Abstract
Background
The association of Helicobacter pylori (H. pylori) babA2 gene with gastric cancer (GC) was reported by several studies, but results were inconsistent. This meta-analysis was performed to investigate the relationship between H. pylori babA2 gene and GC risk.
Methods
Case-control studies involving the association between H. pylori babA2 gene and GC risk were systematically identified from PubMed databases. A meta-analysis was used to pool studies and to estimate odds ratios (ORs) with 95% confidence intervals (CIs) of H. pylori babA2 gene associated with GC risk.
Results
Twenty studies were identified with a total of 1289 GC cases and 1081 controls. H. pylori babA2 gene was associated with an increased risk of GC by 2.05 fold (95% CI, 1.30–3.24, P = 0.002). In subgroup analysis, we found that H. pylori babA2 gene was significantly associated with GC risk in Asian population (OR = 2.63, 95% CI: 1.36–5.09 P = 0.004) but not in South American population (OR = 1.35, 95% CI: 0.69–2.64, P = 0.379).
Conclusions
This meta-analysis indicates that H. pylori babA2 gene may be associated with increased risk of GC, especially in Asian population.
Keywords: Helicobacter pylori, babA2 gene, Gastric cancer, Meta-analysis
Background
Gastric Cancer (GC) is the fifth most common cancer and the third leading cause of mortality worldwide [1–3], with approximately 42.5% of all cases diagnosed in China [4, 5]. About 1 million incident cases of GC are annually projected, with the majority observed in Eastern Asia, Latin America and Eastern Europe [5]. In 2015, GC was the second most common cancer with about 6,791,000 new cases in China [4]. Genetic and environment factors are involved in GC development. H. pylori infection, cigarette smoking, low intake of fresh vegetables and fruits and salty foods are main risk factors of GC [6].
Helicobacter pylori (H. pylori) infection is the most common human infections inhabiting in the stomach. It is a gram-negative bacterium, which has epigenetic effects on gastric epithelial cells and indirect inflammatory response on the gastric mucosa [7]. Some studies showed that H. pylori alone or associated gene were strongly associated with gastric cancer risk [8–10]. According to the International Agency for Research on Cancer, H. pylori was defined as a class I carcinogen [11]. However, only a small fraction of infected patients develop severe diseases [12]. H. pylori is well distinguished to have a high level of genetic variations allowing it to be adapted to the host gastric epithelium [13]. It is well described that different strains of H. pylori showed different degrees of virulence [14–16]. H. pylori strains harboring the cytotoxin-associated antigen (cagA) and the vacuolating toxin A (vacA) have been considered as risk factors for GC [15, 17, 18]. The OipA, one of porin proteins associated with severe neutrophil infiltration in IL-8 induction and gastric colonization [19], was also associated with GC risk [20–22].
The blood-group antigen-binding adhesin (babA) encoded by babA2 gene is a major adhesin on the outer membrane of H. pylori. BabA2 is characterized to be an active gene in the binding activity of Lewis-b blood group antigen on gastric epithelium and host cell and determine H. pylori colonization density [23, 24]. The sequence of the three babA gene alleles have been identified (babA1, babA2 and babB), but only the babA2 is involve d in Lewis-b binding activity. To date, several studies have evaluated the effect of H. pylori babA2 gene on risk of GC [19, 25–42], but the results are conflicting possibly due to small sample size of single studies. In the present study, we conducted a meta-analysis to assess the association between H. pylori babA2 gene and GC risk based on published cases-control studies.
Methods
Search strategies
All relevant studies were identified from PubMed databases. The search strategy included the terms (“babA2” OR “antigen-binding adhesion gene”) AND (“Helicobacter pylori” OR “H. pylori infection”) AND (“genotype” OR “polymorphism”) AND (“gastric cancer” OR “stomach cancer”) in any text field of the database. In addition, we also collected additional studies from references of original and review articles.
Inclusion and exclusion criteria
Inclusion criteria to select studies for this meta-analysis were as follows: (1) study describing the relationship between H. pylori babA2 gene and GC, (2) studies that provided babA2 positive frequencies, (3) studies published in English with full text available. Exclusion criteria were as follow: (1) insufficient data to calculate OR and 95% CI, (2) vivo or experimental studies, and (3) meta-analysis or review studies.
