Abstract
Background
The stem cell factor SALL4 is reactivated in human cancers. SALL4 plays diverse roles in tumor growth, metastasis, and drug resistance, but its role in tumor metabolism has not been well characterized.
Methods
The glycolytic levels of gastric cancer cells were detected by glucose uptake, lactate production, lactate dehydrogenase activity, ATP level, and hexokinase activity. QRT-PCR and western blot were used to detect the changes in the expression of glycolytic genes and proteins. The downstream target genes of SALL4 were identified by microarray. The regulation of hexokinase II (HK-2) by SALL4 was analyzed by luciferase reporter assay and chromatin immunoprecipitation assay. Transwell migration assay, matrigel invasion assay, cell counting assay and colony formation assay were used to study the roles of HK-2 regulation by SALL4 in gastric cancer cells in vitro. The effects of SALL4 on glycolysis and gastric cancer progression in vivo were determined by subcutaneous xenograft and peritoneal metastasis tumor models in nude mice.
Results
SALL4 knockdown inhibited glucose uptake, lactate production, lactate dehydrogenase activity, ATP level and hexokinase activity in gastric cancer cells, and decreased the expression of glycolytic genes and proteins. Microarray analysis showed that SALL4 knockdown affected glycolysis-related pathway. The regulation of HK-2 gene expression by SALL4 was confirmed by luciferase reporter assay and chromatin immunoprecipitation assay. HK-2 knockdown abrogated the promotion of glycolysis by SALL4 in gastric cancer cells, indicating that HK-2 acts as a downstream effector of SALL4. Moreover, HK-2 knockdown reversed the promoting role of SALL4 in gastric cancer cell proliferation, migration and invasion, suggesting that SALL4 drives gastric cancer progression by upregulating HK-2.
Conclusions
SALL4 promotes gastric cancer progression through HK-2-mediated glycolysis, which reveals a new mechanism for the oncogenic roles of SALL4 in cancer.
Keywords: Gastric cancer, SALL4, Hexokinase II, Glycolysis, Progression
Background
Tumor cells are characterized by abnormal glycolysis, accompanied by increased glucose uptake and lactate production, which is called aerobic glycolysis or Warburg effect [1]. Although the ATP produced by tumor glycolysis is not much, it can supply energy quickly and satisfy the high-speed proliferation of tumor cells. The intermediate products generated by glycolysis could be used to synthesize nucleic acids, amino acids and fats required for the proliferation of tumor cells and the acidic microenvironment caused by lactic acid could enhance tumor metastasis and therapy resistance [2]. Therefore, there is a close relationship between glycolysis and various malignant phenotypes of tumor cells.
Transcription factors play a crucial role in the regulation of aerobic glycolysis, such as hypoxia inducible factor-1α (HIF-1α), c-Myc, p53, and sine oculis homeobox 1 (SIX1) [3–7]. SALL4 is a zinc finger protein transcription factor that is crucial in the self-renewal and pluripotency of embryonic stem cells [8, 9]. With the maturation of tissues and organs, the expression of SALL4 is gradually down-regulated. In adult tissues, SALL4 can only be detected in germ cells and hematopoietic stem cells [10]. However, in many cancers including blood cancers and solid tumors, SALL4 expression is restored [11–15]. The previous studies have shown that SALL4 is involved in tumor cell proliferation, migration, invasion, DNA damage repair, and drug resistance, suggesting a high potential of SALL4 as tumor biomarker and therapeutic target [16–18].
Gastric cancer is one of the most common cancers worldwide and ranks second in tumor-related deaths [19, 20]. We have previously shown that SALL4 is highly expressed in gastric cancer and its upregulation is associated with lymph node metastasis and poor prognosis [14]. SALL4 could enhance the proliferation, migration and invasion of gastric cancer cells by regulating CD44, DANCR, and TGF-β1 expression [21–23]. There are few studies on the role of SALL4 in tumor metabolism. Kim et al. demonstrate that SALL4 promotes the ubiquitination of heterochromatin protein 1α (HP1α), which increases the expression of glucose transporter 1 (GLUT1) and hence promotes glycolysis [18]. Whether SALL4 could affect glycolysis through other mechanism still needs further study.
In the present study, we showed that SALL4 overexpression promoted while SALL4 knockdown inhibited glycolysis in gastric cancer cells. SALL4 promoted the glycolysis of gastric cancer cells by activating the expression of HK-2, a key rate-limiting enzyme of glycolysis. HK-2 knockdown reversed the promotion of gastric cancer cell proliferation, migration, and invasion by SALL4. Our results suggest that SALL4 could promote gastric cancer progression through HK-2-mediated glycolysis, which represents a new mechanism for the oncogenic roles of SALL4 in cancer.
Materials and methods
Cell culture
Human normal gastric mucosa epithelial cell line GES-1 was obtained from Gefan Biological Technology (Shanghai, China). Human gastric cancer cell lines HGC-27, AGS, SGC-7901 were purchased from the Institutes for Biological Sciences at the Chinese Academy of Sciences (Shanghai, China). Human gastric cancer cell line MGC-803 was obtained from the Cell Resource Center, Institute of Basic Medicine, Chinese Academy of Medical Sciences (Beijing, China). GES-1, HGC-27 and SGC-7901 cells were cultured in RPMI 1640 medium (Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; Life Technologies) at 37 °C in humidified air with 5% CO2. The other cell lines were cultured in high-glucose DMEM (Life Technologies) supplemented with 10% FBS. Cells have been regularly tested for Mycoplasma and are free of contamination.
Gene transfection
The cells were seeded in 6-well plates at 50% density and incubated overnight. SALL4 overexpression in HGC-27 and AGS cells was achieved by using SALL4 expression plasmid (RC213089) and pCMV6-Entry vector plasmid (PS100001) purchased from OriGene (OriGene, Rockville, MD, USA). The overexpressing plasmids were transfected into the cells by LipoFiter transfection reagent (Hanbio, Shanghai, China) in a serum-free medium. The cells were changed to a complete medium at 6 h after transfection and cultured for another 42 h. Chemically synthesized SALL4 siRNAs and the matching scramble control siRNAs were purchased from Genechem Company (Shanghai, China). The siRNAs were transiently transfected into SGC-7901 and MGC-803 cells at a final concentration of 20 nM by using LipoFiter transfection reagent (Hanbio, Shanghai, China) in a serum-free medium. The cells were changed to a complete medium at 6 h after transfection and cultured for another 42 h. The SALL4-targeting shRNA lentivirus was provided by Genechem (Shanghai, China). MGC-803 cells were transfected with lentivirus at an MOI (multiplicity of infection) of 100 for 24 h and then selected with puromycin (0.8 µg/mL) for 3 days. The sequences of siRNAs and shRNAs were shown in Additional file 1.
