Abstract
It has been over 35 years since the discovery of a special subtype of B cells in mice. These IgM+ B cells are named B-1 cells, whereas conventional B cells are referred to as B-2 cells. B-1 cells express Ly-1 (CD5) and CD11b antigen, which are usually expressed in T cells and myeloid cells, respectively, reside mainly in the peritoneal and pleural cavities, and secrete natural IgM antibodies in a T cell-independent manner. B-1 cells are further categorized into CD5+ B-1a cells and CD5− B-1b cells. B-1 cells may develop through positive selection and secrete natural antibodies, including low-affinity-binding autoantibodies. Transplantation assays have revealed that the fetal liver, not the bone marrow (BM), is a major site for the production of B-1a cells, leading to the concept of a fetal origin for B-1a cells. This review introduces how the origin of B-1a cells has been explored, and describes the current state of knowledge gained through various approaches.
The adult BM progenitors have poor B-1a cell capacity, proved by transplantation and lineage tracing assays
Traditionally, it is known that fetal liver cells repopulate B-1a cells efficiently, whereas adult BM progenitors do not fully repopulate B-1a cells by adoptive transfer assays. Hardy and Hayakawa demonstrated that pro-B cells in the fetal liver and adult BM have different B cell capacities; while BM pro-B cells produce B-2 cells, fetal liver pro-B cells produce more B-1a cells upon transplantation[1]. These results suggest the presence of different B-lymphopoietic waves in the embryo and postnatal animals. Conditional Rag-2 knockout mice by Mx-Cre showed B cell maturation arrest at the pro-B cell level in the BM and the reduction of follicular B (B-2) cells in the spleen, whereas peritoneal B-1a cells were maintained[2]. Thus, this study indicates that the peritoneal B-1a cells are not generated by the BM progenitors in a steady-state situation. The B-1a cell capacity of highly purified long-term hematopoietic stem cells (LT-HSCs) in adult BM was further investigated using single cell transplantation assays, and lineage−Sca-1+c-kit+(LSK)CD150+ LT-HSCs failed to reconstitute peritoneal B-1a cells[3].
Compatible with the above data, recent lineage tracing studies have reported additional evidence that HSCs in adult BM poorly generate B-1a cells. Pdzklip1 was specifically expressed in adult CD150+CD48− LT-HSCs[4]. Pdzklip1-CreERT2: Rosa-tomato mice enable to trace HSC-derived hematopoiesis in vivo; Pdzklip1-expressing HSCs are labeled by tamoxifen injection. Brain microglia is derived from early extra-embryonic yolk sac (YS)[5], not HSCs, and were not marked in the mouse model when tamoxifen was administrated into adult mice. Similarly, fewer than 5% of the peritoneal B-1a cells were labeled while up to 80% of the BM HSCs were labeled at 11 months after tamoxifen injection into the adult mice. Another HSC-lineage tracing study examined the contribution of HSC to normal hematopoiesis by labeling Fgd5 expressing cells[6]. Fgd5 is exclusively expressed in the endothelial cells and HSCs[7]. When tamoxifen was injected into adult Fgd5CreERT2: Rosa-tomato mice, the tomato+ percentage in all hematopoietic lineages gradually increased; however, the peritoneal B-1a cells were not labeled. These studies indicate two important findings: 1) adult HSCs do not differentiate and provide blood cells continuously, instead early progenitors last longer than expected and maintain the steady-state hematopoiesis, and 2) B-1a cells are not generated in adults in the physiological setting.
In contrast, several studies have shown that adult BM progenitors can generate B-1a cells. Lin− BM cells marked with GFP by mouse stem cell virus transduction were transplanted into lethally irradiated recipients and repopulated GFP+ CD5+ B-1a cells in the recipient peritoneal cavity[8]. These donor BM-derived B-1a cells were functional and secreted natural IgM antibodies; however, they expressed significant Ig N-additions shown by single cell PCR. One of the characteristics of fetal B cells is no terminal deoxynucleotidyl transferase (TdT) expression and low to zero N-addition in the immunoglobulin VH region. Another study used an inducible Rag1 knockout-rescue model where the Rag1 gene was knocked and rescued in the mb-1+ B cell lineage by tamoxifen injection[9]. Rag1 is indispensable for the Ig rearrangement and Rag1 knockout mice showed maturation arrest in the BM pro-B progenitor stage and all IgM+ B cells were diminished[10]. By tamoxifen injection into adult mice, the peritoneal B-1a cells were recovered as well as other IgM+ B cells in the spleen and BM. These rescued B-1a cells also showed N-region additions, implying that they were derived from adult progenitors. Therefore, there are some progenitors that can produce a good number of B-1a cells in particular settings; however, it is still unknown what types of progenitors have B-1a cell potential in adult BM.
