Abstract
Hyaluronan (HA) is an extracellular matrix glycosaminoglycan that critically supports the physicochemical and mechanical properties of the skin. Here, we demonstrate that mycosporine-like amino acids (MAAs), which typically function as UV-absorbing compounds, can stimulate HA secretion from normal human fibroblasts. MAA-stimulated HA secretion was associated with significantly increased and decreased levels of mRNAs encoding HA synthase 2 (HAS2) and the HA-binding protein involved in HA depolymerization (designated HYBID), respectively. Using immunoblotting, we found that MAAs at 10 and at 25 μg/ml stimulate the phosphorylation of the mitogen-activated protein kinase (MAPK) p38, extracellular signal-regulated kinase (ERK)/c-Jun, and mitogen- and stress-activated protein kinase 1 (MSK1) (at Thr-581, Ser-360, and Ser-376, respectively) and activation of cAMP-responsive element-binding protein (CREB) and activating transcription factor 2 (ATF2), but not phosphorylation of JUN N-terminal kinase (JNK) or NF-κB (at Ser-276 or Ser-536, respectively), and increased c-Fos protein levels. Moreover, a p38-specific inhibitor, but not inhibitors of MAPK/ERK kinase (MEK), JNK, or NF-κB, significantly abrogated the increased expression of HAS2 mRNA, accompanied by significantly decreased MAA-stimulated HA secretion. These results suggested that the p38–MSK1–CREB–c-Fos–transcription factor AP-1 (AP-1) or the p38–ATF2 signaling cascade is responsible for the MAA-induced stimulation of HAS2 gene expression. Of note, siRNA-mediated ATF2 silencing failed to abrogate MAA-stimulated HAS2 expression, and c-Fos silencing abolished the increased expression of HAS2 mRNA. Our findings suggest that MAAs stimulate HA secretion by up-regulating HAS2 mRNA levels through activation of an intracellular signaling cascade consisting of p38, MSK1, CREB, c-Fos, and AP-1.
Keywords: amino acid, c-Fos, c-Jun N-terminal kinase (JNK), cell signaling, fibroblast, hyaluronan, p38 MAPK, protein phosphorylation, AP-1, CREB, hyaluronan synthase 2, MSK1, mycosporine-like amino acids
Introduction
HA2 is an important component of the extracellular microenvironment that supports the typical physicochemical and mechanical properties of the skin. Thus, HA has important structural functions via mechanisms by which its high polymer length and polyanionic charge enable it to bind water, which in turn supports volume expansion, turgidity, and skin elasticity (1). In photodamaged skin, there is a marked deficiency of HA in the dermis (2, 3) in addition to the fragmentation and loss of type I collagen fibrils due to the up-regulated activity of matrix-metalloprotease (MMP)-1 (4, 5). Furthermore, the three-dimensional configuration of elastic fibers is impaired due to the up-regulated expression of fibroblast-derived elastase neprilysin (6–13). The sum of those effects is associated with the decreased elasticity of the skin as well as the subsequently increased incidence of skin wrinkles and sagging. That association is consistent with our earlier studies, which demonstrated that a significant reduction of cutaneous elastic properties is a prerequisite factor for the initiation of skin wrinkle formation and that there is a close relationship of impaired skin elasticity with both the clinical scores and the depth of wrinkles as revealed by morphometric analysis (14, 15). Consistently, in clinical studies of human skin, the formation or the amelioration of facial wrinkles is distinctly accompanied by a marked loss or recovery of skin elastic properties, respectively (14–16). In support of the relationship between HA content in the dermis and cutaneous photoaging symptoms, Yoshida et al. (17, 18) recently demonstrated that there is a marked loss of HA in the papillary dermis of photoaged facial skin, the diminished level of which is associated with the degree of skin wrinkling and sagging in the same aged skin. Their studies strongly suggested that a deficiency of HA in photoaged skin contributes to the formation of skin wrinkles and sagging.
HA is a linear polymer composed of repeating disaccharides (d-glucuronic acid, 1,3-GlcNAc, and 1,4-GlcNAc) that is assembled from activated nucleotide sugars (UDP-glucuronic acid and UDP-GlcNAc) at the inner plasma membrane by HA synthases (HAS). Three different isoforms of HAS (HAS1, HAS2, and HAS3) are known to reside in the plasma membrane and to extrude the growing HA polymer into the extracellular space (19). Two different isoforms of hyaluronidase (HYAL) (HYAL1 and HYAL2) are known to cleave HA into discrete fragments (20), which in turn activates Toll-like receptors 2 and 4 and thereby modulates inflammatory responses (21, 22). A third isoform of HYAL (HYAL3) is expressed, although its properties are not well-understood. Nevertheless, Yoshida et al. (23) recently ruled out the possible roles of HYALs in the degradation of HA by human fibroblasts and indicated that KIAA1199, a novel HA-binding protein recently designated as “HYBID” (hyaluronan binding protein involved in HA depolymerization), plays a key role in the degradation of HA by fibroblasts in normal human skin. Thus, it has become clear that the balance of HAS and HYBID functions as a key factor in regulating HA content in the dermis.
We considered that if percutaneously-permeable natural compounds with small molecular weights exist that have the potential to stimulate the secretion of HA by human fibroblasts, they would be good candidates for ameliorating or preventing the loss of skin elastic properties as prerequisite factors of wrinkles and sagging formation in photoaged skin. Under this expectation, in a screening for natural compounds that could stimulate the secretion of HA by human fibroblasts, we now report for the first time that mycosporine-like amino acids (MAAs) have a significant potential to stimulate the secretion of HA by normal human fibroblasts without any cytotoxic effect on cellular viability.
MAAs are generally defined as amino acids with cyclohexanone or cyclohexenimine in their basic structure. MAAs are present as small secondary metabolites in different organisms such as aquatic yeasts (24), cyanobacteria (25), marine dinoflagellates (26), and some Antarctic diatoms (27), all of which live in environments with high exposure to sunlight, usually marine environments. The number of compounds within this class of natural products has been discovered, to date, to be around 30 (28, 29). Because the basic cyclohexanone or cyclohexenimine structures act as chromophores responsible for UV absorbance, MAAs are well-known as UV-absorbing compounds, and their filter capacities are similar to synthetic UVA sunscreens such as Parsol® 1789 (ϵ molar 40,000) and Mexoryl® SX (ϵ molar 45,000) (30). MAAs have also recently been shown to act as antioxidants (31) by increasing the ratio of reduced (GSH) to oxidized GSH (GSSG) in UV-exposed cells (32) and as anti-apoptotic agents by abrogating UV-induced increases in active caspase-3 protein (33). However, there have been no reports showing the stimulatory effect of MAAs on HA secretion by any type of mammalian cell in culture.
In this study, using human dermal fibroblasts (HDFs), we characterized the stimulatory effect of MAAs in terms of its biological mechanism as well as the signaling mechanisms involved in the stimulation. Here, we show for the first time that MAAs have a significant potential to stimulate HA secretion by up-regulating HAS2 mRNA levels in HDFs, which results from activation of the intercellular signaling cascade consisting of the p38/MSK1/CREB/c-Fos/AP-1 axis.
Results and discussion
Purification of MAAs and HPLC/LC-mass analysis
MAAs were extracted and purified as detailed under supporting “Experimental procedures” and were identified as porphyra-334 and shinorine (Fig. 1) by HPLC/LC-mass analysis (Figs. S1–S6) as described under supporting Results. The contents of porphyra-334 and shinorine in MAAs used in this study were calculated as 88.8 and 11.2%, respectively.
Figure 1.
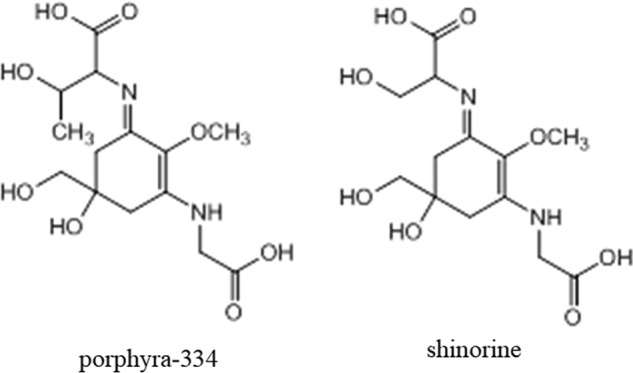
Chemical structures of porphyra-334 and shinorine.
Effects of MAAs on the functional properties of HDFs
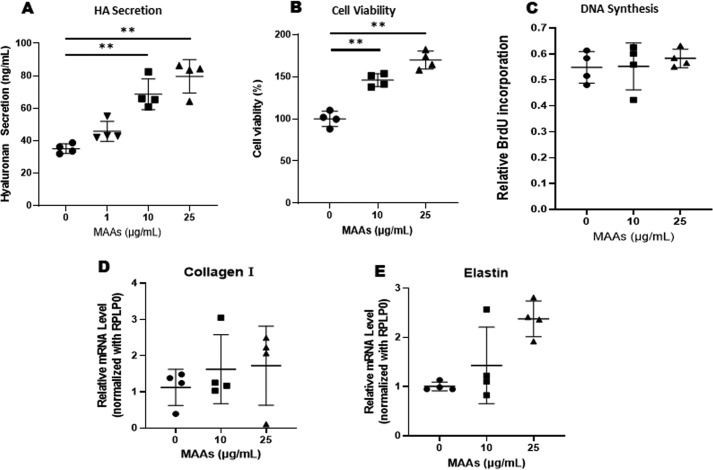
When HDFs were cultured for 72 h in the presence or absence of MAAs at 1, 10, or 25 μg/ml to HDFs in culture, the secretion levels of HA in the medium increased significantly in the presence of 10 or 25 μg/ml MAAs compared with untreated control HDFs (Fig. 2A).
Figure 2.