Luciferase reporter assay
MGC-803 cells or AGS cells were transfected with the luciferase reporter vector containing the promoter region of HK-2 together with SALL4 siRNA or SALL4 overexpressing plasmid as indicated. At 48 h after transfection, the cells were collected and lysed. The luciferase activity was detected by using the dual luciferase assay kit (Promega, Madison, WI, USA).
Microarray analysis
Total RNAs were isolated from control and SALL4-targeting shRNA transfected MGC-803 cells (3 samples/group). TRIzol reagent (Invitrogen). RNA samples were then used to generate Cyanine-3-CTP(Cy3)-labeled cRNA targets for the gene expression analysis by using Agilent Human lncRNA microarray v2.0 4 × 180 K (OE Biotech, Shanghai, China). The labeled cRNA targets were then hybridized in the slides. After hybridization, slides were scanned on the Agilent Scanner G2505C (Agilent Technologies). Data were extracted with Feature Extraction software 10.7.1.1 (Agilent technologies). Genespring software 12.5 (Agilent technologies) were employed to finish the basic analysis with the raw data. Significant differential expressed transcripts were screened by fold change ≥ 2 or ≤ −2 and p value ≤ 0.05. Afterwards, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were applied to determine the roles of these differentially expressed mRNAs.
Chromatin immunoprecipitation assay
The chromatin immunoprecipitation (ChIP) assay was performed in MGC-803 and SGC-7901 cells by using a commercial kit (Millipore, Darmstadt, Germany). After cross-linking with 1% formaldehyde at 37 °C for 10 min, the cells were harvested in sodium dodecyl sulfate lysis buffer and the DNA was shredded to fragments of 200 bp by sonication. The pre-cleared chromatin was incubated with 1 µg anti-SALL4 (ab29112, Abcam) or non-specific IgG overnight. Protein G-agarose beads were added and incubated at 4 °C for 1 h. After reversing the cross-links, the DNA was isolated and used for PCR. The information of the sequences of ChIP primers are listed in Additional file 2.
Transwell migration assay
The transfected cells were plated into the upper chamber (8 µm) at a density of 1 × 105 cells/well in serum-free medium. The lower chamber was filled with 600 µL complete medium. After incubation at 37 °C in 5% CO2 for 12 h, the cells remaining at the upper surface of the membrane were removed with a cotton swab. The cells that migrated through the 8 µm sized pores and adhered to the lower surface of the membranes were fixed with 4% paraformaldehyde, stained with crystal violet and photographed under a light microscope.
Matrigel invasion assay
The matrigel (BD Biosciences, San Jose, CA, USA) was diluted with serum-free medium (1:3) and 50 µL of the diluted matrigel were added into the upper chamber followed by incubation at 37 °C for 1 h. The transfected cells suspended in serum-free medium were seeded into the upper chamber at a density of 2 × 105 cells/well. The lower chamber was filled with 600 µL complete medium. The cells were allowed to invade into the lower membrane through matrigel at 37 °C for 24 h. Subsequently, the invaded cells were fixed with 4% paraformaldehyde, stained with crystal violet and photographed under a light microscope.
Cell counting and colony formation assays
The transfected cells were seeded into 24-well plate (1 × 104 cells/well) and cultured under standard conditions. Cells were collected and counted at the indicated time points. The transfected cells were seeded into 6-well plates at a density of 1000 cells per well. After continuous incubation for 10 days, the cells were fixed with 4% paraformaldehyde and stained with 1% crystal violet for 15 min. All the experiments were performed in triplicates.
RNA extraction and quantitative real-time polymerase chain reaction
Total RNA was extracted from the cells by using Trizol reagent (Life Technologies) and one microgram of RNA was reverse transcribed to cDNA by using reverse transcriptase (Vazyme, Nanjing, China). Quantitative real-time polymerase chain reaction (qRT-PCR) was performed by using a SYBR Green I real-time detection kit (Cwbio, Beijing, China) on a Bio-Rad CFX96 detection system. The relative gene expression was normalized to β-actin. The primers specific for target genes were listed in Additional file 2.
Glucose uptake, lactate production, lactate dehydrogenase activity, ATP level and hexokinase activity
To detect glucose uptake, lactate production, lactate dehydrogenase activity, ATP level, and hexokinase activity in gastric cancer cells, the Glucose Assay Kit (Applygen, Beijing, China), Lactate Acid Assay Kit (Jiancheng Bioengineering Institute, Nanjing, China), Lactate Dehydrogenase Activity Assay Kit (Jiancheng bioengineering institute), luciferase-based ATP Assay Kit (Beyotime, Shanghai, China), and Hexokinase Activity Assay Kit (Comin, Suzhou, China) were used according to the manufacturer’s protocols. All values were normalized to cell number or total protein levels.
Western blot analysis
The cells were washed twice with PBS and lysed with RIPA buffer containing 1% protease inhibitors. Equal amounts of proteins were separated on 12% sodium dodecyl sulfate–polyacrylamide gels and transferred onto polyvinylidene fluoride membranes, followed by blocking with 5% nonfat milk for 1 h. The membranes were incubated with primary antibodies overnight at 4 °C. The following primary antibodies were used: anti-SALL4 (1:500, ab29112, Abcam), anti-HK-2 (1:1000, 2867T, Cell Signaling Technology), anti-LDHA (1:1000, 3582T, Cell Signaling Technology), anti-PKM2 (1:1000, 4053T, Cell Signaling Technology), anti-β-actin (1:1000, 4970T, Cell Signaling Technology). After incubation with the secondary antibodies (Bioworld Technology) at 37 °C for 1 h, the bands were visualized with a chemiluminescent detection system.
Animal study
Male BALB/c nude mice aged 4 weeks were purchased from the Model Animal Research Cancer of Nanjing University (Nanjing, China) and maintained in accordance with the institutional policies. The mice were randomly grouped (five mice/group) as indicated. Cells (2 × 106 per mice) suspended in 100 µL of phosphate-buffered saline were implanted subcutaneously or intraperitoneally into the mice. At 6 weeks after injection, the mice were sacrificed. The protocol was approved by the Animal Use and Care Committee of Jiangsu University.
Statistical analysis
All the results were expressed as mean ± SD. Statistical analyses were performed using Student’s t-test with GraphPad Prism Version 7.0 software (Graphpad Software, La Jolla, CA, USA). P < 0.05 was considered as statistically significant.
