Abstract
Background:
Patients with sarcoidosis can present with cardiac symptoms as the first manifestation of disease in any organ. In these patients, the use of chest imaging modalities may serve as an initial screening tool towards the diagnosis of sarcoidosis through identification of pulmonary/mediastinal involvement; however, the use of chest imaging for this purpose has not been well studied. We assessed the utility of different chest imaging modalities for initial screening for cardiac sarcoidosis (CS).
Methods and Results:
All patients were investigated with chest x-ray, chest computed tomography (CT) and/or cardiac/thorax magnetic resonance imaging (MRI). We then used the final diagnosis (CS versus no CS) and adjudicated imaging reports (normal versus abnormal) to calculate the sensitivity and specificity of individual and combinations of chest imaging modalities. We identified 44 patients (mean age 54 (±8) years, 35.4% female) and a diagnosis of CS was made in 18/44 patients (41%). The sensitivity and specificity for screening for sarcoidosis were 35% and 85% for chest x-ray, respectively (AUC 0.60; 95%CI 0.42-0.78; p value=0.27); 94% and 86% for chest CT (AUC 0.90; 95%CI 0.80-1.00; p value <0.001); 100% and 50% for cardiac/thorax MRI (AUC 0.75; 95%CI 0.56-0.94; p value=0.04).
Conclusions:
During the initial diagnostic workup of patients with suspected CS, chest x-ray was suboptimal as a screening test. In contrast CT chest and cardiac/thorax MRI had excellent sensitivity. Chest CT has the highest specificity among imaging modalities. Cardiac/thorax MRI or chest CT could be used as an initial screening test, depending on local availability.
Key words: cardiac sarcoidosis, sarcoidosis, screening, imaging, cardiomyopathy
Introduction
Sarcoidosis is a multisystem, granulomatous disease of unknown etiology. The lungs are affected in more than 90% of patients and the disease can also involve the heart, liver, spleen, skin, eyes, parotid gland, or other organs and tissues. Clinically manifest cardiac involvement occurs in perhaps 5% of patients with sarcoidosis (1). There is a growing realization that cardiac-related symptoms may be the first manifestation of sarcoidosis in any organ. Between 16% and 35% of patients presenting with complete atrioventricular (AV) block (aged <60) (2, 3) or ventricular tachycardia (VT) of unknown aetiology (4, 5) have previously undiagnosed cardiac sarcoidosis (CS) as the underlying etiology. Also CS as the underlying cause of heart failure is often missed (6).
The diagnosis of CS is often delayed or missed altogether as the symptoms and clinical manifestations are common to many cardiovascular diseases. Perhaps the most sensitive and specific test for active inflammation is positron emission tomography (PET) with 18F-fluorodeoxyglucose (18F-FDG), but this technology is not readily available in many hospitals. We hypothesized that chest imaging modalities may serve as more accessible and practical screening tools to help identify patients who should undergo more comprehensive workup. In the current study, we assessed and compared the utility of different chest imaging modalities for initial screening for CS.
Methods
For the current study we included all consecutive consenting patients presenting to the University of Ottawa Heart Institute who met all of the following criteria:
- Acute presentation with 1 or more of the following:
- age < 60 years old with unexplained, new onset, significant conduction system disease
- idiopathic sustained ventricular arrhythmia (VA), defined as VA not fulfilling any of: outflow tract VA, fascicular VA or VA secondary to other structural heart disease (e.g. coronary artery disease or any cardiomyopathy other than idiopathic).
- non-ischemic cardiomyopathy
No previous history of sarcoidosis in any organ
All patients had a comprehensive work up including chest x-ray, FDG-PET imaging, chest CT and/or cardiac focused MRI with thoracic imaging. Patients with positive imaging suggestive of sarcoidosis underwent biopsies to confirm the diagnosis when possible. All studies were reported clinically. All readers were aware of the possibility of sarcoidosis in the differential diagnosis but were not informed of the final diagnosis. The reports of all chest x-rays, chest CT and or cardiac/thorax MRI were adjudicated by 2 separate investigators (DHB and JJR). Imaging studies were defined as ‘abnormal with features possibly consistent with sarcoidosis’ or ‘abnormal due to other findings not suggestive of sarcoidois’ or ‘normal’.
The protocol was approved by the local institutional ethics committees and all patients provided informed consent.