A: effects of MAAs on the secretion level of HA by HDFs in culture. MAAs at 0, 1, 10, or 25 μg/ml were added to cultures of HDFs for 72 h as noted, and the conditioned medium was measured for HA levels by ELISA. Data represent means ± S.D., n = 4; **, p < 0.01 versus control (0 μg/ml). B: effects of MAAs on the viability of HDFs in culture. HDFs were cultured for 72 h in the presence of MAAs at the indicated concentrations, and their viability was evaluated using the MTT assay. **, p < 0.01 versus control (0 μg/ml). Data represent means ± S.D. n = 4. C–: effects of MAAs on DNA synthesis (C) and collagen 1 (D), and elastin (E) mRNA levels in HDFs. DNA synthesis was measured 48 h after incubation with MAAs at the indicated concentrations using a cell proliferation ELISA kit. Collagen and elastin mRNA levels were measured by qRT-PCR 3 h after incubation with MAAs at the indicated concentrations. Data represent means ± S.D., n = 4.
To determine whether MAAs are cytotoxic to HDFs, we cultured HDFs for 72 h in the absence or presence of MAAs at 10 and 25 μg/ml, and their viability was evaluated by cellular morphology and by the MTT assay. The results showed that there were no morphological changes in HDFs treated with 10 or 25 μg/ml MAAs (data not shown), and there was actually a significant increase in cell viability of HDFs treated with 10 and 25 μg/ml MAAs compared with untreated controls (Fig. 2B).
To determine the effects of MAAs on the functional properties of HDFs, i.e. DNA synthesis and production of collagen 1 and elastin, we cultured HDFs for 72 h in the absence or presence of 10 or 25 μg/ml MAAs. The results showed that MAAs did not stimulate DNA synthesis or expression levels of collagen 1 and elastin mRNAs at 10 or 25 μg/ml (Fig. 2, C–E).
The sum of those findings indicates that MAAs stimulate the secretion of HA by HDFs without any cytotoxic effect on cell viability and that the stimulatory effect is not accompanied by increased levels of other cellular functions of fibroblasts such as the synthesis of collagen 1, elastin, and DNA.
Effects of MAAs on HAS and HYBID mRNA levels in HDFs
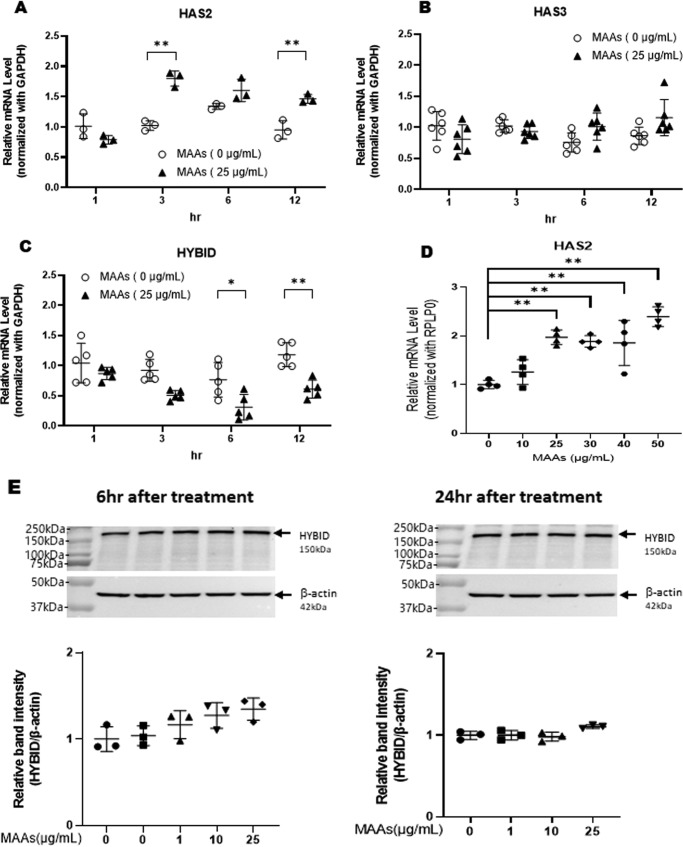
Because the secretion of HA by HDFs is regulated by HAS1, HAS2, and HAS3 and by the HA degradative protein HYBID (23), we then determined the effects of MAAs on the gene expression levels of HAS1, HAS2, and HAS3 and on the gene and protein expression levels of HYBID. When HDFs were treated with MAAs at 25 μg/ml, the gene expression levels of HAS2 (Fig. 3A) and HYBID (Fig. 3C) were significantly increased or decreased, respectively, at 3–12 h post-treatment, but there was no change in the HAS3 mRNA level (Fig. 3B); the level of HAS1 mRNA was undetectable (data not shown). Analysis of the effects of MAAs on mRNA levels of HAS2 revealed that MAAs significantly up-regulated the gene expression level of HAS2 in a dose-dependent fashion at concentrations of 25–50 μg/ml (Fig. 3D). Western blotting using an anti-HYBID antibody revealed that MAAs at 1, 10, and 25 μg/ml did not affect the level of HYBID protein at 6 or 24 h post-treatment (Fig. 3E).
Figure 3.
Effects of MAAs on levels of HAS2 mRNA (A/D), HAS3 mRNA (B), HYBID mRNA (C), and protein (E) in HDFs. HDFs were cultured in the presence or absence of MAAs at 25 μg/ml (A–C) or at 0–50 μg/ml (D and E) for the indicated times (A–C) or 3 h (D) and were then subjected to qRT-PCR analysis for HAS2, HAS3, and HYBID mRNA levels or to Western blotting for HYBID protein levels. Representative immunoblots from three independent experiments are shown (E). Data represent means ± S.D. A: n = 3; B: n = 6; C: n = 5; D: n = 4; E: n = 3; *, p < 0.05; **, p < 0.01 versus the nontreated control (0 μg/ml).
HAS2 enzyme produces HA with a high molecular mass (1,000–10,000 kDa) (34), whereas HYBID is associated with the depolymerization of HA from a high molecular mass to >35 kDa (23). Because the ELISA used in this study detects only HA with >35 kDa (35) and because the expression of HYBID protein is not affected by the treatment with MAAs, the MAA-induced increase in the secretion of HA is predominantly attributed to the increased synthesis of HA, which results from the increased expression of HAS2.
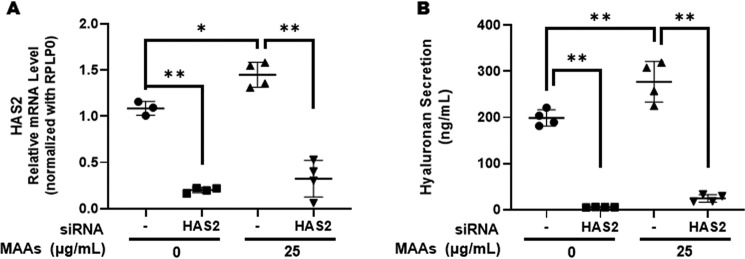
To test that possibility, we determined the effects of transfecting HAS2 siRNA on the MAA-increased secretions of HA. The results indicated that the HAS2 siRNA significantly abolished the secretion of HA both in MAA-treated and -nontreated fibroblasts (Fig. 4). Because the up-regulated gene expression level of HAS2 is mainly responsible for the increased secretion of HA elicited by treatment with MAAs, we next characterized which intracellular signaling cascades are activated by MAAs that result in the up-regulated gene expression level of HAS2.
Figure 4.
Effects of transfecting a HAS2 siRNA on the MAA-increased secretion of HA in HDFs. A: effects on HAS2 mRNA levels. B: effects on HA secretion levels. After transfection of HAS2 siRNA, HDFs were cultured in the presence or absence of MAAs at 25 μg/ml for 72 h and then were subjected to qRT-PCR analysis for mRNA levels of HAS2. The conditioned medium was measured at 72 h post-treatment for HA levels by ELISA. Data represent means ± S.D. n = 4; *, p < 0.05; **, p < 0.01 versus control (0 μg/ml) or negative siRNA.
Effects of MAAs on intracellular signaling cascades in HDFs
It has been reported that HA secretion and synthesis are up-regulated in concert with the increased gene expression level of HAS2 by several chemicals or stimuli via different signaling pathways depending on the cell species and chemicals used. 20-O-β-d-Glucopyranosyl-20(S)-protopanaxadiol enhances the expression of HAS2 in human keratinocytes via the activation of ERK and Akt signaling, which is mediated by Src but not the JNK, p38, EGF receptor, or the Ca2+-related signaling pathways (36). The stimulation of HAS2 expression by transforming growth factor–β1 in human skin fibroblasts is abrogated by blocking MAPK and/or Smad signaling and phosphatidylinositol 3-kinase–Akt signaling (37). All-trans-retinoic acid stimulates HA synthesis in normal human tracheobronchial epithelial cells by activating CREB1 in a nonclassical, retinoic acid receptor–independent fashion via the protein kinase C or the ERK/RSK/CREB cascades (38). UTP specifically up-regulates HAS2 expression among the three HAS genes through the UTP receptor P2Y2, which is accompanied by the increased phosphorylation of p38, ERK, CREB, and STAT3 and by the induced nuclear translocation of pCaMKII, whose inhibitors (for PKC/p38/ERK/CaMKII/STAT3/CREB) partially block the stimulation of HAS2 expression (39). The UDP-glucose–induced up-regulation of HAS2 mRNA levels in human immortalized epidermal keratinocytes is mediated via the JAK2/ERK/STAT3 signaling pathway (40). A neutral sphingomyelinase inhibitor or its deficiency in mouse fibroblast cell lines stimulates HAS2 gene expression via the Akt/mechanistic target of rapamycin pathway (41). Low-dose UVB exposure of rat keratinocytes enhances HA secretion accompanied by the increased expression level of HAS2 mRNA, which is mediated by p38 signaling (42). In contrast, methyl-β-cyclodextrin suppresses HA synthesis by down-regulating HAS2 through the inhibition of Akt (43). It is known that transcription factors that primarily target the HAS2 gene include retinoic acid receptor (44), STAT3, SP1 (45, 46), NF-κB (47), and CREB (48) in a variety of cells.