More recently, the conversion of B-2 cells into CD5+B-1a cells (but not B-1 cells to B-2 cells) has been demonstrated by the inducible transgenic system that changes the BCR that is unique for B-2 cells to B-1 cells, and vice versa[11]. This elegant system clearly indicates the phenotype conversion of B-2 cells into B-1a cells, but there is a caveat. Once the BCR VH chain is determined in a cell, it would be less likely for the BCR to be converted into the usage of different V-chain regions in vivo in a physiological setting.
Fetal liver origin of B-1a cells: Do fetal liver HSCs generate B-1a cells?
Adoptive transplantation assays have shown that the fetal liver is the main source of B-1a cells. Montecino-Rodriguez and colleagues identified B-1 specific progenitors (lin−CD19+B220lo-neg cells) in the fetal liver and neonatal BM and the number of B-1 specific progenitors decreased with age[12, 13]. Because the fetal liver contains HSCs, it was assumed that ultimately the fetal liver HSCs produced B-1 progenitors that mature into peritoneal B-1a cells. However, Ghosn et al. asked the question whether highly purified fetal liver HSCs can produce B-1a cells[14]. Against expectations, CD150+LSK HSCs in the E15.5 fetal liver failed to repopulate the peritoneal B-1a cells in the recipient mice. Instead, the authors found that the CD150−LSK cells repopulated the peritoneal B-1a cells. These results raised a question of what type of cells is the source of B-1 progenitors in the fetal liver.
On the other hand, Kristiansen and colleagues demonstrated that functional HSCs in the E14.5 fetal liver are the main source of peritoneal B-1a cells using barcoding study[15]. They barcoded E14.5 FL LSK cells with a retrovirus and transplanted them into lethally irradiated mice. They analyzed the clonality of B-1, B-2, and T lymphoid subsets as well as myeloid cells by examining shared barcoding in each population. The majority of the peritoneal B-1a cells shared the same barcoding with the B-2 cells and splenic myeloid cells, suggesting these three lineages were derived from the same progenitors.
The presence of “developmentally restricted HSCs (dr-HSCs)” in the fetal liver has been reported, which produces “fetal-derived” innate-type lymphoid cells including Vγ3 T cells and B-1a cells[16]. The dr-HSCs are within the KSL population, expressing FLK2-Cre:GFP+ (that previously expressed Flk2). While GFP+(Flk2+) KSL cells from the fetal liver engrafted in the recipient mice over the long-term, the same population in adult BM never repopulated the irradiated adult recipient over the long-term. Therefore, the dr-HSCs were detectable only in the fetal liver by transplantation assays. It is unknown whether the dr-HSCs were the same population that produced both B-1a and B-2 cells, equivalent to the HSPC demonstrated by Kristiansen[15].
Layered immune model: multiple waves of B-1 lymphopoiesis
Given that B-1a cells are of fetal origin, similar to Vγ3-γδT cells in the skin, Herzenberg proposed in 1989 the layered immune model where each immune cell is derived from different types of stem cells during ontogeny[17]. This proposal was updated by Dorshkind’s group with additional evidence in 2013[18]. Early embryonic tissue [paraaortic splanchnopleural (P-Sp)] and/or YS have B-lymphoid potential in vitro co-culture with stromal cells (OP9 or S-17) and these B cells include B-1a cells[19]. It was also demonstrated that B