Results
SALL4 gene silencing suppresses while gene overexpression promotes glycolysis in gastric cancer cells
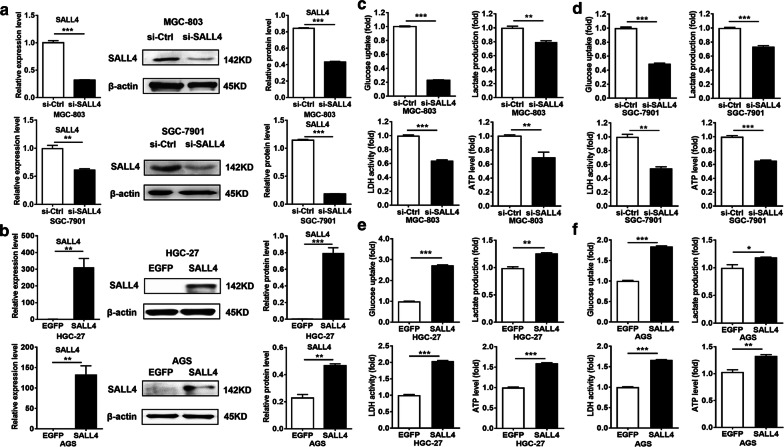
We first wanted to know whether SALL4 modulates the glycolytic phenotype of cultured gastric cancer cells. Since the expression of SALL4 in AGS cells is relatively lower than MGC-803 and SGC-7901 cells but is higher than HGC-27 cells, we chose to use MGC-803 and SGC-7901 cells for knockdown study and HGC-27 and AGS cells for overexpression study. We knocked down SALL4 in MGC-803 and SGC-7901 cells by siRNA and overexpressed SALL4 in HGC-27 and AGS cells by plasmid transfection. The efficiency of gene silencing and overexpression was verified by qRT-PCR and western blot (Fig. 1a, b). Then, we compared glucose uptake, lactate production, lactate dehydrogenase activity and ATP level in gastric cancer cells with SALL4 knockdown or overexpression. As shown in Fig. 1c, d, SALL4 knockdown decreased glucose uptake, lactate production, lactate dehydrogenase activity and ATP level in MGC-803 and SGC-7901 cells. On the contrary, SALL4 overexpression increased glucose uptake, lactate production, lactate dehydrogenase activity and ATP level in HGC-27 and AGS cells (Fig. 1e, f). Taken together, these findings indicate that SALL4 regulates glycolysis in gastric cancer cells.
Fig. 1.
SALL4 gene silencing suppresses while gene overexpression promotes the glycolytic phenotype in gastric cancer cells. a, b The efficiency of SALL4 knockdown and overexpression was verified by qRT-PCR and western blot. c, d The levels of glucose uptake, lactate production, lactate dehydrogenase activity, and ATP level in control and SALL4 knockdown MGC-803 and SGC-7901 cells. e, f The changes in levels of glucose uptake, lactate production, lactate dehydrogenase activity and ATP level in control and SALL4 overexpressing HGC-27 and AGS cells. Vertical bars represented SD of the mean values (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001
SALL4 regulates the expression of glycolytic genes in gastric cancer cells
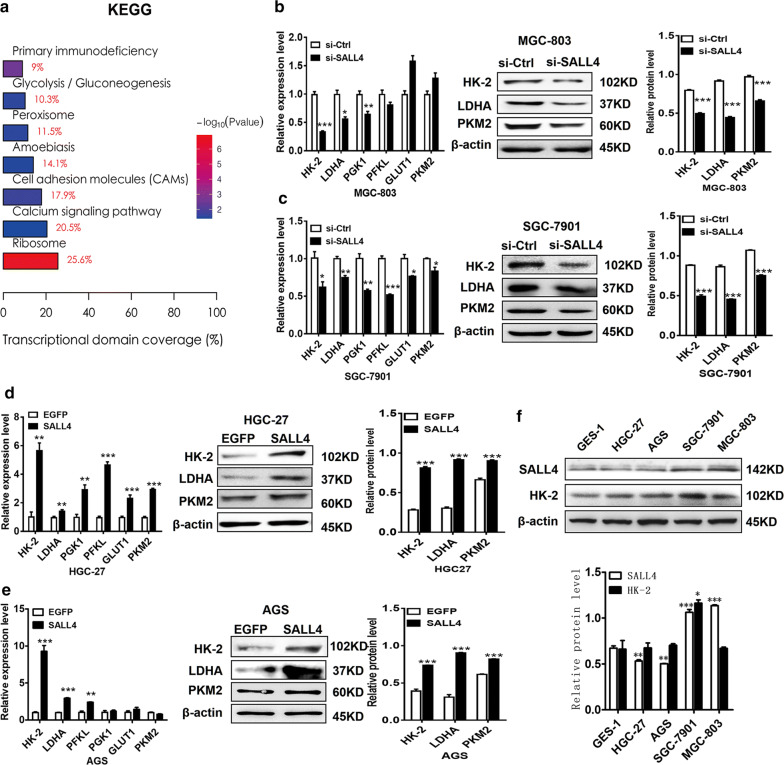
To identify the downstream genes and signaling pathway regulated by SALL4, we have previously performed a microarray to compare the differentially expressed genes between control and SALL4 knockdown gastric cancer cells [23]. The results of KEGG analyses showed that the differentially expressed genes were associated with cell adhesion (P = 0.02), glycolysis/gluconeogenesis (P = 0.03), and calcium signaling pathway (P = 0.04) (Fig. 2a). The regulation of cell adhesion molecules such as integrin by SALL4 has been previously reported in breast cancer cells [24]. The differentially expressed genes involved in glycolysis/gluconeogenesis include hexokinase, phosphofructokinase, lactate dehydrogenase and enolase, among others. Inspired by the microarray data and the previous studies [6, 25], we then detected the expression of several key glycolytic genes in control, SALL4 knockdown, and SALL4-overexpressing gastric cancer cells, including HK-2, lactate dehydrogenase A (LDHA), phosphofructokinase (PFKL), phosphoglycerate kinase 1 (PGK1), Glucose transporter type 1 (GLUT1), and pyruvate kinase M2 (PKM2). As shown in Fig. 2b, c, SALL4 knockdown reduced the expression levels of HK-2, LDHA and PGK1 genes in MGC-803 and SGC-7901 cells (Fig. 2c). SALL4 knockdown also decreased the expression of HK-2 and LDHA proteins in MGC-803 and SGC-7901 cells (Fig. 2b, c). On the contrary, SALL4 overexpression by plasmid transfection increased the expression of HK-2, LDHA, PFKL genes in HGC-27 cells and AGS cells (Fig. 2d, e). SALL4 overexpression also increased the expression of HK-2 and LDHA proteins in HGC-27 and AGS cells (Fig. 2d, e). The gastric cancer cells with high levels of SALL4 seem to also have increased expression of HK-2 (Fig. 2f). Intriguingly, HK-2 has been previously shown to be downregulated in the microarray data of another study whereby SALL4 is knocked down in hepatocellular carcinoma (HCC) cells [26]. Considering that HK-2 is the first rate-limiting enzyme of glycolysis and it showed the most significant change after SALL4 knockdown and overexpression in gastric cancer cells, we hypothesized that SALL4 might promote glycolysis through the regulation of HK-2 in gastric cancer cells.