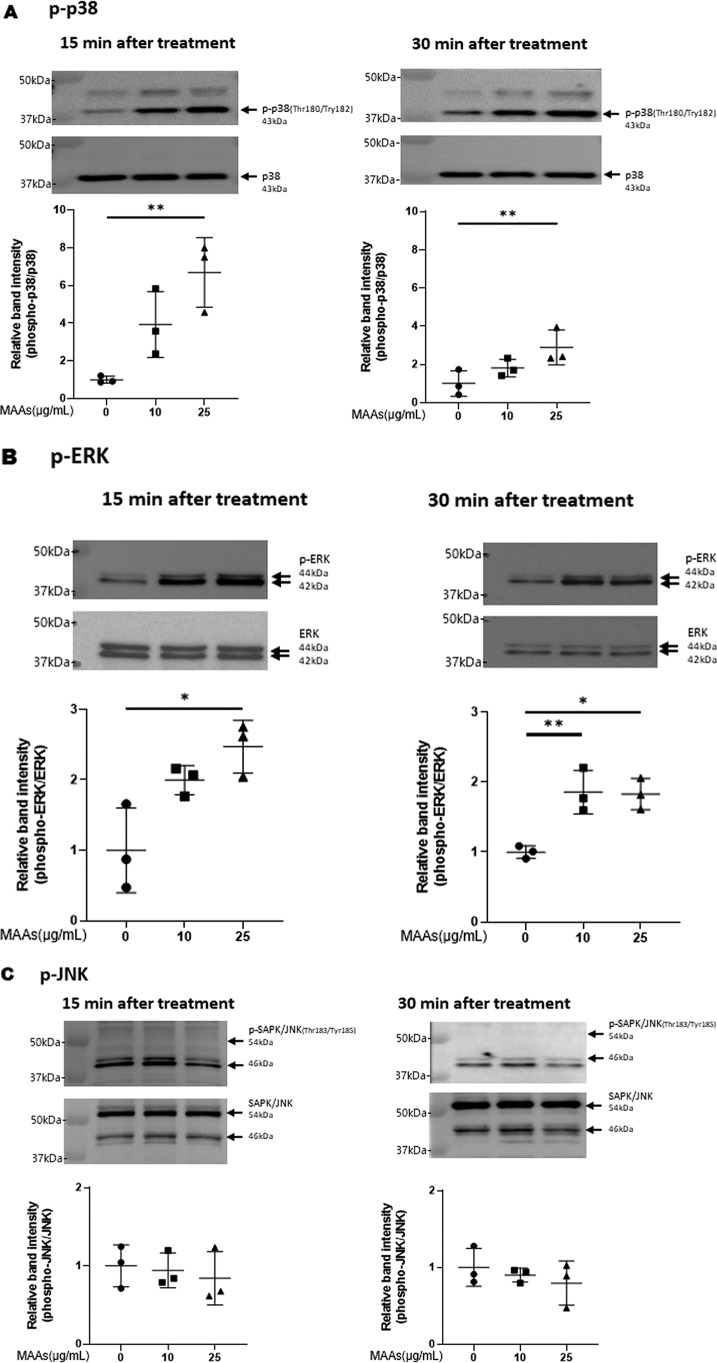
Thus, we first examined the potential effects of MAAs on p38 signaling. Western blot analysis of p38 phosphorylation revealed that MAAs at 25 μg/ml significantly activate p38 in HDFs at 15 and 30 min post-stimulation (Fig. 5A), which suggests that MAA-activated signaling occurs in a similar fashion to UVB-activated signaling (42).
Figure 5.
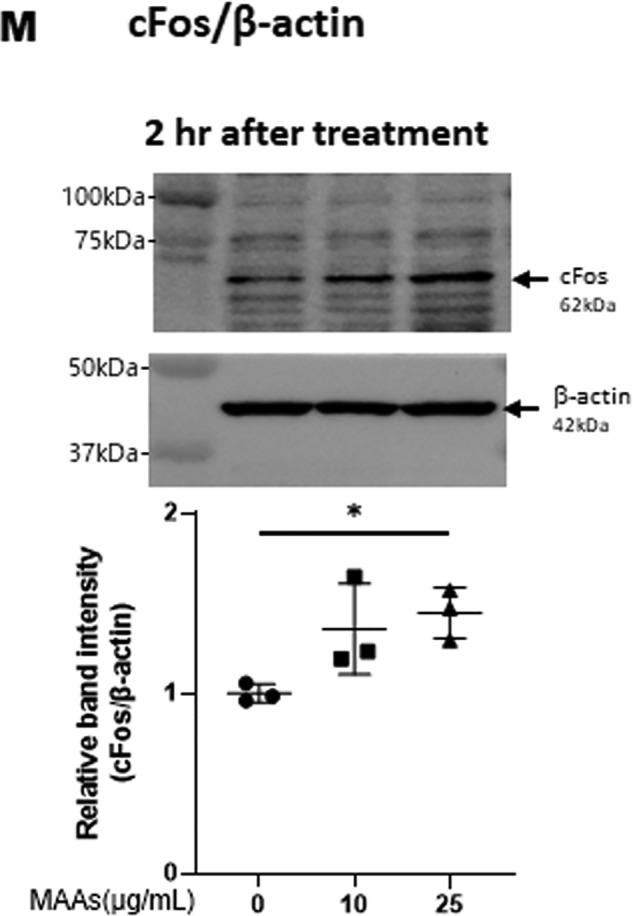
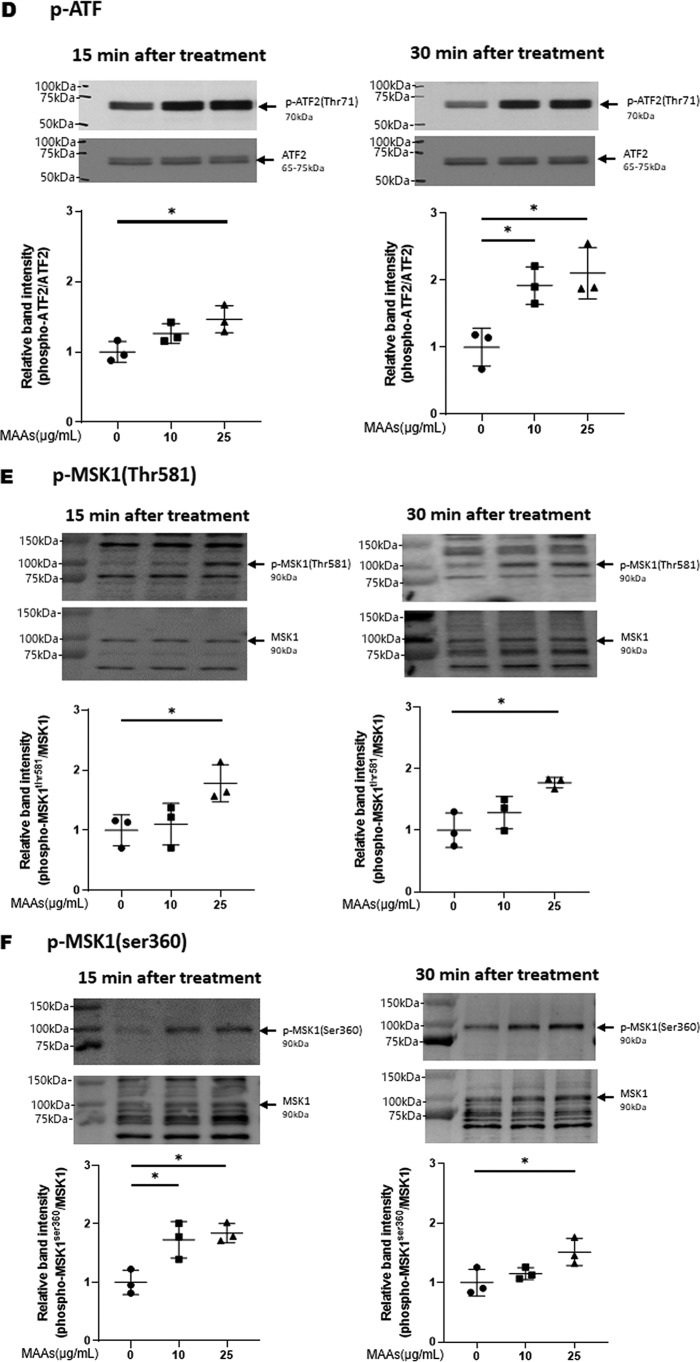
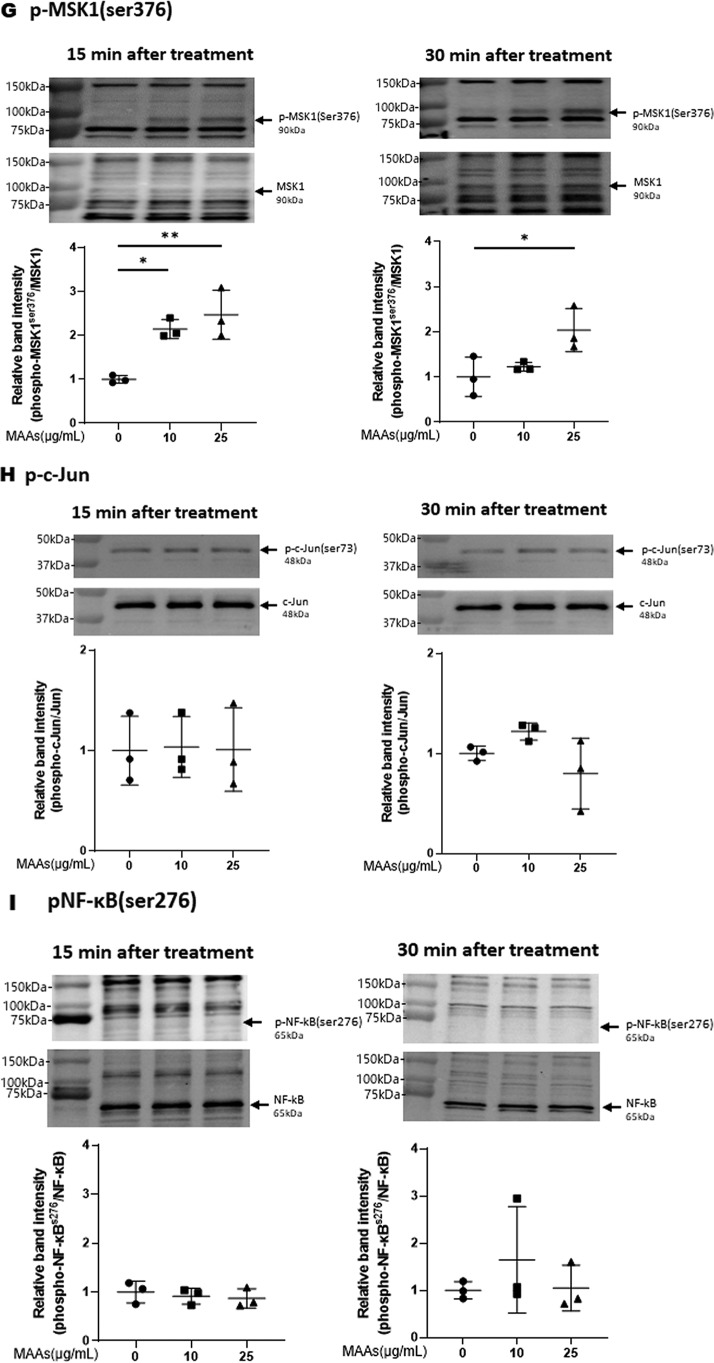
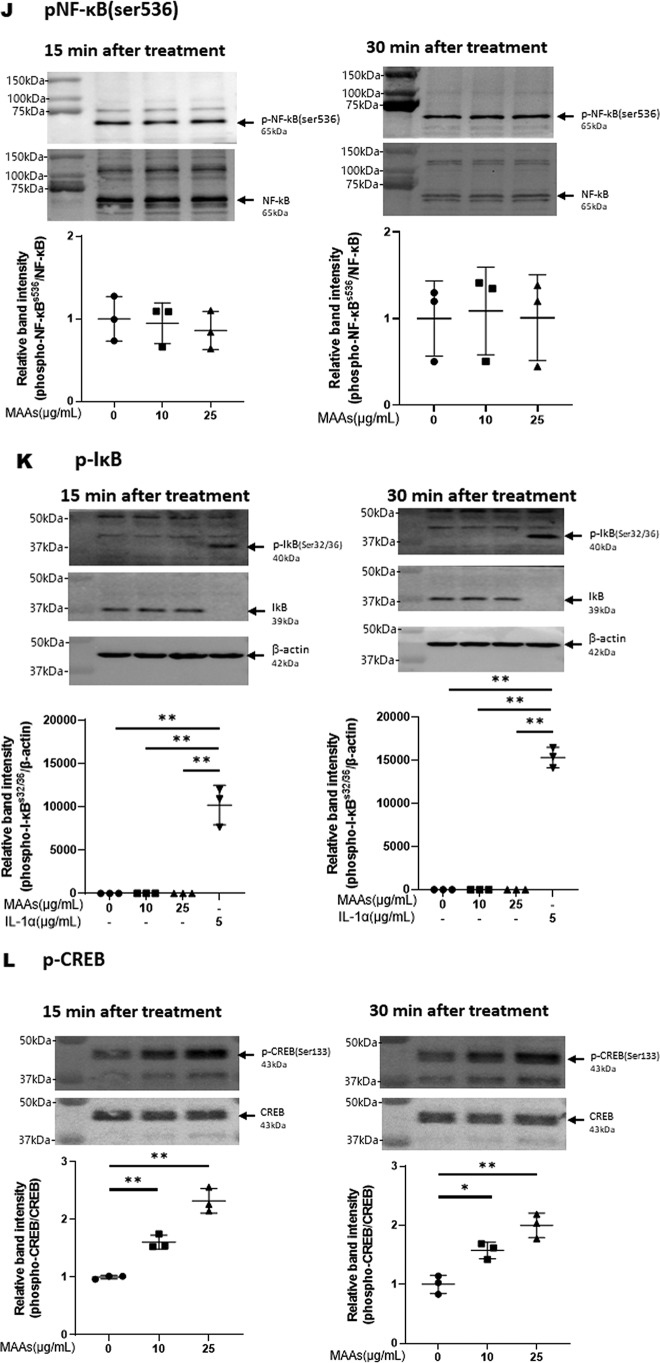
Effects of MAAs on intracellular signaling cascades of p38 (A), ERK (B), JNK (C), ATF2 (D), MSK1(Thr-581/Ser-360/Ser-376) (E–G), c-Jun (H), NF-κB (Ser-276/Ser-536) (I and J), IκB (K), CREB (L), and c-Fos (M) in HDFs. HDFs were incubated with MAAs or with IL-1α at the indicated concentrations and were harvested at 15 and 30 min or 2 h post-treatment and then immunoblotted with antibodies to β-actin and to phosphorylated or nonphosphorylated signaling factors. K: β-actin was used as a loading control because of undetectable levels of nonphosphorylated IκB protein in IL-1α–treated cells. Representative immunoblots from three independent experiments are shown. Data represent means ± S.D., n = 3; *, p < 0.05; **, p < 0.01 versus control (0 μg/ml).
In UVB-exposed human or rat keratinocytes (44, 49, 50) or human melanocytes (51), the activation of the front line of stress-activated signaling factors occurs at p38, JNK, and ERK by which their downstream signaling factors such as MSK1 for p38 and ERK (52, 53), MAPK–APK2 for p38 (54), CK2/i-kB/NF-κB for p38 (55), and ATF2 for p38 (56) as well as c-Jun and c-Fos for ERK (57, 58) are subjected to subsequent and sequential signaling activation. During these downstream cascades, further signaling factors such as CREB for MSK1 (59), NF-κB (Ser-276) for MSK1 (60, 61), and c-Fos for CREB (62) are continuously and sequentially activated. Several functional proteins that are highly expressed in UVB-exposed human keratinocytes or human melanocytes, such as cyclooxygenase (COX)2 (50), interleukin (IL)-8 (50), transglutaminase1 (49) (in human keratinocytes), endothelin B receptor (51, 63), and stem cell factor receptor c-KIT (in human melanocytes) (63, 64), utilize their characteristic and specific signaling cascades to elicit their increased gene expression.
Therefore, based on the known UVB-activated intracellular signaling pathway (49, 51), we characterized the activating or nonactivating effects of MAAs on the front line of stress-activated signaling, JNK and ERK other than p38. Western blot analysis of the phosphorylation of ERK (Fig. 5B) and JNK (Fig. 5C) revealed that MAAs at 10 and/or 25 μg/ml have a significant potential to activate ERK but not JNK at 15 or 30 min post-treatment in HDFs. We next characterized the activating or nonactivating effects of MAAs on downstream signaling factors, MSK1(Thr-581/Ser-360) for p38 and ERK and ATF2 for p38. Western blot analysis of the phosphorylation patterns demonstrated that MAAs at 10 and/or 25 μg/ml have a significant potential to activate ATF2 (Fig. 5D) and MSK1 (Thr-581/Ser-360/Ser-376, the latter of which is an autophosphorylation site after MSK1 phosphorylation at Thr-581/Ser-360) (Fig. 5, E–G) at 15 or 30 min post-treatment. As for the activating or nonactivating effects of MAAs on downstream signaling factors, c-Jun