Data extraction
Data were extracted from each study independently by two investigators and contradictions between them were discussed to obtain agreement. The following information’s were collected: first author’s name, year of publication, country, ethnicity, sample size, type of study, source of sample, study quality assessment, babA2 positive frequencies, OR estimation and 95% CI for the association between H. pylori babA2 gene and GC.
Quality score assessment
Quality score of each included study was assessed by the same two authors independently using the Newcastle-Ottawa Quality Assessment Scale (NOS) for case-control studies [43]. The NOS is a validated quality assessment for case-control studies with three parameters for quality: selection, comparability and exposure. The maximum score of each parameter is 4 for selection, 2 for comparability and 3 for exposure.
Statistical analysis
The pooled ORs with 95% CIs were used to indicate the effect of H. pylori babA2 gene effect on GC risk. χ2 base on Q test and I2 statistics were used to evaluate the statistical heterogeneity among included studies. The fixed-effects model was used when there was no significant heterogeneity (P ≥ 0.10 and I2 ≤ 50%) [44, 45] between studies, otherwise the random effect model was applied to provide more conservative estimates [46]. In addition, we performed subgroup analysis by ethnicity and quality score assessment. Ethnicities were divided into Asian, European, South American and North American. Moreover, sensitivity analyses were performed to estimate the effect of each included study on overall effect. We used Begg’s test and Egger’s test to estimate publication bias [47]. All the statistical analyses were performed using STATA 11.0.
Results
Characteristics of selected studies
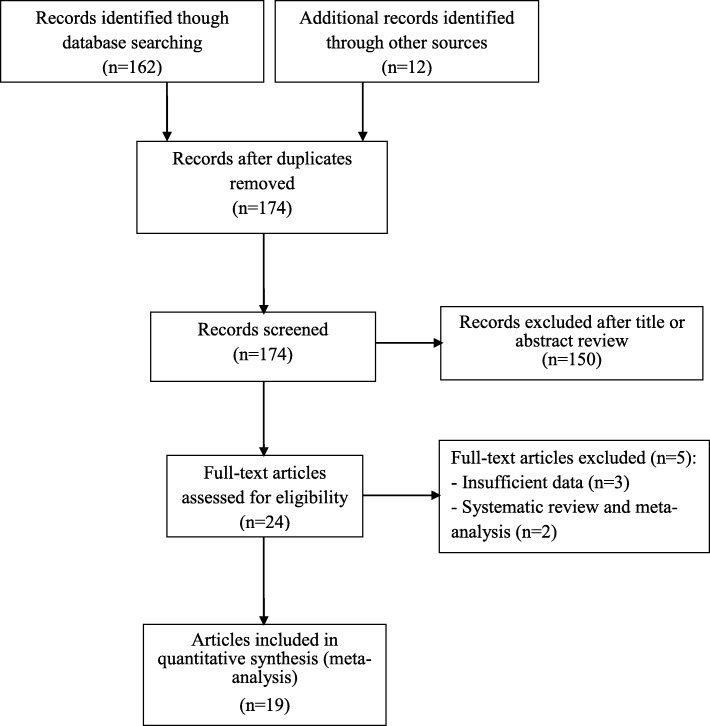
Literature research strategy is detailed in Fig. 1. There were 174 potentially relevant studies. After title and abstract evaluation, 24 articles with full-text assessment were included when duplicated studies were excluded. After full-text reviewed, a total of 19 eligible articles were included in this meta-analysis, and 5 articles were excluded because of the following reasons: two articles were reviews [48, 49], and three articles had insufficient data [50–52]. One article included participants from two countries [19], which were considered as two independent studies for subsequent data extraction and meta-analysis. Among 20 studies, 5 were from South American population [19, 26, 32, 34, 40], 13 from Asian population [25, 27–31, 33, 35–39, 41], one from North American population [19] and one from European population [42] (Table 1).
Fig. 1.
The flowchart of literature search and study inclusion
Table 1.