progenitors that were produced in vitro from early YS and P-Sp differentiated into only peritoneal B-1 and splenic marginal zone B cells after transplantation into NOD-SCIDIl2Rγc−/− (NSG) neonates[20]. This B cell potential that was detectable in early YS and P-Sp regions was assumed to be the first wave of B-lymphopoiesis (Fig. 1). It was also demonstrated that B-1 progenitors (presumably derived from early YS and P-Sp) were present in the HSC-deficient fetal liver, supporting the concept of HSC-independent B-1 cell development and that B-1 progenitors in early fetal liver were YS/P-Sp derived[21]. In the updated layered immune theory, Dorshkind’s group proposes that the second B-lymphopoiesis is derived from FL, which predominantly produce B-1 cells with less B-2 cell production, and that the third wave is derived from BM HSCs that produce mainly B-2 cells with reduced or minimum production of B-1a cells[18]. Supporting this hypothesis, Montecino-Rodriguez and colleagues demonstrated the presence of multiple waves of B-lymphopoiesis using a PU.1 hypomorphic mouse model[22]. PU.1 is expressed in HSPCs and is an important transcriptional factor that regulates B cell development[23]. PU.1 is encoded in the Sfpi1 gene and upstream regulatory element located 14 kb from the Sfpi1 starting site is deleted in the PU.1 hypomorphic mouse. The PU.1 hypomorphic mice display severe B-2 cell reduction while B-1a cells are maintained[24]. However, Montecino-Rodriguez et al. found that B-1 specific progenitors in the fetal liver were diminished in the PU.1 hypomorphic mice, suggesting the impairment of the initial wave of B-1 lymphopoiesis derived from YS. The B-1 progenitors were recovered in the E18.5 fetal and neonatal liver and the peritoneal B-1a cell number resulted in an increased number compared to the wild type. In contrast, B-2 progenitors in the fetal liver was not altered; however, adult-HSC-derived B-2 lymphopoiesis was diminished. The RNA-sequencing of these B-1 and B-2 progenitors at different ages distinguished three waves of B-1 lymphopoiesis and two waves of B-2 lymphopoiesis. These results advanced the concept of the layered immune model, but also raised another question: What is the origin of the second and third waves of B-1 cells and the second wave of B-2 cells? This is still an open question. In the layered immune model (Fig. 1), the second wave in the fetal liver was originally considered to be derived from FL HSCs; however, Ghosn’s study that FL HSC did not generate B-1a cells[14] questioned which cells/tissue provided the B-1 progenitors found in the fetal liver. The dr-HSCs in the fetal liver may be the population responsible for producing both B-1 and B-2 lineages in the PU.1 hypomorphic mouse model, although further investigation is required.
Fig. 1. Updated layered immune model.
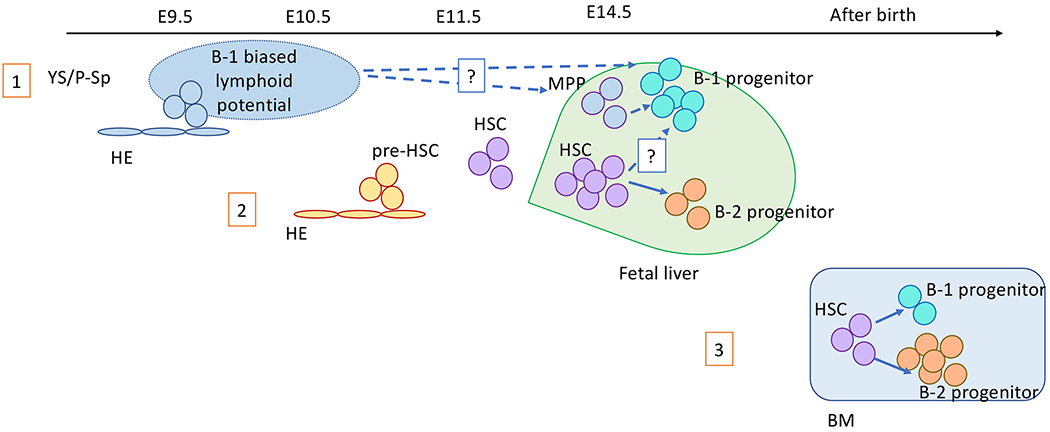
This schema updated and modified the figure proposed by Montecino-Rodriguez and Dorshkind[18]. The first B cell-wave is derived from the hemogenic endothelial cells in the YS/P-Sp at around E8.5-9.5. B cells from this wave are B-1 lymphocyte biased[20]. The second wave is derived from presumably pre-HSCs or HSCs at an early stage of fetal liver. This second wave seems to be the main source of B-1a cells, and also starts to produce B-2 cells. The third wave is derived from HSCs in the post-natal BM, generating mainly B-2 cells with minimum numbers of B-1 cells.