for ERK, NF-κB(Ser-276) for MSK1, NF-κB(Ser-536) for ERK/IKK, and IκB (Ser-32/36) for CK2 or IKK, Western blot analysis of the phosphorylation patterns demonstrated that MAAs at 10 and 25 μg/ml do not have any potential to activate c-Jun (Fig. 5H) or NF-κB(Ser-276/Ser-536) (Fig. 5, I and J) at 15 and 30 min post-treatment in HDFs. Similarly, the phosphorylation of IκB(Ser-32/36) was not stimulated by MAAs at 10 or 25 μg/ml in contrast to their stimulated phosphorylation by IL-1α (Fig. 5K) in which the IκB protein used as a loading control became undetectable probably because of a rapid degradation of phosphorylated IκB.
Finally, we examined the activating or nonactivating effects of MAAs on downstream signaling factors, CREB for MSK1 and c-Fos for CREB. Western blot analysis of the phosphorylation patterns and protein levels demonstrated that MAAs at 10 and/or 25 μg/ml have a significant potential to stimulate CREB phosphorylation (Fig. 5L) and to increase c-Fos protein levels at 15 and 30 min and 2 h post-treatment, respectively, in HDFs (Fig. 5M).
Thus, our intracellular signaling analysis for the phosphorylation and protein levels of various signaling factors revealed that MAAs at 10 and/or 25 μg/ml initiate the activation at the front line of the stress-activated signaling cascade, including ERK and p38, which contribute sequentially to the activation of MSK1(Thr-581/Ser-360/Ser-376) via their downstream lineages. The activation of MSK1 results predominantly in the activation of CREB but not of NF-κB(Ser-276) in the MAA-activated signaling cascades. These findings indicate that the downstream lineage of MSK1 exists as a convergent point distinct from UVB-activated ones. Thus, it is interesting to note that whereas activated MSK1 elicits the increased phosphorylation of both CREB and NF-κB(Ser-276) in the UVB-activated signaling cascades (49), the stimulated phosphorylation of NF-κB at Ser-276 does not occur downstream of activated MSK1 in the MAA-activated signaling cascades. The phosphorylation of NF-κB at Ser-536 and IκBα at Ser-32/36, which simultaneously occurs downstream of IKK/Akt (49, 65), is known to be essentially involved in the disassociation of the complex consisting of IκBα and NF-κB, resulting in its subsequent degradation and translocation from the cytosol to the nucleus, respectively (65). Because the phosphorylation of NF-κB at Ser-536 and IκB at Ser-32/36 was not stimulated in MAA-treated HDFs and because MSK1 is known as a nuclear kinase, functioning only within nuclei (60), it is conceivable that the failure to activate the downstream lineage of MSK1 for NF-κB(Ser-276) in the MAA-activated signaling pathway is due to the lack of IκBα phosphorylation and the subsequent translocation of NF-κB from the cytosol to the nucleus. However, because induction of the c-Fos promoter is known to be mediated by CREB binding to CRE and c-Fos activator protein in UVB-exposed mammalian cells (62), it is likely that the increased protein level of c-Fos in MAA-treated HDFs is directly linked to the activation of CREB, which results directly from the activation of MSK1. The sum of the above findings indicates that treatment of HDFs with MAAs at 25 μg/ml significantly stimulates the signaling cascades of p38/MSK1/CREB/c-Fos and p38/ATF2, but it does not activate the ERK/c-Jun, IKK/IκB/NF-κB, or MSK1/NF-κB signaling cascades.