Characteristics of included studies
| First Author | Year | Country | Ethnicity | Control | Sample Size (case/control) | OR (95%CI) | Quality Assessment |
|---|---|---|---|---|---|---|---|
| Gerhard | 1999 | Germany | European | Gastritis | 39/23 | 3.31 (1.07–10.17) | 5 |
| Mizushima | 2001 | Japan | Asian | NUD | 70/12 | 2.12 (0.58–7.68) | 5 |
| Yamaoka | 2002 | United States | North American | Gastritis | 47/23 | 0.74 (0.27–2.02) | 5 |
| Yamaoka | 2002 | Colombia | South American | Gastritis | 62/19 | 2.08 (0.72–6.00) | 5 |
| Oliveira | 2003 | Brazil | South American | Gastritis | 53/75 | 2.73 (1.32–5.67) | 7 |
| Han | 2004 | Shanghai | Asian | Chronic gastritis | 40/24 | 0.71 (0.25–2.08) | 6 |
| Lee | 2006 | South korea | Asian | Routine gastoscopy | 98/136 | 3.98 (1.94–8.15) | 7 |
| Chomvarin | 2007 | Thailand | Asian | NUG | 72/6 | 1.32 (0.14–12.13) | 6 |
| Zhang | 2008 | China | Asian | Gastritis | 143/69 | 1.07 (0.59–1.94) | 6 |
| Erzin | 2008 | Turkey | Asian | NUD | 36/34 | 31.07 (8.22–117.52) | 6 |
| Bartchewsky | 2009 | Brazil | South American | Gastritis | 142/38 | 0.96 (0.44–2.12) | 6 |
| Safaei | 2010 | Iran | Asian | CAG | 38/16 | 1.87 (0.35–9.96) | 5 |
| Mattar | 2010 | Brazil | South American | Gastritis | 36/32 | 0.38 (0.14–1.02) | 5 |
| Saxena | 2011 | India | Asian | NUD | 45/123 | 1.12 (0.49–2.57) | 7 |
| Abadi | 2011 | Iran | Asian | NUD | 55/50 | 53.65 (11.67–246.69) | 5 |
| Mottaghi | 2014 | Iran | Asian | Chronic gastritis | 60/12 | 0.65 (0.18–2.31) | 6 |
| Abdi | 2016 | Iran | Asian | NAG | 22/61 | 2.81 (1.02–7.74) | 6 |
| Roman-Roman | 2017 | Mexico | South American | Chronic gastritis | 109/282 | 1.93 (0.83–4.50) | 7 |
| Heidari | 2017 | Iran | Asian | Gastritis | 32/22 | 1.12 (0.35–3.57) | 5 |
| Bartpho | 2020 | Thailand | Asian | Chronic gastritis | 90/24 | 7.38 (2.64–20.09) | 6 |
NUD Non-ulcer Dyspepsia, NAG Non-atrophic gastritis, CAG Chronic active gastritis
Meta-analysis
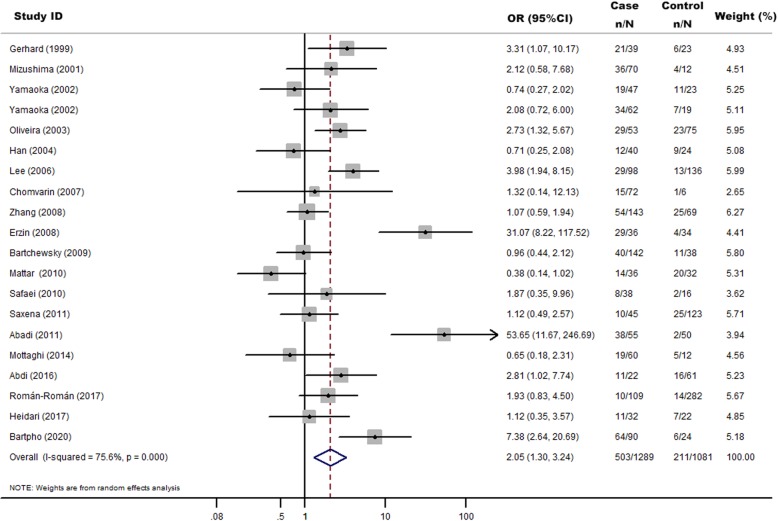
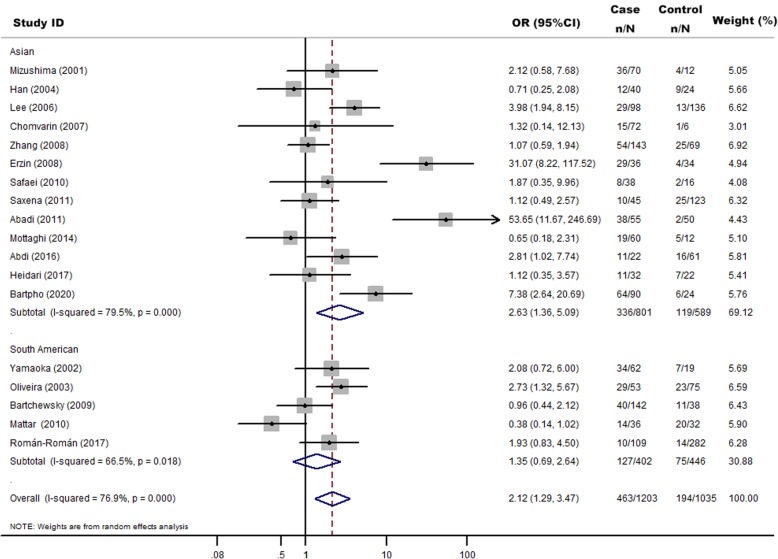
There were 20 studies [19, 25–42] that investigated the association between H. pylori babA2 gene and GC risk. In total, 1289 cases and 1081 controls were included in this meta-analysis (Table 1). The overall proportions of H. pylori babA2 were 39.02% (503/1289) in GC cases and 19.52% (211/1081) in controls. H. pylori babA2 gene was significantly associated with an increased risk of GC (OR = 2.05, 95% CI: 1.30–3.24, P = 0.002) (Fig. 2). In subgroup analysis, we found significant associations in Asian population (OR = 2.63, 95%CI: 1.36–5.09, P = 0.004) but not in South American population (OR = 1.35, 95%CI: 0.69–2.64, P = 0.379) (Fig. 3).