Patients were classified as having active CS (or not) based on consensus criteria (7, 8). The final patient classification and the adjudicated imaging reports (normal versus abnormal) were used to calculate the sensitivity and specificity of individual, and combinations of, imaging modalities. Categorical variables are presented using percentages or frequencies, and continuous variables using means (± standard deviation) or medians (25th, 75th percentiles), when appropriate. We compared categorical variables using the chi-square test (or Fisher’s exact test when appropriate), and continuous variables using one-way analysis of variance or Kruskal-Wallis test for normally and non-normally distributed variables, respectively. Statistical analyses were conducted using SPSS, version 23 (IBM Corp, Armonk, New York). Two-sided p values <0.05 were considered statistically significant.
Results
Of 44 patients undergoing workup for suspected CS included in the current analysis, 18/44 (41%) were ultimately diagnosed with active CS. All 18 patients had abnormal FDG uptake on cardiac PET imaging. Baseline patient and index event characteristics stratified by final diagnosis (CS versus no CS) are provided in Table 1. Table 2 summarizes the frequency of use and results of chest imaging modalities during the initial workup for CS. Chest x-ray, CT thorax, and cardiac and thorax MRI were performed in 100%, 89%, and 57% of patients, respectively.
Table 1.
Baseline patient characteristics
| Characteristic | No sarcoid (n=26) | Sarcoid (n=18) | p value |
| Age (years)* | 54 (±9) | 53 (±7) | 0.63 |
| Female– no. (%) | 9 (35) | 10 (56) | 0.22 |
| BMI (kg/m2)* | 28 (±5) | 31 (±11) | 0.15 |
| Hypertension– no. (%) | 8 (31) | 5 (28) | >0.99 |
| Diabetes– no. (%) | 7 (27) | 1 (5.6) | 0.12 |
| Presenting feature– no. (%) | 0.16 | ||
| AV block | 13 (50) | 10 (56) | |
| Ventricular arrhythmia or cardiac arrest | 13 (50) | 6 (33) | |
| Cardiomyopathy | 0 (0) | 2 (11) | |
| AV block– no. (%) | |||
| 1st degree | 1 (3.8) | 4 (22) | 0.14 |
| 2nd degree | 3 (12) | 2 (11) | >0.99 |
| 3rd degree | 11 (42) | 6 (33) | 0.75 |
*mean (±standard deviation).
Abbreviations: AV, atrioventricular; BMI, body mass index; CHF, congestive heart failure; MI, myocardial infarction; TIA, transient ischemic attack.
Table 2.
Summary of diagnostic imaging performed