Effects of p38, JNK, and MEK inhibitors on MAA-increased gene expression of HAS2 and the stimulated secretion of HA in HDFs
Further studies to identify the activated signaling cascades specific for the increased gene expression level of HAS2 revealed that a p38 inhibitor (SB239063) significantly abrogated the MAA-elicited increase in HAS2 mRNA level in concert with an abrogating effect on the enhanced secretion of HA (Fig. 6, A and B). In contrast, inhibitors of JNK (inhibitor II) and NF-κB (JSH23) did not abolish the MAA-elicited increase in HAS2 mRNA level (Fig. 6C), which is consistent with the lack of any substantial activation of the JNK or NF-κB linkages observed in the MAA-activated signaling pathway. Moreover, a MEK inhibitor (U0126) did not abrogate the MAA-elicited increase in HAS2 mRNA levels (Fig. 6D), which strongly suggests there is no involvement of the ERK/c-Jun axis in the MAA-activated signaling cascade specifically attributable to the MAA-stimulated expression level of HAS mRNA, which is consistent with nonstimulated phosphorylation of c-Jun. Thus, the above detailed analysis of signaling pathways that lead to the stimulated expression level of HAS2 mRNA suggests the possible refinement that the MAA-stimulated expression level of HAS2 mRNA is mediated via either the p38/MSK1/CREB/c-Fos/AP-1 axis or the p38/ATF2 axis.
Figure 6.
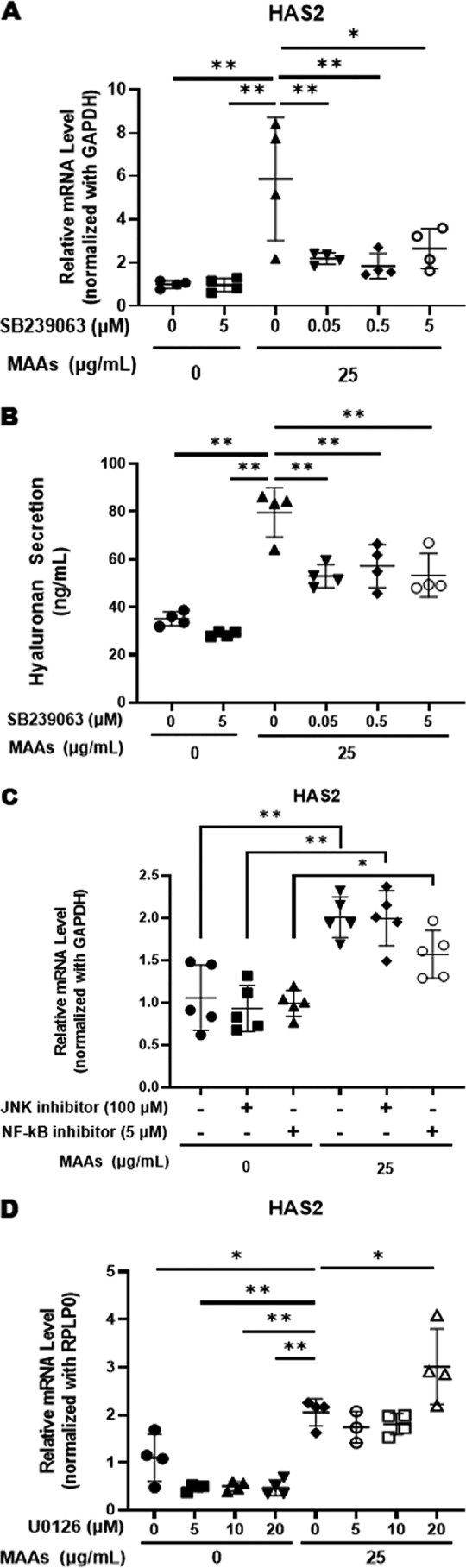
Effects of p38, JNK, NF-κB, and MEK inhibitors on the MAA-increased gene expressions of HAS2 and MAA-stimulated secretion of HA in HDFs. HDFs were incubated with MAAs at 25 μg/ml in the presence or absence of signaling inhibitors for 3, 6, or 72 h, and cell lysates or the conditioned medium was subjected to RT-PCR analysis or ELISA, respectively. A: p38 inhibitor/qRT-PCR, 6 h, data represent means ± S.D., n =; *, p < 0.05; **, p < 0.01, versus MAAs (0 or 25 μg/ml). B: p38 inhibitor/ELISA, 72 h, data represent means ± S.D., n = 4; **, p < 0.01 versus MAAs (0 or 25 μg/ml). C: NF-κB and JNK inhibitors/qRT-PCR, 6 h, data represent means ± S.D., n = 5; *, p < 0.05; **, p < 0.01. D: MEK inhibitor/qRT-PCR, 3 h, data represent means ± S.D., n = 4; *, p < 0.05; **, p < 0.01 versus MAAs 0 μg/ml or MEK inhibitor 0 μm.
Effects of transfecting an ATF2 siRNA or a c-Fos siRNA on the MAA-increased gene expression of HAS2 in HDFs
Although there has been no evidence indicating the involvement of AP-1 or ATF2 as transcription factors regulating HAS2 gene expression, we attempted to use mRNA silencing to identify the terminal point of the intracellular signaling pathway leading to the transcriptional process that increases levels of HAS2 mRNA. In elucidating which transcription factor, AP-1 or ATF2, might be essentially involved in the MAA-stimulated expression level of HAS2 mRNA, although transfection of ATF2 siRNA significantly reduced the level of ATF2 protein and abrogated the MAA-increased phosphorylation level of ATF2 (Fig. 7A), the ATF2 siRNA failed to abrogate the stimulated expression level of HAS2 mRNA by MAAs (Fig. 7C). While the transfection of a c-Fos siRNA significantly reduced the level of c-Fos protein and abrogated the MAA-increased level of c-Fos protein (Fig. 7B), the c-Fos siRNA significantly abolished the increased expression level of HAS2 mRNA by MAAs (Fig. 7C).
Figure 7.
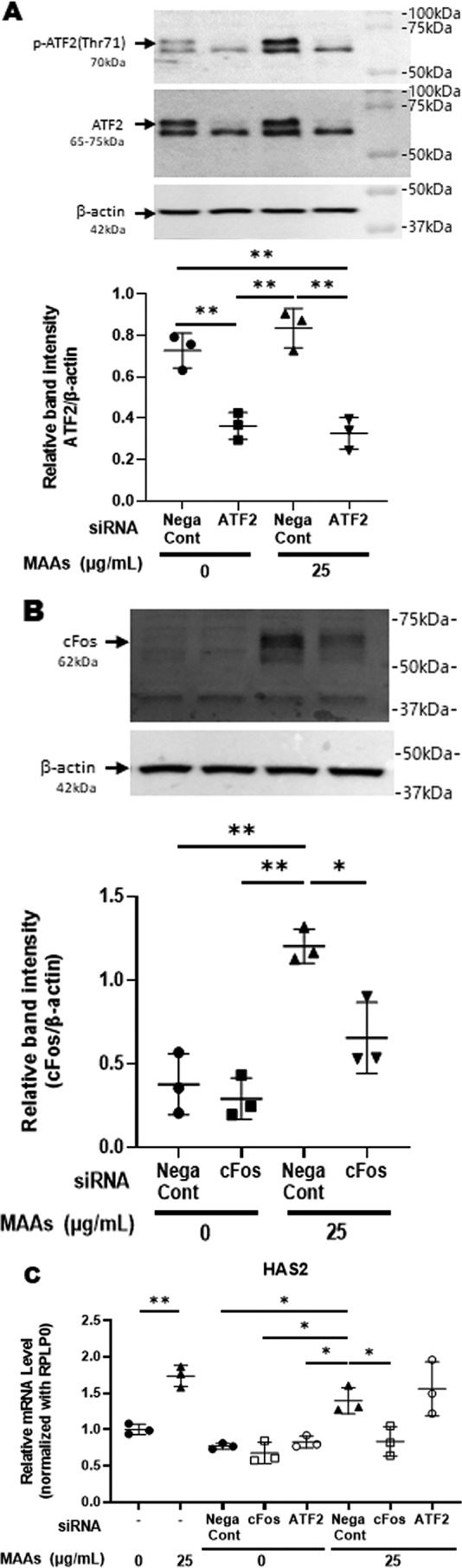
Effects of transfecting an ATF2 siRNA or a c-Fos siRNA. A: effect of transfecting an ATF2 siRNA on the expression of ATF2 protein and its phosphorylation. HDFs were incubated with or without MAAs at 25 μg/ml. Lysates were harvested at 15 min post-treatment and were immunoblotted with antibodies to β-actin and to phosphorylated or nonphosphorylated ATF2. Representative immunoblots from three independent experiments are shown. Data represent means ± S.D., n = 3; **, p < 0.01. B: effect of transfection of a c-Fos siRNA on the MAA-stimulated expressions of c-Fos protein. HDFs were incubated with or without MAAs at 25 μg/ml. Lysates were harvested at 2 h post-treatment and were immunoblotted with antibodies to β-actin and c-Fos. Representative immunoblots from three independent experiments are shown. Data represent means ± S.D., n = 3; *, p < 0.05; **, p < 0.01. C: effects of transfection of an ATF2 siRNA or a c-Fos siRNA on MAA-increased gene expression of HAS2 in HDFs. After transfection of an ATF2 siRNA or a c-Fos siRNA, HDFs were cultured in the presence or absence of MAAs at 25 μg/ml for 3 h and then were subjected to RT-PCR analysis for mRNA levels of HAS2. Data represent means ± S.D., n = 3; *, p < 0.05; **, p < 0.01.
Conclusion
As summarized schematically in Fig. 8, the sum of these findings strongly suggests that MAAs up-regulate the gene expression level of HAS2 via an activation of the intracellular signaling cascade consisting of the p38/MSK1/CREB/c-Fos/AP-1 axis that stimulates the production and secretion of HA by HDFs.
Figure 8.