Fig. 2.
Random-effects meta-analysis forest plot of the odds ratio of gastric cancer according to H.pylori infection with babA2 with respect to without babA2. The studies are sorted by publication year. The solid squares are centered on the odds ratio (OR) point estimate from each study, and the horizontal line through each square indicates the 95% confidence interval (CI) for the study. The area of each square represents the magnitude of association, and the horizontal tips of the diamond represent the 95% CI
Fig. 3.
Random-effects meta-analysis forest plot of the odds ratio of gastric cancer according to H.pylori infection with babA2 with respect to without babA2. The studies are divided into Asian and South American according to participants ethnicities and sorted by publication year. The solid squares are centered on the odds ratio (OR) point estimate from each study, and the horizontal line through each square indicates the 95% confidence interval (CI) for the study. The area of each square represents the magnitude of association, and the horizontal tips of the diamond represent the 95% CI
Heterogeneity analysis and quality assessment
Heterogeneity analysis showed a significant high heterogeneity among studies (I2 = 75.6%, P < 0.001). In sub-group analysis by ethnicity, heterogeneity was high for Asian (I2 = 79.5%, P < 0.001) but moderate for South American (I2 = 66.5%, P = 0.018). By exploring the potential sources of the heterogeneity, we found that the studies by Erzin et al. [36] and Abadi et al. [30] showed larger effect estimates (OR = 31.07, 95% CI: 8.22–117.52) [36], and OR = 53.65, 95% CI: 11.67–246.69 [30], respectively), as compared with other studies. According to the Newcastle-Ottawa study quality assessment scale, we found that studies with score of 7 showed a significant association (OR = 2.27, 95% CI: 1.34–3.85). However, we didn’t find significant association among studies with score of 6 or 5 (OR = 2.07, 95% CI: 0.90–4.77 and OR = 2.01, 95% CI: 0.79–5.11, respectively) (Figure S1).
