer (2%).
AE incidence
The cumulative incidence rates of the initial AE were 9.6% for 1 year following diagnosis, 16.8% for 2 years, 23.9% for 3 years, and 37.3% for 4 years. For the first four years, the incidence of AE–IPF occurred at a consistent rate of 7.1%-13.4% per year (Figure 2). There were 39 patients in whom an AE–IPF occured during the observation period, and there was a significant difference in the prognosis between the group with AE–IPF (median survival time, 3.65 years) and that without (median survival time, not calculable) (Log-rank test, P<0.001) (Figure 3).
Fig. 2.
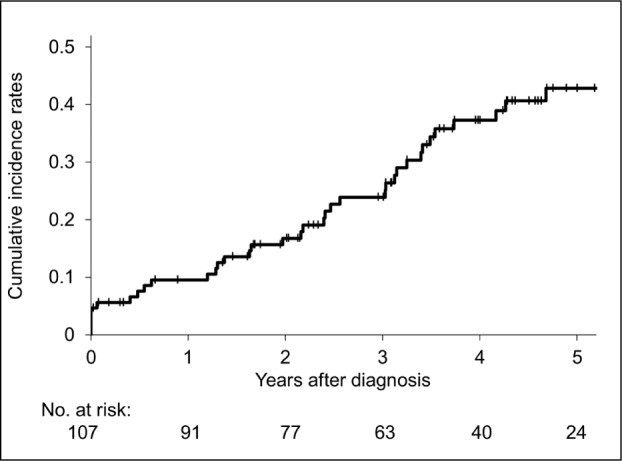
Kaplan-Meier curve for cumulative incidence rates of acute exacerbation of idiopathic pulmonary fibrosis.
Fig. 3.
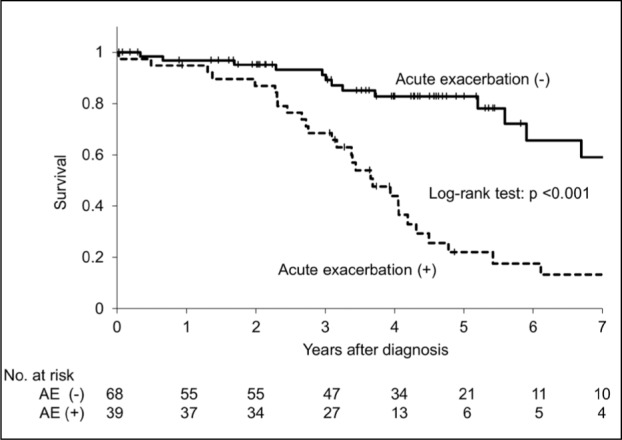
Kaplan-Meier survival curves for cases with acute exacerbation of IPF and those without acute exacerbation of IPF. Median survival time of cases with acute exacerbation was 3.65 years and median survival time of cases without acute exacerbation was unreached
Risk factors for AE
Analysis of the risk factors for the incidence of AE using a univariate Cox proportional hazards model revealed the following factors: 1) the 6-minute walk distance was short; 2) the minimum SpO2 during 6-minte walk test was 88% or less; 3) KL-6, SP-D, and C-reactive protein (CRP) were high; 4) PaO2 was low; 5) FVC was low; 6) there was 10% or more FVC decline in 1 year; and 7) 15% or more DLco decline in 1 year. Furthermore, the multivariate analysis found the following risk factors for the incidence of AE–IPF: 1) the minimum SpO2 during the 6-minute walk test at the time of the diagnosis was 88% or less; 2) 10% or more FVC decline in 1 year; and 3) 15% or more DLco decline in 1 year (Table 2).
Table 2.