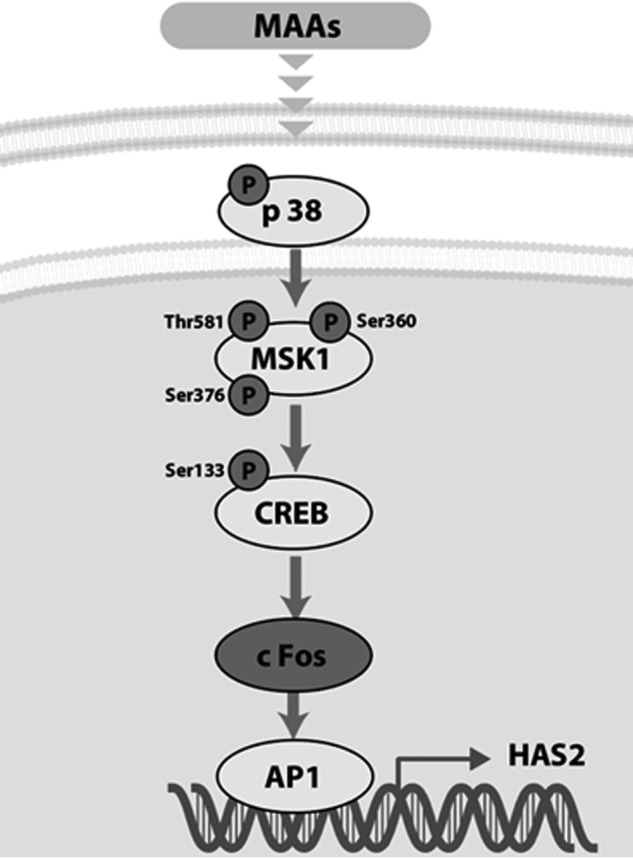
MAA-activated signaling cascades leading to the increased secretion of HA.
Experimental procedures
Materials
Antibodies to ERK, phospho-ERK, p38, phospho-p38, JNK, phospho-JNK, IκB, phospho-IκB, NFκBp65, phospho-Ser-276/Ser-536NFκBp65, CREB, phospho-Ser-133CREB, c-Fos, ATF2, phospho-Thr-71ATF2, MSK1, and phospho-Thr-581/Ser-376/Ser-360MSK1 were purchased from Cell Signaling Technology (Danvers, MA). Antibodies to β-actin and HYBID (anti-KIAA1199) were obtained from Sigma.
The NF-κB activation inhibitor II (JSH-23) and the MEK inhibitor (U0126) were from Calbiochem; the p38 inhibitor (SB239063) was from Santa Cruz Biotechnology, Inc. (Heidelberg, Germany), and the JNK inhibitor (JNK inhibitor II) was from Merck KGaA (Darmstadt, Germany).
Cell cultures
HDFs derived from human foreskins (Thermo Fisher Scientific, Waltham, MA) were cultivated in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) at 37 °C in a 95% air, 5% CO2 atmosphere.
Cell viability assay
Cell viability assays were performed using a cell proliferation kit I (MTT assay) (Roche Diagnostics) according to the manufacturer's instructions. HDFs were cultured for 72 h in the presence of MAAs, and their viability was evaluated using the MTT assay.
DNA synthesis
DNA synthesis was measured using a CytoSelectTM BrdU cell proliferation ELISA kit (Cell Biolabs, Inc., San Diego, CA).
Measurement of HA
HDFs were seeded in DMEM with FBS and were cultured for 12 h. After exchange with fresh DMEM without FBS, HA secreted into the culture medium was measured at the indicated times of culture using an HA assay kit (R&D Systems, Inc., Minneapolis, MN), which can detect HA with molecular mass ≥35 kDa according to the instructions of the manufacturer. Levels of HA are expressed as nanograms/ml.
Real-time qRT-PCR
After exchange with fresh DMEM without FBS, levels of mRNAs encoding HAS1, HAS2, HAS3, HYBID, collagen 1, and elastin in HDFs were measured at the indicated times of culture using real-time qRT-PCR. Total RNA was isolated from HDFs using a ReliaPrepTM RNA Miniprep System (Promega Corp., Madison, WI), followed by reverse transcription to cDNA using ReverTra Ace® qPCR RT Master Mix (Toyobo Co. Ltd., Osaka, Japan). Real-time PCRs were analyzed using SYBR Fast qPCR Mix (Takara Bio, Otsu, Shiga, Japan) and a Light Cycler 96 (Roche Diagnostics). The respective primers are shown in Table 1. The mRNA expression level was corrected by the expression level of GAPDH or Ribosomal Protein Lateral Stalk Subunit P0 (RPLP0).
Table 1.
Primers used in this study
| Primer name | Sequence | |
|---|---|---|
| GAPDH | Forward | 5′-GCACCGTCAAGGCGAGAAC-3′ |
| Reverse | 5′-TGGTGAAGACGCCAGTGGA-3′ | |
| KIAA1199 (HYBID) | Forward | 5′-CCAGGAATGTTGAATGTCT-3′ |
| Reverse | 5′-ATTGGCTCTTGGTGAATG-3′ | |
| HAS1 | Forward | 5′-ATCCTGCATCAGCGGTCCTC-3′ |
| Reverse | 5′-CTGGTTGTACCAGGCCTCAAGAA-3′ | |
| HAS2 | Forward | 5′-TCGCAACACGTAACGCAAT-3′ |
| Reverse | 5′-ACTTCTCTTTTTCCACCCCATTT-3′ | |
| HAS3 | Forward | 5′-AACAAGTACGACTCATGGATTTCCT-3′ |
| Reverse | 5′-GCCCGCTCCACGTTGA-3′ | |
| Elastin | Forward | 5′-AGGTGTATACCCAGGTGGCGTGCT-3′ |
| Reverse | 5′-CAACCCCTGTCCCTGTTGGGTAAC-3′ | |
| Collagen I | Forward | 5′-CTGGTCCCCAAGGCTTCCAAGGTC-3′ |
| Reverse | 5′-CCATCATTTCCACGAGCACCAGCA-3′ | |
| RPLPO | Forward | 5′-TTCGACAATGGCAGCATCTACAA-3′ |
| Reverse | 5′-CTGCAGACAGACACTGGCAACA-3′ | |
siRNA transfection
HDFs were plated at 6.0 × 104 in wells of 12-well plates and were cultured for 1 day in DMEM with 5% FBS. On day 1, HDFs were transfected with a control siRNA (MISSION® siRNA Universal Negative Control, Sigma), an ATF2 siRNA or a c-Fos siRNA (Mission siRNA, Sigma), and an HAS2 siRNA using Lipofectamine RNAiMAX transfection reagent (Invitrogen) following the manufacturer's protocol.
Western blotting
After exchange with fresh DMEM without FBS, HDFs were cultured for the indicated times and were then solubilized in lysis buffer (50 mm Tris, pH 7.6, 150 mm NaCl, and 1% Triton X-100) plus protease inhibitors (Complete mini-tablets; Roche Diagnostics, Mannheim, Germany) and were then centrifuged at 13,000 rpm for 15 min. The supernatants were harvested and lysed in 2× loading buffer (125 mm Tris, pH 6.8, 4.6% SDS, 20% glycerol and 0.04% pyronin Y). The mixture was then boiled for 5 min. Samples were solubilized in SDS sample buffer plus 50 mm DTT and boiled for 5 min. Total proteins from cultures of HDFs were subjected to Western blotting with antibodies to various phosphorylated or nonphosphorylated signaling factors and to β-actin as a loading control. Samples were separated on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, blocked with 5% nonfat dry milk in TBS (TBST), and probed with the above antibodies at room temperature for 1 h. Membranes were washed three times for 15 min each with TBST, probed with peroxidase-conjugated secondary antibodies (GE Healthcare), then washed three times for 30 min each in TBST, and developed by ECL (GE Healthcare). Detection was performed using an imaging system (WSE-6100 LuminoGraph I; ATTO Corp., Tokyo, Japan).
Statistics
All data are expressed as means ± S.D. as indicated in the figure legends. Student's t test was applied for pairwise comparisons. For multiple comparisons, data were tested by one-way ANOVA and were subsequently analyzed using the Tukey multiple comparison test. p values <0.05 are considered statistically significant.
Author contributions
S. T. data curation; S. T. and M. N. formal analysis; S. T., M. N., and G. I. investigation; S. T. methodology; S. T. and G. I. writing-original draft; M. N. and A. Y. resources; M. N. validation; A. Y. supervision; A. Y. funding acquisition; A. Y. project administration; G. I. conceptualization.
Supplementary Material
Acknowledgment
We sincerely acknowledge Dr. Vince Hearing for his critical reading of the manuscript.
The authors declare that they have no conflicts of interest with the contents of this article.
This article contains Figs. S1–S6, supporting Results, supporting Experimental procedures, and supporting Ref. 1.
- HA
- hyaluronan
- MAA
- mycosporine-like amino acid
- HDF
- human dermal fibroblast
- ELISA
- enzyme-linked immunosorbent assay
- qRT
- quantitative RT
- MTT
- 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide
- MAPK
- mitogen-activated protein kinase
- ERK
- extracellular signal-regulated kinase
- MEK
- MAPK/ERK kinase
- DMEM
- Dulbecco's modified Eagle's medium
- FBS
- fetal bovine serum
- CREB
- cAMP-responsive element-binding protein
- IL
- interleukin
- HAS
- HA synthase
- HYAL
- hyaluronidase
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase.