Relationship between the emergence of HSCs and B-1a cells
From the FL transplantation assays, we also confirmed that CD150−CD48+LSK cells (not CD150+ LSK HSCs) in E15.5 fetal liver were responsible for B-1a cell generation upon transplantation, which was compatible with Ghosn’s study[14, 25]. Then, if FL HSCs do not produce B-1a cells, which cells are the precursors of CD150−CD48+LSK cells that produce B-1a cells? As reported previously, the YS and P-Sp hemogenic endothelial cells are strong candidates for this production[20]. However, the first HSCs arising in the aorta-gonado-mesonephros (AGM) region of E10.5-11.5 mouse embryo have not been evaluated for their B-1a production capacity. The first HSC generation from the AGM region is a step-wise process from hemogenic endothelial cells through HSC-precursor (pre-HSC) cells (CD45−VE-cadherin+ cells)[25] (Fig. 2). Pre-HSCs reportedly mature into multi-lineage repopulating cells following co-culture with Akt-expressing AGM-derived endothelial cells (AGM-ECs)[26]. Hadland and colleagues co-cultured a single pre-HSC with AGM-ECs and transplanted the cells that formed a hematopoietic colony derived from a single pre-HSC. All engrafted mice transplanted with single-clone-derived cells displayed both B-1a and B-2 cell engraftment in addition to T and myeloid cell engraftment[27]. Therefore, CD45−VE-cadherin+ pre-HSC population has the capacity to produce B-1a cells and HSCs following AGM-EC co-culture. However, it is still not clear whether the HSCs produced B-1a cells or pre-HSCs produced B-1a cell and HSCs by asymmetric cell division.
Fig. 2. HSCs mature from hemogenic endothelial cells through pre-HSC stages.
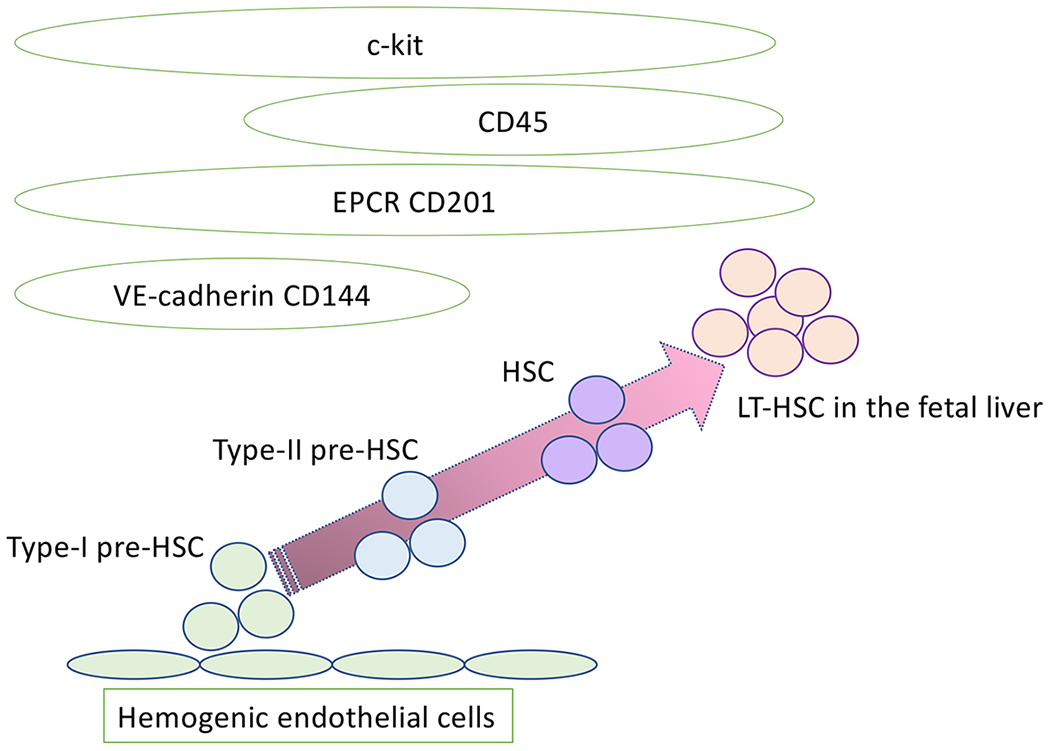
The current evidence supports the concept of pre-HSCs, intermediate stages between hemogenic endothelial cells and HSCs. Pre-HSCs express markers for both endothelial cells and hematopoietic cells.