Fig. 2.
SALL4 regulates the expression of glycolytic genes in gastric cancer cells. a The affected signaling pathways between sh-Ctrl- and sh-SALL4-transfected MGC-803 cells were determined by KEGG analysis. b, c The expression of glycolytic related genes and proteins in control and SALL4 knockdown MGC-803 and SGC-7901 cells was detected by qRT-PCR and western blot. d, e The expression of glycolytic related genes and proteins in control and SALL4 overexpressing HGC-27 and AGS cells was detected by qRT-PCR and western blot. f. The protein levels of SALL4 and HK-2 in GES-1, HGC-27, AGS, SGC-7901 and MGC-803 cells were detected by western blot. Vertical bars represented SD of the mean values (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001
HK-2 is identified as a downstream target of SALL4
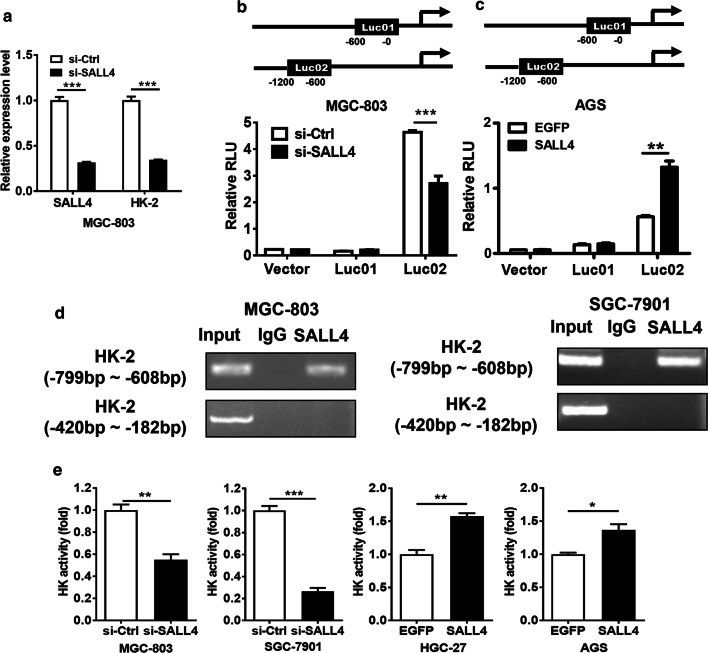
In consistent with the microarray data, our qRT-PCR results confirmed that HK-2 expression was decreased in SALL4 knockdown MGC-803 cells (Fig. 3a). To test whether SALL4 transcriptionally regulates HK-2 gene expression, we searched up to approximately 1200 bp of the promoter region of HK-2 gene for putative SALL4 binding sites and constructed two promoter luciferase reporters. The results of luciferase reporter assay showed that SALL4 knockdown downregulated while SALL4 overexpression upregulated the luciferase activity of HK-2 gene promoter (Fig. 3b, c). The -1200 to -600 bp region in HK-2 gene promoter was critical for SALL4-mediated transactivation. We then performed ChIP assays to test the binding of SALL4 protein to HK-2 gene promoter in MGC-803 and SGC-7901 cells. ChIP assay results showed that SALL4 could bind to the -799 to -608 bp region of the promoter of HK-2 gene (Fig. 3d). There was no binding between SALL4 and the -420 to -182 bp region of the promoter of HK-2 gene (Fig. 3d). Consistent with these results, we observed a decrease of hexokinase activity in SALL4 knockdown MGC-803 and SGC-7901 cells and an increase of hexokinase activity in SALL4- overexpressing HGC-27 and AGS cells (Fig. 3e). Taken together, these results suggest that SALL4 regulates the expression and activity of HK-2 in gastric cancer cells.
Fig. 3.
HK-2 is identified as a downstream target of SALL4. a Verification of microarray results for differentially expressed genes between control and SALL4 knockdown MGC-803 cells. b, c Luciferase reporter assays for the relative luciferase activity of HK-2 gene promoter. d ChIP assay for the binding of SALL4 to the promoter region of HK-2 gene. e The changes in levels of hexokinase activity in control and SALL4 knockdown and overexpressing gastric cancer cells. Vertical bars represented SD of the mean values (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001
HK-2 inhibition abrogates the induction of glycolysis in gastric cancer cells by SALL4 overexpression
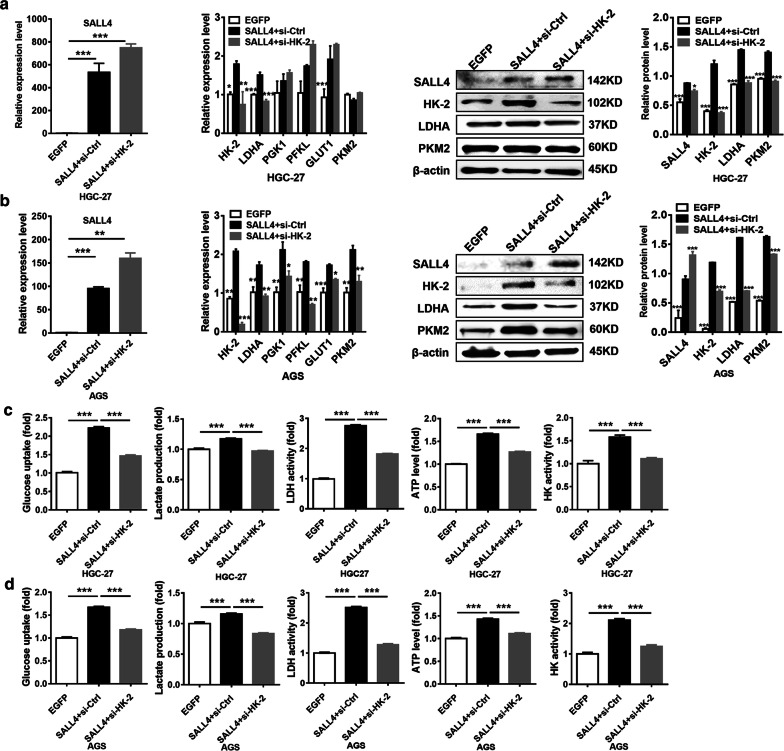
To further confirm that HK-2 is a direct target of SALL4, we determined the effect of SALL4 on glycolysis in HK-2 knockdown HGC-27 and AGS cells. We co-transfected HGC-27 and AGS cells with SALL4-overexpressing plasmid and HK-2 siRNA. As shown in Fig. 4a, b, the upregulation of HK-2 by SALL4 was reversed by HK-2 interference. Consistent with these results, SALL4 overexpression could not further increase the levels of glycolysis, including glucose uptake, lactate production, lactate dehydrogenase activity, ATP level and hexokinase activity in HGC-27 and AGS cells when HK-2 expression was interfered (Fig. 4c, d). These results indicate that HK-2 inhibition abrogates the promotion of glycolysis by SALL4 in gastric cancer cells.