| Characteristic | No sarcoid (n=26) | Sarcoid (n=18) | p value |
| Chest x-ray – performed no. (%) Abnormal | 26/26 (100) 4/26 (15) |
18/18 (100) 6/18 (33) |
0.41 |
| Chest CT - performed no. (%) Abnormal | 22/26 (85) 3/26 (14) |
17/18 (94) 16/18 (94) |
0.63 |
| Cardiac/thorax MRI - performed – no. (%) Abnormal | 14/26 (54) 7/14 (50) |
11/18 (61) 11/11 (100) |
0.76 |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging
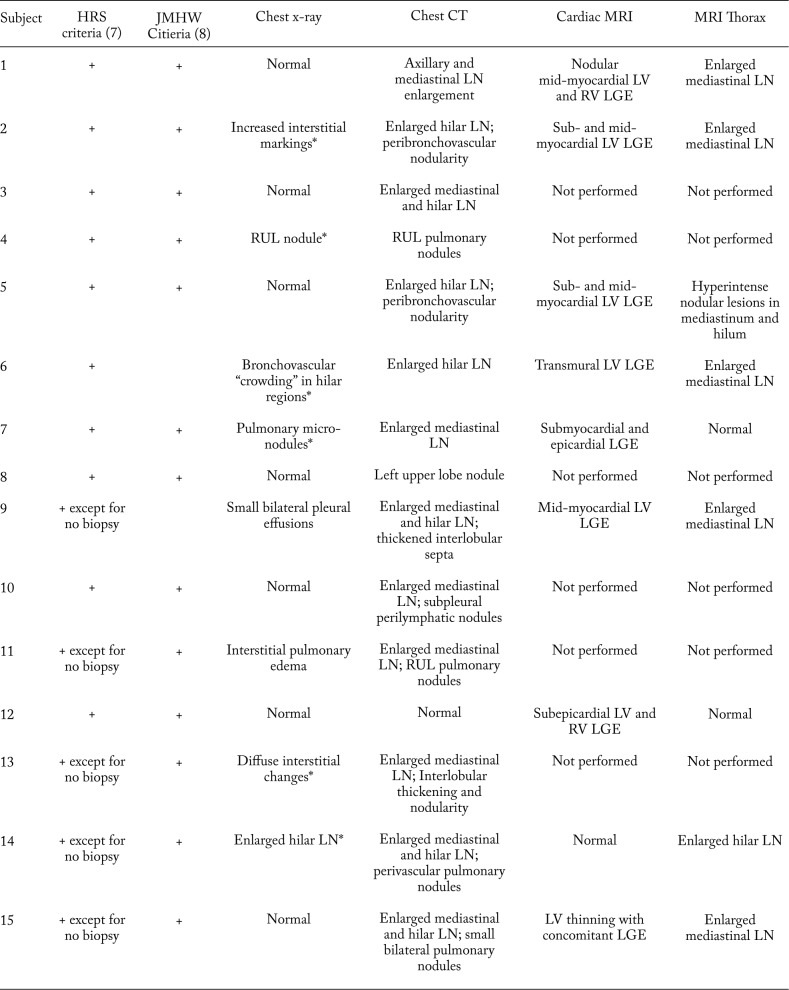
Table 3 details the diagnostic criteria and chest imaging findings of the 18 patients that were ultimately diagnosed with CS. Figure 1 shows initial chest imaging in a 47 year-old male (subject 17) ultimately diagnosed with CS. During initial chest imaging, chest x-ray was normal while the CT of the chest identified mediastinal lymphadenopathy. Figure 2 shows initial chest imaging for a 45-year old patient (subject 14) who was also subsequently diagnosed with CS. For this patient, initial chest x-ray showed hilar lymphadenopathy while CT of the chest identified hilar and mediastinal lymphadenopathy.
Table 3.
Diagnostic Criteria and Findings of initial screening chest imaging modalities in patients subsequently diagnosed with cardiac sarcoidosis
Fig. 1.
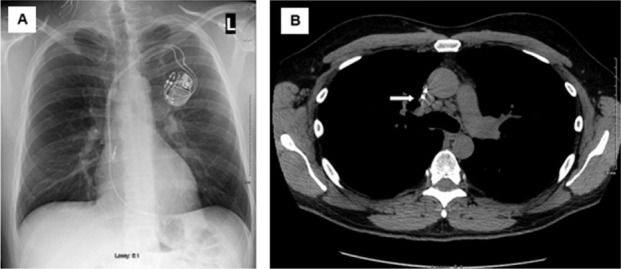
Initial chest imaging in 47-year old male (subject 17) subsequently diagnosed with cardiac sarcoidosis. A. Chest x-ray showing no significant abnormalities. B. Computed tomography of the chest showing mediastinal lymphadenopathy (white arrow)
Fig. 2.
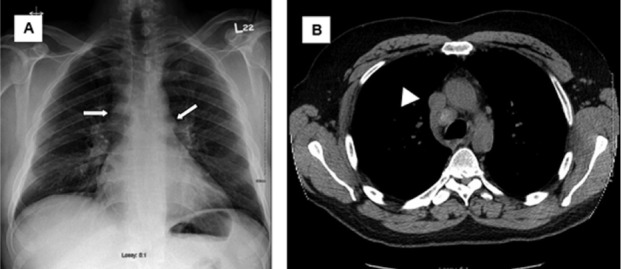
Initial chest imaging in 45-year old male (subject 14) subsequently diagnosed with cardiac sarcoidosis. A. Chest x-ray showing hilar lymphadenopathy (white arrows). B. Computed tomography of the chest showing documenting hilar and mediastinal lymphadenopathy (white arrow head).
Table 4 lists the sensitivities, specificities, and area under the curve (AUC) of individual chest imaging modalities for screening of CS. We include information for permutations of the combination of cardiac/thorax MRI and CT chest. The p-value for the area under the curve was statistically significant for chest CT (AUC 0.90; 95% CI 0.80-1.00; p<0.001), cardiac/thorax MRI (AUC 0.75; 95% CI 0.56-0.94; p=0.04), and the combination of abnormal cardiac/thorax MRI and CT scan (AUC 0.91; 95% CI 0.76-1.00; p=0.002).