Our group further investigated the hematopoietic capacity of the pre-HSC population by direct transplantation assays into NSG neonates[25]. We injected E10.5 CD45−VC+c-kit+ pre-HSC population into sublethally irradiated NSG neonates that are more permissive for embryonic cell engraftment. Surprisingly, there were many recipient mice that displayed donor-derived cell engraftment in the peritoneal cavity but not in the peripheral blood. In these mice, only the peritoneal B-1 and marginal zone B cells were engrafted, similar to HSC-deficient FL transplantation as previously reported[21]. There were several recipient mice that showed multi-lineage repopulation including B-1a cells. However, these recipients were transplanted with more than three embryo equivalents of pre-HSC cells. EPCR has been reported to enrich the pre-HSC population[28] and when only 10 EPCRhighVC+c-kit+ cells were transplanted, only B-1 cells were repopulated in the recipient mice. The frequencies of multi-lineage and B-1a repopulating cells were 1 of 10 e.e and 1 of 0.3 e.e, respectively. Thus, the E10.5 pre-HSC population contains more B-1 biased repopulating cells. When these EPCRhighVC+c-kit+ cells were co-cultured with AGM-ECs, their B-1 progenitor colony forming capacity converted into B-1 and B-2 progenitor capacities at the single cell level. Because pre-HSCs become mutli-lineage repopulating cells after co-culture with AGM-ECs, these results strongly indicate that the pre-HSC population is B-1 cell biased and mature into multi-lineage repopulating HSCs by gaining B-2 cell capacity. Given the higher frequency of B-1 biased repopulating cells among pre-HSCs, it can be speculated that at least a part of the B-1a cells were produced by E10.5 hemogenic endothelial cells directly, separate from the fist HSCs. However, whether B-1a cells are produced via HSCs has yet to be elucidated.
Will lineage tracing studies answer this question? Thus far, there is no report that distinguishes the origin of B-1a cells and HSCs in embryonic stages. However, a study by Pei et al., may provide a hint. They developed an elegant polylox barcoding system that was induced by tamoxifen-inducible Tie-2 Mer-Cre-Mer transgenic mice[29]. In this mouse model, by single 4OHT injection into an E9.5 pregnant dam, more than 95% of HSCs successfully recombined the barcodes and were traceable up to 11 months after birth. They examined the barcoding of HSPC and blood lineages to predict the productive HSC clones. In their analysis, B-1a cells were branched separately from other B-2 and T lymphoid subsets and were isolated from all other blood lineages. Their results implied that B-1a cells developed separately from other blood lineages derived from adult HSCs.
Lin28b: a possible regulator of fetal hematopoiesis
Yuan and colleagues performed global miRNA-expression profiling of FL and adult BM pro-B progenitors (IgM−B220+CD19+CD24+CD42+ cells) because this population in the FL generate more B-1a cells while the same population in the adult BM generate B-2 cells[1], and found that the Lin28b and let-7 family of miRNA were differentially expressed in FL and BM pro-B cells[30]. The transplantation of adult BM HSPC overexpressing resulted in the repopulation of embryonic-type lymphoid cells including B-1a, marginal zone B cells, γδT cells, and NKT cells. Lin28b was also reported as a master regulator of developmentally timed changes in the HSC program[31]. Zhou and colleagues further validated the role of Lin28 and its miRNA, Let-7, on the B-1a cell production from B progenitor cells[32]. They confirmed that Lin28b was highly expressed in fetal liver Pro-B cells while Let-7 was highly expressed in adult BM pro-B cells. Lin28b overexpressing BM pro-B cells repopulated B-1a cells efficiently upon adoptive transfer whereas the control adult BM pro-B cells did not. Similarly, Let-7 overexpressing fetal liver pro-B cells (repressed Lin28b expression) failed to repopulate peritoneal B-1a cells. Taken together, Lin28b regulates B-1a cell development in the fetal liver stage. To understand the origin of B-1a cells more precisely, further investigation of the molecular mechanism that regulates fetal lymphopoiesis in the hemogenic endothelial cells and pre-HSCs will be required.
Acknowledgement
We would like to thank Editage (www.editage.com) for English language editing.
This study is supported by NIAID R01AI121197.
Footnotes
There is no conflict of interest.