Fig. 4.
HK-2 mediates the promotion of glycolysis by SALL4 in gastric cancer cells. a, b qRT-PCR and western blot analyzed the expression of SALL4, HK-2 and glycolytic related genes and proteins in SALL4-overexpressing HGC-27 cells and AGS cells with or without HK-2 knockdown. c, d The levels of glucose uptake, lactate production, lactate dehydrogenase activity, ATP level, and hexokinase activity in SALL4-overexpressing HGC-27 cells and AGS cells with or without HK-2 knockdown. Vertical bars represented SD of the mean values (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001
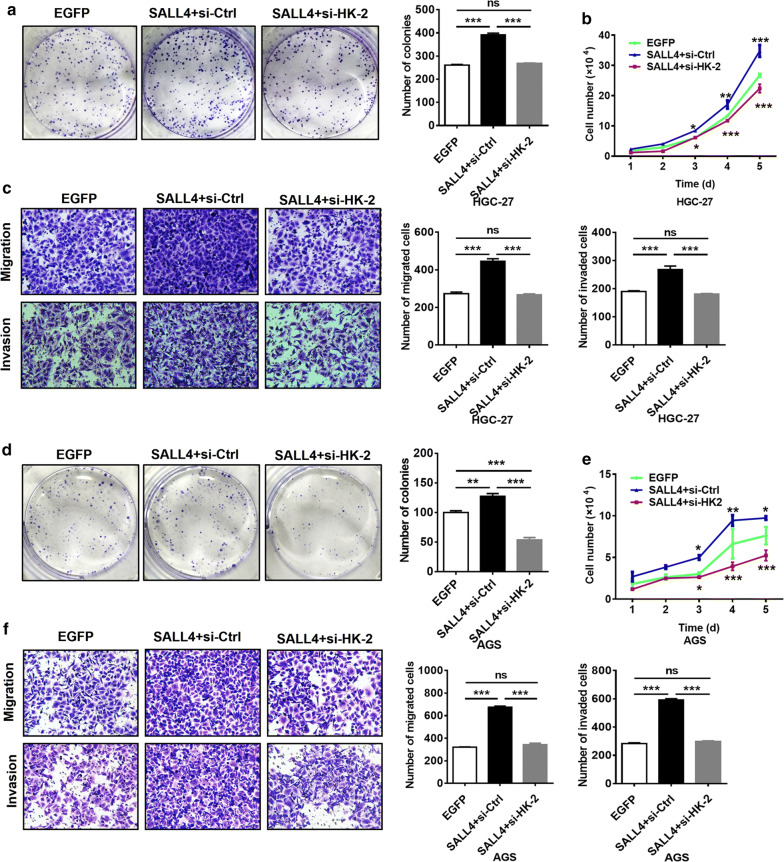
HK-2 inhibition disturbs the promotion of gastric cancer cell proliferation, migration and invasion by SALL4 overexpression
Glycolysis is closely related to many malignant behaviors of tumor cells. The previous studies have shown that SALL4 overexpression promotes gastric cancer cell proliferation, migration, and invasion [21–23]. Therefore, we wanted to know whether HK-2 is related to these effects of SALL4. We overexpressed SALL4 in HGC-27 and AGS cells in the presence or absence of HK-2 interference. We found that the proliferation, migration and invasion abilities of gastric cancer cells, which had been enhanced by SALL4 overexpression, were weakened after interfering with HK-2 expression (Fig. 5). Therefore, SALL4 may maintain the malignant phenotypes of gastric cancer cells by regulating HK-2-mediated glycolysis.
Fig. 5.
HK-2 mediates the promoting role of SALL4 in gastric cancer cells proliferation, migration and invasion. a, d Cell colony formation assay for the growth ability of SALL4-overexpressing HGC-27 and AGS cells with or without HK-2 knockdown. b, e Cell counting assay for the growth ability of SALL4-overexpressing HGC-27 and AGS cells with or without HK-2 knockdown. c, f Transwell migration and matrigel invasion assays for the migration and invasion abilities of SALL4-overexpressing HGC-27 and AGS cells with or without HK-2 knockdown. Vertical bars represented SD of the mean values (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001
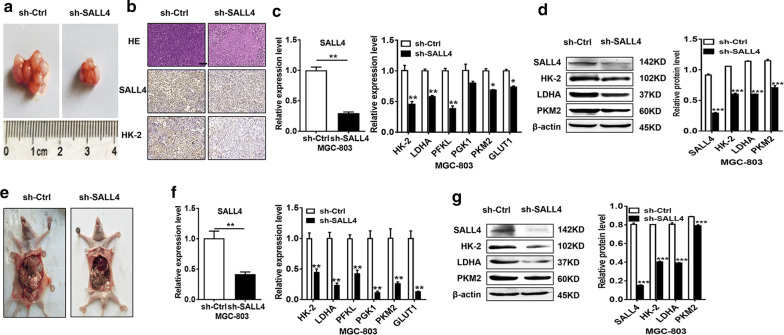
SALL4 knockdown inhibits gastric cancer growth and metastasis though the downregulation HK-2
To verify whether the regulation of HK-2 by SALL4 is critical for gastric carcinogenesis, we established subcutaneous tumor-bearing and peritoneal metastasis tumor mouse models by using control and SALL4 knockdown gastric cancer cells. Consistent with our previous reports, SALL4 knockdown inhibited tumor growth in both models (Fig. 6a, e). Tumor tissues from control and SALL4 knockdown groups were collected for the detection of gene and protein expression. The results of qRT-PCR, western blot, and immunohistochemistry showed that HK-2 expression was decreased in SALL4 knockdown group compared to control group in both models (Fig. 6b, c, d, f and g), suggesting that SALL4 knockdown decreases HK-2 expression, which in turn suppresses glycolysis in gastric cancer cells and disturbs cancer progression.
Fig. 6.