Publication bias and sensibility analysis
Publication bias was evaluated by Begg’s and Egger’s test. The visual inspection of funnel plot revealed that there was no significant evidence of asymmetry distribution. And no significant publication bias was observed based on Begg’s test (P = 0.284) or Egger’s test (P = 0.288) (Figure S2). The impact of each study on the pooled OR was examined by repeating the meta-analysis while excluding individual study, which confirmed the stability of our results (Figure S3).
Discussion
To date, numerous studies have assessed the association between H. pylori babA2 gene and GC risk, but results remained inconsistent. The controversial results of individual studies may be due to relatively small sample size. Meta-analysis is an important approach to pool multiple studies and therefore may result in more precise and robust conclusion. In this meta-analysis, we included 20 studies focusing on H. pylori babA2 gene and GC risk with a total of 1289 patients and 1081 controls. We found that H. pylori babA2 gene was significantly associated with risk of GC. There is evidence that H. pylori increase the risk of GC development through the sequence of atrophy and metaplasia originate from several studies. Chronic H. pylori induced inflammation which can probably lead to loss of normal gastric mucosal, with gastric gland destruction, and replacement by fibrosis [53]. The H. pylori strains virulence factors, host and environmental factors are main factors to contribute in clinical infection manifestations [54]. And it is well showed that gene encoding pathogenic H pylori factors are involved in GC development and colonization properties [29, 49].
The increased GC risk was also associated with co-expression of H pylori vacAs1, cagA and babA2 genes [17, 18, 23, 55]. Furthermore, interaction between host’s immunological defenses and H pylori virulence factors may play an important role in the development of GC [56, 57]. It was showed that babA2 as a virulence marker could predict clinical outcome, which was dependent on the geographic origin of the H. pylori strains [27].
In our study, sub-group analysis according to geographical areas showed that H. pylori babA2 was not significantly associated with the risk of GC among South American. Our results are comparable to previous studies from South America [19, 26, 32, 34, 50]. The difference among populations may be due to the small sample size for each population, heterogeneity between studies and geographical factors. In other hand, H. pylori babA2 gene was closely involved in the risk of GC in Asian population, which was confirmed by original studies from Asian population [29, 30, 36, 38].
Our meta-analysis showed some limitations. Firstly, we didn’t obtain original data, which have limited further evaluation of potential gene-gene and gene-environmental interactions. Secondly, the sample sizes of most included studies are relative small. Thirdly, additional analysis based on other factors, such as age, gender, family history, other virulence factors, environment factors (e.g. alcohol intake, smoking, high BMI) and GC subtypes (e.g. intestinal, diffuse or mixed type), could not be analyzed because of the limited information obtained from included studies. Finally, high heterogeneity among studies indicates that the pooled estimation risk should be interpreted with caution.
Conclusions
Our results suggest that the presence of H. pylori with positive babA2 gene may contribute to increased risk of GC, especially in Asian population. Studies with large sample size are necessary to further elucidate the interaction among environmental factors, bacterial genotype and host factors on GC risk.
Supplementary information
Additional file 1: Figure S1. Sub-group analysis of the association between H. pylori babA2 gene and gastric cancer risk according to study quality assessment. Figure S2. Funnel plot of case–control studies evaluating the association between H. pylori babA2 gene and gastric cancer risk. Each point represents a study to indicate an association. Figure S3. Influence of the summary OR coefficients on the association between H. pylori babA2 gene and gastric cancer risk.
Acknowledgements
Not applicable.
Abbreviations
- babA
Blood-group antigen-binding adhesion
- BMI
Body mass index
- CI
Confidence interval
- GC
Gastric cancer
- H. pylori
Helicobacter pylori
- NOS
Newcastle-Ottawa Scale
- OR
Odds ratio
Authors’ contributions
GJ and MAK conceived and designed the study. MAK extracted and analyzed data, interpreted results, and drafted the manuscript. MAK, JW and TW selected and assessed quality of studies. All authors reviewed the manuscript and approved the submitted version.