Table 4.
Sensitivity and specificity of chest imaging modalities for screening of sarcoidosis
| Imaging modality | Sensitivity* | Specificity* | AUC† | p value‡ |
| Chest x-ray | 35 (14-62) | 85 (65-96) | 0.60 (0.42-0.78) | 0.27 |
| Chest CT | 94 (71-100) | 86 (65-97) | 0.90 (0.80-1.00) | <0.001 |
| Cardiac MRI | 91 (59-100) | 50 (23, 77) | 0.71 (0.50-0.91) | 0.09 |
| Thorax MRI | 73 (39-94) | 93 (66, 100) | 0.83 (0.65-1.00) | 0.006 |
| Cardiac/Thorax MRI | 100 (72-100) | 50 (23-77) | 0.75 (0.56-0.94) | 0.04 |
| Abnormal cardiac/thorax MRI or chest CT§ | 100 (69-100) | 45 (17-77) | 0.73 (0.51-0.95) | 0.09 |
| Abnormal cardiac/thorax MRI and chest CT | 90 (55-100) | 91 (59-100) | 0.91 (0.76-1.00) | 0.002 |
* Percent (95% confidence interval);
† Area under the curve (95% confidence intervals);
‡ p value indicates whether the AUC of the test is statistically different from 0.5;
§ When both tests performed.
Abbrevitation: CT, computed tomography; MRI, magnetic resonance imaging.
Discussion
In the current study, we assessed the utility of different chest imaging modalities for initial screening for CS in patients with clinically suspicious cardiac presentations. The key findings of this study are that chest x-ray was suboptimal as a screening test due to low sensitivity. In contrast chest CT and cardiac/thorax MRI had excellent sensitivity. Chest CT has the highest specificity among imaging modalities.
Studies suggest that CS is becoming more prevalent. However, this is likely due to improvements in imaging and/or more thorough investigation rather than a true increase in prevalence. In Finland the rate of diagnosis of CS increased more than 20-fold between 1988 and 2012 (9). In the US, in patients undergoing cardiac transplantation, CS as the etiology of cardiomyopathy increased from 0.1% (1994-1997) to 0.5% (2010-2014) (10). It is still common for the diagnosis of CS to be delayed or missed altogether; for example, core LV biopsies at the time of left ventricular assist device implantation found previously undiagnosed CS in 6 of 177 patients (3.4%) (6). CS can also present with features similar to arrhythmogenic right ventricular cardiomyopathy (11).
Recent data showed that cardiac presentations can be the first manifestation of sarcoidosis in any organ. In a Finnish study of 72 patients aged <55 years with new onset, unexplained, significant conduction system disease, biopsy-verified CS was found in 14/72 (19%); “probable” CS was found in 4/72 (6%); and giant cell myocarditis was found in 4/72 (6%). The prognosis for CS patients was poorer versus those who had idiopathic complete AV block (2). In a similar study from a tertiary Canadian centre, CS was diagnosed in 11/32 (34%) patients aged <60 years with advanced heart block (3). In a prospective study that screened consecutive patients with VT of unknown etiology for sarcoidosis, 4/14 patients (29%) were diagnosed with CS (4). In a study by Tung et al of 103 patients (85% Caucasian, 7% African American and 8% Asian) with VT and non-ischemic cardiomyopathy, 17/103 (16.5%) had undiagnosed CS (5). In these patients, the diagnosis of CS is often delayed or missed altogether because of limited pulmonary and/or other organ involvement (3, 4, 12, 13).
In this sample of patients routinely undergoing screening tests who met pre-specified criteria for suspicion of CS, we found that the initial chest x-ray had features possibly consistent with sarcoidosis in only 6/18 patients (33%). There are likely 2 reasons for this: 1) in this group, most patients did not have pulmonary sarcoidosis and 2) the absence of lymph node enlargement which can be explained by the pattern of lymphatic drainage from the heart. Although it is not completely understood, the principal lymphatics likely drain from the ventricular muscle to the upper mediastinum (14). The lungs primarily drain to the more central hilar lymph nodes resulting in the classic bilateral hilar lymphadenopathy of pulmonary sarcoidosis.