SALL4 knockdown inhibits glycolysis and suppresses gastric cancer growth and metastasis though the downregulation HK-2 in vivo. a, e Representative images of tumors from mice injected with control and SALL4 knockdown MGC-803 cells subcutaneously and intraperitoneally (n = 5 per group). b Representative images of HE staining and immunohistochemical staining of SALL4 and HK-2. c, d, f, g qRT-PCR and western blot analyses of glycolysis-related genes and proteins in tumors from mice injected with control and SALL4 knockdown MGC-803 cells. Vertical bars represented SD of the mean values (n = 3); *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar: 50 μm
Discussion
Aerobic glycolysis is the main form of energy metabolism in tumor cells [27, 28] and is closely related to cancer development and progression [29, 30]. In recent years, increasing studies suggest that the aberrant activation of multiple signaling pathways [31–33] and transcription factors could promote Warburg effect through the regulation of key glycolytic genes such as GLUT1 and LDHA [3–7]. Increased glycolysis leads to enhanced tumor growth, angiogenesis, metastasis, and drug resistance in various cancers [31, 34, 35]. Moreover, many studies have shown that down-regulation of glycolytic genes or inhibition of glycolysis could suppress tumor growth [36–38]. Increased levels or activities of three rate-limiting enzymes involved in glycolysis, including HK-2, accelerates gastric cancer progression and leads to poor prognosis in cancer patients [39]. In mammals, hexokinase has four subtypes, which are encoded by different genes. HK-2 overexpression is positively related to the high level of tumor glycolysis and the low overall survival of tumor patients [40–42]. In addition to glycolysis, HK-2 is also involved in cell proliferation, apoptosis, and autophagy. For instance, HK-2 could bind to and interact with mitochondrial voltage-dependent anion channels, stabilize the mitochondrial membrane, prevent pro-apoptotic factors from binding to it, and thus inhibit apoptosis [43], suggesting a critical role of HK-2 in caner development and progression through both glycolysis-dependent and -independent mechanisms.
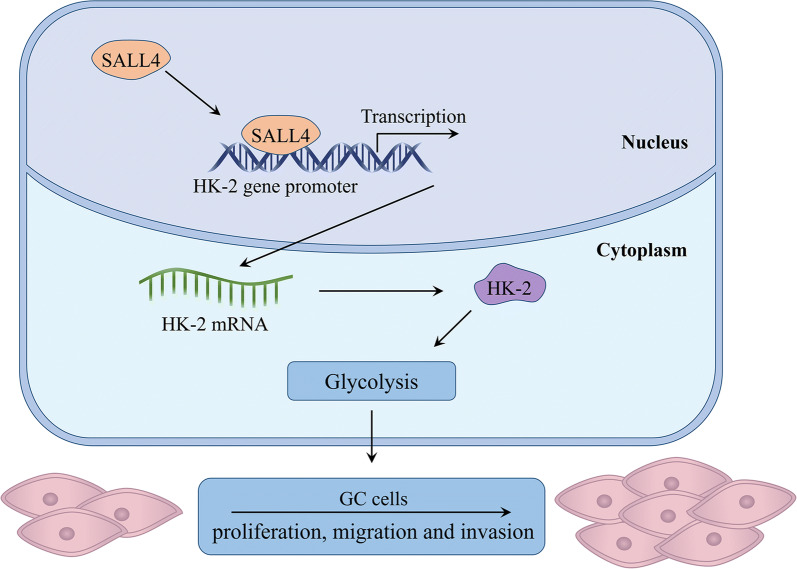
HK-2 expression is regulated at both transcriptional and post-transcriptional levels [44–46]. Several studies have shown that c-Myc binds to the regulatory region of HK-2 gene and plays a pivotal role in glucose metabolism. The transcription factor BACH1 could activate HK-2 transcription and increase glucose uptake, glycolytic rate, and lactate secretion, thereby stimulating glycolysis-dependent metastasis of human lung cancer cells. In epithelial ovarian cancer, FOXM1 promotes reprogramming of glucose metabolism in cancer cells via activation of HK-2 and GLUT1 transcription [25]. The regulation of HK-2 by miRNAs has also been widely reported [36, 41]. In this study, we found that SALL4 could also regulate the transcription of HK-2, thereby promoting glycolysis and gastric cancer progression (Fig. 7). The transcription factor SALL4 has been extensively studied in human cancers. Increasing evidence suggest that SALL4 promotes tumor growth, metastasis, and therapy resistance through the regulation of c-Myc [13], TGF-β1 [23], ATP-binding cassette (ABC) [15], among others. Li et al. demonstrate that SIX1 interacts with histone acetyltransferase HBO1 and AIB1 to induce the expression of glycolytic genes and enhance glycolysis, which ultimately promotes cell malignant transformation and cancer development [6]. Since the consensus binding sequence for SALL4 has not been identified, we analyzed the promoter region of HK-2 gene and found that SALL4 could bind to the -799 to -608 bp region of the promoter of HK-2 gene. Thus, SALL4 may bind to this region and recruit other factors (such as HDACs) to enter into this site, opening the chromatin structure of HK-2 gene and initiating transcription. In addition, this region harbors the binding sites for other transcription factors such as Oct4 and Sox2. Whether other factors cooperate with SALL4 to regulate HK-2 transcription warrants further investigation.
Fig. 7.
The proposed model for SALL4 regulation of glycolysis in gastric cancer. SALL4 upregulates HK-2 transcription and induces glycolysis in a HK-2 dependent manner, thus promoting the proliferation, migration and invasion of gastric cancer cells
Accumulating studies suggest that oncogenes promote gastric cancer progression through glycolysis. For example, AhpC/TSA antioxidant enzyme domain containing 1 (AAED1) enhances the proliferation of gastric cancer cells by promoting glycolysis [47]. Kim et al. demonstrate that SALL4 could induce drug resistance by promoting glycolysis [18]. We found that SALL4 promoted glycolysis by enhancing the expression of HK-2 and interfere with HK-2 expression inhibited the promoting role of SALL4 in gastric cancer cell proliferation, migration and invasion. Shi et al. demonstrate that B7-H3 promotes aerobic glycolysis by promoting HK-2 expression and HK-2 is a key mediator of B7-H3-induced chemoresistance [37]. In consistence with these findings, our results showed that SALL4, at least in part, promoted gastric cancer progression by regulating HK-2-mediated glycolysis. However, it could not be excluded that SALL4 may regulate glycolysis and participate in gastric cancer progression through other mechanisms.
Conclusion
In conclusion, our findings show that SALL4 induces glycolysis via the upregulation of HK-2, thus promoting the proliferation, migration and invasion of gastric cancer cells (Fig. 7). Our study not only reveals a new mechanism for the oncogenic roles of SALL4 in cancer, but also provides evidence for the potential of SALL4 as a therapeutic target for gastric cancer.
Supplementary information
Additional file 1:Table S1. Sequences of shRNA and siRNA
Additional file 2: Table S2. Sequences of PCR primers for target gene detection
Acknowledgements
We would like to acknowledge the reviewers for their helpful comments on this paper.