Risk factors for the occurence of acute exacerbation
| Parameters | Hazard ratio (95% CI) | p value | |
| Univariate Cox analysis | |||
| Males, sex | 0.98 | (0.43-2.24) | NS |
| Age (yrs) | 1.02 | (0.97-1.07) | NS |
| BMI (kg/m^2) | 1.03 | (0.94-1.12) | NS |
| Smoking history | 1.01 | (0.46-2.20) | NS |
| Family history | 1.86 | (0.85-4.08) | NS |
| BAL | |||
| Total cell count (×10^5) | 1.13 | (0.89-1.43) | NS |
| Macrophages (%) | 1.01 | (0.98-1.03) | NS |
| Lymphocytes (%) | 0.99 | (0.97-1.02) | NS |
| Neutrophils (%) | 1.03 | (0.97-1.10) | NS |
| Eosinophils (%) | 1.02 | (0.84-1.24) | NS |
| 6-minute walk test (6MWT) | |||
| Distance (meter) | 0.99 | (0.988-0.99) | 0.02 |
| Minimum SpO2, 88% or less | 0.86 | (0.80-0.93) | <0.001 |
| ΔSpO2, 4% or more | 1.63 | (0.74-3.55) | NS |
| KL-6 (U/mL) | 1.001 | (1.001-1.005) | 0.002 |
| SP-D (ng/mL) | 1.003 | (1.001-1.005) | 0.003 |
| LDH (U/L) | 1.01 | (0.998-1.01) | NS |
| CRP (mg/dL) | 1.20 | (1.06-1.35) | 0.003 |
| PaO2(Torr) | 0.94 | (0.90-0.98) | 0.005 |
| FVC %pred | 0.97 | (0.95-0.99) | 0.007 |
| DLco %pred | 0.99 | (0.99-1.01) | NS |
| 10% or more FVC decline in 1 year | 7.45 | (3.12-17.77) | <0.001 |
| 15% or more DLco decline in 1 year | 2.74 | (1.06-7.10) | 0.038 |
| KL-6 increase in 1 year (U/mL) | 1.21 | (0.54-2.70) | NS |
| SP-D increase in 1 year (ng/mL) | 1.67 | (0.70-3.98) | NS |
| Multivariate Cox analysis | |||
| Minimum SpO2 in 6 MWT, 88% or less | 5.28 | (1.44-19.32) | 0.012 |
| 10% or more FVC decline in 1 year | 4.14 | (1.26-13.65) | 0.020 |
| 15% or more DLco decline in 1 year | 4.66 | (1.19-18.17) | 0.027 |
Cox proportional hazards regression model was used. Definition of abbreviations: BMI: body mass index, BAL: Bronchoalveolar lavage, SpO2: arterial oxygen saturation measured by pulse oximetry, ΔSpO2: difference from the resting SpO2 to the minimum SpO2, KL-6: Krebs von den Lungen-6, SP-D: surfactant protein-D, LDH: lactate dehydrogenase, CRP: C-reactive protein, PaO2: partial pressure of oxygen in arterial blood, FVC: forced vital capacity, %pred: % predicted, DLco diffusion capacity of the lungs for carbon monoxide, NS: not significant.
Second AE–IPF
Among the 39 patients with AE–IPF, eight patients (21%) experienced a second occurence of AE–IPF. Of these eight, two patients experienced a third incidence of AE–IPF.
Prognosis of AE–IPF
Among the 39 patients with AE-IPF, the mean duration from the time of IPF diagnosis until the onset of AE–IPF was 2.1 years, and 14 patients (36%) received no previous treatment before AE onset. At the time of the AE onset, the mean CRP was 9.66 mg/dL, KL-6 was 1625 U/mL, and the PaO2/fraction of inspired oxygen (FiO2) (P/F) ratio was 234. The 90-day survival rate from the onset of AE–IPF was 50.4% (Figure 4).
Fig. 4.
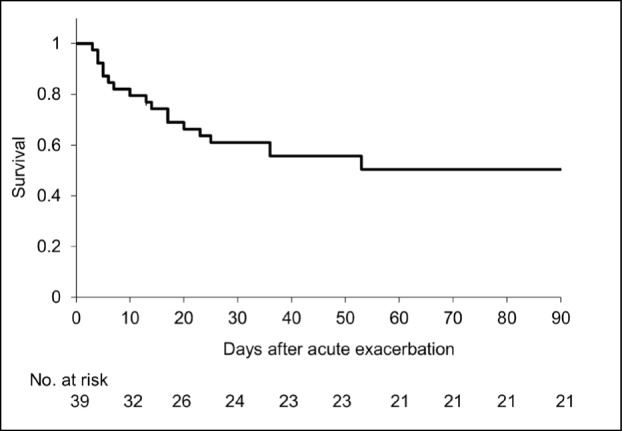
90-day survival curve from the acute exacerbation onset. 90-day survival rate was 50.4%
Comparison between triggered and idiopathic AE
Among the 39 patients with AE, 10 had the following types of triggered AE: infections (6 patients), surgical lung biopsy (2 patients), video-assisted thoracoscopic surgery for lung cancer found during the observation period (1 patient), and alveolar hemorrhage (1 patient). The CRP level at the onset of AE was significantly higher in the triggered AE group compared to the idopathic AE group (Table 3). The median CRP level of triggered AE due to infection was 25.0 mg/dL, while that of triggered AE due to causes other than infection was 8.2 mg/dL. The 90-day survival rates for triggered and idiopathic AEs were 60% and 45%, respectively, with no significant difference in survival between the two groups (Figure 5). In addition, there was no difference in the treatment between the patients with triggered AE and those with idiopathic AE (Table 4).