References
- 1. Manuskiatti W., and Maibach H. I.. 1996) Hyaluronic acid and skin: wound healing and aging. Int. J. Dermatol. 35, 539–544 10.1111/j.1365-4362.1996.tb03650.x [DOI] [PubMed] [Google Scholar]
- 2. Margelin D., Medaisko C., Lombard D., Picard J., and Fourtanier A.. 1996) Hyaluronic acid and dermatan sulfate are selectively stimulated by retinoic acid in irradiated and nonirradiated hairless mouse skin. J. Invest. Dermatol. 106, 505–509 10.1111/1523-1747.ep12343819 [DOI] [PubMed] [Google Scholar]
- 3. Takahashi Y., Ishikawa O., Okada K., Ohnishi K., and Miyachi Y.. 1995) Disaccharide analysis of the skin glycosaminoglycans in chronically ultraviolet light-irradiated hairless mice. J. Dermatol. Sci. 10, 139–144 10.1016/0923-1811(95)00396-A [DOI] [PubMed] [Google Scholar]
- 4. Fisher G. J., Datta S. C., Talwar H. S., Wang Z. Q., Varani J., Kang S., and Voorhees J. J.. 1996) Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 379, 335–339 10.1038/379335a0 [DOI] [PubMed] [Google Scholar]
- 5. Fisher G. J., Quan T., Purohit T., Shao Y., Cho M. K., He T., Varani J., Kang S., and Voorhees J. J.. 2009) Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 174, 101–114 10.2353/ajpath.2009.080599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsukahara K., Takema Y., Moriwaki S., Tsuji N., Suzuki Y., Fujimura T., and Imokawa G.. 2001) Selective inhibition of skin fibroblast elastase elicits a dose-dependent prevention of UVB-induced wrinkle formation. J. Invest. Dermatol. 117, 671–677 10.1046/j.0022-202x.2001.01450.x [DOI] [PubMed] [Google Scholar]
- 7. Tsuji N., Moriwaki S., Suzuki Y., Takema Y., and Imokawa G.. 2001) The role of elastases secreted by fibroblasts in wrinkle formation: implication through selective inhibition of elastase activity. Photochem. Photobiol. 74, 283–290 [DOI] [PubMed] [Google Scholar]
- 8. Tsukahara K., Nakagawa H., Moriwaki S., Kakuo S., Ohuchi A., Takema Y., and Imokawa G.. 2004) Ovariectomy is sufficient to accelerate spontaneous skin ageing and to stimulate ultraviolet irradiation-induced photoaging of murine skin. Br. J. Dermatol. 151, 984–994 10.1111/j.1365-2133.2004.06203.x [DOI] [PubMed] [Google Scholar]
- 9. Morisaki N., Moriwaki S., Sugiyama-Nakagiri Y., Haketa K., Takema Y., and Imokawa G.. 2010) Neprilysin is identical to skin fibroblast elastase–its role in skin ageing and UV responses. J. Biol. Chem. 285, 39819–39827 10.1074/jbc.M110.161547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakajima H., Ezaki Y., Nagai T., Yoshioka R., and Imokawa G.. 2012) Epithelial-mesenchymal interaction during UVB-induced up-regulation of neutral endopeptidase. Biochem. J. 443, 297–305 10.1042/BJ20111876 [DOI] [PubMed] [Google Scholar]
- 11. Imokawa G., and Ishida K.. 2015) Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging. I: reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 16, 7753–7775 10.3390/ijms16047753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Imokawa G., Nakajima H., and Ishida K.. 2015) Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging. II: over-expression of neprilysin plays an essential role. Int. J. Mol. Sci. 16, 7776–7795 10.3390/ijms16047776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imokawa G. 2016) Epithelial-mesenchymal interaction mechanisms leading to the over-expression of neprilysin are involved in the UVB-induced formation of wrinkles in the skin. Exp. Dermatol. 25, Suppl. 3, 2–13 10.1111/exd.13083 [DOI] [PubMed] [Google Scholar]
- 14. Akasaki S., and Imokawa G.. 2001) Mechanical methods for evaluating skin surface architecture in relation to wrinkling. J. Dermatol. Sci. 27, Suppl. 1, S5–S10 10.1016/s0923-1811(01)00115-3 [DOI] [PubMed] [Google Scholar]
- 15. Akazaki S., Nakagawa H., Kazama H., Osanai O., Kawai M., Takema Y., and Imokawa G.. 2002) Age-related changes in skin wrinkles assessed by a novel three-dimensional morphometric analysis. Br. J. Dermatol. 147, 689–695 10.1046/j.1365-2133.2002.04874.x [DOI] [PubMed] [Google Scholar]
- 16. Takema Y., Yorimoto Y., and Kawai M.. 1995) The relationship between age-related changes in the physical properties and development of wrinkles in human facial skin. J. Soc. Cosmet. Chem. 46, 163–173 [Google Scholar]
- 17. Yoshida H., Nagaoka A., Komiya A., Aoki M., Nakamura S., Morikawa T., Ohtsuki R., Sayo T., Okada Y., and Takahashi Y.. 2018) Reduction of hyaluronan and increased expression of HYBID (alias CEMIP and KIAA1199) correlate with clinical symptoms in photoaged skin. Br. J. Dermatol. 179, 136–144 10.1111/bjd.16335 [DOI] [PubMed] [Google Scholar]
- 18. Yoshida H., Komiya A., Ohtsuki R., Kusaka-Kikushima A., Sakai S., Kawabata K., Kobayashi M., Nakamura S., Nagaoka A., Sayo T., Okada Y., and Takahashi Y.. 2018) Relationship of hyaluronan and HYBID (KIAA1199) expression with roughness parameters of photoaged skin in Caucasian women. Skin Res. Technol. 24, 562–569 10.1111/srt.12467 [DOI] [PubMed] [Google Scholar]
- 19. Itano N., and Kimata K.. 1996) Molecular cloning of human hyaluronan synthase. Biochem. Biophys. Res. Commun. 222, 816–820 10.1006/bbrc.1996.0827 [DOI] [PubMed] [Google Scholar]
- 20. Stern R. 2004) Hyaluronan catabolism: a new metabolic pathway. Eur. J. Cell Biol. 83, 317–325 10.1078/0171-9335-00392 [DOI] [PubMed] [Google Scholar]
- 21. Termeer C., Benedix F., Sleeman J., Fieber C., Voith U., Ahrens T., Miyake K., Freudenberg M., Galanos C., and Simon J. C.. 2002) Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 195, 99–111 10.1084/jem.20001858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., and Noble P. W.. 2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 11, 1173–1179 10.1038/nm1315 [DOI] [PubMed] [Google Scholar]
- 23. Yoshida H., Nagaoka A., Kusaka-Kikushima A., Tobiishi M., Kawabata K., Sayo T., Sakai S., Sugiyama Y., Enomoto H., Okada Y., and Inoue S.. 2013) KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc. Natl. Acad. Sci. U.S.A. 110, 5612–5617 10.1073/pnas.1215432110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Libkind D., Pérez P., Sommaruga R., Diéguez Mdel C., Ferraro M., Brizzio S., Zagarese H., and van Broock M.. 2004) Constitutive and UV-inducible synthesis of photoprotective compounds (carotenoids and mycosporines) by freshwater yeasts. Photochem. Photobiol. Sci. 3, 281–286 10.1039/B310608J [DOI] [PubMed] [Google Scholar]
- 25. Portwich A., and Garcia-Pichel F.. 1999) Ultraviolet and osmotic stresses induce and regulate the synthesis of mycosporines in the cyanobacterium chlorogloeopsis PCC 6912. Arch. Microbiol. 172, 187–192 10.1007/s002030050759 [DOI] [PubMed] [Google Scholar]
- 26. Neale P. J., Banaszak A. T., and Jarriel C. R.. 1998) Ultraviolet sunscreens in Gymnodinium sanguineum (Dinophyceae): mycosporine-like amino acids protect against inhibition of photosynthesis. J. Phycol. 34, 928–938 10.1046/j.1529-8817.1998.340928.x [DOI] [Google Scholar]
- 27. Oren A., and Gunde-Cimerman N.. 2007) Mycosporines and mycosporine-like amino acids: UV protectants or multipurpose secondary metabolites? FEMS Microbiol. Lett. 269, 1–10 10.1111/j.1574-6968.2007.00650.x [DOI] [PubMed] [Google Scholar]
- 28. Cardozo K. H., Guaratini T., Barros M. P., Falcão V. R., Tonon A. P., Lopes N. P., Campos S., Torres M. A., Souza A. O., Colepicolo P., and Pinto E.. 2007) Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 146, 60–78 10.1016/j.cbpc.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 29. Wada N., Sakamoto T., and Matsugo S.. 2015) Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 4, 603–646 10.3390/antiox4030603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmid D., Schürch C., Zülli F., Nissen H.-P., and Prieur H.. 2003) Mycosporine-like amino acids: natural UV-screening compounds from red algae to protect the skin against photoaging. SÖFW-J. 129, 38–42 [Google Scholar]
- 31. Lawrence K. P., Gacesa R., Long P. F., and Young A. R.. 2018) Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 178, 1353–1363 10.1111/bjd.16125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim S., You D. H., Han T., and Choi E. M.. 2014) Modulation of viability and apoptosis of UVB-exposed human keratinocyte HaCaT cells by aqueous methanol extract of laver (Porphyra yezoensis). J. Photochem. Photobiol. B. 141, 301–307 10.1016/j.jphotobiol.2014.10.012 [DOI] [PubMed] [Google Scholar]
- 33. Suh S. S., Oh S. K., Lee S. G., Kim I. C., and Kim S.. 2017) Porphyra-334, a mycosporine-like amino acid, attenuates UV-induced apoptosis in HaCaT cells. Acta Pharm. 67, 257–264 10.1515/acph-2017-0015 [DOI] [PubMed] [Google Scholar]
- 34. Itano N., Sawai T., Yoshida M., Lenas P., Yamada Y., Imagawa M., Shinomura T., Hamaguchi M., Yoshida Y., Ohnuki Y., Miyauchi S., Spicer A. P., McDonald J. A., and Kimata K.. 1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 274, 25085–25092 10.1074/jbc.274.35.25085 [DOI] [PubMed] [Google Scholar]
- 35. Kondo T., Chichibu K., Usui H., Matsuura T., Shichijo S., and Yokoyama M.. 1991) Rapid assay of hyaluronic acid in serum. Jpn. J. Clin. Pathol. 39, 536–540 [PubMed] [Google Scholar]
- 36. Lim T. G., Jeon A. J., Yoon J. H., Song D., Kim J. E., Kwon J. Y., Kim J. R., Kang N. J., Park J. S., Yeom M. H., Oh D. K., Lim Y., Lee C. C., Lee C. Y., and Lee K. W.. 2015) 20-O-β-d-glucopyranosyl-20(S)-protopanaxadiol, a metabolite of ginsenoside Rb1, enhances the production of hyaluronic acid through the activation of ERK and Akt mediated by Src tyrosine kinase in human keratinocytes. Int. J. Mol. Med. 35, 1388–1394 10.3892/ijmm.2015.2121 [DOI] [PubMed] [Google Scholar]