Funding
The authors declare that they did not receive funding for this research from any source.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Marce-Amara Kpoghomou and Jinchen Wang contributed equally to this work.
Contributor Information
Marce-Amara Kpoghomou, Email: marceamara@gmail.com.
Jinchen Wang, Email: wjc_njmu@163.com.
Tianpei Wang, Email: wangtianpei1@163.com.
Guanfu Jin, Email: guangfujin@njmu.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-020-06962-7.
References
- 1.Bray F, et al. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132(5):1133–1145. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 2.Bertuccio P, et al. Recent patterns in gastric cancer: a global overview. Int J Cancer. 2009;125(3):666–673. doi: 10.1002/ijc.24290. [DOI] [PubMed] [Google Scholar]
- 3.Sigon R, Canzonieri V, Rossi C. Early gastric cancer: a single-institution experience on 60 cases. Suppl Tumori. 2003;2(5):S23–S26. [PubMed] [Google Scholar]
- 4.Chen W, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 5.Ferlay J, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 6.World Cancer Research Fund International. Diet, nutrition, physical activity and stomach cancer. https://www.wcrf.org/sites/default/files/Stomach-Cancer-2016-Report.pdf. Accessed 24 Dec 2019.
- 7.Cheng XJ, Lin JC, Tu SP. Etiology and prevention of gastric cancer. Gastrointest Tumors. 2016;3(1):25–36. doi: 10.1159/000443995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Correa P, Piazuelo MB. Helicobacter pylori infection and gastric adenocarcinoma. US Gastroenterol Hepatol Rev. 2011;7(1):59–64. [PMC free article] [PubMed] [Google Scholar]
- 9.Wroblewski LE, Peek RM, Wilson KT. Helicobacter pylori and gastric cancer: factors that modulate disease risk. Clin Microbiol Rev. 2010;23(4):713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polk DB, Peek RM. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10(6):403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans Schistosomes, liver flukes and helicobacter pylori. Lyon, 7–14 June 1994. IARC Monogr Eval Carcinog Risks Hum. 1994;61:1–241. [PMC free article] [PubMed] [Google Scholar]
- 12.Shiota S, et al. Systematic review and meta-analysis: the relationship between the helicobacter pylori dupA gene and clinical outcomes. Gut Pathog. 2010;2(1):13. doi: 10.1186/1757-4749-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costa AC, Figueiredo C, Touati E. Pathogenesis of helicobacter pylori infection. Helicobacter. 2009;14(Suppl 1):15–20. doi: 10.1111/j.1523-5378.2009.00702.x. [DOI] [PubMed] [Google Scholar]
- 14.Queiroz DM, et al. Factors associated with helicobacter pylori infection by a cagA-positive strain in children. J Infect Dis. 2000;181(2):626–630. doi: 10.1086/315262. [DOI] [PubMed] [Google Scholar]
- 15.Blaser MJ, et al. Infection with helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55(10):2111–2115. [PubMed] [Google Scholar]
- 16.Atherton JC, et al. Mosaicism in vacuolating cytotoxin alleles of helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270(30):17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 17.Zambon CF, et al. Helicobacter pylori virulence genes and host IL-1RN and IL-1beta genes interplay in favouring the development of peptic ulcer and intestinal metaplasia. Cytokine. 2002;18(5):242–251. doi: 10.1006/cyto.2002.0891. [DOI] [PubMed] [Google Scholar]
- 18.Yamaoka Y, et al. Relationship between helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37(7):2274–2279. doi: 10.1128/jcm.37.7.2274-2279.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaoka Y, et al. Importance of helicobacter pylori oipA in clinical presentation, gastric inflammation, and mucosal interleukin 8 production. Gastroenterology. 2002;123(2):414–424. doi: 10.1053/gast.2002.34781. [DOI] [PubMed] [Google Scholar]