Table 3.
Characteristics at the onset of acute exacerbation
| Characteristics | Triggered AE | Idiopathic AE | p value |
| Subjects | 10 | 29 | NS |
| Male, sex | 9 | 23 | NS |
| Age (yrs) | 70.8±5.7 | 69.2±7.7 | NS |
| Period from IPF diagnosis (yrs) | 3.3±3.5 | 1.7±1.4 | NS |
| PSL before AE | 1 | 11 | NS |
| Immunosuppressants before AE | 0 | 7 | NS |
| Anti-fibrotic agents before AE | 6 | 16 | NS |
| No previous treatment before AE | 4 | 10 | NS |
| History of AE | 1 | 7 | NS |
| Blood tests | |||
| KL-6 (U/mL) | 1175±778 | 1763±828 | NS |
| SP-D (ng/mL) | 251±212 | 253±123 | NS |
| LDH (U/L) | 343±164 | 361±125 | NS |
| Albumin (g/mL) | 3.1±0.6 | 3.3±0.5 | NS |
| CRP (mg/dL) | 17.7±10.2 | 6.8±7.2 | 0.007 |
| P/F ratio | 242±196 | 230±89 | NS |
Data are presented as n or mean±standard deviation. Date from the most recent acute exacerbation were used. Mann-Whitney U test was used. Definition of abbreviations: IPF: idiopathic pulmonary fibrosis, PSL: prednisolone, AE: acute exacerbation, KL-6: Krebs von den Lungen-6, SP-D: surfactant protein-D, LDH: lactate dehydrogenase, CRP: C-reactive protein, P/F ratio: PaO2/FiO2 ratio, NS: not significant.
Fig. 5.
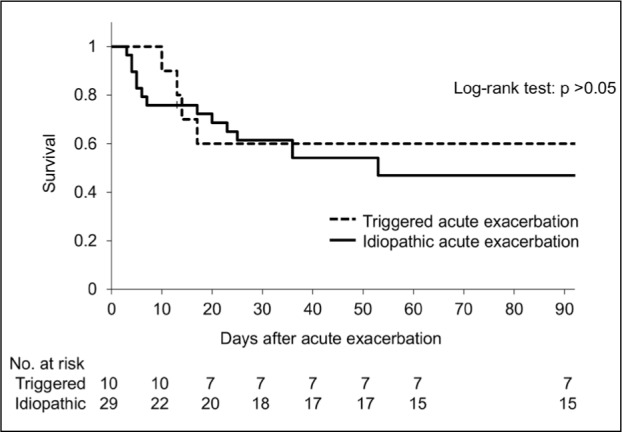
Comparison of survival curves from the acute exacerbation onset between triggered and idiopathic acute exacerbation
Table 4.
Treatment of triggered acute exacerbation and idiopathic acute exacerbation
| Parameters | Triggered AE | Idiopathic AE | p value |
| Cases | 10 | 29 | |
| Steroid pulse | 8 (80) | 26 (90) | NS |
| IVCY | 4 (40) | 6 (21) | NS |
| Oral immunosuppressants | 2 (20) | 9 (31) | NS |
| PMX-DHP | 3 (30) | 6 (21) | NS |
| Antibacterial drugs | 9 (90) | 20 (69) | NS |
Data are presented as n (%). Fisher’s exact test was used.
Definition of abbreviations: AE: acute exacerbation, IVCY: intravenous cyclophosphamide, PMX-DHP: polymyxin B-immobilized fiber column direct hemoperfusion, NS: not significant.
Discussion
This retrospective cohort study was conducted including newly diagnosed IPF patients with UIP, as identified by surgical lung biopsy. This study investigated the incidence rates, risk factors and mortality of patients with newly defined AE. The results indicated that AE–IPF occurred at a consistent rate for the first 4 years following diagnosis. Furthermore, the analysis revealed that risk factors for the incidence of AE included minimum SpO2 during the 6-minute walk test at the time of the diagnosis and annual declines of FVC and DLco. There were no differences in the prognosis or treatment between the triggered AE and idiopathic AE in the clinical setting.