Abbreviations
- AAED1
AhpC/TSA antioxidant enzyme domain containing 1
- ABC
ATP-binding cassette
- ChIP
Chromatin immunoprecipitation
- Cy3
Cyanine-3-CTP
- FBS
Fetal bovine serum
- GLUT1
Glucose transporter 1
- GO
Gene ontology
- HCC
Hepatocellular carcinoma
- HK-2
Hexokinase II
- HIF-1α
Hypoxia inducible factor-1α
- HP1α
Heterochromatin protein 1α
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LDHA
Lactate dehydrogenase A
- MOI
Multiplicity of infection
- PFKL
6-phosphofructokinase, liver type
- PGK1
Phosphoglycerate kinase 1
- PKM2
Pyruvate kinase M2
- qRT-PCR
Quantitative real-time polymerase chain reaction
- SIX1
Sine oculis homeobox 1
Authors’ contributions
XZ and MS conception and design. MS, JZ, HS, RJ, FM, and HQ development of methodology. MS, JZ, HS, and RJ acquisition of data. MS, XZ and WX analysis and interpretation of data. MS and XZ writing, review, and revision of the manuscript. HQ, WX, and XZ administrative, technical, or material support. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81972310, 81672416), Major Natural Science Reasearch Project for Universities in Jiangsu Province (18KJA320001), Key Laboratory of Molecular Diagnostics and Precision Medicine for Surgical Oncology in Gansu Province (2019GSZDSYS01), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data and materials
All data generated or analyzed during this study are included in this article.
Ethics approval and consent to participate
Animal care was according to the Animal Use and Care Committee of Jiangsu University.
Competing interests
We have no competing interests to be declared.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12935-020-01275-y.
References
- 1.Lu J. The Warburg metabolism fuels tumor metastasis. Cancer Metastasis Rev. 2019;38(1–2):157–164. doi: 10.1007/s10555-019-09794-5. [DOI] [PubMed] [Google Scholar]
- 2.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McDonald PC, Chafe SC, Brown WS, Saberi S, Swayampakula M, Venkateswaran G, et al. Regulation of pH by carbonic anhydrase 9 mediates survival of pancreatic cancer cells with activated KRAS in response to hypoxia. Gastroenterology. 2019;157(3):823–837. doi: 10.1053/j.gastro.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Sowa T, Menju T, Chen-Yoshikawa TF, Takahashi K, Nishikawa S, Nakanishi T, et al. Hypoxia-inducible factor 1 promotes chemoresistance of lung cancer by inducing carbonic anhydrase IX expression. Cancer Med. 2017;6(1):288–297. doi: 10.1002/cam4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo P, Zhang C, Liao F, Chen L, Liu Z, Long L, et al. Transcriptional positive cofactor 4 promotes breast cancer proliferation and metastasis through c-Myc mediated Warburg effect. Cell Commun Signal. 2019;17(1):36. doi: 10.1186/s12964-019-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, et al. Transcriptional regulation of the Warburg effect in cancer by SIX1. Cancer Cell. 2018;33(3):368-385 e367. doi: 10.1016/j.ccell.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Nie ZY, Liu XJ, Zhan Y, Liu MH, Zhang XY, Li ZY, et al. miR-140-5p induces cell apoptosis and decreases Warburg effect in chronic myeloid leukemia by targeting SIX1. Biosci Rep. 2019; 39(4). [DOI] [PMC free article] [PubMed]
- 8.Lim CY, Tam WL, Zhang J, Ang HS, Jia H, Lipovich L, et al. Sall4 regulates distinct transcription circuitries in different blastocyst-derived stem cell lineages. Cell Stem Cell. 2008;3(5):543–554. doi: 10.1016/j.stem.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Tam WL, Tong GQ, Wu Q, Chan HY, Soh BS, et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of Pou5f1. Nat Cell Biol. 2006;8(10):1114–1123. doi: 10.1038/ncb1481. [DOI] [PubMed] [Google Scholar]
- 10.Miettinen M, Wang Z, McCue PA, Sarlomo-Rikala M, Rys J, Biernat W, et al. Sall4 expression in germ cell and non-germ cell tumors: a systematic immunohistochemical study of 3215 cases. Am J Surg Pathol. 2014;38(3):410–420. doi: 10.1097/PAS.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ueno S, Lu J, He J, Li A, Zhang X, Ritz J, et al. Aberrant expression of SALL4 in acute B cell lymphoblastic leukemia: mechanism, function, and implication for a potential novel therapeutic target. Exp Hematol. 2014;42(4):307–316 e308. doi: 10.1016/j.exphem.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui W, Kong NR, Ma Y, Amin HM, Lai R, Chai L. Differential expression of the novel oncogene, SALL4, in lymphoma, plasma cell myeloma, and acute lymphoblastic leukemia. Mod Pathol. 2006;19(12):1585–1592. doi: 10.1038/modpathol.3800694. [DOI] [PubMed] [Google Scholar]
- 13.Li A, Jiao Y, Yong KJ, Wang F, Gao C, Yan B, et al. Sall4 is a new target in endometrial cancer. Oncogene. 2015;34(1):63–72. doi: 10.1038/onc.2013.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Xu Z, Xu X, Zhang B, Wu H, Wang M, et al. Sall4, a novel marker for human gastric carcinogenesis and metastasis. Oncogene. 2014;33(48):5491–5500. doi: 10.1038/onc.2013.495. [DOI] [PubMed] [Google Scholar]
- 15.Oikawa T, Kamiya A, Zeniya M, Chikada H, Hyuck AD, Yamazaki Y, et al. Sal-like protein 4 (SALL4), a stem cell biomarker in liver cancers. Hepatology. 2013;57(4):1469–1483. doi: 10.1002/hep.26159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang X, Yuan X, Zhu W, Qian H, Xu W. Sall4: an emerging cancer biomarker and target. Cancer Lett. 2015;357(1):55–62. doi: 10.1016/j.canlet.2014.11.037. [DOI] [PubMed] [Google Scholar]
- 17.Xiong J, Todorova D, Su NY, Kim J, Lee PJ, Shen Z, et al. Stemness factor SALL4 is required for DNA damage response in embryonic stem cells. J Cell Biol. 2015;208(5):513–520. doi: 10.1083/jcb.201408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim J, Xu S, Xiong L, Yu L, Fu X, Xu Y. Sall4 promotes glycolysis and chromatin remodeling via modulating HP1alpha-Glut1 pathway. Oncogene. 2017;36(46):6472–6479. doi: 10.1038/onc.2017.265. [DOI] [PubMed] [Google Scholar]