References
- 1.Hardy RR, Hayakawa K. A developmental switch in B lymphopoiesis. Proc Natl Acad Sci U S A. 1991;88:11550–11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194:1151–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosn EE, Yamamoto R, Hamanaka S, Yang Y, Herzenberg LA, Nakauchi H et al. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci U S A. 2012;109:5394–5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sawai CM, Babovic S, Upadhaya S, Knapp DJ, Lavin Y, Lau CM et al. Hematopoietic Stem Cells Are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity. 2016;45:597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sawen P, Eldeeb M, Erlandsson E, Kristiansen TA, Laterza C, Kokaia Z et al. Murine HSCs contribute actively to native hematopoiesis but with reduced differentiation capacity upon aging. Elife. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gazit R, Mandal PK, Ebina W, Ben-Zvi A, Nombela-Arrieta C, Silberstein LE et al. Fgd5 identifies hematopoietic stem cells in the murine bone marrow. J Exp Med. 2014;211:1315–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holodick NE, Repetny K, Zhong X, Rothstein TL. Adult BM generates CD5+ B1 cells containing abundant N-region additions. Eur J Immunol. 2009;39:2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duber S, Hafner M, Krey M, Lienenklaus S, Roy B, Hobeika E et al. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. [DOI] [PubMed] [Google Scholar]
- 10.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. [DOI] [PubMed] [Google Scholar]
- 11.Graf R, Seagal J, Otipoby KL, Lam KP, Ayoub S, Zhang B et al. BCR-dependent lineage plasticity in mature B cells. Science. 2019;363:748–753. [DOI] [PubMed] [Google Scholar]
- 12.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. [DOI] [PubMed] [Google Scholar]
- 13.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:13700–13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosn EE, Waters J, Phillips M, Yamamoto R, Long BR, Yang Y et al. Fetal Hematopoietic Stem Cell Transplantation Fails to Fully Regenerate the B-Lymphocyte Compartment. Stem Cell Reports. 2016;6:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kristiansen TA, Jaensson Gyllenback E, Zriwil A, Bjorklund T, Daniel JA, Sitnicka E et al. Cellular Barcoding Links B-1a B Cell Potential to a Fetal Hematopoietic Stem Cell State at the Single-Cell Level. Immunity. 2016;45:346–357. [DOI] [PubMed] [Google Scholar]
- 16.Beaudin AE, Boyer SW, Perez-Cunningham J, Hernandez GE, Derderian SC, Jujjavarapu C et al. A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells. Cell Stem Cell. 2016;19:768–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzenberg LA. Toward a layered immune system. Cell. 1989;59:953–954. [DOI] [PubMed] [Google Scholar]
- 18.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Godin I, Dieterlen-Lievre F, Cumano A. Emergence of multipotent hemopoietic cells in the yolk sac and paraaortic splanchnopleura in mouse embryos, beginning at 8.5 days postcoitus. Proc Natl Acad Sci U S A. 1995;92:773–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A. 2011;108:1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobayashi M, Shelley WC, Seo W, Vemula S, Lin Y, Liu Y et al. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfbeta for their development. Proc Natl Acad Sci U S A. 2014;111:12151–12156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montecino-Rodriguez E, Fice M, Casero D, Berent-Maoz B, Barber CL, Dorshkind K. Distinct Genetic Networks Orchestrate the Emergence of Specific Waves of Fetal and Adult B-1 and B-2 Development. Immunity. 2016;45:527–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeKoter RP, Lee HJ, Singh H. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity. 2002;16:297–309. [DOI] [PubMed] [Google Scholar]
- 24.Rosenbauer F, Owens BM, Yu L, Tumang JR, Steidl U, Kutok JL et al. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat Genet. 2006;38:27–37. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Tarnawsky SP, Wei H, Mishra A, Azevedo Portilho N, Wenzel P et al. Hemogenic Endothelial Cells Can Transition to Hematopoietic Stem Cells through a B-1 Lymphocyte-Biased State during Maturation in the Mouse Embryo. Stem Cell Reports. 2019;13:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadland BK, Varnum-Finney B, Poulos MG, Moon RT, Butler JM, Rafii S et al. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J Clin Invest. 2015;125:2032–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadland BK, Varnum-Finney B, Mandal PK, Rossi DJ, Poulos MG, Butler JM et al. A Common Origin for B-1a and B-2 Lymphocytes in Clonal Pre- Hematopoietic Stem Cells. Stem Cell Reports. 2017;8:1563–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Li X, Wang W, Zhu P, Zhou J, He W et al. Tracing haematopoietic stem cell formation at single-cell resolution. Nature. 2016;533:487–492. [DOI] [PubMed] [Google Scholar]
- 29.Pei W, Feyerabend TB, Rossler J, Wang X, Postrach D, Busch K et al. Polylox barcoding reveals haematopoietic stem cell fates realized in vivo. Nature. 2017;548:456–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Copley MR, Babovic S, Benz C, Knapp DJ, Beer PA, Kent DG et al. The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nat Cell Biol. 2013;15:916–925. [DOI] [PubMed] [Google Scholar]
- 32.Zhou Y, Li YS, Bandi SR, Tang L, Shinton SA, Hayakawa K et al. Lin28b promotes fetal B lymphopoiesis through the transcription factor Arid3a. J Exp Med. 2015;212:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]