The 2011 ATS/ERS/Japanese Respiratory Society/Latin American Thoracic Association IPF statement allowed for the diagnosis of IPF if CT images revealed UIP pattern without a surgical lung biopsy, which was previously necessary for the diagnosis of IPF (6). In the 2017 Fleischner Society White Paper, IPF diagnosis by CT images alone became more common (9). However, diagnosing IPF using CT images is difficult and there is often disagreement even among chest radiology specialists (10). We were concerned that cases other than IPF might be diagnosed as IPF in the clinical setting. Additionally, even when CT images show a typical UIP, histopathological analysis occasionally reveals that it is UIP a known cause (e.g., vasculitis and rheumatoid arthritis). Moreover, it is common for the diagnosis of UIP to be made histopathologically when there is no typical UIP pattern on CT images (11). Although the present study has a strong bias, with only surgical lung biopsy cases, we believe that patients with IPF who have a UIP pattern on surgical lung biopsy and are diagnosed through MDD likely have an accurate diagnosis that would be minimally influenced by future changes in IPF diagnostic criteria. As IPF diagnosis by CT images alone became more common, it is expect that the frequency of surgical lung biopsy will decrease in the clinical setting. Therefore, we included IPF patients with surgically-proven UIP as the subjects in this study, even though it is important to consider AE in IPF cases without surgical lung biopsy.
During the first 4 years after the diagnosis of IPF, the cumulative rate of initial AE–IPF was consistently 7.1%-13.4%. For the fifth year, the annual incidence rate was 5.5%, which was slightly lower than that of the first 4 years. This may have occurred because the mean observation period was only 3.9 years. Previous studies have reported that the incidence rate of AE–IPF for 1 year was 4%-9% (12-14). The incidence of AE using the new AE-IPF diagnostic criteria tended to be relatively high. The reason of the increase in AE incident was that the acute respiratory failure induced by identified and unknown causes could be diagnosed as AE.
Previous reports indicated that risk factors for the AE–IPF consisted of respiratory symptoms (modified Medical Research Council breathlessness scores and St. George’s Respiratory Questionnaire scores), PaO2, FVC, DLco, and 6-minute walk distance (2-5). In addition, Kondoh et al. reported that an FVC decline greater than 10% within 6 months was a risk factor for the occurrence of AE–IPF (5) (15). In this study, FVC decline of 10% or greater and DLco decline of 15% or greater within 1 year were risk factors for AE–IPF. Even 4 years after the diagnosis, AE–IPF occurred approximately at the same frequency as 1 year after the diagnosis. Therefore, data from the time of the IPF diagnosis alone is not sufficient for predicting the incidence of AE–IPF over the long-term. The results of this study demonstrated that the amount of change in FVC and DLco in 1 year was a risk factor for the occurrence of AE, suggesting the importance of follow-up after the diagnosis. Furthermore, one study reported that FVC does not accurately reflect the prediction of disease progression in patients who also developed emphysema (16). Therefore, it might be important to check not only FVC but also DLco regularly.
In this study, the AE incidence over 4 years was higher in patients whose the minimum SpO2 was 88% or less during the 6-minute walk test. The 6-minute walk test has been reported to correlate with maximum oxygen uptake (17), quality of life (18), and to have no correlation with spirometry in patient with chronic pulmonary disease (19). Evaluation of total exercise tolerability, such as the 6-minute walk test might be important for the prediction of AE–IPF.
The 90-day survival rate from the onset of an AE was 50.4% in this study, which is better than that described in previous reports from Japan (20, 21). A possible reason is that, unlike the Japanese AE–IPF diagnostic criteria, the 2016 AE–IPF diagnostic criteria do not include “more than 10 Torr decline in PaO2 from baseline” which enables mild acute respiratory failure to be diagnosed as AE–IPF.
There were no differences in the prognosis or treatment (steroid pulse, intravenous cyclophosphamide, oral immunosuppressants, polymyxin B, and antimicrobial drugs) at the time of the AE–IPF between the triggered AE and idiopathic AE groups. The causes of AE–IPF did not impact the prognosis or treatment options in clinical practice; therefore, the new AE–IPF diagnostic criteria appear to be useful in clinical setting. CRP level at the onset of AE was significantly higher in triggered AE group, especially AE due to infection. CRP level might be useful for differentiating between AE caused by infection and other AEs.
This study had several limitations. This study was a retrospective investigation conducted at a single facility with a limited number of AE–IPF patients. The investigation included patients who had undergone a surgical lung biopsy; therefore, mild cases and patients who had positive attitudes toward medical care tended to be selected as the subject group. In addition, because all the patients were Japanese, it was not possible to determine whether there were any inter-racial differences in the incidence and mortality rates of AE–IPF. Despite these limitation, we believe that we could show the incidence, risk factors, and prognosis of newly defined AE in IPF patients.