- 20.Su YL, et al. Combination of OipA, BabA, and SabA as candidate biomarkers for predicting helicobacter pylori-related gastric cancer. Sci Rep. 2016;6:36442. doi: 10.1038/srep36442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Souod N, et al. The study of the oipA and dupA genes in helicobacter pylori strains and their relationship with different gastroduodenal diseases. Gastroenterol Hepatol Bed Bench. 2015;8(Suppl 1):S47–S53. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, et al. Association of presence/absence and on/off patterns of helicobacter pylori oipA gene with peptic ulcer disease and gastric cancer risks: a meta-analysis. BMC Infect Dis. 2013;13:555. doi: 10.1186/1471-2334-13-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilver D, et al. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279(5349):373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 24.Boren T, et al. Attachment of helicobacter pylori to human gastric epithelium mediated by blood group antigens. Science. 1993;262(5141):1892–1895. doi: 10.1126/science.8018146. [DOI] [PubMed] [Google Scholar]
- 25.Bartpho TS, et al. Precancerous gastric lesions with helicobacter pylori vacA (+)/babA2(+)/oipA (+) genotype increase the risk of gastric cancer. Biomed Res Int. 2020;2020:7243029. doi: 10.1155/2020/7243029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roman-Roman A, et al. Helicobacter pylori vacA s1m1 genotype but not cagA or babA2 increase the risk of ulcer and gastric cancer in patients from southern Mexico. Gut Pathog. 2017;9:18. doi: 10.1186/s13099-017-0167-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heidari K, et al. The prevalence of helicobacter pylori virulence related genes (hpa and babA2) in Iranian patients with gastrointestinal disorders. Jundishapur J Microbiol. 2017;10(12):e60947. [Google Scholar]
- 28.Mottaghi B, et al. Helicobacter pylori vacA i region polymorphism but not babA2 status associated to gastric cancer risk in northwestern Iran. Clin Exp Med. 2016;16(1):57–63. doi: 10.1007/s10238-014-0327-0. [DOI] [PubMed] [Google Scholar]
- 29.Abdi E, et al. Helicobacter pylori babA2 positivity predicts risk of gastric cancer in Ardabil, a very high-risk area in Iran. Asian Pac J Cancer Prev. 2016;17(2):733–738. doi: 10.7314/apjcp.2016.17.2.733. [DOI] [PubMed] [Google Scholar]
- 30.Talebi Bezmin Abadi A, et al. High correlation of babA 2-positive strains of helicobacter pylori with the presence of gastric cancer. Intern Emerg Med. 2013;8(6):497–501. doi: 10.1007/s11739-011-0631-6. [DOI] [PubMed] [Google Scholar]
- 31.Saxena A, et al. Virulence attributes of helicobacter pylori isolates & their association with gastroduodenal disease. Indian J Med Res. 2011;133:514–520. [PMC free article] [PubMed] [Google Scholar]
- 32.Mattar R, et al. Association of LEC and tnpA helicobacter pylori genes with gastric cancer in a Brazilian population. Infect Agent Cancer. 2010;5:1. doi: 10.1186/1750-9378-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghasemian Safaei H, et al. Relation of bab A2 genotype of helicobacter pylori infection with chronic active gastritis, duodenal ulcer and non-cardia active gastritis in Alzahra hospital Isfahan, Iran. Jundishapur J Microbiol. 2010;3(3):93–98. [Google Scholar]
- 34.Bartchewsky W, Jr, et al. Effect of helicobacter pylori infection on IL-8, IL-1beta and COX-2 expression in patients with chronic gastritis and gastric cancer. Scand J Gastroenterol. 2009;44(2):153–161. doi: 10.1080/00365520802530853. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, et al. The helicobacter pylori duodenal ulcer promoting gene, dupA in China. BMC Gastroenterol. 2008;8:49. doi: 10.1186/1471-230X-8-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erzin Y, et al. Role of host interleukin 1beta gene (IL-1B) and interleukin 1 receptor antagonist gene (IL-1RN) polymorphisms in clinical outcomes in helicobacter pylori-positive Turkish patients with dyspepsia. J Gastroenterol. 2008;43(9):705–710. doi: 10.1007/s00535-008-2220-7. [DOI] [PubMed] [Google Scholar]
- 37.Chomvarin C, et al. Prevalence of helicobacter pylori vacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int J Infect Dis. 2008;12(1):30–36. doi: 10.1016/j.ijid.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 38.Lee HS, et al. Expression of Lewis antigens and their precursors in gastric mucosa: relationship with helicobacter pylori infection and gastric carcinogenesis. J Pathol. 2006;209(1):88–94. doi: 10.1002/path.1949. [DOI] [PubMed] [Google Scholar]