- 19.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 20.Sitarz R, Skierucha M, Mielko J, Offerhaus GJA, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan X, Zhang X, Zhang W, Liang W, Zhang P, Shi H, et al. Sall4 promotes gastric cancer progression through activating CD44 expression. Oncogenesis. 2016;5(11):e268. doi: 10.1038/oncsis.2016.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan L, Liang W, Gu J, Zang X, Huang Z, Shi H, et al. Long noncoding RNA DANCR is activated by SALL4 and promotes the proliferation and invasion of gastric cancer cells. Oncotarget. 2018;9(2):1915–1930. doi: 10.18632/oncotarget.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Zhang P, Shao M, Zang X, Zhang J, Mao F, et al. Sall4 activates TGF-beta/SMAD signaling pathway to induce EMT and promote gastric cancer metastasis. Cancer Manag Res. 2018;10:4459–4470. doi: 10.2147/CMAR.S177373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Itou J, Tanaka S, Li W, Iida A, Sehara-Fujisawa A, Sato F, et al. The sal-like 4–integrin alpha6beta1 network promotes cell migration for metastasis via activation of focal adhesion dynamics in basal-like breast cancer cells. Biochim Biophys Acta Mol Cell Res. 2017;1864(1):76–88. doi: 10.1016/j.bbamcr.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Cui J, Shi M, Xie D, Wei D, Jia Z, Zheng S, et al. FOXM1 promotes the Warburg effect and pancreatic cancer progression via transactivation of ldha expression. Clin Cancer Res. 2014;20(10):2595–2606. doi: 10.1158/1078-0432.CCR-13-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yong KJ, Gao C, Lim JS, Yan B, Yang H, Dimitrov T, et al. Oncofetal gene SALL4 in aggressive hepatocellular carcinoma. N Engl J Med. 2013;368(24):2266–2276. doi: 10.1056/NEJMoa1300297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Cairns RA, Harris IS, Mak TW. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11(2):85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 29.Hasanpourghadi M, Looi CY, Pandurangan AK, Sethi G, Wong WF, Mustafa MR. Phytometabolites targeting the Warburg effect in cancer cells: a mechanistic review. Curr Drug Targets. 2017;18(9):1086–1094. doi: 10.2174/1389450117666160401124842. [DOI] [PubMed] [Google Scholar]
- 30.Poff A, Koutnik AP, Egan KM, Sahebjam S, D’Agostino D, Kumar NB. Targeting the Warburg effect for cancer treatment: ketogenic diets for management of glioma. Semin Cancer Biol. 2019;56:135–148. doi: 10.1016/j.semcancer.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qian X, Xu W, Xu J, Shi Q, Li J, Weng Y, et al. Enolase 1 stimulates glycolysis to promote chemoresistance in gastric cancer. Oncotarget. 2017;8(29):47691–47708. doi: 10.18632/oncotarget.17868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu H, Pan L, Gao C, Xu H, Li Y, Zhang L, et al. Quercetin inhibits the proliferation of glycolysis-addicted HCC cells by reducing hexokinase 2 and Akt-mTOR pathway. Molecules. 2019;24(10):1993. doi: 10.3390/molecules24101993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wei J, Wu J, Xu W, Nie H, Zhou R, Wang R, et al. Salvianolic acid B inhibits glycolysis in oral squamous cell carcinoma via targeting PI3k/AKT/HIF-1alpha signaling pathway. Cell Death Dis. 2018;9(6):599. doi: 10.1038/s41419-018-0623-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP, et al. Reversal of Warburg effect and reactivation of oxidative phosphorylation by differential inhibition of EGFR signaling pathways in non-small cell lung cancer. Clin Cancer Res. 2015;21(22):5110–5120. doi: 10.1158/1078-0432.CCR-15-0375. [DOI] [PubMed] [Google Scholar]
- 35.Ma S, Jia R, Li D, Shen B. Targeting cellular metabolism chemosensitizes the doxorubicin-resistant human breast adenocarcinoma cells. Biomed Res Int. 2015;2015:453986. doi: 10.1155/2015/453986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Gao F, Ma X, Wang R, Dong X, Wang W. Deguelin inhibits non-small cell lung cancer via down-regulating hexokinases ii-mediated glycolysis. Oncotarget. 2017;8(20):32586–32599. doi: 10.18632/oncotarget.15937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, et al. B7-H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10(4):308. doi: 10.1038/s41419-019-1549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu S, Zhou T, Doh HM, Trinh KR, Catapang A, Lee JT, et al. An HK2 antisense oligonucleotide induces synthetic lethality in HK1(-)HK2(+) multiple myeloma. Cancer Res. 2019;79(10):2748–2760. doi: 10.1158/0008-5472.CAN-18-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tao L, Yu H, Liang R, Jia R, Wang J, Jiang K, et al. Rev-erbalpha inhibits proliferation by reducing glycolytic flux and pentose phosphate pathway in human gastric cancer cells. Oncogenesis. 2019;8(10):57. doi: 10.1038/s41389-019-0168-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu F, Yan JJ, Gan Y, Chang Y, Wang HL, He XX, et al. miR-885-5p negatively regulates Warburg effect by silencing hexokinase 2 in liver cancer. Mol Ther Nucleic Acids. 2019;18:308–319. doi: 10.1016/j.omtn.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu W, Li W, Liu H, Yu X. Xanthohumol inhibits colorectal cancer cells via downregulation of hexokinases II-mediated glycolysis. Int J Biol Sci. 2019;15(11):2497–2508. doi: 10.7150/ijbs.37481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin G, Wu Y, Cai F, Li Z, Su S, Wang J, et al. Matrine promotes human myeloid leukemia cells apoptosis through Warburg effect mediated by hexokinase 2. Front Pharmacol. 2019;10:1069. doi: 10.3389/fphar.2019.01069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim S, Jang JY, Koh J, Kwon D, Kim YA, Paeng JC, et al. Programmed cell death ligand-1-mediated enhancement of hexokinase 2 expression is inversely related to t-cell effector gene expression in non-small-cell lung cancer. J Exp Clin Cancer Res. 2019;38(1):462. doi: 10.1186/s13046-019-1407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu L, Chen X, Wang L, Chen S. The sweet trap in tumors: aerobic glycolysis and potential targets for therapy. Oncotarget. 2016;7(25):38908–38926. doi: 10.18632/oncotarget.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Wang Y, Bai R, Yang K, Tian Z. miR-186 inhibited aerobic glycolysis in gastric cancer via HIF-1alpha regulation. Oncogenesis. 2016;5:e224. doi: 10.1038/oncsis.2016.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li LQ, Yang Y, Chen H, Zhang L, Pan D, Xie WJ. MicroRNA-181b inhibits glycolysis in gastric cancer cells via targeting hexokinase 2 gene. Cancer Biomark. 2016;17(1):75–81. doi: 10.3233/CBM-160619. [DOI] [PubMed] [Google Scholar]
- 47.Zhang B, Wu J, Cai Y, Luo M, Wang B, Gu Y. AAED1 modulates proliferation and glycolysis in gastric cancer. Oncol Rep. 2018;40(2):1156–1164. doi: 10.3892/or.2018.6478. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1:Table S1. Sequences of shRNA and siRNA
Additional file 2: Table S2. Sequences of PCR primers for target gene detection
Data Availability Statement
All data generated or analyzed during this study are included in this article.