Our observations are consistent with a small study from Japan. Otsuka et al investigated 8 patients diagnosed with idiopathic cardiomyopathy who underwent left ventriculoplasty and were later proven to have CS by histological evaluation of the resected myocardium (15). All chest x-rays of the CS patients were normal. However, chest CT demonstrated significant mediastinal lymphadenopathy in 7 (88%) of them (15). Our findings are also similar to observations in patients presenting with possible ocular sarcoidosis. Chung et al studied 44 patients with uveitis who subsequently were diagnosed with biopsy-proven sarcoidosis (16). Chest x-ray was abnormal in 22 patients (50%) and chest CT in 42 (95%) (16).
Our study has some limitations; first, our population was exclusively Caucasian and it is well recognized that sarcoidosis phenotypes have important racial differences and thus our findings need to be replicated in other groups. However, our observations are similar to the small Japanese study referenced above (15).Our sample size is small and our findings should be replicated in a larger cohort. Furthermore not all patients had all scans. Also although we aimed to enroll patients consecutively, there is still a possibility of selection bias. Other types of bias are also possible; however, our study methodology rated as low risk on all 4 domains of the quality assessment of diagnostic accuracy studies checklist (17). The cardiac MRI did not use other techniques like T2 weighted imaging which may have improved diagnostic accuracy (18). Finally it should be noted that these are ‘real world data’ with multiple readers of clinically performed scans. However, this study design was purposeful as we felt that over reading of all tests by physicians aware of the purpose of research may have lead to over-reporting of tests as having findings consistent with sarcoidosis.
Conclusions and clinical implications
During the initial diagnostic workup of patients with suspected CS, chest x-ray was suboptimal as a screening test. In contrast chest CT and cardiac/thorax MRI had excellent sensitivity. Chest CT has the highest specificity among imaging modalities. This has important clinical implications as recent data suggests that sarcoidosis can often present with important cardiac manifestations and diagnosis can be delayed. Chest CT is widely available and could be used as initial screening test. A suggested clinical screening algorithm is shown in figure 3.
Fig. 3.
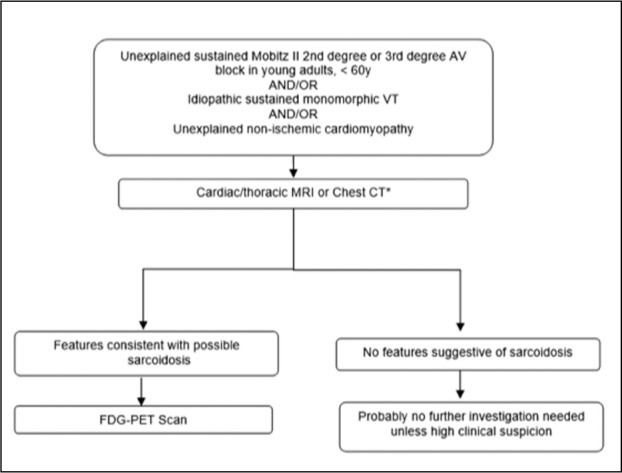
Suggested algorithm for the screening of sarcoidosis in certain cardiac presentations.
*choice dependent on local availability. If both available then cardiac/thoracic MRI suggested as first.
Acknowledgements
We express our gratitude to the Cardiac Sarcoidosis Study project managers (Karen MacDonald and Tammy Knight) and patients.
Funding sources:
The work has been supported in part by a research trial grant from the Ministry of Health and Long Term Care Research (Grant # 06374) for Ontario PET Cardiac Sarcoidosis Trial. This work has also been partially supported by the Canadian Institute of Health Research (D. Birnie, NPI). (Grant no. 342139, NCT01477359).
Funding sources and disclosures:
R.S.B is a Career Investigator supported by the Heart and Stroke Foundation of Ontario (HFSO), the University of Ottawa Heart Institute (UOHI) Vered Chair in Cardiology and the Tier 1 University of Ottawa Chair in Cardiovascular Imaging Research. D.B. is a Mid-career Investigator supported by the HSFO, UOHI Leadership Chair in Electrophysiology and the Tier 1 University of Ottawa Chair in Electrophysiology Research. J.H. is a Mid-career Investigator supported by the HSFO. D.J. is a Cardiac Imaging Fellow and is supported by the UOHI Foundation and the Vered-Beanlands Fellowship in Cardiology Research as well as a grant from the CHUM and CHUM Foundation.