- 37. Nagaoka A., Yoshida H., Nakamura S., Morikawa T., Kawabata K., Kobayashi M., Sakai S., Takahashi Y., Okada Y., and Inoue S.. 2015) Regulation of hyaluronan (HA) metabolism mediated by HYBID (hyaluronan-binding protein involved in HA depolymerization, KIAA1199) and HA synthases in growth factor-stimulated fibroblasts. J. Biol. Chem. 290, 30910–30923 10.1074/jbc.M115.673566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Aggarwal S., Kim S. W., Cheon K., Tabassam F. H., Yoon J. H., and Koo J. S.. 2006) Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol. Biol. Cell 17, 566–575 10.1091/mbc.e05-06-0519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rauhala L., Jokela T., Kärnä R., Bart G., Takabe P., Oikari S., Tammi M. I., Pasonen-Seppänen S., and Tammi R. H.. 2018) Extracellular ATP activates hyaluronan synthase 2 (HAS2) in epidermal keratinocytes via P2Y2, Ca2+ signaling, and MAPK pathways. Biochem. J. 475, 1755–1772 10.1042/BCJ20180054 [DOI] [PubMed] [Google Scholar]
- 40. Jokela T. A., Kärnä R., Makkonen K. M., Laitinen J. T., Tammi R. H., and Tammi M. I.. 2014) Extracellular UDP-glucose activates P2Y14 receptor and induces signal transducer and activator of transcription 3 (STAT3) Tyr-705 phosphorylation and binding to hyaluronan synthase 2 (HAS2) promoter, stimulating hyaluronan synthesis of keratinocytes. J. Biol. Chem. 289, 18569–18581 10.1074/jbc.M114.551804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qin J., Berdyshev E., Poirer C., Schwartz N. B., and Dawson G.. 2012) Neutral sphingomyelinase 2 deficiency increases hyaluronan synthesis by up-regulation of hyaluronan synthase 2 through decreased ceramide production and activation of Akt. J. Biol. Chem. 287, 13620–13632 10.1074/jbc.M111.304857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rauhala L., Hämäläinen L., Salonen P., Bart G., Tammi M., Pasonen-Seppänen S., and Tammi R.. 2013) Low dose ultraviolet B irradiation increases hyaluronan synthesis in epidermal keratinocytes via sequential induction of hyaluronan synthases Has1–3 mediated by p38 and Ca2+/calmodulin-dependent protein kinase II (CaMKII) signaling. J. Biol. Chem. 288, 17999–18012 10.1074/jbc.M113.472530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kultti A., Kärnä R., Rilla K., Nurminen P., Koli E., Makkonen K. M., Si J., Tammi M. I., and Tammi R. H.. 2010) Methyl-β-cyclodextrin suppresses hyaluronan synthesis by down-regulation of hyaluronan synthase 2 through inhibition of Akt. J. Biol. Chem. 285, 22901–22910 10.1074/jbc.M109.088435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Saavalainen K., Pasonen-Seppänen S., Dunlop T. W., Tammi R., Tammi M. I., and Carlberg C.. 2005) The human hyaluronan synthase 2 gene is a primary retinoic acid and epidermal growth factor responding gene. J. Biol. Chem. 280, 14636–14644 10.1074/jbc.M500206200 [DOI] [PubMed] [Google Scholar]
- 45. Monslow J., Williams J. D., Fraser D. J., Michael D. R., Foka P., Kift-Morgan A. P., Luo D. D., Fielding C. A., Craig K. J., Topley N., Jones S. A., Ramji D. P., and Bowen T.. 2006) Sp1 and Sp3 mediate constitutive transcription of the human hyaluronan synthase 2 gene. J. Biol. Chem. 281, 18043–18050 10.1074/jbc.M510467200 [DOI] [PubMed] [Google Scholar]
- 46. Monslow J., Williams J. D., Guy C. A., Price I. K., Craig K. J., Williams H. J., Williams N. M., Martin J., Coleman S. L., Topley N., Spicer A. P., Buckland P. R., Davies M., and Bowen T.. 2004) Identification and analysis of the promoter region of the human hyaluronan synthase 2 gene. J. Biol. Chem. 279, 20576–20581 10.1074/jbc.M312666200 [DOI] [PubMed] [Google Scholar]
- 47. Saavalainen K., Tammi M. I., Bowen T., Schmitz M. L., and Carlberg C.. 2007) Integration of the activation of the human hyaluronan synthase 2 gene promoter by common cofactors of the transcription factors retinoic acid receptor and nuclear factor κB. J. Biol. Chem. 282, 11530–11539 10.1074/jbc.M607871200 [DOI] [PubMed] [Google Scholar]
- 48. Makkonen K. M., Pasonen-Seppänen S., Törrönen K., Tammi M. I., and Carlberg C.. 2009) Regulation of the hyaluronan synthase 2 gene by convergence in cyclic AMP response element-binding protein and retinoid acid receptor signaling. J. Biol. Chem. 284, 18270–18281 10.1074/jbc.M109.012492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Terazawa S., Mori S., Nakajima H., Yasuda M., and Imokawa G.. 2015) The UVB-stimulated expression of transglutaminase 1 is mediated predominantly via the NFκB signaling pathway: new evidence of its significant attenuation through the specific interruption of the p38/MSK1/NFκBp65 Ser276 axis. PLoS ONE 10, e0136311 10.1371/journal.pone.0136311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Terazawa S., Nakajima H., Mori S., Niwano T., and Imokawa G.. 2012) Astaxanthin attenuates the UVB-induced secretion of prostaglandin E2 and interleukin-8 in human keratinocytes by interrupting MSK1 phosphorylation in a ROS depletion-independent manner. Exp. Dermatol. 21, Suppl. 1, 11–17 10.1111/j.1600-0625.2012.01496.x [DOI] [PubMed] [Google Scholar]
- 51. Tagashira H., Miyamoto A., Kitamura S., Tsubata M., Yamaguchi K., Takagaki K., and Imokawa G.. 2015) UVB stimulates the expression of endothelin B receptor in human melanocytes via a sequential activation of the p38/MSK1/CREB/MITF pathway which can be interrupted by a French maritime pine bark extract through a direct inactivation of MSK1. PLoS ONE 10, e0128678 10.1371/journal.pone.0128678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Deak M., Clifton A. D., Lucocq L. M., and Alessi D. R.. 1998) Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 17, 4426–4441 10.1093/emboj/17.15.4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McCoy C. E., Campbell D. G., Deak M., Bloomberg G. B., and Arthur J. S.. 2005) MSK1 activity is controlled by multiple phosphorylation sites. Biochem. J. 387, 507–517 10.1042/BJ20041501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Terazawa S., Nakajima H., Fukasawa K., and Imokawa G.. 2015) Withaferin A abolishes the stem cell factor-stimulated pigmentation of human epidermal equivalents by interrupting the auto-phosphorylation of c-KIT in human melanocytes. Arch. Dermatol. Res. 307, 73–88 10.1007/s00403-014-1518-y [DOI] [PubMed] [Google Scholar]
- 55. Kato T. Jr., Delhase M., Hoffmann A., and Karin M.. 2003) CK2 is a C-terminal IκB kinase responsible for NF-κB activation during the UV response. Mol. Cell 12, 829–839 10.1016/S1097-2765(03)00358-7 [DOI] [PubMed] [Google Scholar]
- 56. Gupta S., Campbell D., Dérijard B., and Davis R.. 1995) Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science 267, 389–393 10.1126/science.7824938 [DOI] [PubMed] [Google Scholar]
- 57. Wisdom R., Johnson R. S., and Moore C.. 1999) c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 18, 188–197 10.1093/emboj/18.1.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lopez-Bergami P., Huang C., Goydos J. S., Yip D., Bar-Eli M., Herlyn M., Smalley K. S., Mahale A., Eroshkin A., Aaronson S., and Ronai Z.. 2007) Re-wired ERK-JNK signaling pathways. Cancer Cell 11, 447–460 10.1016/j.ccr.2007.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Niwano T., Terazawa S., Nakajima H., and Imokawa G.. 2018) The stem cell factor-stimulated melanogenesis in human melanocytes can be abrogated by interrupting the phosphorylation of MSK1: Evidence for involvement of the p38/MSK1/CREB/MITF axis. Arch. Dermatol. Res. 310, 187–196 10.1007/s00403-018-1816-x [DOI] [PubMed] [Google Scholar]
- 60. Vermeulen L., De Wilde G., Van Damme P., Vanden Berghe W., and Haegeman G.. 2003) Transcriptional activation of the NF-kB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 22, 1313–1324 10.1093/emboj/cdg139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reber L., Vermeulen L., Haegeman G., and Frossard N.. 2009) Ser276 phosphorylation of NF-kB p65 by MSK1 controls SCF expression in inflammation. PLoS ONE 4, e4393 10.1371/journal.pone.0004393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gonzales M., and Bowden G. T.. 2002) Ultraviolet B (UVB) induction of the c-Fos promoter is mediated by phospho-cAMP response element binding protein (CREB) binding to CRE and c-Fos activator protein. Gene 293, 169–179 10.1016/S0378-1119(02)00723-0 [DOI] [PubMed] [Google Scholar]
- 63. Terazawa S., and Imokawa G.. 2018) Signaling cascades activated by UVB in human melanocytes lead to the increased expression of melanocyte-receptors, endothelin B receptor and c-KIT. Photochem. Photobiol. 94, 421–431 10.1111/php.12848 [DOI] [PubMed] [Google Scholar]
- 64. Mizutani Y., Hayashi N., Kawashima M., and Imokawa G.. 2010) A single UVB exposure increases the expression of functional KIT in human melanocytes by up-regulating MITF expression through the phosphorylation of p38/CREB. Arch. Dermatol. Res. 302, 283–294 10.1007/s00403-009-1007-x [DOI] [PubMed] [Google Scholar]
- 65. Sakurai H., Chiba H., Miyoshi H., Sugita T., and Toriumi W.. 1999) IκB kinases phosphorylate NF-κBp65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274, 30353–30356 10.1074/jbc.274.43.30353 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.