- 39.Han YH, et al. Clinical relevance of iceA and babA2 genotypes of helicobacter pylori in a Shanghai population. Chin J Dig Dis. 2004;5(4):181–185. doi: 10.1111/j.1443-9573.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira AG, et al. babA2- and cagA-positive helicobacter pylori strains are associated with duodenal ulcer and gastric carcinoma in Brazil. J Clin Microbiol. 2003;41(8):3964–3966. doi: 10.1128/JCM.41.8.3964-3966.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mizushima T, et al. Clinical relevance of the babA2 genotype of helicobacter pylori in Japanese clinical isolates. J Clin Microbiol. 2001;39(7):2463–2465. doi: 10.1128/JCM.39.7.2463-2465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerhard M, et al. Clinical relevance of the helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci U S A. 1999;96(22):12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells G, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of case-control studies in meta-analyses. 2011. pp. 603–605. [Google Scholar]
- 44.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 45.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10(1):101–129. [Google Scholar]
- 46.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 47.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pormohammad A, et al. Risk of gastric cancer in association with helicobacter pylori different virulence factors: a systematic review and meta-analysis. Microb Pathog. 2018;118:214–219. doi: 10.1016/j.micpath.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 49.Chen MY, et al. Association of Helicobacter pylori babA2 with peptic ulcer disease and gastric cancer. World J Gastroenterol. 2013;19(26):4242–4251. doi: 10.3748/wjg.v19.i26.4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Con SA, et al. Role of bacterial and genetic factors in gastric cancer in Costa Rica. World J Gastroenterol. 2009;15(2):211–218. doi: 10.3748/wjg.15.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zagari RM, Bazzoli F. Gastric cancer: who is at risk? Dig Dis. 2004;22(4):302–305. doi: 10.1159/000083590. [DOI] [PubMed] [Google Scholar]
- 52.Maeda S, et al. Relationship between nuclear factor-kappaB activation and virulence factors of helicobacter pylori in Japanese clinical isolates. J Gastroenterol Hepatol. 2002;17(5):556–562. doi: 10.1046/j.1440-1746.2002.02738.x. [DOI] [PubMed] [Google Scholar]
- 53.Kuipers EJ, et al. Long-term sequelae of helicobacter pylori gastritis. Lancet. 1995;345(8964):1525–1528. doi: 10.1016/s0140-6736(95)91084-0. [DOI] [PubMed] [Google Scholar]
- 54.Dhar SK, et al. Molecular mechanism of action of major helicobacter pylori virulence factors. Mol Cell Biochem. 2003;253(1–2):207–215. doi: 10.1023/a:1026051530512. [DOI] [PubMed] [Google Scholar]
- 55.Zambon CF, et al. Helicobacter pylori babA2, cagA, and s1 vacA genes work synergistically in causing intestinal metaplasia. J Clin Pathol. 2003;56(4):287–291. doi: 10.1136/jcp.56.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marshall DG, et al. Genomic DNA fingerprinting of clinical isolates of helicobacter pylori using short oligonucleotide probes containing repetitive sequences. J Appl Bacteriol. 1996;81(5):509–517. doi: 10.1111/j.1365-2672.1996.tb03540.x. [DOI] [PubMed] [Google Scholar]
- 57.Akopyanz N, et al. DNA diversity among clinical isolates of helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20(19):5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Sub-group analysis of the association between H. pylori babA2 gene and gastric cancer risk according to study quality assessment. Figure S2. Funnel plot of case–control studies evaluating the association between H. pylori babA2 gene and gastric cancer risk. Each point represents a study to indicate an association. Figure S3. Influence of the summary OR coefficients on the association between H. pylori babA2 gene and gastric cancer risk.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.