References
- 1.Birnie DH, Nery PB, Ha AC, Beanlands RS. Cardiac sarcoidosis. J Am Coll Cardiol. 2016;68:411–421. doi: 10.1016/j.jacc.2016.03.605. [DOI] [PubMed] [Google Scholar]
- 2.Kandolin R, Lehtonen J, Kupari M. Cardiac sarcoidosis and giant cell myocarditis as causes of atrioventricular block in young and middle-aged adults. Circ Arrhythm Electrophysiol. 2011;4:303–309. doi: 10.1161/CIRCEP.110.959254. [DOI] [PubMed] [Google Scholar]
- 3.Nery PB, Beanlands RS, Nair GM, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol. 2014;25:875–881. doi: 10.1111/jce.12401. [DOI] [PubMed] [Google Scholar]
- 4.Nery PB, Mc Ardle BA, Redpath CJ, et al. Prevalence of cardiac sarcoidosis in patients presenting with monomorphic ventricular tachycardia. Pacing Clin Electrophysiol. 2013:364–374. doi: 10.1111/pace.12277. [DOI] [PubMed] [Google Scholar]
- 5.Tung R, Bauer B, Schelbert H, et al. Incidence of abnormal positron emission tomography in patients with unexplained cardiomyopathy and ventricular arrhythmias: The potential role of occult inflammation in arrhythmogenesis. Heart Rhythm. 2015;12:2488–2498. doi: 10.1016/j.hrthm.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segura AM, Radovancevic R, Demirozu ZT, Frazier OH, Buja LM. Granulomatous myocarditis in severe heart failure patients undergoing implantation of a left ventricular assist device. Cardiovasc Pathol. 2014;23:17–20. doi: 10.1016/j.carpath.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis and Granulomatous Disorders. 2007;27:89–102. [Google Scholar]
- 9.Kandolin R, Lehtonen J, Airaksinen J, et al. Cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131:624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 10.Al-Kindi SG, Oliveira GH. Letter by Al-kindi and Oliveira regarding article “cardiac sarcoidosis: Epidemiology, characteristics, and outcome over 25 years in a nationwide study”. Circulation. 2015;132:e211. doi: 10.1161/CIRCULATIONAHA.115.016258. [DOI] [PubMed] [Google Scholar]
- 11.Philips B, Madhavan S, James CA, et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy and cardiac sarcoidosis: Distinguishing features when the diagnosis is unclear. Circ Arrhythm Electrophysiol. 2014;7:230–236. doi: 10.1161/CIRCEP.113.000932. [DOI] [PubMed] [Google Scholar]
- 12.Kandolin R, Lehtonen J, Graner M, et al. Diagnosing isolated cardiac sarcoidosis. J Intern Med. 2011;270:461–468. doi: 10.1111/j.1365-2796.2011.02396.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharma PS, Lubahn JG, Donsky AS, et al. Diagnosing cardiac sarcoidosis clinically without tissue confirmation. Proc (Bayl Univ Med Cent) 2009;22:236–238. doi: 10.1080/08998280.2009.11928525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loukas M, Abel N, Tubbs RS, Grabska J, Birungi J, Anderson RH. The cardiac lymphatic system. Clin Anat. 2011;24:684–691. doi: 10.1002/ca.21104. [DOI] [PubMed] [Google Scholar]
- 15.Otsuka K, Terasaki F, Eishi Y, et al. Cardiac sarcoidosis underlies idiopathic dilated cardiomyopathy: Importance of mediastinal lymphadenopathy in differential diagnosis. Circ J. 2007;71:1937–1941. doi: 10.1253/circj.71.1937. [DOI] [PubMed] [Google Scholar]
- 16.Chung YM, Lin YC, Liu YT, Chang SC, Liu HN, Hsu WH. Uveitis with biopsy-proven sarcoidosis in chinese--a study of 60 patients in a uveitis clinic over a period of 20 years. J Chin Med Assoc. 2007;70:492–496. doi: 10.1016/S1726-4901(08)70047-9. [DOI] [PubMed] [Google Scholar]
- 17.Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 18.Friedrich MG, Larose E, Patton D, et al. Canadian society for cardiovascular magnetic resonance (CANSCMR) recommendations for cardiovascular magnetic resonance image analysis and reporting. Can J Cardiol. 2013;29:260–265. doi: 10.1016/j.cjca.2012.07.007. [DOI] [PubMed] [Google Scholar]