In conclusion, the newly defined AE–IPF occurred at a consistent rate each year following diagnosis. Predictive factors for the incidence of AE included the minimum SpO2 during the 6-mintue walk test, and an annual decline in FVC and DLco. The causes of AE–IPF did not impact the prognosis or treatment options in clinical practice.
References
- 1.Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 2.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 3.Collard HR, Yow E, Richeldi L, Anstrom KJ, Glazer C. Suspected acute exacerbation of idiopathic pulmonary fibrosis as an outcome measure in clinical trials. Respir Res. 2013;14:73. doi: 10.1186/1465-9921-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohsimo S, Ishikawa N, Horimasu Y, et al. Baseline KL-6 predicts increased risk for acute exacerbation of idiopathic pulmonary fibrosis. Respir Med. 2014;108:1031–1039. doi: 10.1016/j.rmed.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2010;27:103–110. [PubMed] [Google Scholar]
- 6.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lama VN, Flaherty KR, Toews GB, et al. Prognostic value of desaturation during a 6-minute walk test in idiopathic interstitial pneumonia. Am J Respir Crit Care Med. 2013;168:1084–1090. doi: 10.1164/rccm.200302-219OC. [DOI] [PubMed] [Google Scholar]
- 8.American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS) Am J Respir Crit Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 9.Lynch DA, Sverzellati N, Travis WD, et al. Diagnostic criteria for idiopathic pulmonary fibrosis: a Fleischner Society White Paper. Lancet Respir Med. 2018;6:138–153. doi: 10.1016/S2213-2600(17)30433-2. [DOI] [PubMed] [Google Scholar]
- 10.Watadani T, Sakai F, Johkoh T, Noma S, et al. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936–944. doi: 10.1148/radiol.12112516. [DOI] [PubMed] [Google Scholar]
- 11.Yagihashi K, Huckleberry J, Colby TV, et al. Radiologic-pathologic discordance in biopsy-proven usual interstitial pneumonia. Eur Respir J. 2016;47:1189–1197. doi: 10.1183/13993003.01680-2015. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi H, Ebina M, Kondoh Y, et al. Pifenidone in idiopathic pulmonary fibrosis. Eur Respir J. 2010;35:821–829. doi: 10.1183/09031936.00005209. [DOI] [PubMed] [Google Scholar]
- 13.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 14.Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27:143–150. doi: 10.1183/09031936.06.00114004. [DOI] [PubMed] [Google Scholar]
- 15.Kondoh Y, Taniguchi H, Ebina M, et al. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis--Extended analysis of pirfenidone trial in Japan. Respir Investig. 2015;53:271–278. doi: 10.1016/j.resinv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Cottin V, Hansell DM, Sverzellati N, et al. Effect of Emphysema Extent on Serial Lung Function in Patients with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med. 2017;196:1162–1171. doi: 10.1164/rccm.201612-2492OC. [DOI] [PubMed] [Google Scholar]
- 17.Cahalin LP, Mathier MA, Semigran MJ, Dec GW, DiSalvo TG. The six-minute walk test predicts peak oxygen uptake and survival in patients with advanced heart failure. Chest. 1996;110:325–332. doi: 10.1378/chest.110.2.325. [DOI] [PubMed] [Google Scholar]
- 18.Brown CD, Benditt JO, Sciurba FC, et al. Exercise testing in severe emphysema: association with quality of life and lung function. COPD. 2008;5:117–124. doi: 10.1080/15412550801941265. [DOI] [PubMed] [Google Scholar]
- 19.Karanth MS, Awad NT. Six minute walk test: a tool for predicting mortality in chronic pulmonary diseases. J Clin Diagn Res. 2017;11:OC34–38. doi: 10.7860/JCDR/2017/24707.9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arai T, Tachibana K, Sugimoto C, et al. High-dose prednisolone after intravenous methylprednisolone improves prognosis of acute exacerbation in idiopathic interstitial pneumonias. Respirology. 2017;22:1363–1370. doi: 10.1111/resp.13065. [DOI] [PubMed] [Google Scholar]
- 21.Kishaba T, Tamaki H, Shimaoka Y, Fukuyama H, Yamashiro S. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung. 2014;192:141–149. doi: 10.1007/s00408-013-9530-0. [DOI] [PubMed] [Google Scholar]


